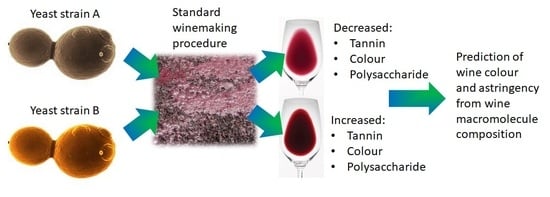
Commercial Saccharomyces cerevisiae Yeast Strains Significantly Impact Shiraz Tannin and Polysaccharide Composition with Implications for Wine Colour and Astringency
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Yeast Strains
2.3. Grape Samples and Winemaking Treatments
2.4. Model Fermentations with Added Tannin and Anthocyanin
2.5. Basic Must and Wine Compositional Analysis
2.6. Marc and Lees Extraction
2.7. Tannin Concentration and Colour Analysis
2.8. Tannin Composition
2.9. Polysaccharide Preparation and Hydrolysis
2.10. Monosaccharide Analysis
2.11. Wine Volatiles
2.12. Quantitative Descriptive Sensory Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Fermentation and Basic Wine Composition
3.2. Yeast-Derived Flavour Compound Production
3.3. Wine Tannin Composition and Colour Properties
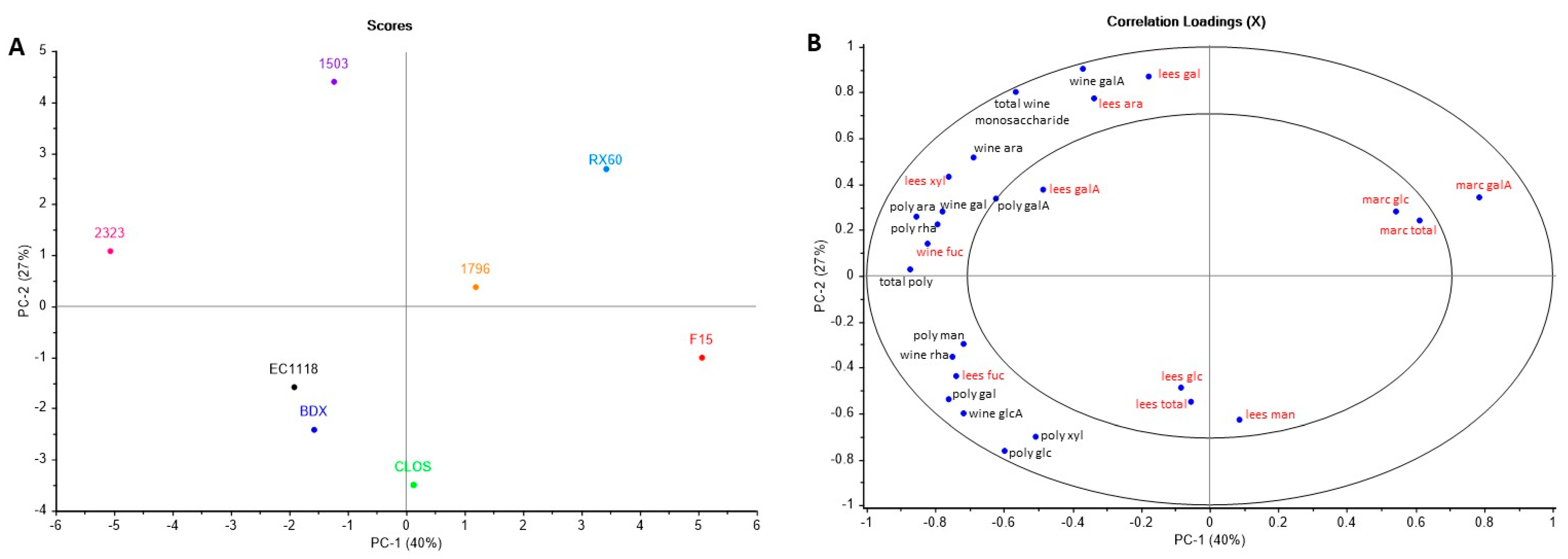
3.4. Wine Polysaccharide and Monosaccharide Composition
3.5. Model Fermentations
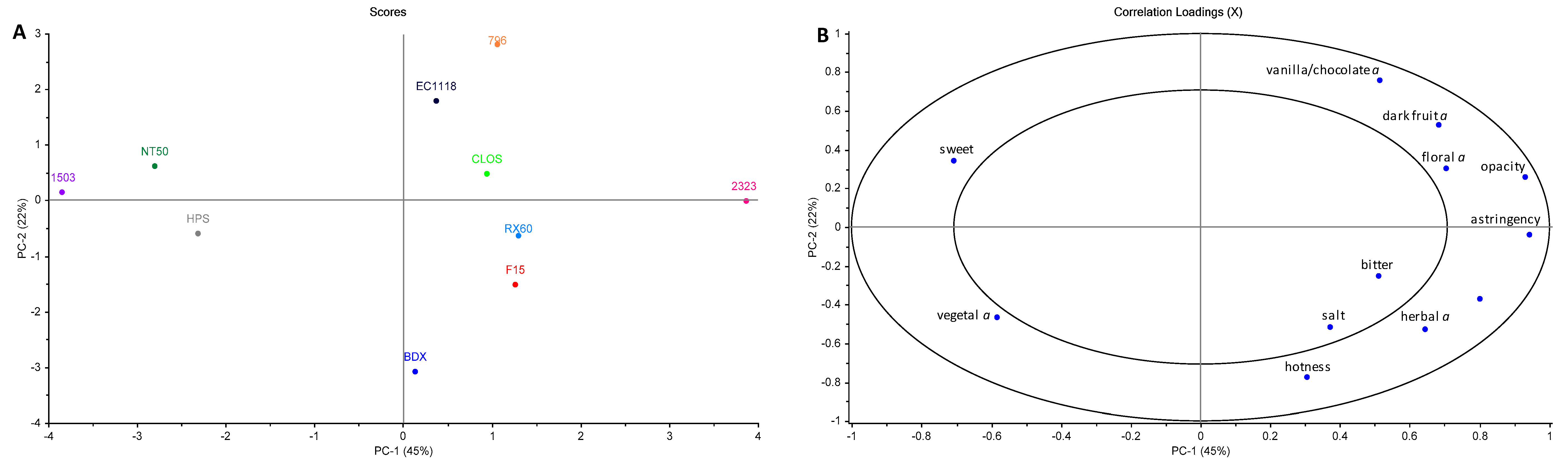
3.6. Wine Sensory Properties
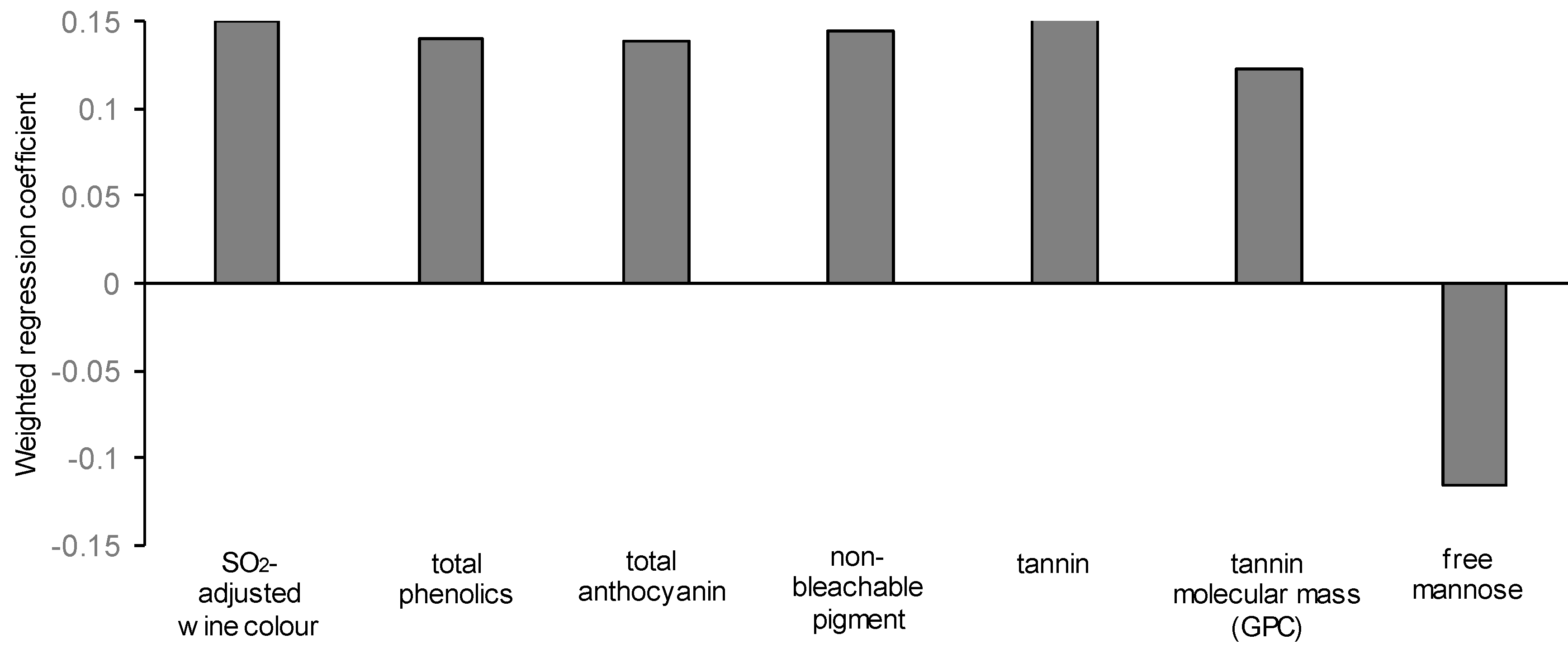
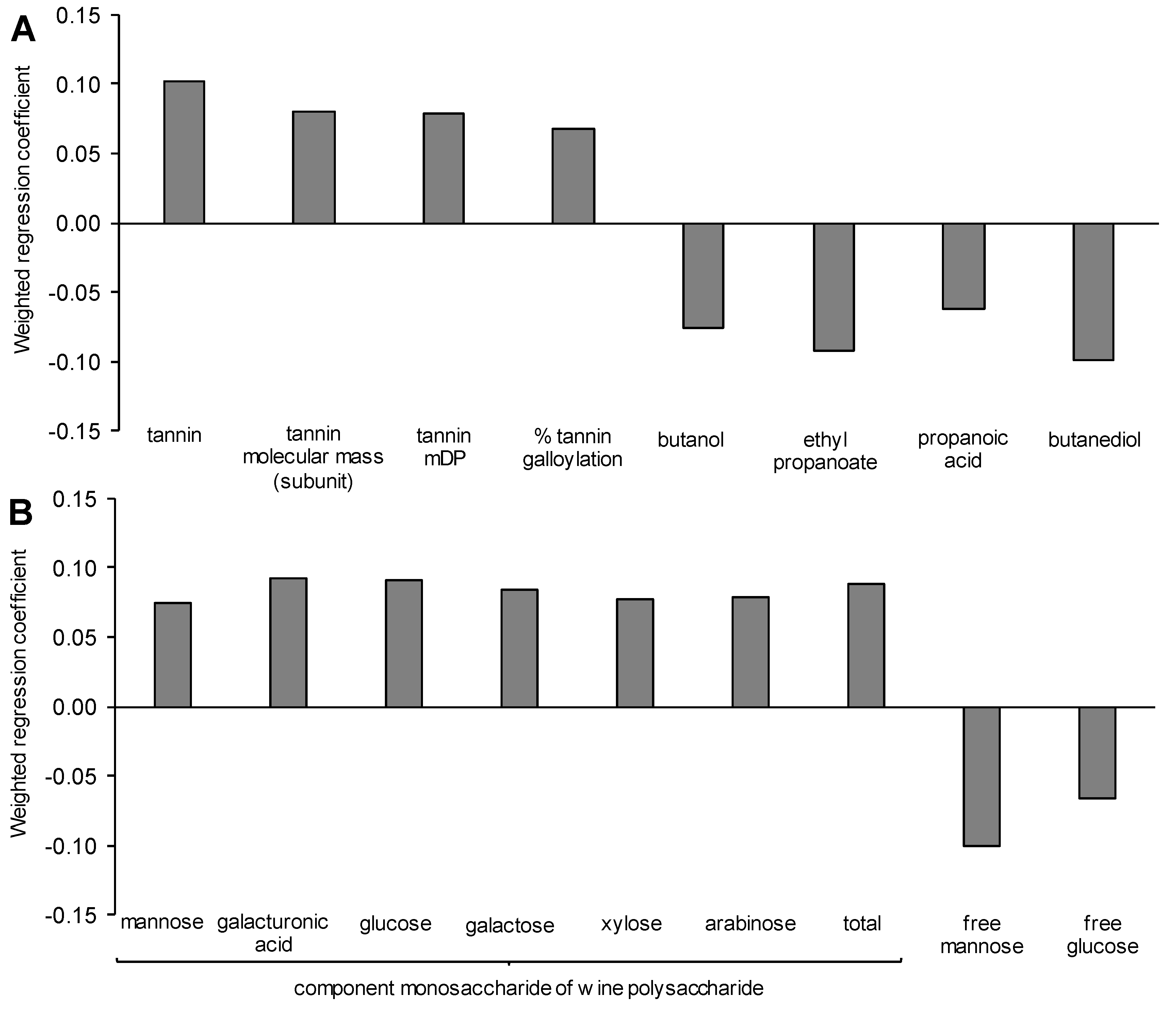
3.7. Modelling the Yeast-Derived Predictors of Wine Sensory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Hyma, K.E.; Saerens, S.M.; Verstrepen, K.J.; Fay, J.C. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Casey, G.P.; Ingledew, W.M.M. Ethanol tolerance in yeasts. CRC Crit. Rev. Microbiol. 1986, 13, 219–280. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Swiegers, J.H.; Travis, B.; Francis, I.L.; Bastian, S.E.P.; Pretorius, I.S. Coinoculated Fermentations Using Saccharomyces Yeasts Affect the Volatile Composition and Sensory Properties of Vitis Vinifera L. cv. Sauvignon Blanc Wines. J. Agric. Food Chem. 2008, 56, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L.; King, E.S.; Pretorius, I.S. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009, 26, 204–211. [Google Scholar] [CrossRef]
- Lattey, K.A.; Bramley, B.R.; Francis, I.L. Understanding consumer preferences of Shiraz and Cabernet Sauvignon wines. In Maximising the Value—Maximise Returns through Quality and Process Efficiency; Allen, M., Cameron, W., Francis, M., Goodman, K., Wall, G., Waters, E., Quarisa, J., Eds.; Australian Society of Oenology and Viticulture: Adelaide, Australia, 2007; pp. 7–10. [Google Scholar]
- Lattey, K.A.; Bramley, B.R.; Francis, I.L. Consumer acceptability, sensory properties and expert quality judgements of Australian Cabernet Sauvignon and Shiraz wines. Aust. J. Grape Wine Res. 2010, 16, 189–202. [Google Scholar] [CrossRef]
- Callejon, R.M.; Margulies, B.; Hirson, G.D.; Ebeler, S.E. Dynamic Changes in Volatile Compounds during Fermentation of Cabernet Sauvignon Grapes with and without Skins. Am. J. Enol. Vitic. 2012, 63, 301–312. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Influence of Yeast Strain, Canopy Management, and Site on the Volatile Composition and Sensory Attributes of Cabernet Sauvignon Wines from Western Australia. J. Agric. Food Chem. 2011, 59, 3273–3284. [Google Scholar] [CrossRef]
