The Molecular Mechanisms Underlying Zucchini-Induced Changes in the Host Adaptation of Cotton- and Cucumber-Type Aphis gossypii
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Insects and Plants
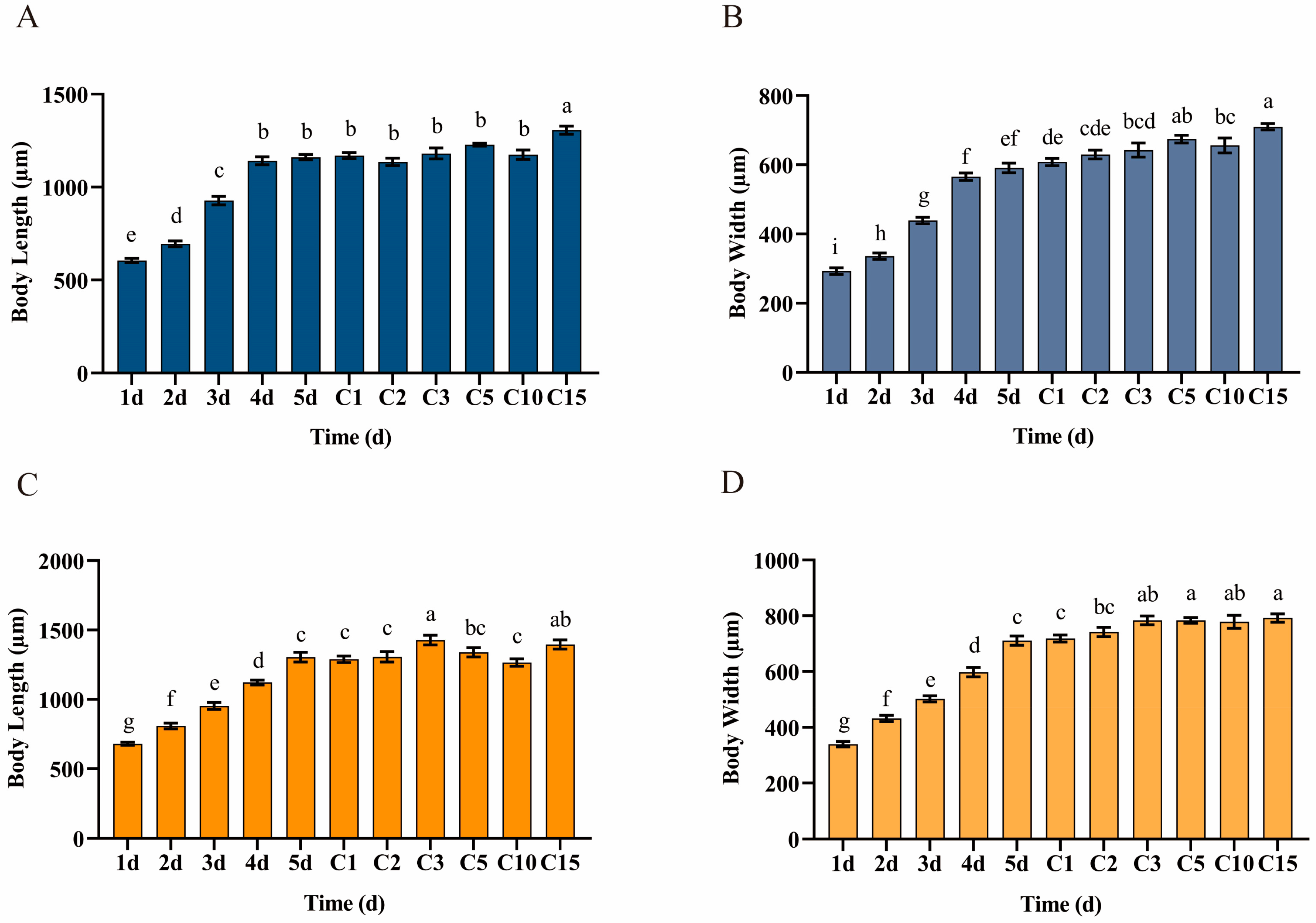
2.3. Changes in the Body Length and Width of Hap1 and Hap3 A. gossypii on Their Original Hosts and After Transfer to Zucchini
2.4. RNA-Seq Sample Preparation
2.5. Processing, Assembly, and Functional Annotation of Transcriptome Data
2.6. Statistical Analysis
3. Results
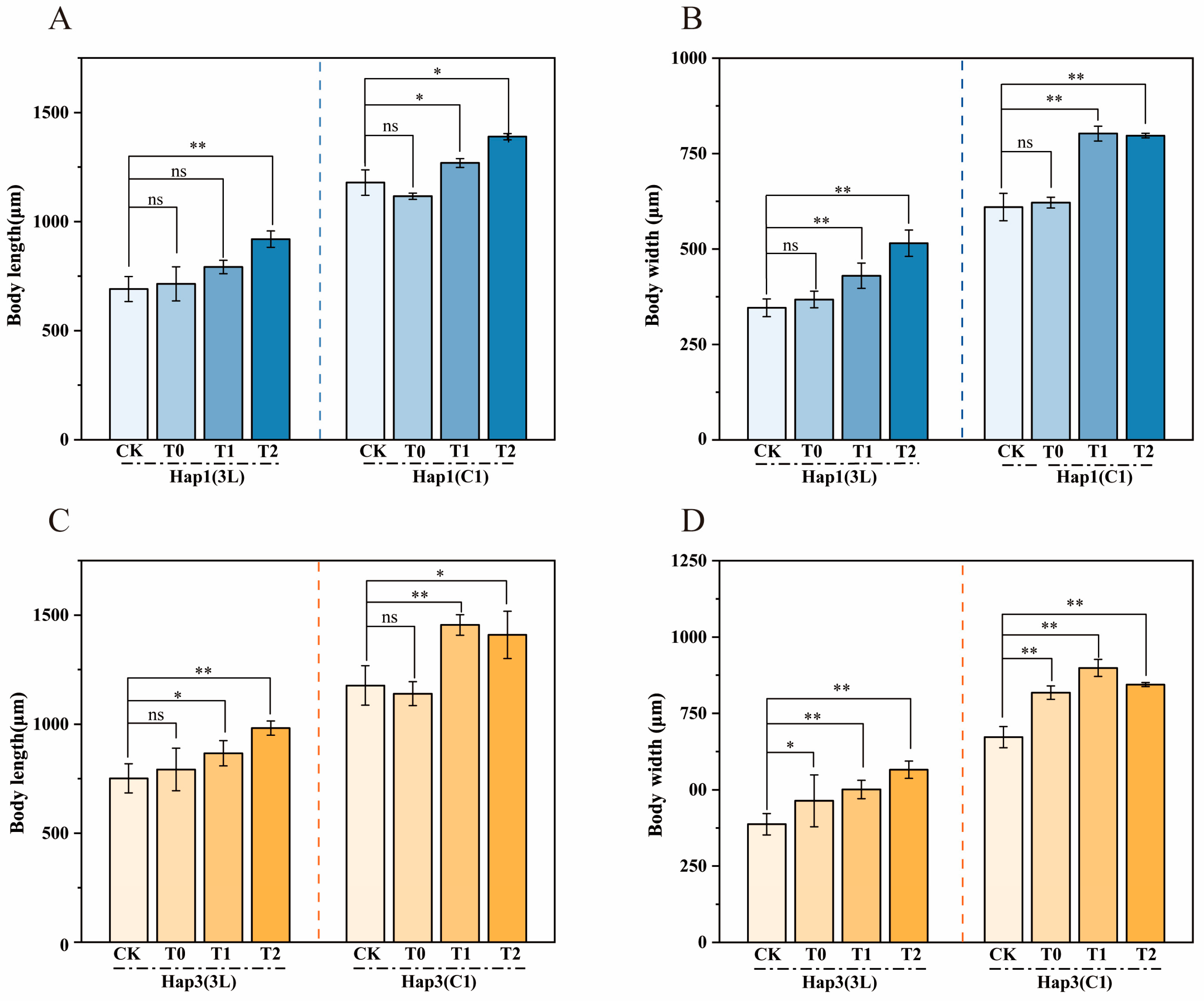
3.1. Changes in Body Length and Width
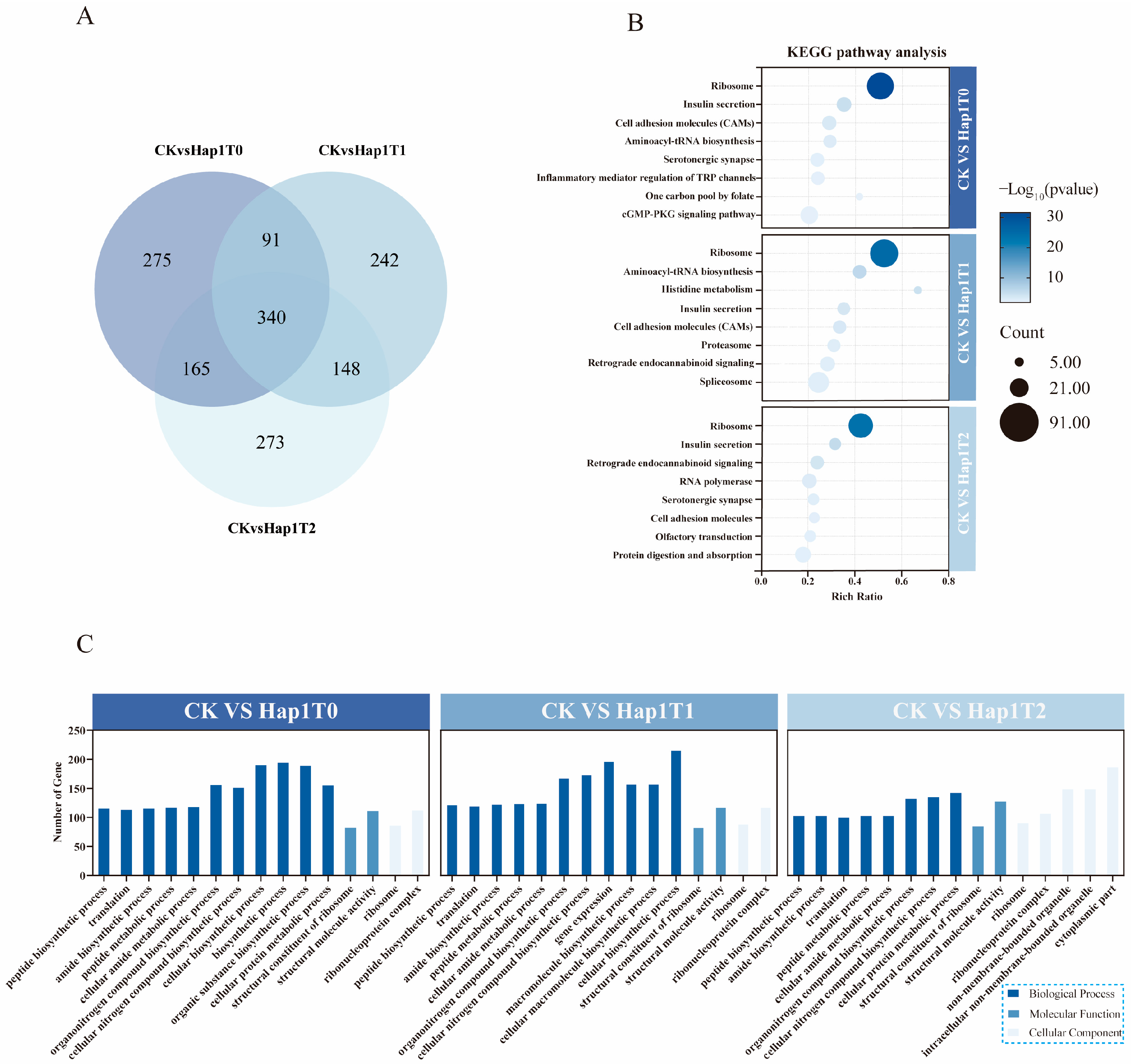
3.2. Transcriptome Analysis of the T0-T2 Generation of Hap1 A. gossypii Transferred to Zucchini
3.3. Transcriptome Analysis of the T0-T2 Generations of Hap3 A. gossypii Transfer to Zucchini
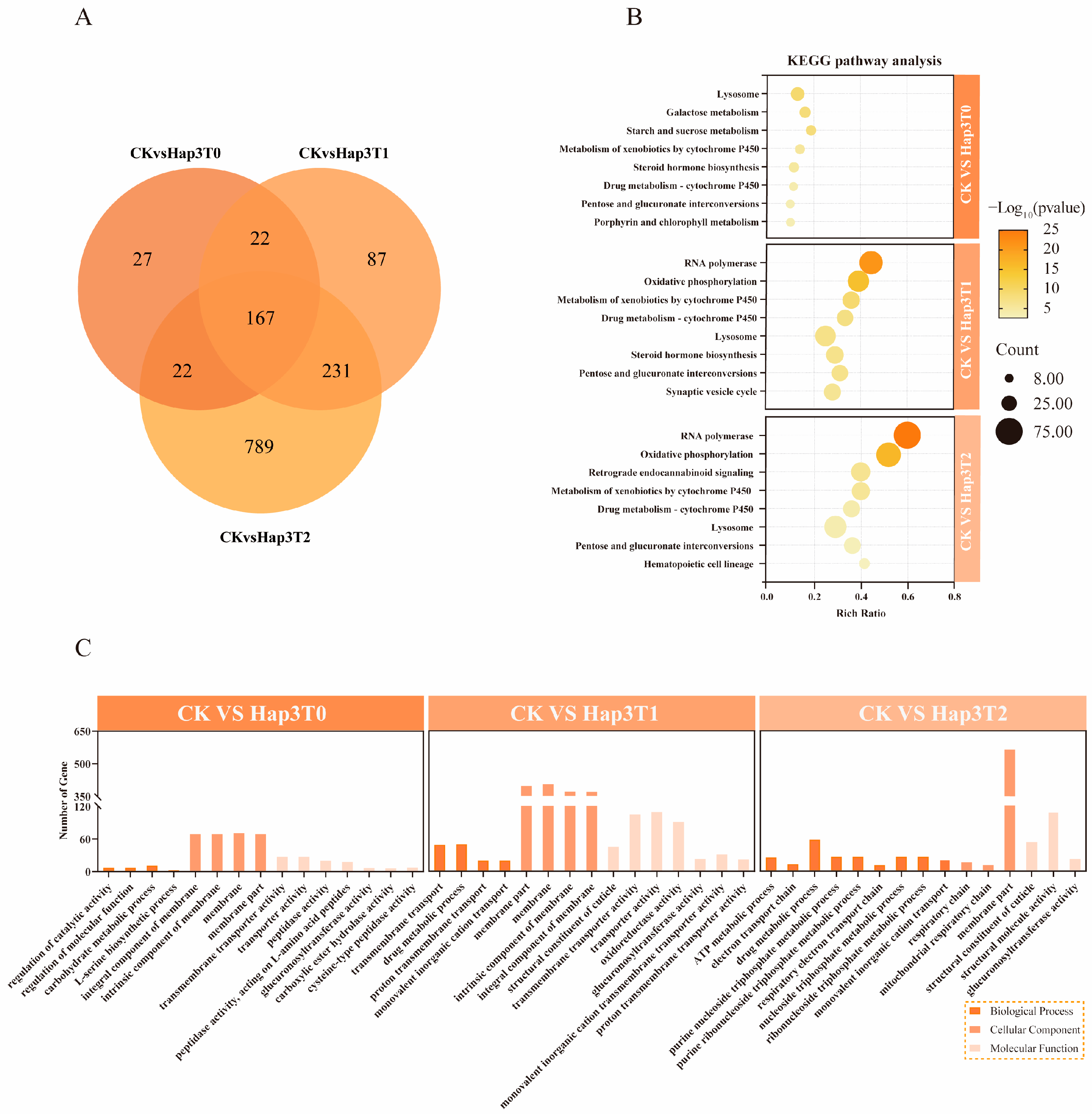
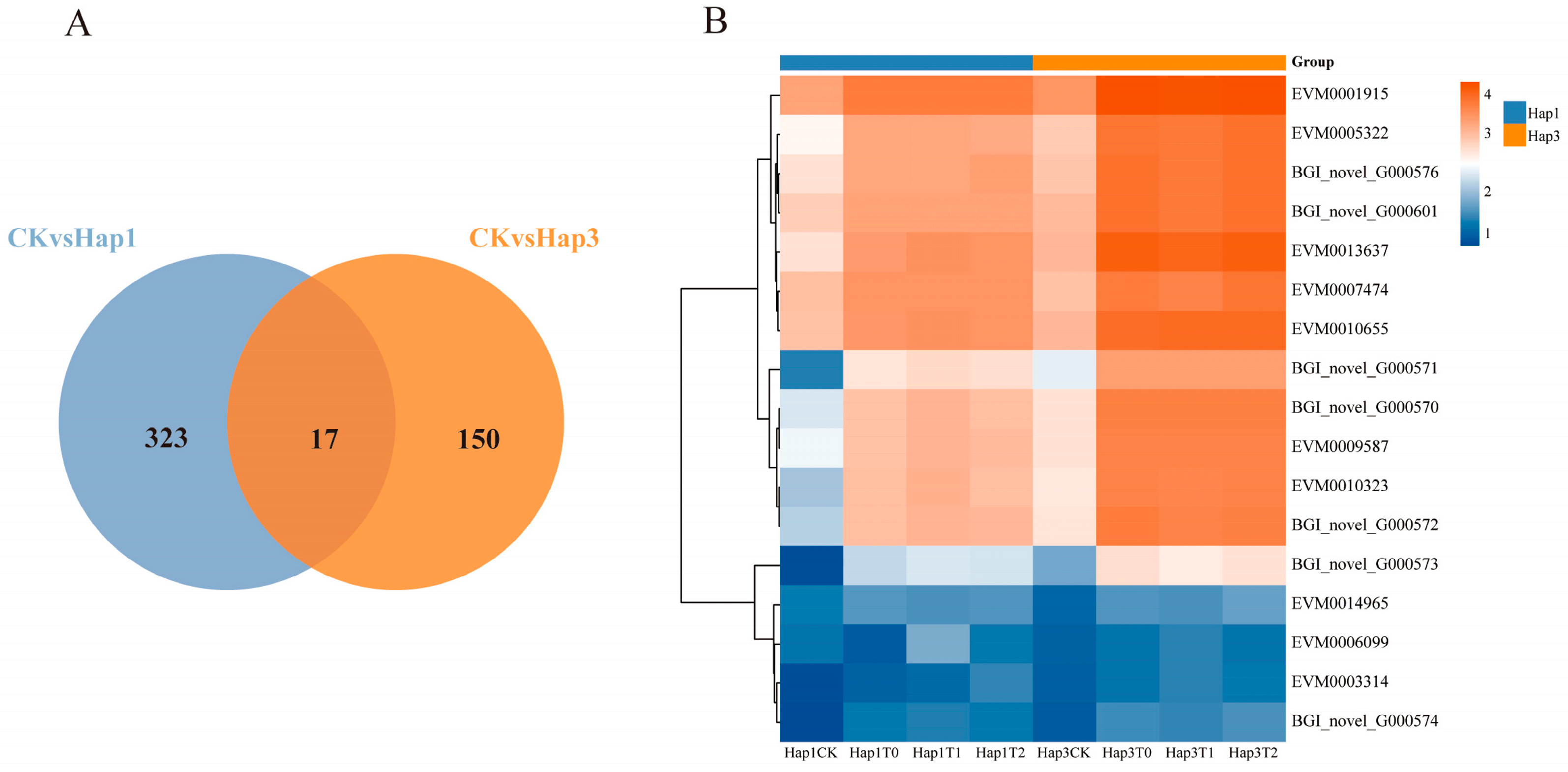
3.4. Analysis of Differentially Expressed Genes Shared Between Hap1 and Hap3 A. gossypii After Host Transfer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, T.J. Interplay between insects and plants: Dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte host preferences and host traits: Mechanisms for species-specific interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef]
- Liu, X.D.; Xu, T.T.; Lei, H.X. Refuges and host shift pathways of host-specialized aphids Aphis gossypii. Sci. Rep. 2017, 7, 2008. [Google Scholar] [CrossRef]
