Abstract
Developmental and epileptic encephalopathies (DEEs) are a group of neuropediatric diseases associated with epileptic seizures, severe delay or regression of psychomotor development, and cognitive and behavioral deficits. What sets DEEs apart is their complex interplay of epilepsy and developmental delay, often driven by genetic factors. These two aspects influence one another but can develop independently, creating diagnostic and therapeutic challenges. Intellectual disability is severe and complicates potential treatment. Pathogenic variants are found in 30–50% of patients with DEE. Many genes mutated in DEEs encode ion channels, causing current conduction disruptions known as channelopathies. Although channelopathies indeed make up a significant proportion of DEE cases, many other mechanisms have been identified: impaired neurogenesis, metabolic disorders, disruption of dendrite and axon growth, maintenance and synapse formation abnormalities —synaptopathies. Here, we review recent publications on non-channelopathies in DEE with an emphasis on the mechanisms linking epileptiform activity with intellectual disability. We focus on three major mechanisms of intellectual disability in DEE and describe several recently identified genes involved in the pathogenesis of DEE.
1. Introduction
About 40% of epileptic seizures in the first years of life are caused by developmental and epileptic encephalopathy (DEE) [1,2]. DEEs are a group of diseases characterized by epileptic seizures or epileptiform activity, a severe delay or regression of psychomotor development, and cognitive and behavioral deficits [3]. Seizures and developmental delay in DEEs have a common, usually genetic, etiology and affect each other but progress independently. Often, onset of epilepsy is so early that it is impossible to determine the underlying cause. Thus, the consequences for neurodevelopment in DEEs are associated with a combination of the direct effects of the genetic variant and the impact of epileptiform activity, both of which can contribute to pathogenesis to varying degrees [4,5,6,7].
Both seizures and the progression of cognitive impairment cause severe consequences, compromising the quality of life and burdening families with financial and emotional difficulties. Depending on the DEE variant, the mortality before the age of 20 can reach 25% in some syndromes, and the remaining patients suffer from mental, behavioral, and movement disorders [8,9,10]. The situation is aggravated by the limited efficiency of existing drug therapy in controlling seizures and improving the neurological condition [9]. In our days, early diagnosis is key in managing the outcome of DEE—timely detection of the disease and its etiology directly correlates with a more favorable treatment outcome and long-term prognosis [5].
Pathogenic variants are found in 30–50% of patients with DEE [11,12,13]. Next-generation sequencing technologies have significantly accelerated the identification of genetic alterations in DEE patients [14]. The identification and characterization of such variants provide insights into the molecular mechanisms of the disease. The dissection of the underlying mechanisms can provide the basis for personalized therapies that will not only alleviate the severity of attacks but also improve the cognitive outcome of affected children [15]. Equally important is to understand the genetic etiology and dissect genotype–phenotype correlations in order to facilitate diagnosis and counseling of patient families [16,17].
The literature on DEE in the last decade has focused mainly on channelopathies. Although channelopathies indeed make up a significant proportion of DEE cases with pathogenic gene variants, many others associated with DEE have been identified: these variants cause disruption of different aspects of the brain development and function such as metabolism, progenitor proliferation, neuronal migration, dendrite and axon formation and synaptogenesis. The vast majority of reviews on DEE are devoted to epilepsy and encephalopathy in DEE and often do not discuss mechanisms of cognitive impairment. Intellectual disability, however, is no less severe for patients and complicates potential treatment. Therefore, it is important to consider the mechanisms of seizures and retardation in conjunction with each other. The goal of this review is to assess recent publications on non-channelopathies in DEE with an emphasis on the mechanisms linking epilepsy with intellectual disability.
2. Pathogenesis of Developmental Delay and Intellectual Disability in Developmental and Epileptic Encephalopathy
Developmental and epileptic encephalopathies (DEEs) are characterized by associated neurological pathologies such as developmental delay and intellectual disability. Cognitive deficits in DEE are commonly diagnosed in infancy or early childhood [18]. Cognitive impairment in DEE is a consequence of both the underlying encephalopathy and the accompanying seizures or epileptiform activity detectable on EEG [4,5]. Prolonged neuronal hyperexcitation during seizures, regardless of the pathway, contributes to cognitive decline [19,20,21].
Normally, cognitive functions depend on the coordinated work of neural networks that ensure the effective transfer of information between different brain regions. In epileptic encephalopathies, seizures and epileptiform activity lead to chaotic discharges that disrupt this coordination and destroy the functional connections between neurons [22]. As a result of this process, the integration of sensory information, executive control, and memory is disrupted. This subsequently impairs the development of cognitive abilities. Chronic disruptions in neural synchrony exacerbate developmental delay and contribute to the formation of persistent intellectual disability [23].
Pathogenic gene variants are the main cause of intellectual disability and developmental delay in most cases. Many of these variants are associated with dysfunction of voltage-gated ion channels. Since ion channels affect the generation, propagation, and control of action potentials, such changes often also lead to epileptic activity [6,24]. Ion balance disruption causes hyperexcitation of neurons leading to a distortion of neural function and subsequently cognitive impairment [25,26,27,28]. Most DEEs are characterized by early onset. The nervous system is most vulnerable to abnormal electrical activity during early development. Therefore, ion channel dysfunctions contribute to neuronal damage or death, further exacerbating cognitive and developmental deficits [21].
In addition, some studies describe the disruptions in synaptic plasticity due to pathogenic gene variants [29]. Indeed, neuronal plasticity is a key mechanism underlying higher cognitive abilities such as memory and learning [30]. In epileptic encephalopathies, changes in the expression of genes regulating synaptic plasticity can exacerbate cognitive deficits, since neurons lose the ability to adapt to new conditions or form new connections [31]. This further highlights the complexity and multifactoriality of cognitive impairment in DEE, since ion channels, synaptic plasticity, and neural networks in general can be simultaneously involved in the DEE pathogenesis [32].
Metabolic disturbances in neurons cause severe consequences due to excessive accumulation of metabolic products, disruption of energy metabolism, and decreased inhibition. Energy imbalance and accumulated metabolites disrupt signaling between cells, contribute to neuroinflammation, or even lead to neuronal death. Epileptic activity disrupts metabolism in the focal seizure area, as well as in the neighboring regions [33].
Increased excitability can impair neuronal migration during development. It was shown that temporary activation of migrating projection neurons (PNs) in the developing cerebral cortex causes changes in metabotropic glutamate receptors transcription, premature dendritic branching, and retention of neurons in deeper cortical layers [6,34]. On the other hand, hyperpolarization of neuronal progenitors in the ventricular zone of the mouse neocortex induced changes in transcription and cell division characteristics at later stages of development: they acquired unusual morphological and molecular features. On the other hand, intermediate progenitors expressing transcription factor Tbr2 were formed prematurely. All this indicates that changes in bioelectrical activity during neurogenesis can disrupt temporal programs of neuronal differentiation, causing abnormal neuronal function [35].
Epileptic seizures and epileptiform activity damage neural networks, which are the main substrates of cognitive functions. The basis for the functioning of neural networks in the cerebral cortex and hippocampus is long-term potentiation (LTP), a process of enhancing of conduction of nerve impulses in synaptic transmission over a long period of time [36]. It plays a major role in learning, memory, and the development of sensory systems. LTP is responsible for the stable operation and strengthening of synaptic connections [37]. Chronic seizures, in turn, cause impairment of LTP [18,38].
The location of epileptic activity within the brain is a decisive factor for the cognitive outcome of seizures—damage to functional areas causes their impairment. The hippocampus is considered to be one of the most important structures in memory formation. After seizures, the pyramidal cells of the hippocampus form abnormal neural connections, which leads to impairment of long-term, short-term, and spatial memory [21,39]. The frontal lobe of the neocortex is often damaged too, resulting in impairment of logical thinking, working memory, control of emotions, and voluntary movements [20].
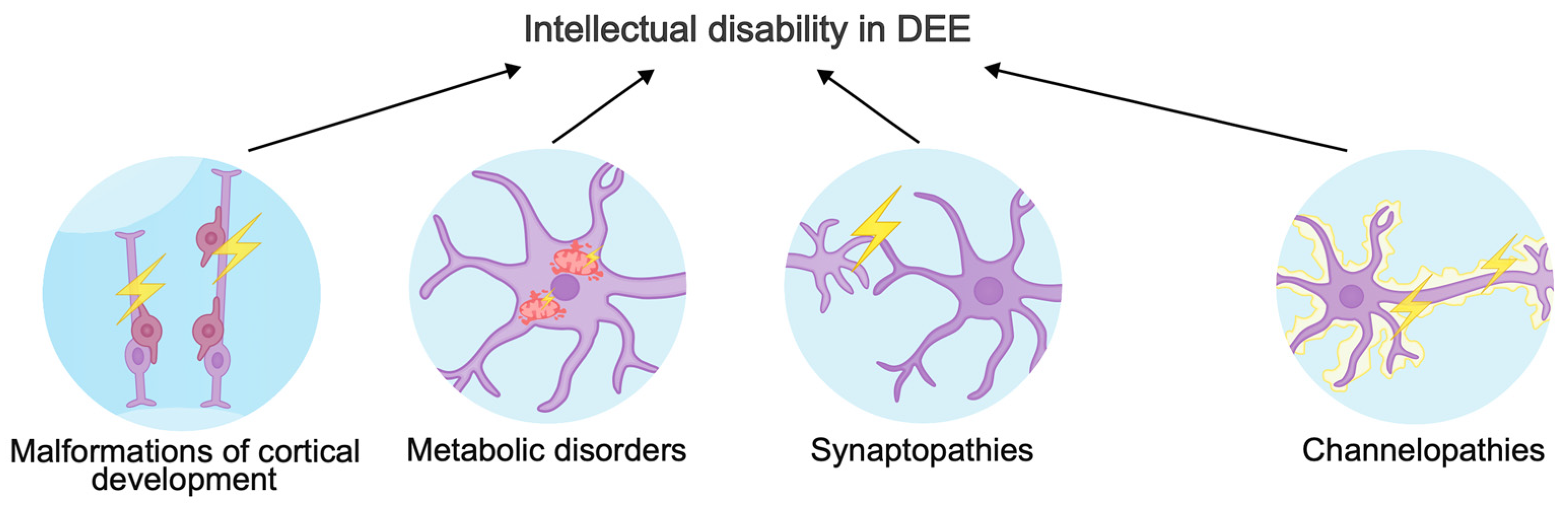
The phenotypic spectrum of gene variants causing DEE is very broad. Different versions in a single gene can cause different consequences for a protein: “gain-of-function” variants most often result in early-onset DEE, while “loss-of-function” variants lead to late-onset DEE, intellectual disability, and ASD [40]. In this review, we will focus on three major mechanisms of intellectual disability in DEE (Figure 1). In addition, we will describe new genes involved in the pathogenic molecular cascades and ignored by other reviews about DEEs.
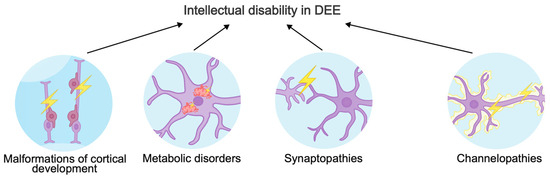
Figure 1.
Mechanisms underlying intellectual disability in developmental and epileptic encephalopathies.
3. Molecular Mechanisms Underlying Developmental and Epileptic Encephalopathy
The most frequent reasons for DEE have a genetic etiology [10,17]. The diseases are often monogenic, but oligogenic variants also occur [41,42]. According to exome sequencing and whole-genome analysis, de novo variants are the main causes, but there are also other inherited forms: autosomal recessive, dominant, and X-linked variants [12,42,43,44]. The majority of pathogenic variants are associated with channelopathies, metabolic disorders, membrane transport, and progenitor growth and proliferation during neurogenesis [45]. There is a short description of genes discussed below in Table 1.

Table 1.
Pathogenic variants associated with intellectual disability in developmental and epileptic encephalopathies.
3.1. Malformations of Cortical Development as a Cause of DEE
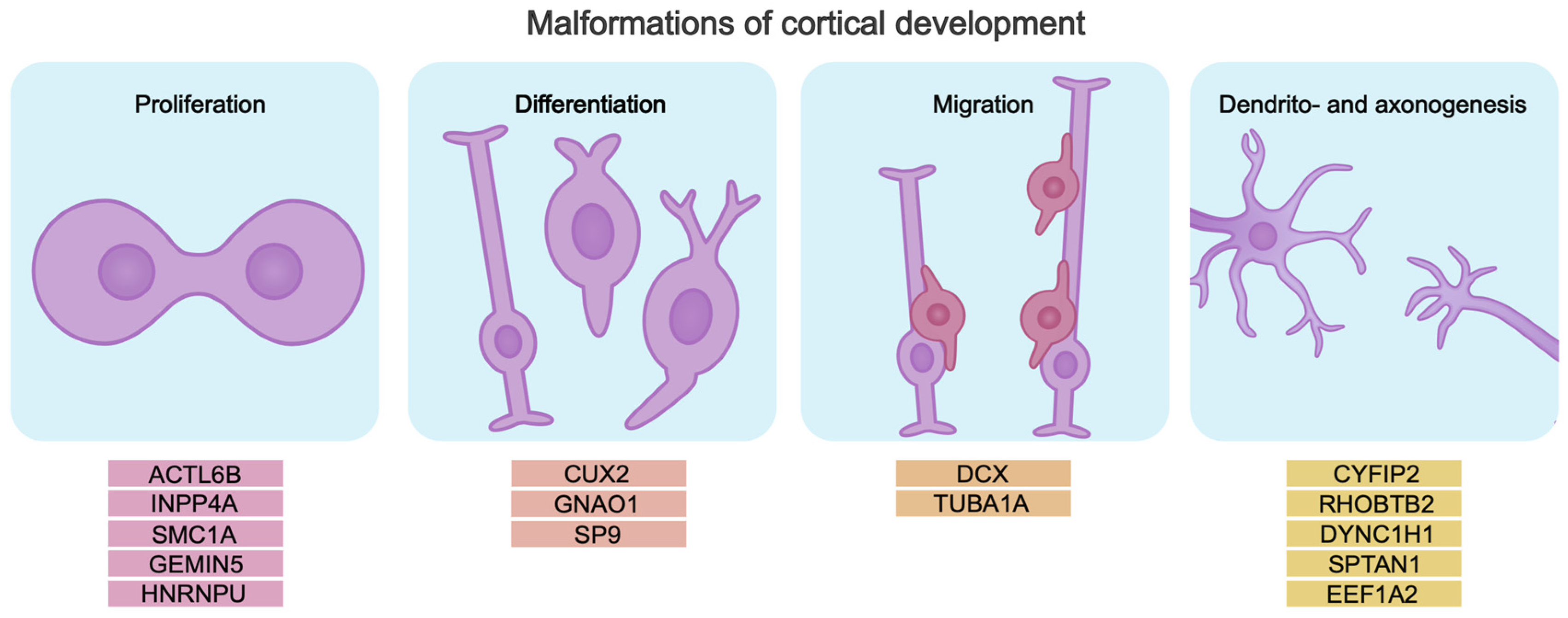
Cerebral cortex development relies on correct temporal activation and inactivation of tightly regulated genetic programs that control the proliferation and differentiation of neuronal progenitors, specification, migration, and formation of neuronal circuits. All this determines the formation of a brain that functions properly. Malformations of cortical development (MCDs) are associated with impaired cerebral cortex development. Pathogenic gene variants disrupting these processes cause abnormalities in brain morphology and function. Pathogenic variants can be associated with genes encoding chromatin modifiers, transcription factors, and RNA-binding proteins that control the process of neurogenesis. Mutations in such genes cause neurodevelopmental disorders including DEEs [75,76,77,78,79,80,81] (Figure 2).
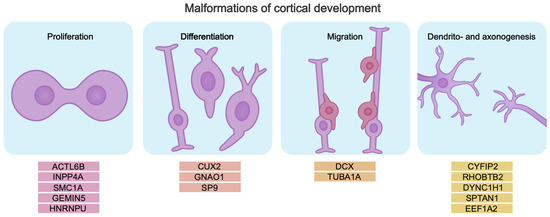
Figure 2.
Pathogenic gene variants lead to disruption of key neurogenesis processes, which may be the cause of intellectual disability in DEE. The genes are divided into subgroups by mechanism, based on their role in the pathogenesis of intellectual disability in DEE.
3.1.1. Neuronal Progenitor Proliferation Disruption
Pathogenic variants of the neuron-specific chromatin remodeling complex (BAF), which regulate the expression of genes involved in the control of neocortical lamination, dendritic branching, and synapse formation, have been described [82,83,84,85]. Pathogenic variants of BAF, ACTL6B, are associated with severe forms of DEE with profound developmental delay and intellectual disability [86,87,88,89,90]. ACTL6B protein controls DNA transcription accessibility and is required for the maintenance of neuronal progenitor cell (NPC) proliferation balance. The proliferative state is maintained by the (np)BAF complex with ACTL6A during neurogenesis, whereas the differentiation of NPCs into mature postmitotic neurons requires a switch from ACTL6A to ACTL6B in the (n)BAF complex [83,89,91,92]. The most common pathogenic variants have been found in the actin-like domains of the protein, causing loss of protein function and disruption of the BAF complex assembly. They cause dysregulation of genes associated with the self-renewal of neuronal progenitors, causing abnormal cytoarchitecture of the neocortex and subsequently intellectual disability. Thus, among the clinical signs of ACTL6B variants are intellectual disability, developmental delay, lack of speech, hypomyelination, agenesis of the corpus callosum, and severe epilepsy [86,88,89,93,94,95,96]. Neuronal cell culture experiments demonstrated disrupted synapse formation, supporting the important influence of ACTL6B in neuronal development [89]. Thus, loss of ACTL6B function reduces the ability of neurons to form synaptic connections and leads to impaired neuronal differentiation, which plays a critical role in DEE pathology and intellectual disability.
Pathogenic variants of INPP4A are associated with disruption of intracellular signaling pathways. Biallelic truncated variants cause a spectrum of neurodevelopmental disorders from mild intellectual disability to DEE and microcephaly [56]. The INPP4A gene encodes the enzyme inositol polyphosphate-4-phosphatase, which is involved in the metabolism of inositol in phosphoinositide signaling pathways and regulates vesicle transport, which is crucial for neuronal function. Mouse models demonstrate elevated neuronal death due to defective proliferation [97,98,99,100]. Moreover, mice with a pathogenic variant of Inpp4a have defects in the development of the striatum, which is important for normal motor and cognitive behavior. In addition, in neuronal cultures, INPP4A has been shown to regulate NMDAR synaptic localization, protect neurons from excitotoxic death, and thereby maintain the functional integrity of the brain [101]. In sum, INPP4A is critical for the development of the nervous system, since the product of this gene influences the functioning of multiple signaling pathways, maintains cellular homeostasis and neurogenesis, and plays a role in cell proliferation and suppression of glutamate excitotoxicity [101,102,103,104].
The next interesting player in neurogenesis disorders is SMC1A. This gene encodes a component of the cohesin complex, which is involved in chromosome segregation during replication, DNA repair, and transcriptional regulation [105,106,107,108]. Pathogenic variants of this gene can lead to Cornelia de Lange syndrome with specific developmental delay and can also induce early DEE. Moreover, DEE associated with SMC1A is characterized by global developmental delay and occurs exclusively in women, due to the probable male lethality [106,109,110].