- Liang, H.Y.; Chen, J.Y.; Reeves, M.; Han, B.Z. Aromatic and sensorial profiles of young Cabernet Sauvignon wines fermented by different Chinese autochthonous Saccharomyces cerevisiae strains. Food Res. Int. 2013, 51, 855–865. [Google Scholar] [CrossRef]
- Blanco, P.; Miras-Avalos, J.M.; Pereira, E.; Fornos, D.; Orriols, I. Modulation of chemical and sensory characteristics of red wine from mencia by using indigenous Saccharomyces cerevisiae yeast strains. J. Int. Des Sci. De La Vigne Et Du Vin 2014, 48, 63–74. [Google Scholar] [CrossRef]
- Del Barrio-Galan, R.; Caceres-Mella, A.; Medel-Maraboli, M.; Pena-Neira, A. Effect of selected Saccharomyces cerevisiae yeast strains and different aging techniques on the polysaccharide and polyphenolic composition and sensorial characteristics of Cabernet Sauvignon red wines. J. Sci. Food Agric. 2015, 95, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Capece, A.; Serafino, V.; Romaniello, R.; Poeta, C. Biodiversity of wild strains of Saccharomyces cerevisiae as tool to complement and optimize wine quality. World J. Microbiol. Biotechnol. 2008, 24, 1797–1802. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Holt, H.; Cozzolino, D.; McCarthy, J.; Abrahamse, C.; Holt, S.; Solomon, M.; Smith, P.; Chambers, P.J.; Curtin, C. Influence of yeast strain on Shiraz wine quality indicators. Int. J. Food Microbiol. 2013, 165, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Blazquez Rojas, I.; Smith, P.; Bartowsky, E. Influence of choice of yeasts on volatile fermentation-derived compounds, colour and phenolics composition in Cabernet Sauvignon wine. World J. Microbiol. Biotechnol. 2012, 28, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast Effects on Pinot noir Wine Phenolics, Color, and Tannin Composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascues, E.; Marquina, D.; Santos, A. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Birse, M.; Eglinton, J.; Herderich, M. The effect of Saccharomyces cerevisiae and Saccharomyces bayanus yeast on colour properties and pigment profiles of a Cabernet Sauvignon red wine. Aust. J. Grape Wine Res. 2007, 13, 176–185. [Google Scholar] [CrossRef]
- Ristic, R.; Bindon, K.; Francis, L.I.; Herderich, M.J.; Iland, P.G. Flavonoids and C-13-norisoprenoids in Vitis vinifera L. cv. Shiraz: Relationships between grape and wine composition, wine colour and wine sensory properties. Aust. J. Grape Wine Res. 2010, 16, 369–388. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Cozzolino, D.; Herderich, M.J.; Smith, P.A. Relationship between Red Wine Grades and Phenolics. 1. Tannin and Total Phenolics Concentrations. J. Agric. Food Chem. 2010, 58, 12313–12319. [Google Scholar] [CrossRef] [PubMed]