- Quan, Q.; Hu, X.; Pan, B.; Zeng, B.; Wu, N.; Fang, G.; Cao, Y.; Chen, X.; Li, X.; Huang, Y.; et al. Draft genome of the cotton aphid Aphis gossypii. Insect Biochem. Mol. Biol. 2019, 105, 25–32. [Google Scholar] [CrossRef]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. Comptes Rendus Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bang, H.; Yang, H.; Zhao, J.; Farhan, M.; Ma, X.; Zhang, S. Adaptability of Aphis gossypii glover to different Capsicum annuum varieties. Bull. Entomol. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Su, H.; Zhou, F.; Jing, T.; Yang, Y.; Zhang, S. Host adaptation of hap4 cotton aphid after feeding on zucchini. Plant Prot. 2023, 49, 222–229. [Google Scholar]
- Jing, H.; Fucai, Z.; Honghua, S.; Bin, Z.; Yidong, S.; Aimin, Y.; Xinning, Z.; Sen, H.; Haibo, Z.; Qiuxia, X. Population growth of Bemisia tabaci (gennadius) on different varieties of pepper. Chin. J. Appl. Entomol. 2017, 54, 629–638. [Google Scholar]
- Tian, P.P.; Chang, C.Y.; Miao, N.H.; Li, M.Y.; Liu, X.D. Infections with arsenophonus facultative endosymbionts alter performance of aphids (Aphis gossypii) on an amino-acid-deficient diet. Appl. Environ. Microbiol. 2019, 85, e01407-19. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, Y.; Wang, L.; Zhu, X.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Cui, J.; Luo, J.; et al. Regulation of amino acid metabolism in Aphis gossypii parasitized by binodoxys communis. Front. Nutr. 2022, 9, 1006253. [Google Scholar] [CrossRef]