Changes in this gene in embryonic brain stem cells caused decreased DNA loops, loss of cohesin on promoters and enhancers, changes in gene expression, and proliferation defects. Supposedly the defects in SMC1A lead to chromosomal instability and gene expression disorders in the early stages of brain development, contributing to the neurodevelopmental pathologies [105,106,107,108,111].
Impaired neurogenesis may also be caused by other mechanisms. For example, the formation of the correct pool of mRNA isoforms is necessary for neuronal progenitors to exit the cell cycle. The disruption of RNA splicing programs during early brain development plays an important role in the etiology of NDDs [112,113,114,115]. The gene GEMIN5 encodes a multifunctional protein involved in the assembly of small nuclear ribonucleoproteins (snRNPs), the regulation of pre-mRNA splicing, and, in general, translation [116,117,118,119,120,121,122,123,124]. Defects in GEMIN5 are associated with cerebellar atrophy, intellectual disability, movement disorders, and early infantile developmental epileptic encephalopathy (EIDEE) [3,125,126]. Pathogenic variants of GEMIN5 impair the ability of GEMIN5 to interact with other proteins of the SMN complex or to bind snRNA. “Loss-of-function” variants of GEMIN5 are the most common and result in impaired translation and decreased binding of the internal ribosome entry site (IRES), which causes defects in the expression of genes essential for nervous system development [122,124,127,128,129]. “Loss-of-function” variants of GEMIN5 increase the activity of pathways associated with postsynaptic membrane signaling and neurotransmitter secretion and decrease the activity of pathways associated with cell development, the extracellular matrix, and nuclear transport [129]. Gemin5 is thought to play a critical role in early mammalian development. Homozygous knockout models are embryonic lethal [130,131]. Biallelic variants in GEMIN5 are also known to cause developmental delay, motor dysfunction, and cerebellar atrophy. This is likely due to decreased levels of snRNP complex assembly proteins and defects in target RNA regulation [129].
The HNRNPU gene is one of the genes regulating RNA processing. It encodes heterogeneous nuclear ribonucleoprotein U (hnRNP U), a protein that plays a key role in maintaining the three-dimensional genome structure [81,132,133,134,135,136,137,138]. HNRNPU is widely expressed in the brain, especially in the cortex, hippocampus, and cerebellum [139]. Pathogenic variants are recognized as causes of NDD, intellectual disability, ASD, and early DEE (EIEE54) [55,114,115,140,141]. HNRNPU-associated developmental pathologies are mostly caused by loss-of-function defects, which lead to a spectrum of neural pathologies: abnormal neuronal migration, enlargement of the lateral ventricles, and defects in the formation of the corpus callosum [81,142,143,144]. Mouse models with Hnrnpu haploinsufficiency demonstrate abnormalities in brain organization and pathologies of neuronal projection and migration pathways. Since all reported human variants are heterozygous, homozygous HNRNPU ones probably lead to prenatal death in humans, similar to mice [80,81,115,137,145]. HNRNPU haploinsufficiency supposedly prevents neuronal progenitors from exiting the cell cycle and initiating differentiation, disrupting the neuronal developmental trajectory. This leads to impaired neural development and causes a spectrum of neurological disorders [115,146,147].
3.1.2. Neuronal Differentiation Disruption
Another gene implicated in DEE known to be important for neurogenesis is CUX2 [148]. CUX2 encodes a transcription factor regulating the proliferation of neuronal progenitors in the subventricular zone (SVZ) and their differentiation and exit from the cell cycle. CUX2 is expressed late in the cell cycle, before the final mitosis of neuronal progenitors in the SVZ [149,150,151]. Cux-2 with Cux-1 together are early markers of neuronal differentiation: the Cux1 gene is involved in proliferation, and the Cux2 gene controls cell type specification and neuronal differentiation. It is also known that Cux gene expression is required for the differentiation and development of interneurons [149,150,152,153,154]. Delayed CUX2 expression can lead to abnormal cell cycle exit, causing defects in corticogenesis and subsequent neurodevelopmental pathologies [149,150,151].
Another group of genes whose pathogenic variants cause intellectual disability is involved in intracellular signaling cascades. Thus, in recent years, de novo variants of the G-protein subunits have been identified. For example, pathogenic variants of the GNAO1 gene are associated with severe neurological syndromes, ranging from developmental delay with movement disorders to EIEE [155,156,157,158,159,160,161,162]. GNAO1 encodes the alpha subunit of Gα, a heterotrimeric G protein that regulates intracellular signaling. The highest level of GNAO1 is observed in the growth cone of differentiating neurons. Gα is responsible for molecular signaling that directs the growth cone navigation lead by external signals. This process is key for correct neural circuit formation [162,163,164,165]. Defects in Gα disrupt the protein’s ability to bind and hydrolyze GTP, reduce interactions with partner proteins, and cause a loss of the protein in the cytoplasmic membrane. Because of a key role in multiple neuronal signaling systems, Gα variants cause various defects in development. For example, they lead to impaired neurite growth and extension [166,167,168,169,170]. Mouse models of Gnao1 exhibited early postnatal lethality, decreased numbers of cortical neuronal progenitors, and enlarged lateral ventricles [171]. In contrast, patients with impaired GNAO1 had decreased levels of neurogenesis genes, increased expression of astrocyte markers, differentiation defects, and abnormal neural network formation. They had low intracellular free calcium concentrations and impaired neurotransmitter responsiveness. Thus, pathogenic GNAO1 variants impair neural communication [172].
The next example is the SP9 gene, which encodes a transcription factor of the Sp/KLF family, which is necessary for the regulation of gene expression in neurogenesis. SP9 is expressed during embryogenesis in the cerebral cortex and basal ganglia, where it is necessary for the correct differentiation, migration of neurons, and the formation of neural circuits [59,173]. Several studies have reported two main types of NDD caused by defects in the SP9 gene. A loss of function in the third C2H2 binding domain results in developmental delay, epilepsy, and autistic disorders, while changes in the second domain result in EE [59,174]. SP9 is involved in the development of the corticospinal tract and tangential migration of GABAergic neurons. The gene also plays an important role in the proliferation and differentiation of striatopallidal projection neurons. Without SP9, cortical interneurons do not migrate to the cortex or striatum. Sp9-knockout animal models exhibit reduced cortical interneuron density, abnormal network organization, and defective axonal growth. Thus, Sp9 knockout results in cognitive and motor impairments similar to those seen in patients with DEE [59]. It appears that loss-of-function SP9 disrupts the transcriptional control of genes critical for corticogenesis, causing neuronal mislocalization, defective circuit formation, and altered synaptic plasticity [59,175].
3.1.3. Neuronal Migration Disorders
Disruption of neuron migration during brain development may be the cause of DEE [160,176]. Appropriate regulation of cytoskeletal dynamics, particularly microtubules, is essential for neuronal migration [177]. Tubulins play an important role in this process, being essential for mitosis, axonal transport, neuronal migration, and synapse formation [178,179,180]. One of these genes, TUBA1A, encodes the α-tubulin isotype, which is highly expressed in postmitotic neuronal cells but absent in neuronal precursors [181,182,183,184]. α-tubulin forms heterodimers with β-tubulin to form microtubule polymers. Microtubule dysfunction can lead to various disorders of neural development referred to as tubulinopathies [180,185,186].
Pathogenic variants of TUBA1A are the main genetic cause of lissencephaly and can also lead to microcephaly, corpus callosum abnormalities, gray matter heterotypes, and DEE [61,187,188,189]. Variants that cause a loss of function (LoF) of TUBA1A result in a lack of tubulin in cells, as these variants are unable to polymerize microtubules. On the other hand, gain-of-function (GoF) variants are able to form microtubules but are unable to interact with dynein [180,185,190,191].
Tuba1a mutants have impaired radial neuronal migration. Mouse models of the pathogenic Tuba1a variant exhibit perinatal mortality in the homozygous state and severe brain malformations by E16.5. These mice show a decrease in the thickness of CTIP2+ and PAX6+ neuronal layers and apoptotic neuron death. The severe phenotype of neurodevelopment is associated with a decrease in postmitotic and apical neuronal precursors [180,192,193].
Another key gene for neuron migration is DCX. It encodes the doublecortin protein, which is involved in organizing microtubules during neuronal differentiation and the migration of interneurons to the cerebral cortex [194,195,196,197,198,199]. Pathogenic variants of DCX disrupt the structure of the N- and C-terminal regions of the protein, which are necessary for binding to microtubules and unpolymerized tubulin. These changes in the DCX protein prevent neurons from interacting properly, leading to impaired neuron migration and defects in the architecture of the developing brain cortex [195,200,201].
These pathogenic variants have been clinically associated with severe brain malformations, subcortical band heterotopia, lissencephaly, intellectual disability, epilepsy, and DEE [195,197,202]. The most severe variants of the phenotype are associated with de novo frameshift variants, while missense variants cause milder developmental defects. DCX is located on the X chromosome. Therefore, the most severe consequences of pathogenic variants of this gene occur in males, manifesting as severe MCD, lissencephaly, developmental delay, intellectual disability, and seizures. Females, on the other hand, have a milder phenotype in the form of heterotopia [195,203,204,205].
3.1.4. Dendrito- and Axonogenesis Disorders
Neuronal morphogenesis which includes the formation of dendritic trees and axons, depends on the action of multiple molecules that control cytoskeleton structure and maintenance. One such factor is the CYFIP2 gene, which plays an important role in regulating the actin cytoskeleton via the WAVE complex [206]. When the small Rho GTPase Rac1 binds to the CYFIP2 protein, the WAVE complex is activated, and it interacts with Arp2/3 [207]. This interaction promotes actin filament polymerization and maintains polymerization/depolymerization dynamics required for neurite outgrowth and branching [208]. Defects in CYFIP2 disrupt this process, leading to actin filament destabilization and impaired outgrowth [209]. This is manifested by a reduced ability of neurons to form leaf-like lamellipodia and synaptic contacts, which entails defects in synaptic plasticity and impaired neuronal migration [210]. In patients, a pathogenic variant of CYFIP2 leads to severe DEE, psychomotor delay, intellectual impairment, hypotonia, and behavioral disorders and may be associated with fragile X syndrome [211,212].
SPTAN1 is another gene important for maintaining the structural integrity of neurons too. SPTAN1 encodes the spectrin αII protein, which is also involved in the actin organization and membrane structure stabilization. SPTAN1 ensures the structural integrity of the cytoskeleton and the normal functioning of neurons [213]. Spectrin αII binds to actin filaments, forming a supporting network under the cell membrane, which is important for maintaining the mechanical stability of membranes and synaptic plasticity [60]. This protein is also necessary for the assembly of nodes of Ranvier [214]. Pathogenic variants of SPTAN1 result in axonal defects and disrupted cellular architecture, leading to epilepsy, developmental delay, ASD, microcephaly, spastic paraplegia, and West syndrome [213,215,216].
Cytoskeletal dynamics is also regulated by the RHOBTB2 gene, which encodes a protein of the Rho-type GTPase family. RHOBTB2 is involved in the regulation of cytoskeletal dynamics, cell migration, and vesicular transport, influencing cell differentiation and apoptosis [217]. Interaction of RhoBTB with the Cullin3 protein, which is part of the ubiquitin–proteasome complex, can regulate the levels of specific proteins required for normal dendritic development and synaptic plasticity. In the context of RHOBTB2, the association with Cullin3 suggests that missense variants may disrupt the degradation machinery, affecting the stability of proteins required for normal dendritic development and neuronal function. RHOBTB2 has an important role in cell cycle control, participating in the regulation of cellular differentiation and apoptosis [218,219]. Knockout of RhoBTB in Drosophila dendritic neurons highlighted the critical role of the formation of dendritic architecture, decreasing the number of dendritic branches. Missense variants in the coding region of the BTB domain of RHOBTB2 are associated with DEE, indicating importance in neuronal development and possibly in the regulation of dendritogenesis [220]. However, the precise molecular mechanisms linking missense variants with neurodevelopment remain poorly understood, requiring further studies to characterize their role in neuropathology.
The DYNC1H1 gene regulates cytoskeleton functions too. It contains the cytoplasmic dynein heavy chain, which mediates the binding of dynein complexes to microtubules [221]. This process is critical for maintaining neuronal homeostasis and delivering key components involved in synaptic activity, such as neurotransmitter receptors, synaptic vesicle precursors, and others [222,223]. Disruptions in DYNC1H1 function can lead to defects in protein folding and microtubule bundling [224]. Patients with pathological variants of the DYNC1H1 exhibit neurodevelopmental delay, DEE, and, in some cases, abnormal brain morphology, including microcephaly and other phenotypes [221].
Impaired inhibitory neuron function in DEE may be due to decreased levels of the Caspr2 protein, encoded by the CNTNAP2 gene [225]. This gene encodes contactin-associated protein-like 2, a member of the neurexin family—cell adhesion molecules involved in the formation of synaptic contacts [226,227]. CNTNAP2 is necessary for myelination, axon guidance, organization of dendritic branching, and spine formation, and therefore, it controls the formation of neural networks in general [228]. CNTNAP2 deficiency causes increased neuronal excitability [229]. In particular, recessive variants in the CNTNAP2 gene affect the levels and functions of GluA1, a subunit of AMPA receptors regulating excitatory synaptic transmission [230]. Disruption of CNTNAP2 leads to altered expression, surface localization, and endocytosis of GluA1, attenuating synaptic plasticity and modulating the activity of calcium-dependent signaling pathways [231]. Patients with a recessive variant in the CNTNAP2 gene demonstrated cognitive impairment, language disorders, seizures, and focal cortical dysplasia epilepsy syndrome (CDFE) and also had a decrease in the number of GABAergic interneurons and neuronal migration abnormalities, indicating profound defects in the formation and functioning of neural networks [47].
The EEF1A2 gene plays an important role in the translation and organization of the neuronal cytoskeleton. It encodes eukaryotic translation elongation factor 1A2, which affects the process of protein synthesis. EEF1A2 binds to amino acids and tRNAs and participates in the transfer of tRNA to the A-site of the ribosome, which is necessary for the elongation of the polypeptide chain during translation. Pathogenic variants of EEF1A2 are associated with DEE, developmental delay, and microcephaly [232] because they disrupt translation (due to increased tRNA binding), reducing the translation velocity. This affects the morphological development of cortical neurons. Pathogenic EEF1A2 has lower actin-binding activity. Thus, EEF1A2 has two functions: translation regulation and organization of the neuronal cytoskeleton [233]. When EEF1A2 was knocked out in human glioblastoma cells, the process of cell proliferation and differentiation was impaired [234].
In summary, as the cerebral cortex develops, multiple molecular pathways interact to produce a complex neuronal network making up the cerebral cortex. The disruption of any of these pathways can lead to serious pathologic conditions. This, in turn, can cause the development of intense and sometimes multifocal epileptic activity associated with DEE.
3.2. Synaptopathies—Synaptic Transmission Disorders
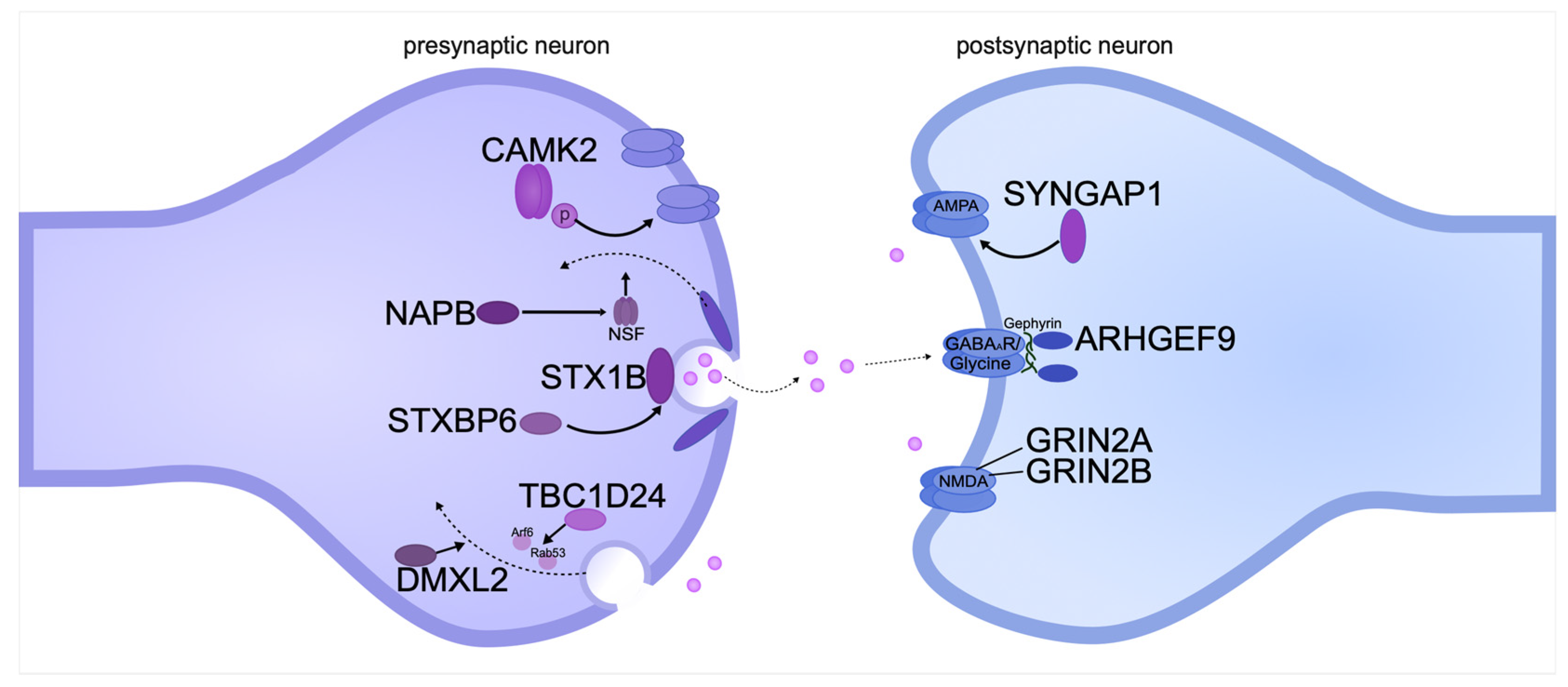
Pathogenic gene variants affecting pre- and postsynaptic transmembrane proteins can lead to DEE both directly and indirectly. Pre- and postsynaptic membranes are involved in the transport of synaptic vesicles in axons and action potential initiation in dendrites (Figure 3).
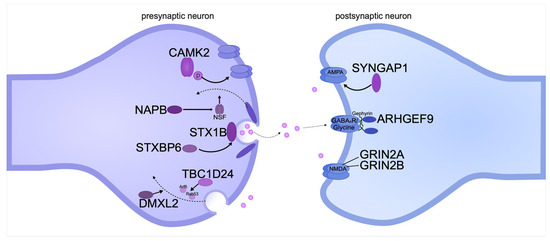
Figure 3.
Pathogenic gene variants encoding pre- and postsynaptic transmembrane proteins can cause intellectual disability in DEE. The diagram shows the location of proteins relative to the synaptic cleft and the functions they perform. The proteins are systematized based on recent publications on DEE.