- Kassara, S.; Kennedy, J.A. Relationship between Red Wine Grade and Phenolics. 2. Tannin Composition and Size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.P.; Salmon, J.M. Interactions between Yeast Lees and Wine Polyphenols during Simulation of Wine Aging: I. Analysis of Remnant Polyphenolic Compounds in the Resulting Wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Gomez-Cordoves, C.; Bartolome, B. Evaluation of different Saccharomyces cerevisiae strains for red winemaking. Influence on the anthocyanin, pyranoanthocyanin and non-anthocyanin phenolic content and colour characteristics of wines. Food Chem. 2007, 104, 814–823. [Google Scholar] [CrossRef]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.D.; Harbertson, J.F.; Osborne, J.P.; Freitag, M.; Lim, J.; Bakalinsky, A.T. Systematic Identification of Yeast Proteins Extracted into Model Wine during Aging on the Yeast Lees. J. Agric. Food Chem. 2010, 58, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Ayestarán, B. Effect of Commercial Mannoprotein Addition on Polysaccharide, Polyphenolic, and Color Composition in Red Wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Li, S.J.; Bindon, K.; Bastian, S.E.P.; Jiranek, V.; Wilkinson, K.L. Use of Winemaking Supplements to Modify the Composition and Sensory Properties of Shiraz Wine. J. Agric. Food Chem. 2017, 65, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramos, D.; Munoz, A.; Ortiz-Julien, A.; Palacios, A.; Heras, J.M.; Gonzalez, R. A Saccharomyces cerevisiae wine yeast strain overproducing mannoproteins selected through classical genetic methods. J. Int. Des Sci. De La Vigne Et Du Vin 2010, 44, 243–249. [Google Scholar] [CrossRef]
- Gonzalez-Royo, E.; Urtasun, A.; Gil, M.; Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Effect of Yeast Strain and Supplementation with Inactive Yeast during Alcoholic Fermentation on Wine Polysaccharides. Am. J. Enol. Vitic. 2013, 64, 268–273. [Google Scholar] [CrossRef]
- Alimardani-Theuil, P.; Gainvors-Claisse, A.; Duchiron, F. Yeasts: An attractive source of pectinases-From gene expression to potential applications: A review. Process Biochem. 2011, 46, 1525–1537. [Google Scholar] [CrossRef]
- Radoi, F.; Kishida, M.; Kawasaki, H. Polygalacturonase production in wine yeast strains and its carbon source regulation. Yeast 2003, 20, S318. [Google Scholar]
- Radoi, F.; Kishida, M.; Kawasaki, H. Endo-polygalacturonase in Saccharomyces wine yeasts: Effect of carbon source on enzyme production. FEMS Yeast Res. 2005, 5, 663–668. [Google Scholar] [CrossRef][Green Version]
- Radoi, F.; Kishida, M.; Kawasaki, H. Characteristics of wines made by Saccharomyces mutants which produce a polygalacturonase under wine-making conditions. Biosci. Biotechnol. Biochem. 2005, 69, 2224–2226. [Google Scholar] [CrossRef]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High Throughput Analysis of Red Wine and Grape Phenolics-Adaptation and Validation of Methyl Cellulose Precipitable Tannin Assay and Modified Somers Color Assay to a Rapid 96 Well Plate Format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Jeffery, D.W.; Mercurio, M.D.; Herderich, M.J.; Hayasaka, Y.; Smith, P.A. Rapid Isolation of Red Wine Polymeric Polyphenols by Solid-Phase Extraction. J. Agric. Food Chem. 2008, 56, 2571–2580. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 1. Effect on Proanthocyanidin Composition and Molecular Mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kennedy, J.A. Ripening-Induced Changes in Grape Skin Proanthocyanidins Modify Their Interaction with Cell Walls. J. Agric. Food Chem. 2011, 59, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Carew, A.L.; Mierczynska-Vasilev, A.; Kassara, S.; Kerslake, F.; Smith, P.A. Characterization of macromolecular complexes in red wine: Composition, molecular mass distribution and particle size. Food Chem. 2016, 199, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid-chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwohner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective Determination of Volatile Sulfur Compounds in Wine by Gas Chromatography with Sulfur Chemiluminescence Detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]
- Borneman, A.R.; Forgan, A.H.; Kolouchova, R.; Fraser, J.A.; Schmidt, S.A. Whole Genome Comparison Reveals High Levels of Inbreeding and Strain Redundancy Across the Spectrum of Commercial Wine Strains of Saccharomyces cerevisiae. G3-Genes Genomes Genet. 2016, 6, 957–971. [Google Scholar]
- Morata, A.; Gomez-Cordoves, M.C.; Suberviola, J.; Bartolome, B.; Colomo, B.; Suarez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Morata, A.; Gomez-Cordoves, M.C.; Colomo, B.; Suarez, J. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Vernhet, A.; Sieczkowski, N.; Brillouet, J.M. Interactions of Condensed Tannins with Saccharomyces cerevisiae Yeast Cells and Cell Walls: Tannin Location by Microscopy. J. Agric. Food Chem. 2015, 63, 7539–7545. [Google Scholar] [CrossRef] [PubMed]
- Doco, T.; Williams, P.; Cheynier, V. Effect of flash release and pectinolytic enzyme treatments on wine polysaccharide composition. J. Agric. Food Chem. 2007, 55, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Eschstruth, A.; Divol, B. Comparative characterization of endo-polygalacturonase (Pgu1) from Saccharomyces cerevisiae and Saccharomyces paradoxus under winemaking conditions. Appl. Microbiol. Biotechnol. 2011, 91, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Gainvors, A.; Frezier, V.; Lemaresquier, H.; Lequart, C.; Aigle, M.; Belarbi, A. Detection of polygalacturonase, pectin-lyase and pectin-esterase activities in a saccharomyces-cerevisiae strain. Yeast 1994, 10, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Louw, C.; La Grange, D.; Pretorius, I.S.; van Rensburg, P. The effect of polysaccharide-degrading wine yeast transformants on the efficiency of wine processing and wine flavour. J. Biotechnol. 2006, 125, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Divol, B.; van Rensburg, P. PGU1 gene natural deletion is responsible for the absence of endo-polygalacturonase activity in some wine strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2007, 7, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Ferrier, J.; Harbertson, J.F.; Gachons, C.P.D. Analysis of tannins in red wine using multiple methods: Correlation with perceived astringency. Am. J. Enol. Vitic. 2006, 57, 481–485. [Google Scholar]