- Dar, S.A.; Hasan, W.; Devi, Y.K.; Gajger, I.T.; John, J. Enzyme-mediated adaptation of herbivorous insects to host phytochemicals. Phytochem. Rev. 2024, 23, 1–24. [Google Scholar] [CrossRef]
- Eyres, I.; Jaquiéry, J.; Sugio, A.; Duvaux, L.; Gharbi, K.; Zhou, J.J.; Legeai, F.; Nelson, M.; Simon, J.C.; Smadja, C.M.; et al. Differential gene expression according to race and host plant in the pea aphid. Mol. Ecol. 2016, 25, 4197–4215. [Google Scholar] [CrossRef]
- Nouhaud, P.; Gautier, M.; Gouin, A.; Jaquiéry, J.; Peccoud, J.; Legeai, F.; Mieuzet, L.; Smadja, C.M.; Lemaitre, C.; Vitalis, R.; et al. Identifying genomic hotspots of differentiation and candidate genes involved in the adaptive divergence of pea aphid host races. Mol. Ecol. 2018, 27, 3287–3300. [Google Scholar] [CrossRef] [PubMed]
- Carolan, J.C.; Caragea, D.; Reardon, K.T.; Mutti, N.S.; Dittmer, N.; Pappan, K.; Cui, F.; Castaneto, M.; Poulain, J.; Dossat, C.; et al. Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): A dual transcriptomic/proteomic approach. J. Proteome Res. 2011, 10, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.J.; Hartson, S.D.; Puterka, G.J. Proteomic analysis of secreted saliva from russian wheat aphid (Diuraphis noxia kurd.) biotypes that differ in virulence to wheat. J. Proteom. 2012, 75, 2252–2268. [Google Scholar] [CrossRef]