The SNARE protein complex plays an important role in the presynaptic membrane [235]. One of the members of the complex is syntaxin-1B, which is encoded by the STX1B gene. The main role of the protein is to anchor synaptic vesicles to the presynaptic membrane [236]. Syntaxin-1B has two conformations: open, which is necessary for the formation of the SNARE complex, and closed, which initiates the vesicle fusion reaction [237,238]. De novo STX1B pathogenic variants are clinically associated with DEE and generalized epilepsy with febrile seizures [67]. Most often, missense variants are loss-of-function variants of the open conformation of the protein, resulting in disruption of the assembly of the complex and vesicle transport. Pathogenic variants of the closed conformation of the protein lead to the disruption of protein–protein interactions and normal fusion of presynaptic vesicles [239]. Mice with a gene knockout of Stx1b exhibit severe seizures and premature death associated with dysfunction of neurotransmitter release at GABA and glutamatergic synapses [240]. In addition to STX1B, other regulatory proteins, such as the product of the STXBP6 gene, are involved in maintaining the fidelity of vesicle–membrane fusion processes. STXBP6, encoding syntaxin-binding protein 6, also known as amysin, is involved in modulating syntaxin activity and controlling membrane interactions, which is necessary for the normal functioning of the synaptic apparatus, namely the movement of neuronal vesicles [241]. A patient with epileptic encephalopathy and autism spectrum disorder (ASD) was found to have a truncated variant of the protein encoded by the STXBP6 gene [242]. Mice with a deletion of this gene had reduced body weight, which is also one of the phenotypes in some ASD patients. However cognitive skills were not impaired in these mice [243].
Neurotransmitter release occurs through Ca(2+)-induced synaptic vesicle fusion mediated by the SNARE complex [244]. The SNARE complex is associated with the βSNAP protein, which is a product of the NAPB gene. βSNAP is one of the cofactors of NSF-ATPase, which is essential for synaptic transmission, since this enzyme is involved in the disassembly and utilization of SNARE complex proteins [245]. Whole-exome sequencing of three siblings with severe intellectual disability and DEE revealed a seven-base-pair deletion in the NAPB gene, resulting in a 46% truncation of the protein. The children developed epileptic seizures before 6 months of age and severe developmental regression by 2 years of age [246]. Recently, whole-exome sequencing of an Arab-Palestinian consanguineous family of three identical twins diagnosed with Cohen syndrome was performed. The twins suffered from early-onset epileptic encephalopathy, autism, and intellectual disability. Analysis of the sequencing data identified a pathogenic variant affecting the splice site of the NAPB gene [247]. Mice with reduced βSnap expression showed epileptic seizures, followed by ataxia and, in some cases, death [245].
Among the postsynaptic membrane proteins, pathogenic variants of SYNGAP1 are most frequently associated with DEE [248]. This protein is a key mediator in the RAS signaling cascade activated by the NMDA receptor. During LTP, SYNGAP1 activates RAS-GTPase (SynGAP) in glutamatergic neurons, resulting in the insertion of AMPA receptors and an increase in synaptic surface area [249,250,251]. SYNGAP1 pathogenic variants affect glutamatergic synapses and enhance glutamate receptor activity, increasing the probability of epileptogenesis [252]. SYNGAP1 mRNA has multiple alternatively spliced variants encoding different protein isoforms that differ in structure, function, and temporal expression. Four C-terminal isoforms have been identified: α1, α2, β, and γ. The β isoform is expressed early in postnatal development, while α2 is expressed at higher levels in the mature brain [253,254]. This explains the differences in phenotypic severity; for example, nonsense-mediated decay caused by defects in early isoforms leads to complete loss of the gene product. A milder outcome of SYNGAP1-DEE is observed in patients with splice site variants in exons 1 through 4 [68].
Another gene associated with synaptopathy is TBC1D24. This gene encodes a protein that activates the small GTPases Arf6 and Rab35, which act antagonistically. They are required for membrane transport at synapses, as well as between the plasma membrane and endocytic compartments [255]. TBC1D24 has a broad expression pattern and is found in all layers of the cerebral cortex and the hippocampus. Pathogenic variants of TBC1D24 cause dysregulation of synaptic vesicles, which causes excessive neurotransmission. In addition, it interferes with the normal disposal of defective proteins through endosomal pathways, leading to their accumulation and neuronal dysfunction. These alterations contribute to the development of a wide range of epileptic phenotypes and other neurodevelopmental disorders in patients [69]. Knockout of the Tbc1d24 gene in rat primary cortical neurons revealed impaired axon initial segment formation and neuronal excitability. This phenotype was associated with increased activation of the GTPase Arf6, which is required for axon specification and neurite extension [256].
The DMXL2 gene encodes a large protein that is associated with vesicular transport and plays a key role in the regulation of synaptic transmission. Disruptions in the function of the DMXL2 protein can lead to disruptions in synaptic endocytosis and vesicle recycling. This is due to the fact that DMXL2 regulates the acidification of intracellular compartments via the vacuolar proton pump (V-ATPase) [257]. In addition, the DMXL2 protein acts as a modulator of the Notch signaling pathway and is required for chromatin recruitment of Notch-dependent transcription factors [258]. Pathogenic variants of DMXL2 can disrupt these processes, which leads to an imbalance of excitation/inhibition in the nervous system, causing neuronal hyperexcitability. This, in turn, is associated with the development of epileptic seizures and severe developmental delay, characteristic of DEE [6]. The neuronal hyperexcitability underlying DEE may also be associated with dysfunction of glutamate receptors. In particular, the GRIN2A and GRIN2B genes encode subunits of NMDA (N-methyl-D-aspartate) receptors, which are subtypes of glutamate receptors. They play a key role in synaptic plasticity, learning, and memory. These receptors control the entry of calcium, sodium, and potassium ions across the neuronal membrane, which is necessary for the transmission of excitatory signals in the brain [65,259]. Increased activity of NMDA receptors leads to excessive calcium influx into cells, which can cause neuronal hyperactivity and, as a result, neuronal death [260]. There are multiple rare variants of GRIN2A and GRIN2B genes associated with neurological diseases. Currently, 304 variants that cause DEE have been reported in GRIN2A, and 273 variants that cause DEE have been reported in GRIN2B (ClinVar) [261]. The phenotypic manifestations in these genes have been studied in detail in a number of clinical studies. Patients with pathogenic variants of GRIN2A/GRIN2B exhibit severe forms of epileptic encephalopathy, accompanied by delayed motor and cognitive development. These clinical manifestations correlate with disturbances observed at the level of synaptic transmission and neuronal activity [262].
The ARHGEF9 gene encodes the protein collibostin (Cb), which regulates the actin cytoskeleton dynamics and synaptic activity through activation of Rho GTPases, in particular Cdc42 [263]. It interacts directly with the scaffold protein gephyrin and is required for the formation of gephyrin-dependent GABA A clusters on the postsynaptic membrane [264]. Cb interaction occurs due to the presence of the SH3 domain, which binds to the large intracellular loop of the α2 subunit of GABA A receptors [265]. Point mutations in ARHGEF9 disrupt inhibitory synaptic transmission through interaction with GABA and glycine receptors, which leads to neuronal hyperexcitability and cognitive impairment. It is associated with the development of epilepsy, ASD, intellectual disability, and, in some cases, certain facial dysmorphism [62,147].
Ca 2+/calmodulin-dependent protein kinase II (CAMK2) is one of the most important enzymes in synaptic plasticity and memory formation [266]. The protein consists of two predominant subunits, alpha (CAMK2A) and beta (CAMK2B), that are highly homologous to each other and can probably substitute each other’s functions when one is inactivated [267]. Pathogenic variants of CAMK2A or CAMK2B cause intellectual disability, ASD, and DEE in humans [268]. The CAMK2 enzyme is part of the Ca-dependent signaling pathway and phosphorylates various substrates responsible for LTP [269]. When activated, CAMK2A exerts significant effects on dendritic spines and postsynaptic density by interacting with enzyme-associated proteins, particularly the GluN2B subunits of the NMDA receptor [270]. When the CaMK2A autophosphorylation site is disrupted in mice, defects in spatial learning and memory are observed [271].
Thus, synaptopathies are one of the key mechanisms underlying epileptic encephalopathies and neurodevelopmental disorders in general. Disturbances in synaptic plasticity lead to dysregulation of neural connections, which causes epileptic activity in the brain and significant cognitive and motor deficits. This undoubtedly emphasizes the importance of studying synaptopathies for understanding the pathogenesis of epileptic disorders and associated developmental delays [31].
3.3. Metabolic Disorders
The mammalian brain has a high energy demand. Most of the energy is utilized for the activation of action potentials and synaptic transmission. It is provided by glycolysis and mitochondrial respiration. On the other hand, energy demand during neurogenesis is extremely high as well. It is not surprising therefore that abnormal bioenergetics and mitochondrial dysfunction in neurons cause cognitive disorders [272].
One such disorder is caused by pathogenic variants of HK1, which encodes hexokinase HK1. This enzyme carries out ATP-dependent phosphorylation of glucose to glucose-6-phosphate (G6P) in glycolysis [273]. HK1 is predominantly expressed in neurons and astrocytes in the brain, and gene dysfunction has been associated with multiple developmental disabilities, including neurodevelopmental disorders (NDDs) and DEE [72,273].
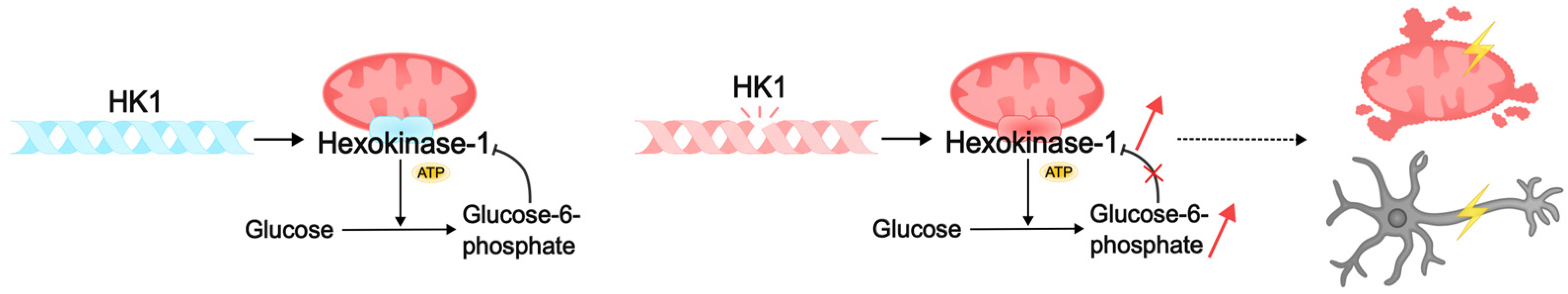
Pathogenic variants of HK1 can cause intellectual disability through several mechanisms related to the essential functions of hexokinase in cellular metabolism and neuronal activity. HK1 consists of two symmetrical monomers that contain an alpha-helix-linked regulatory N-terminal domain and a catalytic C-terminal domain [274,275]. The phosphorylation product of this hexokinase, G6P, binds to both domains of the enzyme, resulting in competitive inhibition of ATP binding and inhibition of kinase activity [276,277]. This process is disrupted by missense variants in the alpha helix and regulatory domain of the enzyme, which makes binding of G6P to the HK1 domains impossible, and the enzyme loses its ability to self-regulate. Such defects result in HK1 “gain of function”: the enzyme continues to constitutively phosphorylate glucose, leading to the accumulation of metabolites and mitochondrial damage [278]. It is thought to result in the accumulation of misfolded proteins, endoplasmic reticulum stress, mitochondrial dysfunction, apoptosis, and cell death [72,279]. This may cause neuronal loss in brain regions responsible for learning, memory, and cognition, such as the hippocampus and prefrontal cortex. Pathogenic variants of HK1 reduce energy availability in brain cells, and energy deficiency leads to impaired neuronal activity. This, in turn, may lead to defects in the formation of neural networks during critical periods of development and, consequently, cognitive impairment and intellectual disability [6,72] (Figure 4).
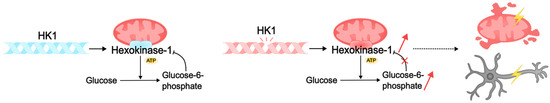
Figure 4.
Pathogenic variants of HK1 can lead to intellectual disability by disrupting cellular metabolism. Normally, HK1 hexokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate (G6P), which binds to the enzyme domains. Competitive inhibition with ATP blocks kinase activity. Pathogenic HK1 disrupts the reverse binding of G6P to the enzyme domains. As a result, the kinase continues to constitutively phosphorylate glucose, which leads to the accumulation of metabolites, damage to mitochondria, and death of neurons. Lack of energy and dysfunction of neural networks can subsequently lead to intellectual disability in DEE.
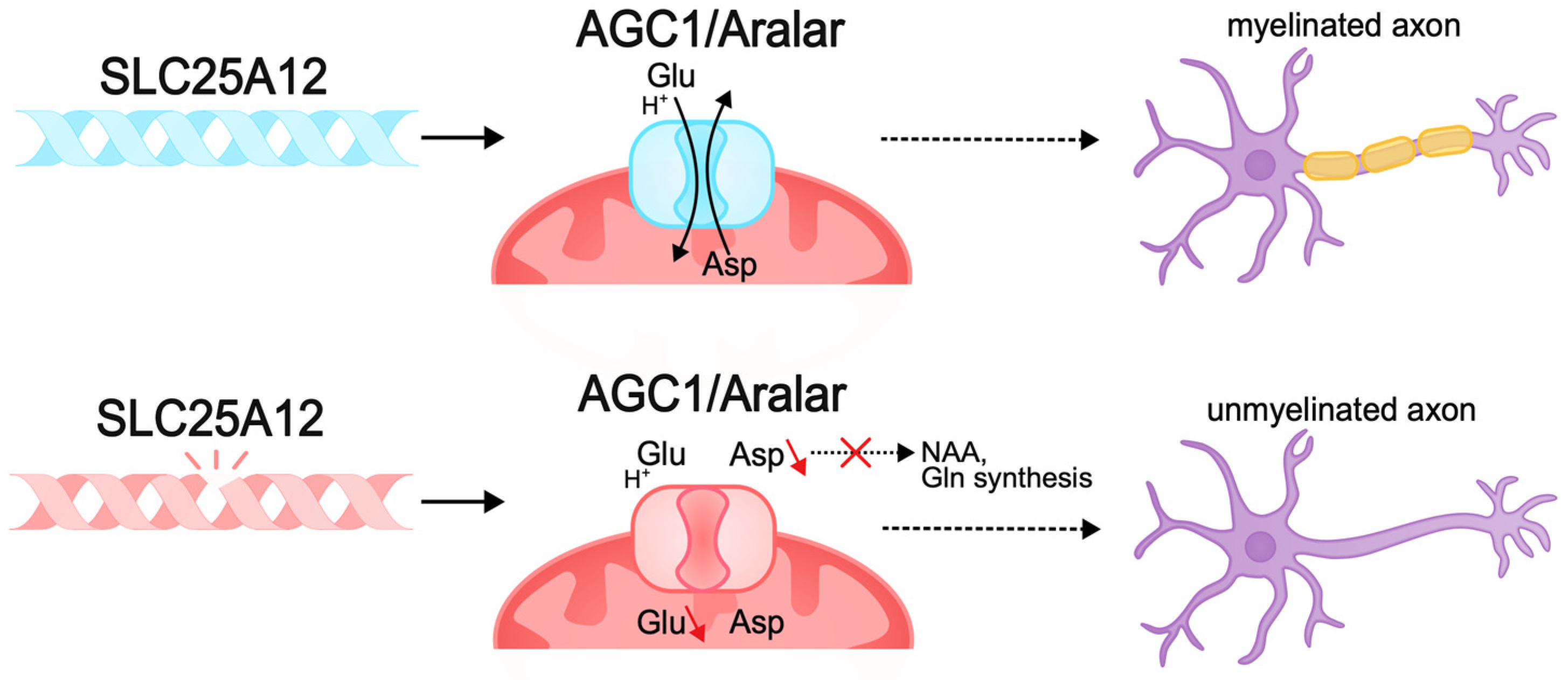
Other pathogenic variants, such as those causing membrane transporter dysfunction, can also lead to disruption of neuronal bioenergetics. The SLC25A12 gene encodes the mitochondrial aspartate–glutamate transporter (AGC1/Aralar), a component of the malate–aspartate shuttle (MAS), mainly expressed in the nervous system and muscles. This transporter carries out the antiport of cytosolic glutamate and protons in exchange for intramitochondrial aspartate. MAS function is necessary to maintain the redox balance between cytosolic glycolysis and mitochondrial respiration and ensures ATP synthesis, which is important for neurons, which have high energy requirements [280,281,282,283]. Pathogenic variants of SLC25A12 result in AGC1 deficiency, which causes infantile epileptic encephalopathy with global psychomotor retardation and brain hypomyelination [284]. Pathogenic variants result in the disruption of the transporter gating, which limits conformational changes in the protein for substrate release in the mitochondrial matrix and impairs cellular metabolic activity [285,286,287,288]. Studies in Agc1-knockout mouse models demonstrate decreased cellular respiration in the brain, decreased aspartate levels, and impaired glutamate metabolism. As a result, neurons lacking AGC1 are unable to maintain normal metabolic activity [282,288]. AGC1 plays a central role in neuronal bioenergetics, and since neuronal growth and differentiation require increased energy production, this protein is very important during neurogenesis [272,289].
In addition, AGC1 pathologies in the nervous system lead to aspartate deficiency and limited biosynthesis of N-acetylaspartate (NAA), which is necessary for myelin synthesis. Reduced myelination disrupts normal axon development and the ability of axons to transmit signals, causing pathologies of neurotransmission, in particular glutamatergic, which explains the intellectual deficit observed in patients [70,290,291,292] (Figure 5).
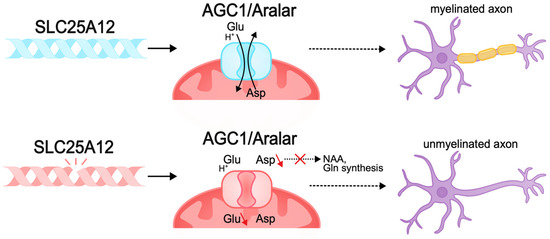
Figure 5.
Pathogenic variants of SLC25A12 can lead to the disruption of neuronal bioenergetics and axonal myelination. The SLC25A12 gene encodes the mitochondrial aspartate–glutamate transporter (AGC1/Aralar). Pathogenic variants of SLC25A12 lead to disruption of the functioning of the transporter gate and the inability to antiport aspartate and glutamate. The lack of aspartate in the nervous system causes a deficiency in the biosynthesis of N-acetylaspartate (NAA), which is necessary for the synthesis of myelin and myelination of axons. Decreased myelination disrupts the normal development of axons and their ability to transmit signals, which may be the cause of intellectual disability in DEE.
Mouse models of pathologies of this transporter have pronounced hypomyelination, as well as impaired development of cortical axons and postnatal development of cortico-hippocampal neurons [282,293,294,295,296]. Thus, pathogenic variants of SLC25A12 lead to disruptions in neuronal function and the development of severe forms of epileptic encephalopathy with concomitant intellectual deficit, which is associated with disruptions of corticogenesis, myelination, and glutamatergic transmission due to metabolic disorder.