- Mercurio, M.D.; Smith, P.A. Tannin Quantification in Red Grapes and Wine: Comparison of Polysaccharide- and Protein-Based Tannin Precipitation Techniques and Their Ability to Model Wine Astringency. J. Agric. Food Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef]
- Caceres-Mella, A.; Pena-Neira, A.; Narvaez-Bastias, J.; Jara-Campos, C.; Lopez-Solis, R.; Canals, J.M. Comparison of analytical methods for measuring proanthocyanidins in wines and their relationship with perceived astringency. Int. J. Food Sci. Technol. 2013, 48, 2588–2594. [Google Scholar] [CrossRef]
- Quijada-Morin, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailon, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, M.U.; Klionsky, D.J. Vacuolar localization of oligomeric α-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 20491–20498. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Saenz-Navajas, M.P.; Campo, E.; Herrero, P.; de la Fuente, A.; Fernandez-Zurbano, P. Sensory interactions between six common aroma vectors explain four main red wine aroma nuances. Food Chem. 2016, 199, 447–456. [Google Scholar] [CrossRef]
| Strain | Code | Strain | Supplier |
|---|---|---|---|
| Rhone 2323 | 2323 | Saccharomyces cerevisiae var cerevisiae | Lallemand |
| AWRI 1503 | 1503 | Saccharomyces cerevisiae × Saccharomyces kudriavzevii hybrid | AB Mauri |
| AWRI 796 | 796 | Saccharomyces cerevisiae var. cerevisiae | AB Mauri |
| Enoferm BDX | BDX | Saccharomyces cerevisiae var. cerevisiae | Lallemand |
| Lalvin CLOS | CLOS | Saccharomyces cerevisiae var. cerevisiae | Lallemand |
| Lalvin EC1118 | EC1118 | Saccharomyces cerevisiae var bayanus | Lallemand |
| Zymaflore F15 | F15 | Saccharomyces cerevisiae var. cerevisiae | Laffort |
| Anchor NT50 | NT50 | Saccharomyces cerevisiae hybrid | Oenobrands |
| Zymaflore RX60 | RX60 | Saccharomyces cerevisiae var cerevisiae | Laffort |
| Uvaferm HPS | HPS | Saccharomyces cerevisiae var cerevisiae | Lallemand |
| Basic Wine Composition | Yeast Strain | ANOVA p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2323 | 1503 | 1796 | BDX | CLOS | EC1118 | F15 | NT50 | RX60 | HPS | ||
| Alcohol (%) | 15.8 bc | 15.7 bcd | 15.2 e | 15.4 cde | 15.9 b | 15.9 b | 15.7 bcd | 15.3 de | 16.4 a | 15.2 e | 0.0006 |
| Residual sugar (g/L) | 0.45 b | 1.20 a | 0.48 b | 0.41 b | 0.51 b | 0.45 b | 0.51 b | 0.48 b | 0.38 b | 0.40 b | <0.05 |
| Glycerol (g/L) | 9.9 f | 12.6 a | 11.0 d | 12.3 ab | 10.3 ef | 10.6 de | 11.5 c | 12.2 b | 10.0 f | 9.2 g | <0.0001 |
| Malic acid (g/L) | 0.03 | 0.03 | 0.03 | 0.06 | 0.07 | 0.02 | 0.03 | 0.06 | 0.03 | 0.02 | ns |