- Agarwala, B.K.; Choudhury, P.R. Host races of the cotton aphid, Aphis gossypii, in asexual populations from wild plants of taro and brinjal. J. Insect Sci. 2013, 13, 34. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Luo, J.Y.; Wang, C.Y.; Lv, L.M.; Zhu, X.Z.; Li, C.H.; Cui, J.J. Identification of Aphis gossypii glover (Hemiptera: Aphididae) biotypes from different host plants in north china. PLoS ONE 2016, 11, e0146345. [Google Scholar] [CrossRef]
- Guildemond, J.A.; Tigges, W.T.; De Vrijer, P.W.F. Host races of Aphis gossypii (homoptera: Aphididae) on cucumber and chrysanthemum. Environ. Entomol. 1994, 23, 1235–1240. [Google Scholar] [CrossRef]
- Satar, S.; Kersting, U.; Yokomi, R. Presence of two host races of Aphis gossypii glover (homoptera: Aphididae) collected in turkey. Ann. Appl. Biol. 2013, 162, 41–49. [Google Scholar] [CrossRef]
- Wu, W.; Liang, X.L.; Zhao, H.Y.; Xu, T.T.; Liu, X.D. Special plant species determines diet breadth of phytophagous insects: A study on host plant expansion of the host-specialized Aphis gossypii glover. PLoS ONE 2013, 8, e60832. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. Rna-seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jankielsohn, A. Distribution and diversity of russian wheat aphid (homoptera: Aphididae) biotypes in south africa and lesotho. J. Econ. Entomol. 