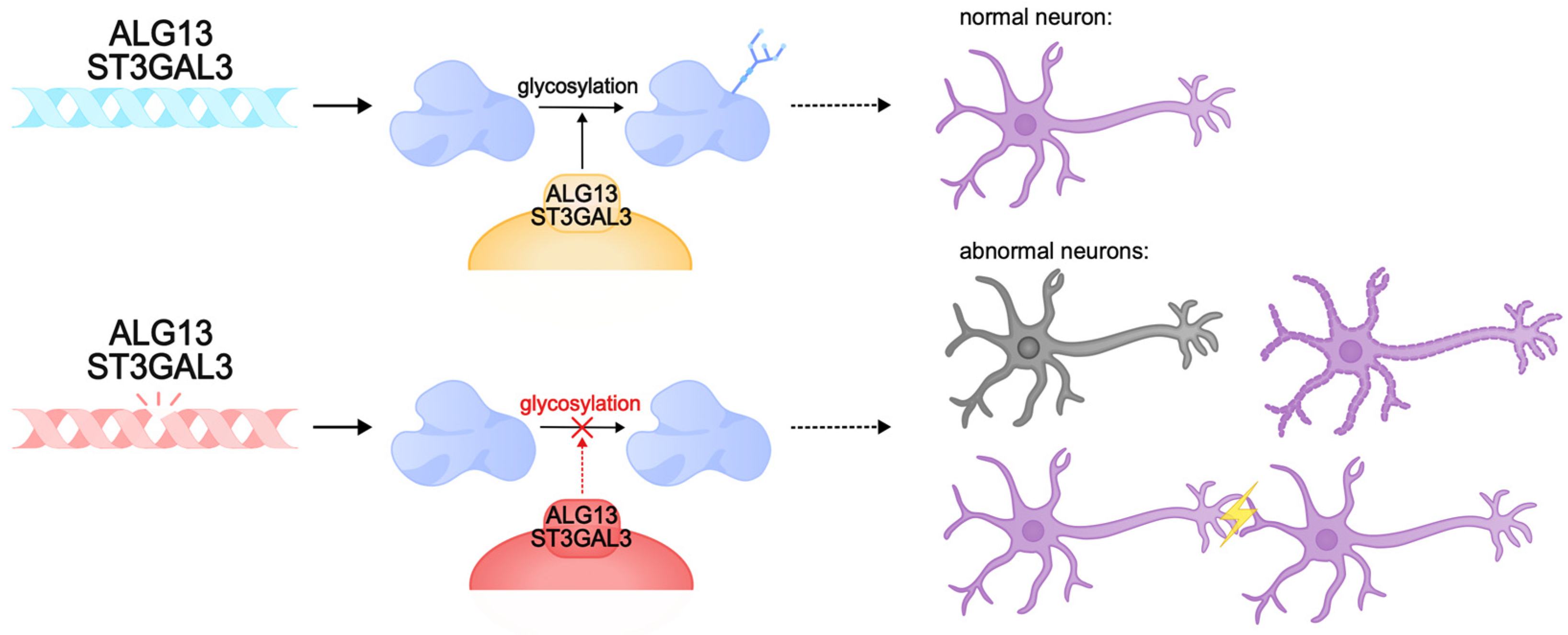
There are cases of intellectual disability caused by protein glycosylation defects. This post-translational modification of proteins plays an important role in many intracellular processes, including synaptic plasticity. Animal models of glycosylation disorders in the nervous system demonstrate synaptogenesis disorders, hippocampal developmental abnormalities, and intellectual disability [297,298,299,300]. One of the genes involved in the control of glycosylation is ALG13. The product of this gene is involved in post-translational modification of proteins by N-glycosylation, and gene expression is predominantly observed in neurons of the cerebral cortex and hippocampus [301]. Pathogenic variants of ALG13 lead to congenital disorders of glycosylation and DEE. They are characterized by global developmental delay with regression, hypotonia, and movement disorders. They are mainly diagnosed in females [302,303,304]. Inside the cell, ALG13 forms a heterodimeric complex with the ALG14 protein, which performs an auxiliary function for anchoring ALG13 to the endoplasmic reticulum membrane. Together, they form a functional glycosyltransferase UDP-GlcNAc, which transfers N-acetylglucosamine to asparagine residues of proteins [305,306,307,308,309]. This process is necessary for the correct folding of proteins and the formation of functional glycoproteins, which ensures their stability, sorting, and transport, and is also important for the implementation of intercellular interactions [301,310,311,312]. The ALG13 protein has several isoforms: long (ALG13-is1) and short (ALG13is2). These isoforms are identical in the catalytic domain of the N-terminal region but are significantly different in the C-terminal region, the part of the protein responsible for the transport of proteins to the endoplasmic reticulum. Pathologies are caused by mutations in both the catalytic and C-terminal domains, which disrupt the activity of the protein, its interactions with the endoplasmic reticulum membrane, and the ability to glycosylate proteins. Defects in the C-terminal region of the long isoform of ALG13 are known to cause developmental and epileptic encephalopathy, intellectual disability, and type I glycosylation disorders [308,313,314,315]. The Alg13KO mouse model exhibited cognitive deficits, decreased dendritic complexity and length, and dendritic spine density in the hippocampus. It is likely that cognitive decline in ALG13 pathology is caused by the failure to form correct synaptic connections [316,317,318,319]. Furthermore, ALG13 loss was found to be characterized by neuronal death and reactive astrogliosis and may reduce inhibitory synaptic transmission by regulating the transcription of the GABA A R α2 subunit, which aggravates synaptic plasticity pathologies [301]. Collectively, pathogenic variants of ALG13 cause profound cognitive impairment, developmental delay, and a severe DEE phenotype due to glycosylation and neuronal plasticity disorders.
Other examples of glycosylation disorders are pathogenic variants of ST3GAL3, which encodes the Golgi transmembrane enzyme sigleosyltransferase ST3Gal-III. This enzyme catalyzes the transfer of sialic acid to galactose in gangliosides and glycoproteins. Sialoglycans are critical for the nervous system, as they are required for normal neuronal function, intercellular communication, myelination, and synaptic plasticity [320,321,322,323,324]. Disruptions in the ST3Gal-III enzyme result in decreased levels of sialoglycans, which impair nervous system function, affecting cognitive development and learning ability. Sialoglycan deficiency disrupts the stability and function of membrane proteins, which interferes with normal neuronal signaling [325,326].
ST3GAL3 loss-of-function variants result in West syndrome, a DEE syndrome with developmental regression and intellectual disability, and severe nonsyndromic autosomal recessive intellectual disability (NSARID) [324,325,327,328,329].
Studies in the St3gal3-null and St3gal2/3-null mouse models showed that gene disruptions lead to a lack of glycoprotein sialylation and, subsequently, to hypomyelination, impaired oligodendrocyte proliferation, and abnormal formation of nodes of Ranvier. Similar to the human phenotype, these mice exhibited severe cognitive deficits, decreased motor coordination, and hyperactive behavior. In addition, the lack of adequate sialylation disrupted the proper functioning of synapses, which reduced synaptic plasticity and impaired learning and memory. Taken together, pathogenic variants of ST3GAL3 lead to glycosylation deficiency of gangliosides and glycoproteins, and subsequently to cognitive dysfunction, and patients with pathogenic ST3GAL3 exhibit severe intellectual disability, developmental delay, and DEE [323,325] (Figure 6).
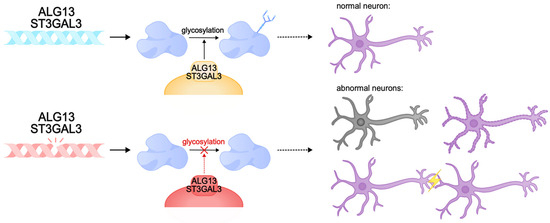
Figure 6.
Pathogenic variants of ALG13 and ST3GAL3 can lead to congenital glycosylation disorders. These genes encode transmembrane glycosylation enzymes that play a critical role in the nervous system. Pathogenic variants can cause abnormalities in synapse function, neuronal membrane formation, and neuronal death. These abnormalities can result in intellectual disability, delayed development, and DEE.
Thus, metabolic disorders in the developing nervous system are one of the mechanisms for the development of intellectual disability in DEE. Among the causes we considered, pathogenic variants of some enzymes and transporters cause defects in bioenergetics and post-translational modification, leading to synaptic transmission and myelination pathologies, neural network formation pathologies, and neuronal death. Ultimately, all this comes down to the disruption of interneuronal communication and subsequent intellectual disability.
4. Conclusions
Developmental and epileptic encephalopathies (DEEs) are a group of diseases characterized by epileptic seizures, interictal epileptiform activity, and severe developmental delay with cognitive deficits. These pathologies often have a common etiology and influence each other but develop in parallel and in different ways.
A genetic etiology often underlies DEEs. In the last decade, due to the development of next-generation sequencing, many research groups around the world have discovered many pathogenic variants that cause DEE. These are often monogenic disorders that either occur de novo or are inherited recessively. The most frequent variants that cause DDE, associated with channelopathies, disrupt the function of the genes that encode voltage-dependent sodium and potassium channels, such as, for example, SCN2A and KCNQ2.
However, many DEE-causing variants have been described recently whose gene products control processes other than current conductance: metabolic disorders, membrane transport, and growth and proliferation during neurogenesis. These findings demonstrate that the pathogenesis of DEE extends far beyond neuronal transmission and any disruption of the correct numbers and proportions of different types of neurons, their positioning, synaptic input and output, axonal and dendritic transport, and energy consumption can disrupt the correct excitation/inhibition balance and cause very severe consequences in brain function that will be manifested in epileptiform activity and cause intellectual disability.
The identification and detailed investigation of the genetic causes of DEE and the molecular cascades involved are important for understanding the molecular basis of pathogenesis responsible for the occurrence of these disorders. Understanding these pathways and determining the genotype–phenotype correlation can help in the diagnosis and genetic counseling of patients’ families. Although in many cases, by the time when the disease has been diagnosed, the cytoarchitecture of the brain has been terminally malformed and treatment is no longer possible, there are DEE cases where the brain structure has not been dramatically changed. Such cases could potentially be treated individually, depending on the molecular cascade affected by the pathogenic gene variant. For example, if the cause is a metabolic disorder, a replacement therapy in combination with gene therapy can be used. Gene constructs or mRNAs that would replace malfunctioning proteins can be delivered to the brain. On the other hand, if the main cause of the disease is a “gain of function” of a certain gene, a small inhibitor molecule can be identified that attenuates the hyperactivity of the gene product. A common feature and problem of many DEEs is pharmacoresistance to antiepileptic drugs. Here, animal models replicating the pathology can be used in individual cases in order to select a treatment with a combination of antiepileptic drugs.
Uncovering the molecular mechanisms of the pathogenesis of intellectual disability in DEE can become the basis for personalized therapy that will improve not only the severity of seizures, but also the cognitive outcome in affected children.
Author Contributions
Conceptualization A.D.M., A.O.K., and E.V.K.; writing—original draft preparation, A.D.M., P.E.A., A.O.K., and E.V.K.; writing—review and editing, A.D.M., P.E.A., A.O.K., E.V.K., and V.S.T.; visualization, A.D.M.; supervision, V.S.T. and E.V.K.; funding acquisition, E.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (project No. FSWR-2023-0029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Engel, J. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Symonds, J.D.; Elliott, K.S.; Shetty, J.; Armstrong, M.; Brunklaus, A.; Cutcutache, I.; A Diver, L.; Dorris, L.; Gardiner, S.; Jollands, A.; et al. Early childhood epilepsies: Epidemiology, classification, aetiology, and socio-economic determinants. Brain 2021, 144, 2879–2891. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52. [Google Scholar] [CrossRef]
- Guerrini, R.; Conti, V.; Mantegazza, M.; Balestrini, S.; Galanopoulou, A.S.; Benfenati, F. Developmental and epileptic encephalopathies: From genetic heterogeneity to phenotypic continuum. Physiol. Rev. 2023, 103, 433–513. [Google Scholar] [CrossRef]
- Surdi, P.; Trivisano, M.; De Dominicis, A.; Mercier, M.; Piscitello, L.M.; Pavia, G.C.; Calabrese, C.; Cappelletti, S.; Correale, C.; Mazzone, L.; et al. Unveiling the Disease Progression in Developmental and Epileptic Encephalopathies: Insights from EEG and Neuropsychology. Epilepsia 2024, 65, 3279–3292. [Google Scholar] [CrossRef]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of Epilepsy: Current Concepts and Future Perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Nariai, H.; Duberstein, S.; Shinnar, S. Treatment of Epileptic Encephalopathies: Current State of the Art. J. Child Neurol. 2017, 33, 41–54. [Google Scholar] [CrossRef]
- Jeffrey, J.S.; Leathem, J.; King, C.; Mefford, H.C.; Ross, K.; Sadleir, L.G. Developmental and epileptic encephalopathy: Personal utility of a genetic diagnosis for families. Epilepsia Open 2020, 6, 149–159. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhao, Y.; Bao, X.; Wei, L.; Wang, J. Gene mutation analysis of 175 Chinese patients with early-onset epileptic encephalopathy. Clin. Genet. 2017, 91, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.B.; Eggers, S.; Dalziel, K.; Riseley, J.; Mandelstam, S.; Myers, C.T.; McMahon, J.M.; Schneider, A.; Carvill, G.L.; Mefford, H.C.; et al. A population-based cost-effectiveness study of early genetic testing in severe epilepsies of infancy. Epilepsia 2018, 59, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Happ, H.C.; Carvill, G.L. A 2020 View on the Genetics of Developmental and Epileptic Encephalopathies. Epilepsy Curr. 2020, 20, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Borowicz-Reutt, K.; Czernia, J.; Krawczyk, M. Genetic Background of Epilepsy and Antiepileptic Treatments. Int. J. Mol. Sci. 2023, 24, 16280. [Google Scholar] [CrossRef] [PubMed]
- Syrbe, S. Developmental and epileptic encephalopathies–therapeutic consequences of genetic testing. Med. Genet. 2022, 34, 215–224. [Google Scholar] [CrossRef]
- Nieh, S.E.; Sherr, E.H. Epileptic Encephalopathies: New Genes and New Pathways. Neurotherapeutics 2014, 11, 796–806. [Google Scholar] [CrossRef]
- Symonds, J.D.; McTague, A. Epilepsy and developmental disorders: Next generation sequencing in the clinic. Eur. J. Paediatr. Neurol. 2019, 24, 15–23. [Google Scholar] [CrossRef]
- Nickels, K.C.; Wirrell, E.C. Cognitive and Social Outcomes of Epileptic Encephalopathies. Semin. Pediatr. Neurol. 2017, 24, 264–275. [Google Scholar] [CrossRef]
- Raspall-Chaure, M.; Chin, R.F.M.; Neville, B.G.; Bedford, H.; Scott, R.C. The Epidemiology of Convulsive Status Epilepticus in Children: A Critical Review. Epilepsia 2007, 48, 1652–1663. [Google Scholar] [CrossRef]
- Holmes, G.L. Cognitive impairment in epilepsy: The role of network abnormalities. Epileptic Disord. 2015, 17, 101–116. [Google Scholar] [CrossRef]
- Lenck-Santini, P.-P.; Scott, R.C. Mechanisms Responsible for Cognitive Impairment in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022772. [Google Scholar] [CrossRef] [PubMed]
- Lado, F.A.; Moshé, S.L. How Do Seizures Stop? Epilepsia 2008, 49, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, D.H.; Sohal, V.S. Neural Oscillations and Synchrony in Brain Dysfunction and Neuropsychiatric Disorders It’s about Time. JAMA Psychiatry 2015, 72, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ademuwagun, I.A.; Rotimi, S.O.; Syrbe, S.; Ajamma, Y.U.; Adebiyi, E. Voltage Gated Sodium Channel Genes in Epilepsy: Mutations, Functional Studies, and Treatment Dimensions. Front. Neurol. 2021, 12, 600050. [Google Scholar] [CrossRef]
- Ohba, C.; Kato, M.; Takahashi, S.; Lerman-Sagie, T.; Lev, D.; Terashima, H.; Kubota, M.; Kawawaki, H.; Matsufuji, M.; Kojima, Y.; et al. Early onset epileptic encephalopathy caused by de novo SCN8A mutations. Epilepsia 2014, 55, 994–1000. [Google Scholar] [CrossRef]
- Lemke, J.R.; Hendrickx, R.; Geider, K.; Laube, B.; Schwake, M.; Harvey, R.J.; James, V.M.; Pepler, A.; Steiner, I.; Hörtnagel, K.; et al. GRIN2B mutations in west syndrome and intellectual disability with focal epilepsy. Ann. Neurol. 2014, 75, 147–154. [Google Scholar] [CrossRef]
- Johannessen, K.; Marini, C.; Pfeffer, S.; Møller, R.S.; Dorn, T.; Niturad, C.; Gardella, E.; Weber, Y.; Søndergård, M.; Hjalgrim, H.; et al. Phenotypic Spectrum of GABRA1: From Generalized Epilepsies to Severe Epileptic Encephalopathies. Neurology 2016, 87, 1140–1151. [Google Scholar] [CrossRef]
- Menezes, L.F.S.; Sabiá Júnior, E.F.; Tibery, D.V.; dos Carneiro, L.A.; Schwartz, E.F. Epilepsy-Related Volt-age-Gated Sodium Channelopathies: A review. Front. Pharmacol. 2020, 11, 1276. [Google Scholar] [CrossRef]
- Bonsi, P.; De Jaco, A.; Fasano, L.; Gubellini, P. Postsynaptic autism spectrum disorder genes and synaptic dysfunction. Neurobiol. Dis. 2021, 162, 105564. [Google Scholar] [CrossRef]
- Goto, A. Synaptic plasticity during systems memory consolidation. Neurosci. Res. 2022, 183, 1–6. [Google Scholar] [CrossRef]
- Spoto, G.; Valentini, G.; Saia, M.C.; Butera, A.; Amore, G.; Salpietro, V.; Nicotera, A.G.; Di Rosa, G. Synapto-pathies in Developmental and Epileptic Encephalopathies: A Focus on Pre-Synaptic Dysfunction. Front. Neurol. 2022, 13, 826211. [Google Scholar] [CrossRef] [PubMed]
- Berecki, G.; Bryson, A.; Terhag, J.; Maljevic, S.; Gazina, E.V.; Hill, S.L.; Petrou, S. SCN1A gain of function in early infantile encephalopathy. Ann. Neurol. 2019, 85, 514–525. [Google Scholar] [CrossRef]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [PubMed]