| Succinic acid (g/L) | 1.01 e | 1.28 bc | 1.47 a | 1.47 a | 1.43 ab | 1.04 de | 1.32 abc | 1.19 cd | 1.07 de | 1.01 e | <0.0001 |
| Acetic acid (g/L) | 0.24 bc | 0.20 cd | 0.30 a | 0.21 bcd | 0.24 bc | 0.24 b | 0.29 a | 0.19 d | 0.33 a | 0.31 a | <0.0001 |
| Titratable acidity (g/L) | 5.97 | 6.00 | 5.87 | 6.37 | 6.07 | 6.03 | 5.80 | 6.27 | 5.87 | 6.03 | ns |
| pH | 3.57 abc | 3.48 cd | 3.65 ab | 3.54 bcd | 3.62 ab | 3.55 abcd | 3.64 ab | 3.46 d | 3.65 a | 3.45 d | 0.002 |
| Compositional measure of wine tannin or colour | Yeast Strain | ANOVA p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2323 | 1503 | 1796 | BDX | CLOS | EC1118 | F15 | NT50 | RX60 | HPS | ||
| Tannin | |||||||||||
| Tannin concentration (mg/L) | 1508 a | 906 d | 1120 bcd | 976 cd | 1361 ab | 1238 ab | 1273 ab | 902 d | 1175 bc | 1129 bcd | <0.01 |
| Molecular mass (g/mol, subunit) 1 | 1936 a | 1603 c | 1895 a | 1788 ab | 1883 a | 1759 abc | 1890 a | 1798 ab | 1712 bc | 1883 a | <0.05 |
| Molecular mass (g/mol, 50% elution by GPC) 2 | 1693 ab | 1553 bcd | 1575 bcd | 1536 cd | 1673 ab | 1730 a | 1631 abcd | 1501 d | 1614 abcd | 1655 abc | <0.05 |
| mDP (no units) 3 | 6.51 a | 5.41 c | 6.38 a | 6.02 ab | 6.33 a | 5.92 abc | 6.36 a | 6.06 ab | 5.76 bc | 6.33 a | <0.01 |
| Epigallocatechin (%) | 30.1 a | 24.0 c | 29.2 a | 28.9 a | 28.8 a | 28.0 ab | 27.2 abc | 29.6 a | 24.7 bc | 29.7 a | <0.05 |
| Galloylation (%) | 1.61 abc | 1.53 bc | 1.53 bc | 1.43 cd | 1.67 ab | 1.55 bc | 1.72 ab | 1.23 d | 1.80 a | 1.44 cd | <0.01 |
| Tannin mass conversion (%) 4 | 26.8 | 30.2 | 28.6 | 26.7 | 26.0 | 24.8 | 32.1 | 31.8 | 26.3 | 27.1 | ns |
| Colour 5 | |||||||||||
| Total anthocyanin (mg/L) | 338 a | 253 d | 321 ab | 284 cd | 307 abc | 289 bcd | 317 abc | 289 bcd | 284 bcd | 288 bcd | <0.05 |
| Wine colour density (SO2 corrected, A.U.) | 12.53 a | 9.91 d | 11.46 abc | 10.45 bcd | 11.4 abc | 11.44 abc | 11.80 ab | 11.20 ab | 10.94 bcd | 10.34 cd | <0.05 |
| Hue (no units) | 0.71 | 0.72 | 0.72 | 0.72 | 0.71 | 0.73 | 0.72 | 0.72 | 0.72 | 0.72 | ns |
| Non-bleachable pigment (A.U.) | 3.66 a | 2.87 cd | 3.12 bcd | 2.79 d | 3.15 bcd | 3.32 ab | 3.19 abc | 3.23 abc | 3.24 abc | 2.82 cd | <0.05 |
| Free and Soluble Polysaccharide-Associated Monosaccharide Composition | Yeast Strain | ANOVA p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2323 | 1503 | 1796 | BDX | CLOS | EC1118 | F15 | NT50 | RX60 | HPS | ||
| Polysaccharide | |||||||||||
| Total polysaccharide (mg/L) | 678 a | 486 cd | 576 b | 511 bcd | 430 cd | 474 cd | 427 d | 451 cd | 518 bc | 467 cd | <0.01 |
| Mannose (mg/L) | 140 a | 109 bc | 131 a | 127 ab | 93 c | 99 c | 97 c | 102 c | 103 c | 106 c | <0.01 |
| Rhamnose (mg/L) | 63.6 a | 48.4 c | 56.3 ab | 49.3 bc | 41.0 de | 44.8 cde | 38.8 e | 46.8 cd | 49.3 bc | 47.8 cd | <0.001 |
| Glucuronic acid (mg/L) | 14.2 a | 12.1 abc | 13.5 a | 11.9 abc | 10.1 c | 10.9 bc | 10.5 bc | 13.8 a | 12.3 ab | 12.5 ab | <0.05 |