2011, 104, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thieme, T.; Kim, H. Complex evolution in Aphis gossypii group (homoptera: Aphididae), evidence of primary host shift and hybridization between sympatric species. PLoS ONE 2021, 16, e0245604. [Google Scholar]
- Nevo, E.; Coll, M. Effect of nitrogen fertilization on Aphis gossypii (homoptera: Aphididae): Variation in size, color, and reproduction. J. Econ. Entomol. 2001, 94, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, X.D. Adaptive changes in morph and preference induced by novel host plants mediate host specialization of the cotton-melon aphid. Arthropod Plant Interact. 2020, 14, 453–462. [Google Scholar] [CrossRef]
- Collar, D.C.; Schulte, J.A., 2nd; Losos, J.B. Evolution of extreme body size disparity in monitor lizards (Varanus). Evol. Int. J. Org. Evol. 2011, 65, 2664–2680. [Google Scholar] [CrossRef]
- Ruiz-Montoya, L.; Núñez-Farfán, J. Testing local host adaptation and phenotypic plasticity in a herbivore when alternative related host plants occur sympatrically. PLoS ONE 2013, 8, e79070. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhao, N.; Yang, X.; Chen, X.; Zhang, G. Ecological adaptation of Aphis gossypii (homoptera: Aphididae) to cotton developmental stage and temperature. J. Nanjing Agric. Univ. 2000, 23, 29–32. [Google Scholar]
- Mondor, E.B.; Tremblay, M.N.; Messing, R.H. Morphological and ecological traits promoting aphid colonization of the hawaiian islands. Biol. Invasions 2007, 9, 87–100. [Google Scholar] [CrossRef]