- Hurni, N.; Kolodziejczak, M.; Tomasello, U.; Badia, J.; Jacobshagen, M.; Prados, J.; Dayer, A. Transient Cell-intrinsic Activity Regulates the Migration and Laminar Positioning of Cortical Projection Neurons. Cereb. Cortex 2017, 27, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Vitaliti, G.; Pavone, P.; Marino, S.; Saporito, M.A.N.; Corsello, G.; Falsaperla, R. Molecular Mechanism In-volved in the Pathogenesis of Early-Onset Epileptic Encephalopathy. Front. Mol. Neurosci. 2019, 12, 118. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Langille, J.J.; Brown, R.E. The Synaptic Theory of Memory: A Historical Survey and Reconciliation of Recent Opposition. Front. Syst. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Han, T.; Qin, Y.; Mou, C.; Wang, M.; Jiang, M.; Liu, B. Seizure Induced Synaptic Plasticity Alteration in Hip-pocampus Is Mediated by IL-1β Receptor through PI3K/Akt Pathway. Am. J. Transl. Res. 2016, 8, 4499. [Google Scholar]
- Lin, H.; Hangya, B.; Fox, S.E.; Muller, R.U. Repetitive Convulsant-Induced Seizures Reduce the Number But Not Precision of Hippocampal Place Cells. J. Neurosci. 2012, 32, 4163–4178. [Google Scholar] [CrossRef]
- Mitsui, Y.; Sato, H.; Togi, S.; Ura, H.; Niida, Y. A case of SCN8A-related developmental epileptic encephalopathy diagnosed by clinical speculation driven targeted DNA sequencing and remission of epilepsy by sodium channel blockers combination therapy. Brain Dev. Case Rep. 2024, 2, 100015. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Liao, J. When Monogenic Isn’t Monogenic—Unravelling the Oligogenic Architecture of the Developmental and Epileptic Encephalopathies. Epilepsy Curr. 2019, 19, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Hong, S.-Y.; Lin, W.-D.; Lin, C.-H.; Lin, S.-S.; Tsai, F.-J.; Chou, I.-C. Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics. Children 2023, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Papuc, S.M.; Abela, L.; Steindl, K.; Begemann, A.; Simmons, T.L.; Schmitt, B.; Zweier, M.; Oneda, B.; Socher, E.; Crowther, L.M.; et al. The role of recessive inheritance in early-onset epileptic encephalopathies: A combined whole-exome sequencing and copy number study. Eur. J. Hum. Genet. 2018, 27, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.S.; Loddenkemper, T.; Gaínza-Lein, M.; Sheidley, B.R.; Poduri, A. Diagnostic yield of genetic tests in epilepsy. Neurology 2019, 92, E418–E428. [Google Scholar] [CrossRef]
- Bartolini, E. Inherited Developmental and Epileptic Encephalopathies. Neurol. Int. 2021, 13, 555–568. [Google Scholar] [CrossRef]
- Han, X.; Deng, J.; Chen, C.; Wang, X.; Fang, F.; Li, H.; Luo, J.; Wu, J. Developmental and Epileptic Encephalopathy 76: Case Report and Review of Literature. Children 2022, 9, 1967. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Geschwind, D.H. What Does CNTNAP2 Reveal about Autism Spectrum Disorder? Trends Mol. Med. 2012, 18, 156–163. [Google Scholar] [CrossRef]
- Chatron, N.; Møller, R.S.; Champaigne, N.L.; Schneider, A.L.; Kuechler, A.; Labalme, A.; Simonet, T.; Baggett, L.; Bardel, C.; Kamsteeg, E.J.; et al. The epilepsy phenotypic spectrum associated with a recurrent CUX2 variant. Ann. Neurol. 2018, 83, 926–934. [Google Scholar] [CrossRef]
- Begemann, A.; Sticht, H.; Begtrup, A.; Vitobello, A.; Faivre, L.; Banka, S.; Alhaddad, B.; Asadollahi, R.; Becker, J.; Bierhals, T.; et al. New insights into the clinical and molecular spectrum of the novel CYFIP2-related neurodevelopmental disorder and impairment of the WRC-mediated actin dynamics. Anesth. Analg. 2021, 23, 543–554. [Google Scholar] [CrossRef]
- Reiner, O.; Coquelle, F.M.; Peter, B.; Levy, T.; Kaplan, A.; Sapir, T.; Orr, I.; Barkai, N.; Eichele, G.; Bergmann, S. The evolving doublecortin (DCX) superfamily. BMC Genom. 2006, 7, 188. [Google Scholar] [CrossRef]
- Scoto, M.; Rossor, A.M.; Harms, M.B.; Cirak, S.; Calissano, M.; Robb, S.; Manzur, A.Y.; Arroyo, A.M.; Sanz, A.R.; Mansour, S.; et al. Novel mutations expand the clinical spectrum of DYNC1H1 -associated spinal muscular atrophy. Neurology 2015, 84, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.W.; Millichap, J.J.; Soares, D.C.; Chin, R.; McLellan, A.; FitzPatrick, D.R.; Elmslie, F.; Lees, M.M.; Schaefer, G.B.; DDD Study; et al. Novel de novo EEF1A2 missense mutations causing epilepsy and intellectual disability. Mol. Genet. Genom. Med. 2016, 4, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.H.; Pandey, U.B. Function and Dysfunction of GEMIN5: Understanding a Novel Neurodevelopmental Disorder. Neural Regen. Res. 2024, 19, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Al Masseri, Z.; AlSayed, M. Gonadal mosaicism in GNAO1 causing neurodevelopmental disorder with involuntary movements; two additional variants. Mol. Genet. Metab. Rep. 2022, 31, 100864. [Google Scholar] [CrossRef]
- Taylor, J.; Spiller, M.; Ranguin, K.; Vitobello, A.; Philippe, C.; Bruel, A.; Cappuccio, G.; Brunetti-Pierri, N.; Willems, M.; Isidor, B.; et al. Expanding the phenotype of HNRNPU-related neurodevelopmental disorder with emphasis on seizure phenotype and review of literature. Am. J. Med. Genet. Part A 2022, 188, 1497–1514. [Google Scholar] [CrossRef]
- Hecher, L.; Harms, F.L.; Lisfeld, J.; Alawi, M.; Denecke, J.; Kutsche, K. INPP4A-related genetic and phenotypic spectrum and functional relevance of subcellular targeting of INPP4A isoforms. Neurogenetics 2023, 24, 79–93. [Google Scholar] [CrossRef]
- Langhammer, F.; Maroofian, R.; Badar, R.; Gregor, A.; Rochman, M.; Ratliff, J.B.; Koopmans, M.; Herget, T.; Hempel, M.; Kortüm, F.; et al. Genotype-phenotype correlations in RHOBTB2-associated neurodevelopmental disorders. Anesth. Analg. 2023, 25, 100885. [Google Scholar] [CrossRef]
- Liu, J.; Feldman, R.; Zhang, Z.; Deardorff, M.A.; Haverfield, E.V.; Kaur, M.; Li, J.R.; Clark, D.; Kline, A.D.; Waggoner, D.J.; et al. SMC1A expression and mechanism of pathogenicity in probands with X-Linked Cornelia de Lange syndrome. Hum. Mutat. 2009, 30, 1535–1542. [Google Scholar] [CrossRef]
- Tessarech, M.; Friocourt, G.; Marguet, F.; Lecointre, M.; Le Mao, M.; Díaz, R.M.; Mignot, C.; Keren, B.; Héron, B.; De Bie, C.; et al. De novo variants in SP9 cause a novel form of interneuronopathy characterized by intellectual disability, autism spectrum disorder, and epilepsy with variable expressivity. Anesthesia Analg. 2024, 26, 101087. [Google Scholar] [CrossRef]
- Van de Vondel, L.; De Winter, J.; Beijer, D.; Coarelli, G.; Wayand, M.; Palvadeau, R.; Pauly, M.G.; Klein, K.; Rautenberg, M.; Guillot-Noël, L.; et al. De Novo and Dominantly Inherited SPTAN1 Mutations Cause Spastic Paraplegia and Cerebellar Ataxia. Mov. Disord. 2022, 37, 1175–1186. [Google Scholar] [CrossRef]
- Hebebrand, M.; Hüffmeier, U.; Trollmann, R.; Hehr, U.; Uebe, S.; Ekici, A.B.; Kraus, C.; Krumbiegel, M.; Reis, A.; Thiel, C.T.; et al. The mutational and phenotypic spectrum of TUBA1A-associated tubulinopathy. Orphanet J. Rare Dis. 2019, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Striano, P.; Zara, F. ARHGEF9 mutations cause a specific recognizable X-linked intellectual disability syndrome. Neurol. Genet. 2017, 3, e159. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, B.K.; Veenma, D.C.M.; Chang, K.; Schulman, H.; Van Woerden, G.M. Case Report: Developmental Delay and Acute Neuropsychiatric Episodes Associated With a de novo Mutation in the CAMK2B Gene (c.328G>A p.Glu110Lys). Front. Pharmacol. 2022, 13, 794008. [Google Scholar] [CrossRef] [PubMed]
- Wonkam-Tingang, E.; Schrauwen, I.; Esoh, K.K.; Bharadwaj, T.; Nouel-Saied, L.M.; Acharya, A.; Nasir, A.; Leal, S.M.; Wonkam, A. A novel variant in DMXL2 gene is associated with autosomal dominant non-syndromic hearing impairment (DFNA71) in a Cameroonian family. Exp. Biol. Med. 2021, 246, 1524–1532. [Google Scholar] [CrossRef]
- Strehlow, V.; O Heyne, H.; Vlaskamp, D.R.M.; Marwick, K.F.M.; Rudolf, G.; de Bellescize, J.; Biskup, S.; Brilstra, E.H.; Brouwer, O.F.; Callenbach, P.M.C.; et al. GRIN2A-related disorders: Genotype and functional consequence predict phenotype. Brain 2018, 142, 80–92. [Google Scholar] [CrossRef]
- Conroy, J.; Allen, N.M.; Gorman, K.; Shahwan, A.; Ennis, S.; Lynch, S.A.; King, M.D.; King, M.D. NAPB–a novel SNARE-associated protein for early-onset epileptic encephalopathy. Clin. Genet. 2015, 89, E1–E3. [Google Scholar] [CrossRef]
- Schubert, J.; Siekierska, A.; Langlois, M.; May, P.; Huneau, C.; Becker, F.; Muhle, H.; Suls, A.; Lemke, J.R.; de Kovel, C.G.F.; et al. Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat. Genet. 2014, 46, 1327–1332. [Google Scholar] [CrossRef]
- Vlaskamp, D.R.; Shaw, B.J.; Burgess, R.; Mei, D.; Montomoli, M.; Xie, H.; Myers, C.T.; Bennett, M.F.; XiangWei, W.; Williams, D.; et al. SYNGAP1 encephalopathy. Neurology 2019, 92, e96–e107. [Google Scholar] [CrossRef]
- Balestrini, S.; Milh, M.; Castiglioni, C.; Lüthy, K.; Finelli, M.J.; Verstreken, P.; Cardon, A.; Stražišar, B.G.; Holder, J.L.; Lesca, G.; et al. TBC1D24 genotype–phenotype correlation: Epilepsies and other neurologic features. Neurology 2016, 87, 77–85. [Google Scholar] [CrossRef]
- Falk, M.J.; Li, D.; Gai, X.; McCormick, E.; Place, E.; Lasorsa, F.M.; Otieno, F.G.; Hou, C.; Kim, C.E.; Abdel-Magid, N.; et al. AGC1 Deficiency Causes Infantile Epilepsy, Abnormal Myelination, and Reduced N-Acetylaspartate. In JIMD Reports; SSIEM and Springer: Berlin/Heidelberg, Germany, 2014; Volume 14. [Google Scholar]
- Alsharhan, H.; He, M.; Edmondson, A.C.; Daniel, E.J.P.; Chen, J.; Donald, T.; Bakhtiari, S.; Amor, D.J.; Jones, E.A.; Vassallo, G.; et al. ALG13 X-linked intellectual disability: New variants, glycosylation analysis, and expanded phenotypes. J. Inherit. Metab. Dis. 2021, 44, 1001–1012. [Google Scholar] [CrossRef]
- Okur, V.; Cho, M.T.; van Wijk, R.; van Oirschot, B.; Picker, J.; Coury, S.A.; Grange, D.; Manwaring, L.; Krantz, I.; Muraresku, C.C.; et al. De novo variants in HK1 associated with neurodevelopmental abnormalities and visual impairment. Eur. J. Hum. Genet. 2019, 27, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Pronicka, E.; Piekutowska-Abramczuk, D.; Ciara, E.; Trubicka, J.; Rokicki, D.; Karkucińska-Więckowska, A.; Pajdowska, M.; Jurkiewicz, E.; Halat, P.; Kosińska, J.; et al. New perspective in diagnostics of mitochondrial disorders: Two years’ experience with whole-exome sequencing at a national paediatric centre. J. Transl. Med. 2016, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J.; Guo, C.; Duan, Y.; Liu, C.; Tan, Y.; Pan, Y. Clinical report and genetic analysis of a Chinese patient with developmental and epileptic encephalopathy associated with novel biallelic variants in the ST3GAL3 gene. Mol. Genet. Genom. Med. 2023, 12, e2322. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.T.; Wilkinson, K.D.; Reines, D.; Warren, S.T. FMR1 Protein: Conserved RNP Family Domains and Selective RNA Binding. Science 1993, 262, 563–566. [Google Scholar] [CrossRef]
- Zweier, M.; Gregor, A.; Zweier, C.; Engels, H.; Sticht, H.; Wohlleber, E.; Bijlsma, E.K.; Holder, S.E.; Zenker, M.; Rossier, E.; et al. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Hum. Mutat. 2010, 31, 722–733. [Google Scholar] [CrossRef]
- Carvill, G.L.; Heavin, S.B.; Yendle, S.C.; McMahon, J.M.; O’Roak, B.J.; Cook, J.; Khan, A.; Dorschner, M.O.; Weaver, M.; Calvert, S.; et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP. Nat. Genet. 2013, 45, 825–830. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Cassina, M.; Cappellari, A.; Toldo, I.; Nosadini, M.; Rigon, C.; Suppiej, A.; Sartori, S.; Bertossi, C. Forkhead Box G1 Gene Haploinsufficiency: An Emerging Cause of Dyskinetic Encephalopathy of Infancy. Neuropediatrics 2015, 46, 056–064. [Google Scholar] [CrossRef]
- Sapir, T.; Kshirsagar, A.; Gorelik, A.; Olender, T.; Porat, Z.; Scheffer, I.E.; Goldstein, D.B.; Devinsky, O.; Reiner, O. Heterogeneous nuclear ribonucleoprotein U (HNRNPU) safeguards the developing mouse cortex. Nat. Commun. 2022, 13, 4209. [Google Scholar] [CrossRef]
- Dugger, S.A.; Dhindsa, R.S.; Sampaio, G.D.A.; Ressler, A.K.; Rafikian, E.E.; Petri, S.; Letts, V.A.; Teoh, J.; Ye, J.; Colombo, S.; et al. Neurodevelopmental deficits and cell-type-specific transcriptomic perturbations in a mouse model of HNRNPU haploinsufficiency. PLoS Genet. 2023, 19, e1010952. [Google Scholar] [CrossRef]
- Wu, J.I.; Lessard, J.; Olave, I.A.; Qiu, Z.; Ghosh, A.; Graef, I.A.; Crabtree, G.R. Regulation of Dendritic Development by Neuron-Specific Chromatin Remodeling Complexes. Neuron 2007, 56, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Staahl, B.T.; Tang, J.; Wu, W.; Sun, A.; Gitler, A.D.; Yoo, A.S.; Crabtree, G.R. Kinetic Analysis of npBAF to nBAF Switching Reveals Exchange of SS18 with CREST and Integration with Neural Developmental Pathways. J. Neurosci. 2013, 33, 10348–10361. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Ciernia, A.; Matheos, D.P.; Barrett, R.M.; A Kramár, E.; Azzawi, S.; Chen, Y.; Magnan, C.N.; Zeller, M.; Sylvain, A.; Haettig, J.; et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat. Neurosci. 2013, 16, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Ciernia, A.; Wood, M.A. Neuron-specific chromatin remodeling: A missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology 2013, 80, 18–27. [Google Scholar] [CrossRef]
- Bell, S.; Rousseau, J.; Peng, H.; Aouabed, Z.; Priam, P.; Theroux, J.-F.; Jefri, M.; Tanti, A.; Wu, H.; Kolobova, I.; et al. Mutations in ACTL6B Cause Neurodevelopmental Deficits and Epilepsy and Lead to Loss of Dendrites in Human Neurons. Am. J. Hum. Genet. 2019, 104, 815–834. [Google Scholar] [CrossRef]
- Karaca, E.; Harel, T.; Pehlivan, D.; Jhangiani, S.N.; Gambin, T.; Akdemir, Z.C.; Gonzaga-Jauregui, C.; Erdin, S.; Bayram, Y.; Campbell, I.M.; et al. Genes that Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease. Neuron 2015, 88, 499–513. [Google Scholar] [CrossRef]
- Yüksel, Z.; Yazol, M.; Gümüş, E. Pathogenic homozygous variations in ACTL6B cause DECAM syndrome: Developmental delay, Epileptic encephalopathy, Cerebral Atrophy, and abnormal Myelination. Am. J. Med. Genet. Part A 2019, 179, 1603–1608. [Google Scholar] [CrossRef]
- Wenderski, W.; Wang, L.; Krokhotin, A.; Walsh, J.J.; Li, H.; Shoji, H.; Ghosh, S.; George, R.D.; Miller, E.L.; Elias, L.; et al. Loss of the neural-specific BAF subunit ACTL6B relieves repression of early response genes and causes recessive autism. Proc. Natl. Acad. Sci. USA 2020, 117, 10055–10066. [Google Scholar] [CrossRef]
- Ahn, L.Y.; Coatti, G.C.; Liu, J.; Gumus, E.; Schaffer, A.E.; Miranda, H.C. An epilepsy-associated ACTL6B variant captures neuronal hyperexcitability in a human induced pluripotent stem cell model. J. Neurosci. Res. 2021, 99, 110–123. [Google Scholar] [CrossRef]
- Olave, I.; Wang, W.; Xue, Y.; Kuo, A.; Crabtree, G.R. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 2002, 16, 2509–2517. [Google Scholar] [CrossRef]
- Lessard, J.; Wu, J.I.; Ranish, J.A.; Wan, M.; Winslow, M.M.; Staahl, B.T.; Wu, H.; Aebersold, R.; Graef, I.A.; Crabtree, G.R. An Essential Switch in Subunit Composition of a Chromatin Remodeling Complex during Neural Development. Neuron 2007, 55, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.C.; Popp, D.; Gebhard, W.; Kabsch, W. Atomic model of the actin filament. Nature 1990, 347, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Fichera, M.; Failla, P.; Saccuzzo, L.; Miceli, M.; Salvo, E.; Castiglia, L.; Galesi, O.; Grillo, L.; Calì, F.; Greco, D.; et al. Mutations in ACTL6B, coding for a subunit of the neuron-specific chromatin remodeling complex nBAF, cause early onset severe developmental and epileptic encephalopathy with brain hypomyelination and cerebellar atrophy. Hum. Genet. 2019, 138, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Maddirevula, S.; Alzahrani, F.; Al-Owain, M.; Al Muhaizea, M.A.; Kayyali, H.R.; AlHashem, A.; Rahbeeni, Z.; Al-Otaibi, M.; Alzaidan, H.I.; Balobaid, A.; et al. Autozygome and high throughput confirmation of disease genes candidacy. Genet. Med. 2018, 21, 736–742. [Google Scholar] [CrossRef]
- Al-Ubaidi, M.R.; Hollyfield, J.G.; A Overbeek, P.; Baehr, W. Photoreceptor degeneration induced by the expression of simian virus 40 large tumor antigen in the retina of transgenic mice. Proc. Natl. Acad. Sci. USA 1992, 89, 1194–1198. [Google Scholar] [CrossRef]
- Dudek, H.; Datta, S.R.; Franke, T.F.; Birnbaum, M.J.; Yao, R.; Cooper, G.M.; Segal, R.A.; Kaplan, D.R.; Greenberg, M.E. Regulation of Neuronal Survival by the Serine-Threonine Protein Kinase Akt. Science 1997, 275, 661–665. [Google Scholar] [CrossRef]