| Galacturonic acid (mg/L) | 116 a | 58 bcd | 64 bcd | 56 cd | 51 cd | 69 bc | 46 d | 49 d | 75 b | 54 cd | <0.001 |
| Glucose (mg/L) | 45.0 ab | 25.8 c | 45.7 a | 35.7 abc | 33.4 bc | 31.9 c | 32.0 c | 29.6 c | 31.3 c | 28.8 c | <0.05 |
| Galactose (mg/L) | 129 a | 103 bc | 120 ab | 111 abc | 96 c | 100 c | 94 c | 99 c | 104 bc | 101 c | <0.05 |
| Xylose (mg/L) | 4.36 a | 2.51 bcd | 3.03 bc | 2.86 bcd | 2.22 cd | 2.79 bcd | 3.33 b | 2.72 bcd | 3.20 b | 2.10 d | <0.01 |
| Arabinose (mg/L) | 163 a | 124 bc | 140 b | 115 cd | 101 d | 112 cd | 102 d | 106 cd | 136 b | 112 cd | <0.001 |
| Fucose (mg/L) | 2.75 | 2.73 | 3.56 | 2.47 | 2.67 | 2.64 | 2.95 | 3.02 | 3.22 | 2.78 | ns |
| Free Monosaccharides1 | |||||||||||
| Mannose (mg/L) | 13.8 cde | 20.4 a | 16.8 bc | 16.7 bc | 17.0 b | 16.4 bcd | 13.6 de | 16.5 bc | 12.4 e | 17.4 b | <0.001 |
| Ribose (mg/L) | 19.6 abc | 21.1 ab | 24.1 a | 21.2 ab | 12.8 d | 14.5 cd | 18.5 bc | 23.9 a | 17.9 bc | 12.2 d | <0.001 |
| Rhamnose (mg/L) | 20.9 abcd | 17.2 bcde | 22.1 ab | 26.5 a | 14.1 e | 14.7 de | 17.1 bcde | 21.0 abc | 15.4 cde | 17.1 bcde | <0.01 |
| Galacturonic acid (mg/L) | 1161 a | 1055 b | 879 c | 633 d | 511 e | 874 c | 543 e | 842 c | 1135 ab | 875 c | <0.0001 |
| Galactose (mg/L) | 89.0 | 88.9 | 92.3 | 83.9 | 82.1 | 83.7 | 85.8 | 87.3 | 85.6 | 84.2 | ns |
| Xylose (mg/L) | 6.4 bcd | 7.3 abc | 6.3 cd | 5.6 d | 6.7 bcd | 8.1 ab | 8.6 a | 6.3 cd | 8.8 a | 5.3 d | <0.01 |
| Arabinose (mg/L) | 19.1 bc | 17.8 bc | 21.5 ab | 22.1 ab | 16.4 bcd | 17.1 bc | 12.7 cd | 27.0 a | 10.8 d | 17.6 bc | <0.001 |
| Fucose (mg/L) | 8.72 ab | 9.67 a | 8.34 ab | 8.63 ab | 7.52 bc | 8.12 b | 7.27 bc | 8.29 ab | 6.41 c | 7.99 b | <0.01 |
| Glucose (mg/L) | 39.0 f | 76.3 cd | 99.2 ab | 88.7 bc | 48.2 ef | 53.9 de | 58.9 a | 109.1 a | 0.0 g | 60.6 de | <0.0001 |
| Fructose (mg/L) | 372 b | 1118 a | 382 b | 325 b | 459 b | 452 b | 447 b | 366 b | 376 b | 343 b | <0.05 |
| Monosaccharide Composition of Insoluble Polysaccharides | Yeast Strain | ANOVA p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2323 | 1503 | 1796 | BDX | CLOS | EC1118 | F15 | RX60 | ||
| Marc skin cell wall composition 1 | |||||||||
| Total polysaccharide | 286 bcd | 266 def | 309 ab | 277 cde | 260 ef | 249 f | 314 a | 302 abc | <0.001 |
| Mannose | 27.2 b | 25.9 b | 37.2 a | 29.3 b | 28.5 b | 28.7 b | 29.8 b | 26.5 b | <0.05 |
| Rhamnose | 7.39 | 7.72 | 9.11 | 8.44 | 7.73 | 7.41 | 7.85 | 6.50 | ns |
| Glucuronic acid | 9.07 a | 5.75 bc | 8.51 a | 8.60 a | 4.50 c | 6.94 abc | 7.39 ab | 5.66 bc | <0.05 |
| Galacturonic acid | 38.2 c | 40.9 bc | 40.2 bc | 37.2 c | 35.6 c | 38.0 c | 50.3 a | 45.2 ab | <0.01 |
| Glucose | 140 ab | 128 b | 156 a | 138 ab | 124 bc | 104 c | 154 a | 152 a | <0.001 |
| Galactose | 20.4 | 18.3 | 18.5 | 17.7 | 18.5 | 19.4 | 20.0 | 20.8 | ns |
| Xylose | 16.4 | 15.7 | 14.7 | 15.3 | 15.4 | 16.8 | 17.5 | 17.7 | ns |
| Arabinose | 27.5 ab | 23.8 c | 24.7 bc | 23.1 c | 25.7 abc | 27.6 a | 27.0 ab | 26.8 ab | <0.05 |