- Jiang, W.; Nasir, M.; Zhao, C. Variation of insulin-related peptides accompanying the differentiation of Aphis gossypii biotypes and their expression profiles. Ecol. Evol. 2023, 13, e10306. [Google Scholar] [CrossRef]
- Nässel, D.R.; Vanden Broeck, J. Insulin/igf signaling in drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Wang, X.P.; Jin, K.Y.; Dong, D.J.; Reiff, T.; Zhao, X.F. Insulin-like growth factor 2 promotes tissue-specific cell growth, proliferation and survival during development of Helicoverpa armigera. Cells 2022, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, L.E.; Figueroa, C.C.; Fuentes-Contreras, E.; Niemeyer, H.M.; Nespolo, R.F. Energetic costs of detoxification systems in herbivores feeding on chemically defended host plants: A correlational study in the grain aphid, Sitobion avenae. J. Exp. Biol. 2009, 212, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Bai-Zhong, Z.; Xu, S.; Cong-Ai, Z.; Liu-Yang, L.; Ya-She, L.; Xing, G.; Dong-Mei, C.; Zhang, P.; Ming-Wang, S.; Xi-Ling, C. Silencing of cytochrome P450 in Spodoptera frugiperda (lepidoptera: Noctuidae) by RNA interference enhances susceptibility to chlorantraniliprole. J. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef]