- Feddersen, R.M.; Yunis, W.S.; O’Donnell, M.A.; Ebner, T.J.; Shenb, L.; Iadecolac, C.; Orr, H.T.; Clarka, H.B. Susceptibility to Cell Death Induced by Mutant SV40 T-Antigen Correlates with Purkinje Neuron Functional Development. Mol. Cell Neurosci. 1997, 9, 42–62. [Google Scholar] [CrossRef]
- Nystuen, A.; Legare, M.E.; Shultz, L.D.; Frankel, W.N. A Null Mutation in Inositol Polyphosphate 4-Phosphatase Type I Causes Selective Neuronal Loss in Weeble Mutant Mice. Neuron 2001, 32, 203–212. [Google Scholar] [CrossRef]
- Sasaki, J.; Kofuji, S.; Itoh, R.; Momiyama, T.; Takayama, K.; Murakami, H.; Chida, S.; Tsuya, Y.; Takasuga, S.; Eguchi, S.; et al. The PtdIns(3,4)P2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature 2010, 465, 497–501. [Google Scholar] [CrossRef]
- Hiebert, S.W.; Lutterbach, B.; Amann, J. Role of co-repressors in transcriptional repression mediated by the t(8;21), t(16;21), t(12;21), and inv(16) fusion proteins. Curr. Opin. Hematol. 2001, 8, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Batra, J.; Mabalirajan, U.; Sharma, S.; Nagarkatti, R.; Aich, J.; Sharma, S.K.; Niphadkar, P.V.; Ghosh, B. A Genetic Variation in Inositol Polyphosphate 4 Phosphatase A Enhances Susceptibility to Asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Duan, C.; Yang, Q. Inositol Phosphatase INPP4A Inhibits the Apoptosis of in Vitro Neu-rons with Characteristic of Intractable Epilepsy by Reducing Intracellular Ca 2+ Concentration. Int. J. Clin. Exp. Pathol. 2018, 11, 1999. [Google Scholar] [PubMed]
- Verrotti, A.; Agostinelli, S.; Prezioso, G.; Coppola, G.; Capovilla, G.; Romeo, A.; Striano, P.; Parisi, P.; Grosso, S.; Spalice, A.; et al. Epilepsy in patients with Cornelia de Lange syndrome: A clinical series. Seizre 2013, 22, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Huisman, S.; Mulder, P.A.; Redeker, E.; Bader, I.; Bisgaard, A.-M.; Brooks, A.; Cereda, A.; Cinca, C.; Clark, D.; Cormier-Daire, V.; et al. Phenotypes and genotypes in individuals with SMC1A variants. Am. J. Med. Genet. Part A 2017, 173, 2108–2125. [Google Scholar] [CrossRef]
- Musio, A. The Multiple Facets of the SMC1A Gene. Gene 2020, 743, 144612. [Google Scholar] [CrossRef]
- Elwan, M.; Fowkes, R.; Lewis-Smith, D.; Winder, A.; Baker, M.R.; Thomas, R.H. Late-onset cluster seizures and intellectual disability associated with a novel truncation variant in SMC1A. Epilepsy Behav. Rep. 2022, 19, 100556. [Google Scholar] [CrossRef]
- Bozarth, X.L.; Lopez, J.; Fang, H.; Lee-Eng, J.; Duan, Z.; Deng, X. Phenotypes and Genotypes in Patients with SMC1A-Related Developmental and Epileptic Encephalopathy. Genes 2023, 14, 852. [Google Scholar] [CrossRef]
- Parmeggiani, L.; Stanzial, F.; Menna, E.; Boni, E.; Manzoni, F.; Benedicenti, F.; Pellegrin, S. Early onset developmental and epileptic encephalopathy and Rett-like phenotype in a 15-year-old girl affected by Cornelia de Lange syndrome type 2 due to a SMC1A gene mutation. Epilepsy Behav. Rep. 2023, 24, 100634. [Google Scholar] [CrossRef]
- Carico, Z.M.; Stefan, H.C.; Justice, M.; Yimit, A.; Dowen, J.M. A cohesin cancer mutation reveals a role for the hinge domain in genome organization and gene expression. PLoS Genet. 2021, 17, e1009435. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.H.; Wu, X.; Kodani, A.; Fan, J.; Doan, R.; Ozawa, M.; Ma, J.; Yoshida, N.; Reiter, J.F.; et al. Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex. Cell 2016, 166, 1147–1162.e15. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; Van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef] [PubMed]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Mastropasqua, F.; Oksanen, M.; Soldini, C.; Alatar, S.; Arora, A.; Ballarino, R.; Molinari, M.; Agostini, F.; Poulet, A.; Watts, M.; et al. Deficiency of the Heterogeneous Nuclear Ribonucleoprotein U locus leads to delayed hindbrain neurogenesis. Biol. Open 2023, 12, bio060113. [Google Scholar] [CrossRef]
- Pacheco, A.; Lopez de Quinto, S.; Ramajo, J.; Fernández, N.; Martínez-Salas, E. A novel role for Gemin5 in mRNA translation. Nucleic Acids Res. 2009, 37, 582–590. [Google Scholar] [CrossRef]
- Yong, J.; Kasim, M.; Bachorik, J.L.; Wan, L.; Dreyfuss, G. Gemin5 Delivers snRNA Precursors to the SMN Complex for snRNP Biogenesis. Mol. Cell 2010, 38, 551–562. [Google Scholar] [CrossRef]
- Workman, E.; Kalda, C.; Patel, A.; Battle, D.J. Gemin5 Binds to the Survival Motor Neuron mRNA to Regulate SMN Expression. J. Biol. Chem. 2015, 290, 15662–15669. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Y.; Liu, C.-P.; Yang, N.; Jin, M.; Cong, Y.; Wang, M.; Xu, R.-M. Structural basis for snRNA recognition by the double-WD40 repeat domain of Gemin. Genes Dev. 2016, 30, 2391–2403. [Google Scholar] [CrossRef]
- Xu, C.; Ishikawa, H.; Izumikawa, K.; Li, L.; He, H.; Nobe, Y.; Yamauchi, Y.; Shahjee, H.M.; Wu, X.-H.; Yu, Y.-T.; et al. Structural insights into Gemin5-guided selection of pre-snRNAs for snRNP assembly. Genes Dev. 2016, 30, 2376–2390. [Google Scholar] [CrossRef]
- Francisco-Velilla, R.; Fernandez-Chamorro, J.; Dotu, I.; Martinez-Salas, E. The landscape of the non-canonical RNA-binding site of Gemin5 unveils a feedback loop counteracting the negative effect on translation. Nucleic Acids Res. 2018, 46, 7339–7353. [Google Scholar] [CrossRef]
- Moreno-Morcillo, M.; Francisco-Velilla, R.; Embarc-Buh, A.; Fernández-Chamorro, J.; Ramón-Maiques, S.; Martinez-Salas, E. Structural basis for the dimerization of Gemin5 and its role in protein recruitment and translation control. Nucleic Acids Res. 2019, 48, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, S.; Francisco-Velilla, R.; Zhang, J.; Embarc-Buh, A.; Abellan, S.; Lv, M.; Tang, P.; Gong, Q.; Shen, H.; et al. Structural basis for Gemin5 decamer-mediated mRNA binding. Nat. Commun. 2022, 13, 5166. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Velilla, R.; Abellan, S.; Oliveros, J.C.; Martinez-Salas, E. Alternative Splicing Events Driven by Altered Levels of GEMIN5 Undergo Translation. RNA Biol. 2024, 21, 23–24. [Google Scholar] [CrossRef]
- Ibrahim, N.; Naz, S.; Mattioli, F.; Guex, N.; Sharif, S.; Iqbal, A.; Ansar, M.; Reymond, A. A Biallelic Truncating Variant in the TPR Domain of GEMIN5 Associated with Intellectual Disability and Cerebral Atrophy. Genes 2023, 14, 707. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhu, G.; Wan, L.; Liang, Y.; Huang, M.; Yang, G. Expand-ing the Clinical Phenotype and Genetic Spectrum of GEMIN5 Disorders: Early-Infantile Developmental and Epileptic Encephalopathies. Brain Behav. 2024, 14, e3535. [Google Scholar] [CrossRef]
- Gubitz, A.K.; Mourelatos, Z.; Abel, L.; Rappsilber, J.; Mann, M.; Dreyfuss, G. Gemin5, a Novel WD Repeat Protein Component of the SMN Complex That Binds Sm Proteins. J. Biol. Chem. 2002, 277, 5631–5636. [Google Scholar] [CrossRef]
- Fernandez-Chamorro, J.; Piñeiro, D.; Gordon, J.M.B.; Ramajo, J.; Francisco-Velilla, R.; Macias, M.J.; Martinez-Salas, E. Identification of novel non-canonical RNA-binding sites in Gemin5 involved in internal initiation of translation. Nucleic Acids Res. 2014, 42, 5742–5754. [Google Scholar] [CrossRef]
- Kour, S.; Rajan, D.S.; Fortuna, T.R.; Anderson, E.N.; Ward, C.; Lee, Y.; Lee, S.; Shin, Y.B.; Chae, J.-H.; Choi, M.; et al. Loss of function mutations in GEMIN5 cause a neurodevelopmental disorder. Nat. Commun. 2021, 12, 2558. [Google Scholar] [CrossRef]
- Gates, J.; Lam, G.; Ortiz, J.A.; Losson, R.; Thummel, C.S. Rigor Mortis Encodes a Novel Nuclear Receptor In-teracting Protein Required for Ecdysone Signaling during Drosophila Larval Development. Development 2004, 131, 25–36. [Google Scholar] [CrossRef]
- Rajan, D.S.; Kour, S.; Fortuna, T.R.; Cousin, M.A.; Barnett, S.S.; Niu, Z.; Babovic-Vuksanovic, D.; Klee, E.W.; Kirmse, B.; Innes, M.; et al. Autosomal Recessive Cerebellar Atrophy and Spastic Ataxia in Patients With Pathogenic Biallelic Variants in GEMIN. Front. Cell Dev. Biol. 2022, 10, 783762. [Google Scholar] [CrossRef]
- Kamma, H.; Portman, D.S.; Dreyfuss, G. Cell Type-Specific Expression of hnRNP Proteins. Exp. Cell Res. 1995, 221, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kukalev, A.; Nord, Y.; Palmberg, C.; Bergman, T.; Percipalle, P. Actin and hnRNP U cooperate for productive transcription by RNA polymerase II. Nat. Struct. Mol. Biol. 2005, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Obrdlik, A.; Kukalev, A.; Louvet, E.; Farrants, A.-K.; Caputo, L.; Percipalle, P. The Histone Acetyltransferase PCAF Associates with Actin and hnRNP U for RNA Polymerase II Transcription. Mol. Cell Biol. 2008, 28, 6342–6357. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Miyaji, M.; Ichiyasu, S.; Tsutsui, K.M.; Tsutsui, K. Regulation of DNA Topoisomerase IIβ through RNA-dependent Association with Heterogeneous Nuclear Ribonucleoprotein U (hnRNP U). J. Biol. Chem. 2010, 285, 26451–26460. [Google Scholar] [CrossRef]
- Huelga, S.C.; Vu, A.Q.; Arnold, J.D.; Liang, T.Y.; Liu, P.P.; Yan, B.Y.; Donohue, J.P.; Shiue, L.; Hoon, S.; Brenner, S.; et al. Integrative Genome-wide Analysis Reveals Cooperative Regulation of Alternative Splicing by hnRNP Proteins. Cell Rep. 2012, 1, 167–178. [Google Scholar] [CrossRef]
- Ye, J.; Beetz, N.; O’keeffe, S.; Tapia, J.C.; Macpherson, L.; Chen, W.V.; Bassel-Duby, R.; Olson, E.N.; Maniatis, T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc. Natl. Acad. Sci. USA 2015, 112, E3020–E3029. [Google Scholar] [CrossRef]
- Fan, H.; Lv, P.; Huo, X.; Wu, J.; Wang, Q.; Cheng, L.; Liu, Y.; Tang, Q.-Q.; Zhang, L.; Zhang, F.; et al. The nuclear matrix protein HNRNPU maintains 3D genome architecture globally in mouse hepatocytes. Genome Res. 2017, 28, 192–202. [Google Scholar] [CrossRef]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef]
- Depienne, C.; DDD Study; Nava, C.; Keren, B.; Heide, S.; Rastetter, A.; Passemard, S.; Chantot-Bastaraud, S.; Moutard, M.-L.; Agrawal, P.B.; et al. Genetic and phenotypic dissection of 1q43q44 microdeletion syndrome and neurodevelopmental phenotypes associated with mutations in ZBTB18 and HNRNPU. Hum. Genet. 2017, 136, 463–479. [Google Scholar] [CrossRef]
- I, D.V.; Aysina, V.A. Early infantile epileptic encephalopathy type 54: Clinical and neurophysiological aspects. Epilepsy Paroxysmal Cond. 2021, 13, 132–139. [Google Scholar] [CrossRef]
- Bramswig, N.C.; Lüdecke, H.-J.; Hamdan, F.F.; Altmüller, J.; Beleggia, F.; Elcioglu, N.H.; Freyer, C.; Gerkes, E.H.; Demirkol, Y.K.; Knupp, K.G.; et al. Heterozygous HNRNPU variants cause early onset epilepsy and severe intellectual disability. Hum. Genet. 2017, 136, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.M.; Vasudevan, P.C.; Chandler, K.E.; E Donnelly, D.; Stark, Z.; Sadedin, S.; Willoughby, J.; Genomics, B.C.F.M.; DDD Study; Balasubramanian, M. De novo mutations in HNRNPU result in a neurodevelopmental syndrome. Am. J. Med. Genet. Part A 2017, 173, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Leduc, M.S.; Chao, H.; Qu, C.; Walkiewicz, M.; Xiao, R.; Magoulas, P.; Pan, S.; Beuten, J.; He, W.; Bernstein, J.A.; et al. Clinical and molecular characterization of de novo loss of function variants in HNRNPU. Am. J. Med. Genet. Part A 2017, 173, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Roshon, M.J.; Ruley, H.E. Hypomorphic mutation in hnRNP U results in post-implantation lethality. Transgenic Res. 2005, 14, 179–192. [Google Scholar] [CrossRef]
- Lalli, M.A.; Avey, D.; Dougherty, J.D.; Milbrandt, J.; Mitra, R.D. High-throughput single-cell functional elucidation of neurodevelopmental disease–associated genes reveals convergent mechanisms altering neuronal differentiation. Genome Res. 2020, 30, 1317–1331. [Google Scholar] [CrossRef]
- Pilaz, L.-J.; McMahon, J.J.; Miller, E.E.; Lennox, A.L.; Suzuki, A.; Salmon, E.; Silver, D.L. Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain. Neuron 2016, 89, 83–99. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Chen, F.; Huang, J.; Luo, Q.; Du, X.; Zhou, J.; Gu, W.; Xu, K. Novel Variant Expands the Clinical Spectrum of CUX2-Associated Developmental and Epileptic Encephalopathies. Front. Genet. 2022, 13, 808181. [Google Scholar] [CrossRef]
- Cubelos, B.; Sebastián-Serrano, A.; Kim, S.; Moreno-Ortiz, C.; Redondo, J.M.; Walsh, C.A.; Nieto, M. Cux-2 Controls the Proliferation of Neuronal Intermediate Precursors of the Cortical Subventricular Zone. Cereb. Cortex 2007, 18, 1758–1770. [Google Scholar] [CrossRef]
- Cubelos, B.; Sebastián-Serrano, A.; Beccari, L.; Calcagnotto, M.E.; Cisneros, E.; Kim, S.; Dopazo, A.; Alvarez-Dolado, M.; Redondo, J.M.; Bovolenta, P.; et al. Cux1 and Cux2 Regulate Dendritic Branching, Spine Morphology, and Synapses of the Upper Layer Neurons of the Cortex. Neuron 2010, 66, 523–535. [Google Scholar] [CrossRef]
- Magno, L.; Asgarian, Z.; Pendolino, V.; Velona, T.; Mackintosh, A.; Lee, F.; Stryjewska, A.; Zimmer, C.; Guillemot, F.; Farrant, M.; et al. Transient developmental imbalance of cortical interneuron subtypes presages long-term changes in behavior. Cell Rep. 2021, 35, 109249. [Google Scholar] [CrossRef]
- Nieto, M.; Monuki, E.S.; Tang, H.; Imitola, J.; Haubst, N.; Khoury, S.J.; Cunningham, J.; Gotz, M.; Walsh, C.A. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J. Comp. Neurol. 2004, 479, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C.; Tiveron, M.-C.; Bodmer, R.; Cremer, H. Dynamics of Cux2 Expression Suggests that an Early Pool of SVZ Precursors is Fated to Become Upper Cortical Layer Neurons. Cereb. Cortex 2004, 14, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Iulianella, A.; Sharma, M.; Durnin, M.; Heuvel, G.B.V.; Trainor, P.A. Cux2(Cutl2) integrates neural progenitor development with cell-cycle progression during spinal cord neurogenesis. Development 2008, 135, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kodera, H.; Akita, T.; Shiina, M.; Kato, M.; Hoshino, H.; Terashima, H.; Osaka, H.; Nakamura, S.; Tohyama, J.; et al. De Novo Mutations in GNAO1, Encoding a Gαo Subunit of Heterotrimeric G Proteins, Cause Epileptic Encephalopathy. Am. J. Hum. Genet. 2013, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, H.; Fukai, R.; Ben-Zeev, B.; Sakai, Y.; Mimaki, M.; Okamoto, N.; Suzuki, Y.; Monden, Y.; Saito, H.; Tziperman, B.; et al. Phenotypic spectrum of GNAO1 variants: Epileptic encephalopathy to involuntary movements with severe developmental delay. Eur. J. Hum. Genet. 2015, 24, 129–134. [Google Scholar] [CrossRef]
- Schirinzi, T.; Garone, G.; Travaglini, L.; Vasco, G.; Galosi, S.; Rios, L.; Castiglioni, C.; Barassi, C.; Battaglia, D.; Gambardella, M.L.; et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Park. Relat. Disord. 2019, 61, 19–25. [Google Scholar] [CrossRef]
- Morrison-Levy, N.; Borlot, F.; Jain, P.; Whitney, R. Early-Onset Developmental and Epileptic Encephalopathies of Infancy: An Overview of the Genetic Basis and Clinical Features. Pediatric Neurol. 2021, 116, 85–94. [Google Scholar] [CrossRef]
- Solis, G.P.; Kozhanova, T.V.; Koval, A.; Zhilina, S.S.; Mescheryakova, T.I.; Abramov, A.A.; Ishmuratov, E.V.; Bolshakova, E.S.; Osipova, K.V.; Ayvazyan, S.O.; et al. Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease. Cells 2021, 10, 2749. [Google Scholar] [CrossRef]
- Pérez-Dueñas, B.; Gorman, K.; Marcé-Grau, A.; Ortigoza-Escobar, J.D.; Macaya, A.; Danti, F.R.; Barwick, K.; Papandreou, A.; Ng, J.; Meyer, E.; et al. The Genetic Landscape of Complex Childhood-Onset Hyperkinetic Movement Disorders. Mov. Disord. 2022, 37, 2197–2209. [Google Scholar] [CrossRef]
- Thiel, M.; Bamborschke, D.; Janzarik, W.G.; Assmann, B.; Zittel, S.; Patzer, S.; Auhuber, A.; Opp, J.; Matzker, E.; Bevot, A.; et al. Genotype–phenotype correlation and treatment effects in young patients with GNAO1-associated disorders. J. Neurol. Neurosurg. Psychiatry 2023, 94, 806–815. [Google Scholar] [CrossRef]