| Lees composition | |||||||||
| Total polysaccharide | 205 a | 142 b | 210 a | 197 a | 194 a | 161 b | 192 a | 162 b | <0.0001 |
| Mannose | 77 bcd | 55 e | 93 a | 86 ab | 80 abc | 68 cde | 80 abcd | 65 de | <0.01 |
| Rhamnose | 2.58 | 2.31 | 2.51 | 2.44 | 2.20 | 2.47 | 2.06 | 2.19 | ns |
| Glucuronic acid | 1.87 abc | 1.99 ab | 1.69 bcd | 1.89 abc | 2.18 a | 1.57 cd | 1.52 cd | 1.32 d | <0.01 |
| Galacturonic acid | 3.42 ab | 4.18 a | 3.46 ab | 3.53 ab | 3.14 bc | 2.53 c | 2.63 c | 2.57 c | <0.01 |
| Glucose | 104 a | 60 d | 93 ab | 88 b | 92 b | 72 cd | 93 ab | 75 c | <0.0001 |
| Galactose | 6.98 | 8.01 | 6.85 | 6.33 | 6.34 | 5.97 | 6.28 | 6.85 | ns |
| Xylose | 1.72 ab | 1.83 a | 1.34 b | 1.44 b | 1.42 b | 1.59 ab | 0.71 c | 1.50 ab | <0.001 |
| Arabinose | 7.12 ab | 7.64 a | 7.19 ab | 6.66 bc | 6.28 bc | 6.10 c | 6.17 c | 6.73 abc | <0.05 |
| Fucose | 0.87 abc | 0.87 abc | 0.71 bcd | 0.91 ab | 0.91 ab | 1.03 a | 0.69 cd | 0.57d | <0.01 |
| Variables Included in PLS1 Model | Opacity PLS1 Model | Astringency PLS1 Model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC No | R2cal | R2val | RSMEcal | RSMEval | X (%) | Y (%) | PC No | R2cal | R2val | RSMEcal | RSMEval | X (%) | Y (%) | |
| All variables | 2 | 0.93 | 0.70 | 0.17 | 0.39 | 31 | 93 | 2 | 0.88 | 0.60 | 0.08 | 0.15 | 32 | 87 |
| Significant variables * | 1 | 0.91 | 0.89 | 0.20 | 0.23 | 66 | 91 | 1 | 0.82 | 0.76 | 0.10 | 0.12 | 37 | 82 |
| Significant variables * excluding pH and TA | 1 | 0.90 | 0.88 | 0.21 | 0.24 | 72 | 90 | 1 | 0.81 | 0.75 | 0.10 | 0.12 | 38 | 81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindon, K.A.; Kassara, S.; Solomon, M.; Bartel, C.; Smith, P.A.; Barker, A.; Curtin, C. Commercial Saccharomyces cerevisiae Yeast Strains Significantly Impact Shiraz Tannin and Polysaccharide Composition with Implications for Wine Colour and Astringency. Biomolecules 2019, 9, 466. https://doi.org/10.3390/biom9090466
Bindon KA, Kassara S, Solomon M, Bartel C, Smith PA, Barker A, Curtin C. Commercial Saccharomyces cerevisiae Yeast Strains Significantly Impact Shiraz Tannin and Polysaccharide Composition with Implications for Wine Colour and Astringency. Biomolecules. 2019; 9(9):466. https://doi.org/10.3390/biom9090466
Chicago/Turabian StyleBindon, Keren A., Stella Kassara, Mark Solomon, Caroline Bartel, Paul A. Smith, Alice Barker, and Chris Curtin. 2019. "Commercial Saccharomyces cerevisiae Yeast Strains Significantly Impact Shiraz Tannin and Polysaccharide Composition with Implications for Wine Colour and Astringency" Biomolecules 9, no. 9: 466. https://doi.org/10.3390/biom9090466
APA StyleBindon, K. A., Kassara, S., Solomon, M., Bartel, C., Smith, P. A., Barker, A., & Curtin, C. (2019). Commercial Saccharomyces cerevisiae Yeast Strains Significantly Impact Shiraz Tannin and Polysaccharide Composition with Implications for Wine Colour and Astringency. Biomolecules, 9(9), 466. https://doi.org/10.3390/biom9090466