- Barracco, V.; Moschini, R.; Renzone, G.; Cappiello, M.; Balestri, F.; Scaloni, A.; Mura, U.; Del-Corso, A. Dehydrogenase/reductase activity of human carbonyl reductase 1 with nadp(h) acting as a prosthetic group. Biochem. Biophys. Res. Commun. 2020, 522, 259–263. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Liang, P.; Li, J.; Gao, X. UDP-glycosyltransferases from the UGT344 family are involved in sulfoxaflor resistance in Aphis gossypii glover. Insects 2021, 12, 356. [Google Scholar] [CrossRef]
- Bock, K.W. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem. Pharmacol. 2016, 99, 11–17. [Google Scholar] [CrossRef]
- Bansal, R.; Michel, A. Molecular adaptations of aphid biotypes in overcoming host-plant resistance. In Short Views on Insect Genomics and Proteomics, Vol. 1: Insect Genomics; Raman, C., Goldsmith, M.R., Agunbiade, T.A., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2015; Volume 3, pp. 75–93. [Google Scholar]
- Chiacchiera, F.; Rossi, A.; Jammula, S.; Zanotti, M.; Pasini, D. PRC2 preserves intestinal progenitors and restricts secretory lineage commitment. EMBO J. 2016, 35, 2301–2314. [Google Scholar] [CrossRef]
- Vitiello, A.; Molisso, D.; Digilio, M.C.; Giorgini, M.; Corrado, G.; Bruce, T.J.A.; D’Agostino, N.; Rao, R. Zucchini plants alter gene expression and emission of (e)-β-caryophyllene following Aphis gossypii infestation. Front. Plant Sci. 2020, 11, 592603. [Google Scholar] [CrossRef]