- Taira, R.; Akamine, S.; Okuzono, S.; Fujii, F.; Hatai, E.; Yonemoto, K.; Takemoto, R.; Kato, H.; Masuda, K.; Kato, T.A.; et al. Gnao1 Is a Molecular Switch That Regulates the Rho Signaling Pathway in Differentiating Neurons. Sci. Rep. 2024, 14, 17097. [Google Scholar] [CrossRef] [PubMed]
- Ghil, S.; Kim, B.; Lee, Y.; Suh-Kim, H. Neurite Outgrowth Induced by Cyclic AMP Can Be Modulated by the α Subunit of Go. J. Neurochem. 2000, 74, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Kozasa, T. Functional Characterization of Gαo Signaling through G Protein-Regulated Inducer of Neurite Outgrowth. Mol. Pharmacol. 2004, 67, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Hwangpo, T.A.; Jordan, J.D.; Premsrirut, P.K.; Jayamaran, G.; Licht, J.D.; Iyengar, R.; Neves, S.R. G Protein-regulated Inducer of Neurite Outgrowth (GRIN) Modulates Sprouty Protein Repression of Mitogen-activated Protein Kinase (MAPK) Activation by Growth Factor Stimulation. J. Biol. Chem. 2012, 287, 13674–13685. [Google Scholar] [CrossRef]
- Wettschureck, N.; Offermanns, S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef]
- Huang, Y.; Thathiah, A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015, 589, 1607–1619. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Savitsky, M.; Koval, A.; Solis, G.P.; Valnohova, J.; Katanaev, V.L. Restoration of the GTPase activity and cellular interactions of Gαo mutants by Zn2+ in GNAO1 encephalopathy models. Sci. Adv. 2022, 8, eabn9350. [Google Scholar] [CrossRef]
- Akamine, S.; Okuzono, S.; Yamamoto, H.; Setoyama, D.; Sagata, N.; Ohgidani, M.; Kato, T.A.; Ishitani, T.; Kato, H.; Masuda, K.; et al. GNAO1 organizes the cytoskeletal remodeling and firing of developing neurons. FASEB J. 2020, 34, 16601–16621. [Google Scholar] [CrossRef]
- Domínguez-Carral, J.; Ludlam, W.G.; Segarra, M.J.; Marti, M.F.; Balsells, S.; Muchart, J.; Petrović, D.; Espinoza, I.; Ortigoza-Escobar, J.D.; Martemyanov, K.A.; et al. Severity of GNAO1-Related Disorder Correlates with Changes in G-Protein Function. Ann. Neurol. 2023, 94, 987–1004. [Google Scholar] [CrossRef]
- Silachev, D.; Koval, A.; Savitsky, M.; Padmasola, G.; Quairiaux, C.; Thorel, F.; Katanaev, V.L. Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: From a severe G203R to a milder C215Y mutation. Acta Neuropathol. Commun. 2022, 10, 9. [Google Scholar] [CrossRef]
- Benedetti, M.C.; D’Andrea, T.; Colantoni, A.; Silachev, D.; de Turris, V.; Boussadia, Z.; Babenko, V.A.; Volovikov, E.A.; Belikova, L.; Bogomazova, A.N.; et al. Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties. Heliyon 2024, 10, e26656. [Google Scholar] [CrossRef] [PubMed]
- Suske, G.; Bruford, E.; Philipsen, S. Mammalian SP/KLF transcription factors: Bring in the family. Genomics 2005, 85, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Friocourt, G.; Parnavelas, J.G. Mutations in ARX result in several defects involving GABAergic neurons. Front. Cell Neurosci. 2010, 4, 1437. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Lindtner, S.; Li, Z.; Xu, Z.; Wei, S.; Liang, Q.; Wen, Y.; Tao, G.; You, Y.; et al. Sp9 Regulates Medial Ganglionic Eminence-Derived Cortical Interneuron Development. Cereb. Cortex 2018, 29, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Dobyns, W.B. Malformations of cortical development: Clinical features and genetic causes. Lancet Neurol. 2014, 13, 710–726. [Google Scholar] [CrossRef]
- Lasser, M.; Tiber, J.; Lowery, L.A. The Role of the Microtubule Cytoskeleton in Neurodevelopmental Disor-ders. Front. Cell Neurosci. 2018, 12, 165. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the De-veloping Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef]
- Romaniello, R.; Arrigoni, F.; Fry, A.E.; Bassi, M.T.; Rees, M.I.; Borgatti, R.; Pilz, D.T.; Cushion, T.D. Tubulin genes and malformations of cortical development. Eur. J. Med. Genet. 2018, 61, 744–754. [Google Scholar] [CrossRef]
- Hoff, K.J.; E Aiken, J.; A Gutierrez, M.; Franco, S.J.; Moore, J.K.; Biology, D.; States, U. TUBA1A tubulinopathy mutants disrupt neuron morphogenesis and override XMAP215/Stu2 regulation of microtubule dynamics. eLife 2022, 11, e76189. [Google Scholar] [CrossRef]
- Gloster, A.; El-Bizri, H.; Bamji, S.X.; Rogers, D.; Miller, F.D. Early Induction of Tα1 α-Tubulin Transcription in Neurons of the Developing Nervous System. J. Comp. Neurol. 1999, 405, 45–60. [Google Scholar] [CrossRef]
- Schröter, J.; Döring, J.H.; Garbade, S.F.; Hoffmann, G.F.; Kölker, S.; Ries, M.; Syrbe, S. Cross-sectional quantitative analysis of the natural history of TUBA1A and TUBB2B tubulinopathies. Anesth. Analg. 2021, 23, 516–523. [Google Scholar] [CrossRef]
- Aiken, J.; Buscaglia, G.; Bates, E.A.; Moore, J.K. The α-Tubulin gene TUBA1A in Brain Development: A Key Ingredient in the Neuronal Isotype Blend. J. Dev. Biol. 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Bahi-Buisson, N.; Poirier, K.; Boddaert, N.; Saillour, Y.; Castelnau, L.; Philip, N.; Buyse, G.; Villard, L.; Joriot, S.; Marret, S.; et al. Refinement of cortical dysgeneses spectrum associated with TUBA1A mutations. J. Med. Genet. 2008, 45, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Keays, D.A.; Tian, G.; Poirier, K.; Huang, G.-J.; Siebold, C.; Cleak, J.; Oliver, P.L.; Fray, M.; Harvey, R.J.; Molnár, Z.; et al. Mutations in α-Tubulin Cause Abnormal Neuronal Migration in Mice and Lissencephaly in Humans. Cell 2007, 128, 45–57. [Google Scholar] [CrossRef]
- Bodakuntla, S.; Jijumon, A.S.; Villablanca, C.; Gonzalez-Billault, C.; Janke, C. Microtubule-Associated Pro-teins: Structuring the Cytoskeleton. Trends Cell Biol. 2019, 29, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Pilz, D.T.; Babatz, T.D.; Cushion, T.D.; Harvey, K.; Topf, M.; Yates, L.; Robb, S.; Uyanik, G.; Mancini, G.M.; et al. TUBA1A mutations cause wide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on alpha tubulins. Hum. Mol. Genet. 2010, 19, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Fallet-Bianco, C.; Laquerrière, A.; Poirier, K.; Razavi, F.; Guimiot, F.; Dias, P.; Loeuillet, L.; Lascelles, K.; Beldjord, C.; Carion, N.; et al. Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol. Commun. 2014, 2, 69. [Google Scholar] [CrossRef]
- Hung, K.-L.; Lu, J.-F.; Su, D.-J.; Hsu, S.-J.; Wang, L.-C. Tubulinopathy Presenting as Developmental and Epileptic Encephalopathy. Children 2022, 9, 1105. [Google Scholar] [CrossRef]
- Belvindrah, R.; Natarajan, K.; Shabajee, P.; Bruel-Jungerman, E.; Bernard, J.; Goutierre, M.; Moutkine, I.; Jaglin, X.H.; Savariradjane, M.; Irinopoulou, T.; et al. Mutation of the α-tubulin Tuba1a leads to straighter microtubules and perturbs neuronal migration. J. Cell Biol. 2017, 216, 2443–2461. [Google Scholar] [CrossRef]
- Aiken, J.; Buscaglia, G.; Aiken, A.S.; Moore, J.K.; Bates, E.A. Tubulin mutations in brain development disorders: Why haploinsufficiency does not explain TUBA1A tubulinopathies. Cytoskeleton 2020, 77, 40–54. [Google Scholar] [CrossRef]
- Bittermann, E.; Abdelhamed, Z.; Liegel, R.P.; Menke, C.; Timms, A.; Beier, D.R.; Stottmann, R.W. Differential requirements of tubulin genes in mammalian forebrain development. PLoS Genet. 2019, 15, e1008243. [Google Scholar] [CrossRef] [PubMed]
- Leca, I.; Phillips, A.W.; Ushakova, L.; Cushion, T.D.; Keays, D.A. Codon modification of Tuba1a alters mRNA levels and causes a severe neurodevelopmental phenotype in mice. Sci. Rep. 2023, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.-C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin Is a Developmentally Regulated, Microtubule-Associated Protein Expressed in Migrating and Differentiating Neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; A Flanagan, L.; A Walsh, C. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ramos, R.L.; Ackman, J.B.; Thomas, A.M.; Lee, R.V.; LoTurco, J.J. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 2003, 6, 1277–1283. [Google Scholar] [CrossRef]
- Bahi-Buisson, N.; Souville, I.; Fourniol, F.J.; Toussaint, A.; Moores, C.A.; Houdusse, A.; Lemaitre, J.Y.; Poirier, K.; Khalaf-Nazzal, R.; Hully, M.; et al. New insights into genotype–phenotype correlations for the doublecortin-related lissencephaly spectrum. Brain 2013, 136, 223–244. [Google Scholar] [CrossRef]
- Katsarou, A.; Moshé, S.L.; Galanopoulou, A.S. Interneuronopathies and their role in early life epilepsies and neurodevelopmental disorders. Epilepsia Open 2017, 2, 284–306. [Google Scholar] [CrossRef]
- Procopio, R.; Fortunato, F.; Gagliardi, M.; Talarico, M.; Sammarra, I.; Sarubbi, M.C.; Malanga, D.; Annesi, G.; Gambardell, A. Phenotypic Variability in Novel Doublecortin Gene Variants Associated with Subcortical Band Heterotopia. Int. J. Mol. Sci. 2024, 25, 5505. [Google Scholar] [CrossRef]
- Mahmud, R. Subcortical Band Heterotopia Presented With Refractory Epilepsy and Reversible Aphasia. Cureus 2021, 13, e16990. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J.; Liu, Y.; Hsu, T.; Lin, K.; Chen, C.; Lin, C.; Tsai, M.; Tsai, J. Novel lissencephaly-associated DCX variants in the C-terminal DCX domain affect microtubule binding and dynamics. Epilepsia 2022, 63, 1253–1265. [Google Scholar] [CrossRef]
- Stouffer, M.; Khalaf-Nazzal, R.; Cifuentes-Diaz, C.; Albertini, G.; Bandet, E.; Grannec, G.; Lavilla, V.; Deleuze, J.-F.; Olaso, R.; Nosten-Bertrand, M.; et al. Doublecortin mutation leads to persistent defects in the Golgi apparatus and mitochondria in adult hippocampal pyramidal cells. Neurobiol. Dis. 2022, 168, 105702. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Leventer, R.; Kuc, J.; Mewborn, S.; Dudlicek, L.L.; Ramocki, M.B.; Pilz, D.T.; Mills, P.L.; Das, S.; Ross, M.E.; et al. Mutation analysis of the DCX gene and genotype/phenotype correlation in subcortical band heterotopia. Eur. J. Hum. Genet. 2001, 9, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Leventer, R.J. Genotype-Phenotype Correlation in Lissencephaly and Subcortical Band Heterotopia: The Key Questions Answered. J. Child Neurol. 2005, 20, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Cuccurullo, C.; Rubino, M.; De Vita, G.; Terrone, G.; Bilo, L.; Coppola, A.-N. X-Linked Epilepsies: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4110. [Google Scholar] [CrossRef]
- Cioni, J.-M.; Wong, H.H.-W.; Bressan, D.; Kodama, L.; Harris, W.A.; Holt, C.E. Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron 2018, 97, 1078–1093.e6. [Google Scholar] [CrossRef]
- Chen, B.; Chou, H.-T.; Brautigam, C.A.; Xing, W.; Yang, S.; Henry, L.; Doolittle, L.K.; Walz, T.; Rosen, M.K. Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. eLife 2017, 6, e29795. [Google Scholar] [CrossRef]
- Schaks, M.; Reinke, M.; Witke, W.; Rottner, K. Molecular Dissection of Neurodevelopmental Disorder-Causing Mutations in CYFIP. Cells 2020, 9, 1355. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, D.; Ryu, J.R.; Zhang, Y.; Kim, S.; Kim, Y.; Lee, B.; Sun, W.; Han, K. Phosphorylation of CYFIP2, a component of the WAVE-regulatory complex, regulates dendritic spine density and neurite outgrowth in cultured hippocampal neurons potentially by affecting the complex assembly. NeuroReport 2017, 28, 749–754. [Google Scholar] [CrossRef]
- Schaks, M.; Singh, S.P.; Kage, F.; Thomason, P.; Klünemann, T.; Steffen, A.; Blankenfeldt, W.; Stradal, T.E.; Insall, R.H.; Rottner, K. Distinct Interaction Sites of Rac GTPase with WAVE Regulatory Complex Have Non-redundant Functions in Vivo. Curr. Biol. 2018, 28, 3674–3684.e6. [Google Scholar] [CrossRef]
- Abekhoukh, S.; Bardoni, B. CYFIP family proteins between autism and intellectual disability: Links with Fragile X syndrome. Front. Cell Neurosci. 2014, 8, 81. [Google Scholar] [CrossRef]
- Nakashima, M.; Kato, M.; Aoto, K.; Shiina, M.; Belal, H.; Mukaida, S.; Kumada, S.; Sato, A.; Zerem, A.; Lerman-Sagie, T.; et al. De novo hotspot variants in CYFIP2 cause early-onset epileptic encephalopathy. Ann. Neurol. 2018, 83, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.A.; Xiao, R.; Bekheirnia, M.R.; Kanani, F.; Parker, M.J.; Koenig, M.K.; van Haeringen, A.; Ruivenkamp, C.; Rosmaninho-Salgado, J.; Almeida, P.M.; et al. Heterozygous variants in SPTBN1 cause intellectual disability and autism. Am. J. Med. Genet. Part A 2021, 185, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.-M.; Zhang, C.; Zollinger, D.R.; Leterrier, C.; Rasband, M.N. An αII Spectrin-Based Cytoskeleton Protects Large-Diameter Myelinated Axons from Degeneration. J. Neurosci. 2017, 37, 11323–11334. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, J.; Nakashima, M.; Nabatame, S.; Gaik-Siew, C.; Miyata, R.; Rener-Primec, Z.; Kato, M.; Matsumoto, N.; Saitsu, H. SPTAN1 encephalopathy: Distinct phenotypes and genotypes. J. Hum. Genet. 2015, 60, 167–173. [Google Scholar] [CrossRef]
- Leveille, E.; Estiar, M.A.; Krohn, L.; Spiegelman, D.; Dionne-Laporte, A.; Dupré, N.; Trempe, J.F.; Rouleau, G.A.; Gan-Or, Z. SPTAN1 variants as a potential cause for autosomal recessive hereditary spastic paraplegia. J. Hum. Genet. 2019, 64, 1145–1151. [Google Scholar] [CrossRef]
- Siripurapu, V.; Meth, J.; Kobayashi, N.; Hamaguchi, M. DBC2 Significantly Influences Cell-cycle, Apoptosis, Cytoskeleton and Membrane-trafficking Pathways. J. Mol. Biol. 2005, 346, 83–89. [Google Scholar] [CrossRef]
- Freeman, S.N.; Ma, Y.; Cress, W.D. RhoBTB2 (DBC2) Is a Mitotic E2F1 Target Gene with a Novel Role in Apoptosis. J. Biol. Chem. 2008, 283, 2353–2362. [Google Scholar] [CrossRef]
- Santos, P.K.F.; Araujo, N.d.S.; Françoso, E.; Zuntini, A.R.; Arias, M.C. Diapause in a tropical oil-collecting bee: Molecular basis unveiled by RNA-Seq. BMC Genom. 2018, 19, 305. [Google Scholar] [CrossRef]
- Straub, J.; Konrad, E.D.; Grüner, J.; Toutain, A.; Bok, L.A.; Cho, M.T.; Crawford, H.P.; Dubbs, H.; Douglas, G.; Jobling, R.; et al. Missense Variants in RHOBTB2 Cause a Developmental and Epileptic Encephalopathy in Humans, and Altered Levels Cause Neurological Defects in Drosophila. Am. J. Hum. Genet. 2017, 102, 44–57. [Google Scholar] [CrossRef]
- Chung, C.-T.; Lee, N.-C.; Fan, S.-P.; Hung, M.-Z.; Lin, Y.-H.; Chen, C.-H.; Jao, T. DYNC1H1 variant associated with epilepsy: Expanding the phenotypic spectrum. Epilepsy Behav. Rep. 2022, 21, 100580. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.-F.; Vlachakis, D.; Papakonstantinou, E.; Zaganas, I.; Patrinos, G.P.; Chrousos, G.P.; Dardiotis, E. A novel variant in DYNC1H1 could contribute to human amyotrophic lateral sclerosis-frontotemporal dementia spectrum. Mol. Case Stud. 2021, 8, a006096. [Google Scholar] [CrossRef] [PubMed]
- Poirier, K.; Lebrun, N.; Broix, L.; Tian, G.; Saillour, Y.; Boscheron, C.; Parrini, E.; Valence, S.; Pierre, B.S.; Oger, M.; et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 2013, 45, 639–647. [Google Scholar] [CrossRef]
- Gandhi, T.; Canepa, C.R.; Adeyelu, T.T.; Adeniyi, P.A.; Lee, C.C. Neuroanatomical Alterations in the CNTNAP2 Mouse Model of Autism Spectrum Disorder. Brain Sci. 2023, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Macrì, S.; Onori, M.P.; Roessner, V.; Laviola, G. Animal models recapitulating the multifactorial origin of Tourette syndrome. Int. Rev. Neurobiol. 2013, 112, 211–237. [Google Scholar]
- Ramanathan, S.; Al-Diwani, A.; Waters , P.; Irania, S.R. The autoantibody-mediated encephalitides: From clinical observations to molecular pathogenesis. J. Neurol. 2021, 268, 1689–1707. [Google Scholar]
- Poot, M. Intragenic CNTNAP2 Deletions: A Bridge Too Far? Mol. Syndromol. 2017, 8, 118–130. [Google Scholar]
- Mann, R.S.; Allman, B.L.; Schmid, S. Developmental changes in electrophysiological properties of auditory cortical neurons in the Cntnap2 knockout rat. J. Neurophysiol. 2023, 129, 937–947. [Google Scholar] [CrossRef]
- Gao, R.; Zaccard, C.R.; Shapiro, L.P.; Dionisio, L.E.; Martin-De-Saavedra, M.D.; Piguel, N.H.; Pratt, C.P.; Horan, K.E.; Penzes, P. The CNTNAP2-CASK complex modulates GluA1 subcellular distribution in interneurons. Neurosci. Lett. 2019, 701, 92–99. [Google Scholar] [CrossRef]
- Martín-De-Saavedra, M.D.; Dos Santos, M.; Culotta, L.; Varea, O.; Spielman, B.P.; Parnell, E.; Forrest, M.P.; Gao, R.; Yoon, S.; McCoig, E.; et al. Shed CNTNAP2 ectodomain is detectable in CSF and regulates Ca2+ homeostasis and network synchrony via PMCA2/ATP2B. Neuron 2021, 110, 627–643.e9. [Google Scholar] [CrossRef]