- Kriticos, D.J.; De Barro, P.J.; Yonow, T.; Ota, N.; Sutherst, R.W. The potential geographical distribution and phenology of Bemisia tabaci middle east/asia minor 1, considering irrigation and glasshouse production. Bull. Entomol. Res. 2020, 110, 567–576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Xu, W.; Wang, L.; Zhang, K.; Ji, J.; Li, D.; Zhu, X.; Gao, X.; Luo, J.; Cui, J. The Molecular Mechanisms Underlying Zucchini-Induced Changes in the Host Adaptation of Cotton- and Cucumber-Type Aphis gossypii. Biomolecules 2025, 15, 791. https://doi.org/10.3390/biom15060791
Pan Y, Xu W, Wang L, Zhang K, Ji J, Li D, Zhu X, Gao X, Luo J, Cui J. The Molecular Mechanisms Underlying Zucchini-Induced Changes in the Host Adaptation of Cotton- and Cucumber-Type Aphis gossypii. Biomolecules. 2025; 15(6):791. https://doi.org/10.3390/biom15060791
Chicago/Turabian StylePan, Yibin, Weili Xu, Li Wang, Kaixin Zhang, Jichao Ji, Dongyang Li, Xiangzhen Zhu, Xueke Gao, Junyu Luo, and Jinjie Cui. 2025. "The Molecular Mechanisms Underlying Zucchini-Induced Changes in the Host Adaptation of Cotton- and Cucumber-Type Aphis gossypii" Biomolecules 15, no. 6: 791. https://doi.org/10.3390/biom15060791
APA StylePan, Y., Xu, W., Wang, L., Zhang, K., Ji, J., Li, D., Zhu, X., Gao, X., Luo, J., & Cui, J. (2025). The Molecular Mechanisms Underlying Zucchini-Induced Changes in the Host Adaptation of Cotton- and Cucumber-Type Aphis gossypii. Biomolecules, 15(6), 791. https://doi.org/10.3390/biom15060791