- Vogt, L.M.; Lorenzo, M.; Prendergast, D.B.; Jobling, R.; Gill, P.J. EEF1A2 pathogenic variant presenting in an infant with failure to thrive and frequent apneas requiring respiratory support. Am. J. Med. Genet. Part A 2022, 188, 3106–3109. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Klann, E. Autism- and epilepsy-associated EEF1A2 mutations lead to translational dysfunction and altered actin bundling. Proc. Natl. Acad. Sci. USA 2023, 120, e2307704120. [Google Scholar] [CrossRef]
- Rumpansuwon, K.; Prommahom, A.; Dharmasaroja, P. eEF1A2 knockdown impairs neuronal proliferation and inhibits neurite outgrowth of differentiating neurons. NeuroReport 2022, 33, 336–344. [Google Scholar] [CrossRef]
- Cali, E.; Rocca, C.; Salpietro, V.; Houlden, H. Epileptic Phenotypes Associated with SNAREs and Related Synaptic Vesicle Exocytosis Machinery. Front. Neurol. 2022, 12, 806506. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Khvotchev, M.; Dulubova, I.; Sun, J.; Dai, H.; Rizo, J.; Südhof, T.C. Dual Modes of Munc18-1/SNARE Interactions Are Coupled by Functionally Critical Binding to Syntaxin-1 N Terminus. J. Neurosci. 2007, 27, 12147–12155. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.H.; Rah, J.-C.; Min, S.-W.; Liu, X.; de Wit, H.; Dulubova, I.; Meyer, A.C.; Rizo, J.; Arancillo, M.; Hammer, R.E.; et al. Conformational Switch of Syntaxin-1 Controls Synaptic Vesicle Fusion. Science 2008, 321, 1507–1510. [Google Scholar] [CrossRef]
- Wolking, S.; May, P.; Mei, D.; Møller, R.S.; Balestrini, S.; Helbig, K.L.; Altuzarra, C.D.; Chatron, N.; Kaiwar, C.; Stöhr, K.; et al. Clinical spectrum of STX1B -related epileptic disorders. Neurology 2019, 92, e1238–e1249. [Google Scholar] [CrossRef]
- Mishima, T.; Fujiwara, T.; Sanada, M.; Kofuji, T.; Kanai-Azuma, M.; Akagawa, K. Syntaxin 1B, but Not Syntaxin 1A, Is Necessary for the Regulation of Synaptic Vesicle Exocytosis and of the Readily Releasable Pool at Central Synapses. PLoS ONE 2014, 9, e90004. [Google Scholar] [CrossRef]
- Constable, J.R.L.; Graham, M.E.; Morgan, A.; Burgoyne, R.D. Amisyn Regulates Exocytosis and Fusion Pore Stability by Both Syntaxin-dependent and Syntaxin-independent Mechanisms. J. Biol. Chem. 2005, 280, 31615–31623. [Google Scholar] [CrossRef]
- Vinci, M.; Costanza, C.; Rando, R.G.; Treccarichi, S.; Saccone, S.; Carotenuto, M.; Roccella, M.; Calì, F.; Elia, M.; Vetri, L. STXBP6 Gene Mutation: A New Form of SNAREopathy Leads to Developmental Epileptic Encephalopathy. Int. J. Mol. Sci. 2023, 24, 16436. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Q.; Chen, Y.; Wu, L.; Liu, X.; Liang, D. Behavioral and Gene Expression Analysis of Stxbp6-Knockout Mice. Brain Sci. 2021, 11, 436. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs-Engines for Membrane Fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Burgalossi, A.; Jung, S.; Meyer, G.; Jockusch, W.J.; Jahn, O.; Taschenberger, H.; O’Connor, V.M.; Nishiki, T.-I.; Takahashi, M.; Brose, N.; et al. SNARE Protein Recycling by αSNAP and βSNAP Supports Synaptic Vesicle Priming. Neuron 2010, 68, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Mignon-Ravix, C.; Riccardi, F.; Daquin, G.; Cacciagli, P.; Lamoureux-Toth, S.; Villard, L.; Villeneuve, N.; Molinari, F. NAPB and developmental and epileptic encephalopathy: Description of the electroclinical profile associated with a novel pathogenic variant. Epilepsia 2023, 64, E127–E134. [Google Scholar] [CrossRef]
- AbdelAleem, A.; Haddad, N.; Al-Ettribi, G.; Crunk, A.; Elsotouhy, A. Cohen syndrome and early-onset epileptic encephalopathy in male triplets: Two disease-causing mutations in VPS13B and NAPB. Neurogenetics 2023, 24, 103–112. [Google Scholar] [CrossRef]
- Chen, H.-J.; Rojas-Soto, M.; Oguni, A.; Kennedy, M.B. A Synaptic Ras-GTPase Activating Protein (p135 SynGAP) Inhibited by CaM Kinase II. Neuron 1998, 20, 895–904. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.-K.; Takamiya, K.; Huganir, R.L. The Role of Synaptic GTPase-Activating Protein in Neuronal Development and Synaptic Plasticity. J. Neurosci. 2003, 23, 1119–1124. [Google Scholar] [CrossRef]
- Carlisle, H.J.; Manzerra, P.; Marcora, E.; Kennedy, M.B. SynGAP Regulates Steady-State and Activity-Dependent Phosphorylation of Cofilin. J. Neurosci. 2008, 28, 13673–13683. [Google Scholar] [CrossRef]
- Araki, Y.; Zeng, M.; Zhang, M.; Huganir, R.L. Rapid Dispersion of SynGAP from Synaptic Spines Triggers AMPA Receptor Insertion and Spine Enlargement during LTP. Neuron 2015, 85, 173–189. [Google Scholar] [CrossRef]
- Clement, J.P.; Aceti, M.; Creson, T.K.; Ozkan, E.D.; Shi, Y.; Reish, N.J.; Almonte, A.G.; Miller, B.H.; Wiltgen, B.J.; Miller, C.A.; et al. Pathogenic SYNGAP1 Mutations Impair Cognitive Development by Disrupting Maturation of Dendritic Spine Synapses. Cell 2012, 151, 709–723. [Google Scholar] [CrossRef]
- McMahon, A.; Barnett, M.; O’Leary, T.; Stoney, P.; Collins, M.; Papadia, S.; Choudhary, J.; Komiyama, N.; Grant, S.; Hardingham, G.; et al. SynGAP isoforms exert opposing effects on synaptic strength. Nat. Commun. 2012, 3, 900. [Google Scholar] [CrossRef]
- Araki, Y.; Hong, I.; Gamache, T.R.; Ju, S.; Collado-Torres, L.; Shin, J.H.; Huganir, R.L.; States, U. SynGAP isoforms differentially regulate synaptic plasticity and dendritic development. eLife 2020, 9, e56273. [Google Scholar] [CrossRef]
- Morimune, T.; Tano, A.; Tanaka, Y.; Yukiue, H.; Yamamoto, T.; Tooyama, I.; Maruo, Y.; Nishimura, M.; Mori, M. Gm14230 controls Tbc1d24 cytoophidia and neuronal cellular juvenescence. PLoS ONE 2021, 16, e0248517. [Google Scholar] [CrossRef] [PubMed]
- Aprile, D.; Fruscione, F.; Baldassari, S.; Fadda, M.; Ferrante, D.; Falace, A.; Buhler, E.; Sartorelli, J.; Represa, A.; Baldelli, P.; et al. TBC1D24 regulates axonal outgrowth and membrane trafficking at the growth cone in rodent and human neurons. Cell Death Differ. 2019, 26, 2464–2478. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Denef, N.; Schüpbach, T. The Vacuolar Proton Pump, V-ATPase, Is Required for Notch Signaling and Endosomal Trafficking in Drosophila. Dev. Cell 2009, 17, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Faronato, M.; Nguyen, V.T.; Patten, D.K.; Lombardo, Y.; Steel, J.H.; Patel, N.; Woodley, L.; Shousha, S.; Pruneri, G.; Coombes, R.C.; et al. DMXL2 drives epithelial to mesenchymal transition in hormonal therapy resistant breast cancer through notch hyper-activation. Oncotarget 2015, 6, 22467–22479. [Google Scholar] [CrossRef]
- Shah, A.A.; Amjad, M.; Hassan, J.-U.; Ullah, A.; Mahmood, A.; Deng, H.; Ali, Y.; Gul, F.; Xia, K. Molecular Insights into the Role of Pathogenic nsSNPs in GRIN2B Gene Provoking Neurodevelopmental Disorders. Genes 2022, 13, 1332. [Google Scholar] [CrossRef]
- Zong, P.; Feng, J.; Yue, Z.; Li, Y.; Wu, G.; Sun, B.; He, Y.; Miller, B.; Yu, A.S.; Su, Z.; et al. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron 2022, 110, 1944–1958.e8. [Google Scholar] [CrossRef]
- Myers, S.J.; Yuan, H.; Kang, J.-Q.; Tan, F.C.K.; Traynelis, S.F.; Low, C.-M. Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Research 2019, 8, 1940. [Google Scholar] [CrossRef]
- Korinek, M.; Serra, M.C.; Abdelrahman, F.E.S.; Dobrovolski, M.; Kuchtiak, V.; Abramova, V.; Fili, K.; Tomovic; Krausova, B.H.; Krusek, J.; et al. Dis-ease-Associated Variants in GRIN1, GRIN2A and GRIN2B Genes: Insights into NMDA Receptor Structure, Function, and Pathophysiology. Physiol. Res. 2024, 73, 413–434. [Google Scholar] [CrossRef]
- Hines, D.J.; Contreras, A.; Garcia, B.; Barker, J.S.; Boren, A.J.; El Achkar, C.M.; Moss, S.J.; Hines, R.M. Human ARHGEF9 intellectual disability syndrome is phenocopied by a mutation that disrupts collybistin binding to the GABAA receptor α2 subunit. Mol. Psychiatry 2022, 27, 1729–1741. [Google Scholar] [CrossRef]
- Alber, M.; Kalscheuer, V.M.; Marco, E.; Sherr, E.; Lesca, G.; Till, M.; Gradek, G.; Wiesener, A.; Korenke, C.; Mercier, S.; et al. ARHGEF9 disease. Neurol. Genet. 2017, 3, e148. [Google Scholar] [CrossRef]
- Hines, R.M.; Maric, H.M.; Hines, D.J.; Modgil, A.; Panzanelli, P.; Nakamura, Y.; Nathanson, A.J.; Cross, A.; Deeb, T.; Brandon, N.J.; et al. Developmental seizures and mortality result from reducing GABAA receptor α2-subunit interaction with collybistin. Nat. Commun. 2018, 9, 3130. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.J.; Onori, M.P.; Borgesius, N.Z.; van de Bree, J.E.; Elgersma-Hooisma, M.; Nio, E.; Bezstarosti, K.; Buitendijk, G.H.; Jolfaei, M.A.; Demmers, J.A.; et al. CAMK2-Dependent Signaling in Neurons Is Essential for Survival. J. Neurosci. 2019, 39, 5424–5439. [Google Scholar] [CrossRef] [PubMed]
- Küry, S.; van Woerden, G.M.; Besnard, T.; Onori, M.P.; Latypova, X.; Towne, M.C.; Cho, M.T.; Prescott, T.E.; Ploeg, M.A.; Sanders, S.; et al. De Novo Mutations in Protein Kinase Genes CAMK2A and CAMK2B Cause Intellectual Disability. Am. J. Hum. Genet. 2017, 101, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Akita, T.; Aoto, K.; Kato, M.; Shiina, M.; Mutoh, H.; Nakashima, M.; Kuki, I.; Okazaki, S.; Magara, S.; Shiihara, T.; et al. De novo variants in CAMK2A and CAMK2B cause neurodevelopmental disorders. Ann. Clin. Transl. Neurol. 2018, 5, 280–296. [Google Scholar] [CrossRef]
- Rumian, N.L.; Freund, R.K.; Dell’acqua, M.L.; Coultrap, S.J.; Bayer, K.U. Decreased nitrosylation of CaMKII causes aging-associated impairments in memory and synaptic plasticity in mice. Sci. Signal. 2023, 16, eade5892. [Google Scholar] [CrossRef]
- Stephenson, J.R.; Wang, X.; Perfitt, T.L.; Parrish, W.P.; Shonesy, B.C.; Marks, C.R.; Mortlock, D.P.; Nakagawa, T.; Sutcliffe, J.S.; Colbran, R.J. A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors. J. Neurosci. 2017, 37, 2216–2233. [Google Scholar] [CrossRef]
- Lee, L.-C.; Su, M.-T.; Huang, H.-Y.; Cho, Y.-C.; Yeh, T.-K.; Chang, C.-Y. Association of CaMK2A and MeCP2 signaling pathways with cognitive ability in adolescents. Mol. Brain 2021, 14. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, 243–256. [Google Scholar] [CrossRef]
- Murakami, K.; Kanno, H.; Tancabelic, J.; Fujii, H. Gene Expression and Biological Significance of Hexokinase in Erythroid Cells. Acta Haematol. 2002, 108, 204–209. [Google Scholar] [CrossRef]
- E Aleshin, A.; Zeng, C.; Bourenkov, G.P.; Bartunik, H.D.; Fromm, H.J.; Honzatko, R.B. The mechanism of regulation of hexokinase: New insights from the crystal structure of recombinant human brain hexokinase complexed with glucose and glucose-6-phosphate. Structure 1998, 6, 39–50. [Google Scholar] [CrossRef]
- Rosano, C.; Sabini, E.; Rizzi, M.; Deriu, D.; Murshudov, G.; Bianchi, M.; Serafini, G.; Magnani, M.; Bolognesi, M. Binding of non-catalytic ATP to human hexokinase I highlights the structural components for enzyme–membrane association control. Structure 1999, 7, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.-Y.; Alechina, O.; Aleshin, A.E.; Fromm, H.J.; Honzatko, R.B. Identification of a Phosphate Regulatory Site and a Low Affinity Binding Site for Glucose 6-Phosphate in the N-terminal Half of Human Brain Hexokinase. J. Biol. Chem. 1998, 273, 19548–19553. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; E Wilson, J. Functional interactions between the noncovalently associated N- and C-terminal halves of mammalian Type I hexokinase. Arch. Biochem. Biophys. 2002, 401, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.L.; Badonyi, M.; Cozens, A.; Foulds, N.; Marsh, J.A.; Rahman, S.; Ross, A.; Schooley, J.; Straub, V.; Quigley, A.J.; et al. Expanding the neurodevelopmental phenotype associated with HK1 de novo heterozygous missense variants. Eur. J. Med. Genet. 2023, 66, 104696. [Google Scholar] [CrossRef]
- Kane, M.S.; Alban, J.; Desquiret-Dumas, V.; Gueguen, N.; Ishak, L.; Ferre, M.; Amati-Bonneau, P.; Procaccio, V.; Bonneau, D.; Lenaers, G.; et al. Autophagy controls the pathogenicity of OPA1 mutations in dominant optic atrophy. J. Cell Mol. Med. 2017, 21, 2284–2297. [Google Scholar] [CrossRef]
- Indiveri, C.; Krämer, R.; Palmieri, F. Reconstitution of the malate/aspartate shuttle from mitochondria. J. Biol. Chem. 1987, 262, 15979–15983. [Google Scholar] [CrossRef]
- Palmieri, L.; Pardo, B.; Lasorsa, F.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.; Walker, J.; Saheki, T.; Satrústegui, J.; et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef]
- Llorente-Folch, I.; Rueda, C.B.; Amigo, I.; del Arco, A.; Saheki, T.; Pardo, B.; Satrústegui, J. Calcium-Regulation of Mitochondrial Respiration Maintains ATP Homeostasis and Requires ARALAR/AGC1-Malate Aspartate Shuttle in Intact Cortical Neurons. J. Neurosci. 2013, 33, 13957–13971. [Google Scholar] [CrossRef]
- Poeta, E.; Petralla, S.; Babini, G.; Renzi, B.; Celauro, L.; Magnifico, M.C.; Barile, S.N.; Masotti, M.; De Chirico, F.; Massenzio, F.; et al. Histone Acetylation Defects in Brain Precursor Cells: A Potential Pathogenic Mechanism Causing Proliferation and Differentiation Dysfunctions in Mitochondrial Aspartate-Glutamate Carrier Isoform 1 Deficiency. Front. Cell Neurosci. 2022, 15, 773709. [Google Scholar] [CrossRef]
- Prasun, P.; Young, S.; Salomons, G.; Werneke, A.; Jiang, Y.; Struys, E.; Paige, M.; Avantaggiati, M.L.; McDonald, M. Expanding the Clinical Spectrum of Mitochondrial Citrate Carrier (SLC25A1) Deficiency: Facial Dysmorphism in Siblings with Epileptic Encephalopathy and Combined D,L-2-Hydroxyglutaric Aciduria. JIMD Rep. 2015, 19, 111–115. [Google Scholar]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trézéguet, V.; Lauquin, G.J.-M.; Brandolin, G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef]
- Thangaratnarajah, C.; Ruprecht, J.J.; Kunji, E.R. Calcium-induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers. Nat. Commun. 2014, 5, 5491. [Google Scholar] [CrossRef] [PubMed]
- Pierri, C.L.; Palmieri, F.; De Grassi, A. Single-nucleotide evolution quantifies the importance of each site along the structure of mitochondrial carriers. Cell Mol. Life Sci. 2013, 71, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim. Biophys. Acta 2016, 1863, 2394–2412. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.; Basu, S.; Bogenhagen, D.F. A Quantitative Proteomic Analysis of Mitochondrial Participation in P19 Cell Neuronal Differentiation. J. Proteome Res. 2007, 7, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Mekala, P.; Yahya, D.; Wu, G.; Ledeen, R.W. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: Evidence for myelin-associated aspartoacylase. J. Neurochem. 2001, 78, 736–745. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wang, J.; Wu, G.; Lu, Z.H.; Chakraborty, G.; Meyenhofer, M.; Tyring, S.K.; Matalon, R. Physiological role of N-acetylaspartate: Contribution to myelinogenesis. Adv. Exp. Med. Biol. 2006, 576, 131–143, discussion 361–363. [Google Scholar]
- Wibom, R.; Lasorsa, F.M.; Töhönen, V.; Barbaro, M.; Sterky, F.H.; Kucinski, T.; Naess, K.; Jonsson, M.; Pierri, C.L.; Palmieri, F.; et al. AGC1 Deficiency Associated with Global Cerebral Hypomyelination. Engl. J. Med. 2009, 361, 489–495. [Google Scholar] [CrossRef]
- Ramos, M.; Pardo, B.; Llorente-Folch, I.; Saheki, T.; del Arco, A.; Satrústegui, J. Deficiency of the mitochondrial transporter of aspartate/glutamate aralar/AGC1 causes hypomyelination and neuronal defects unrelated to myelin deficits in mouse brain. J. Neurosci. Res. 2011, 89, 2008–2017. [Google Scholar] [CrossRef]
- Gómez-Galán, M.; Makarova, J.; Llorente-Folch, I.; Saheki, T.; Pardo, B.; Satrústegui, J.; Herreras, O. Altered Postnatal Development of Cortico—Hippocampal Neuronal Electric Activity in Mice Deficient for the Mitochondrial Aspartate—Glutamate Transporter. J. Cereb. Blood Flow Metab. 2011, 32, 306–317. [Google Scholar] [CrossRef]
- Contreras, L.; Ramirez, L.; Du, J.; Hurley, J.B.; Satrústegui, J.; de la Villa, P. Deficient Glucose and Glutamine Metabolism in Aralar/AGC1/Slc25a12 Knockout Mice Contributes to Altered Visual Function. Mol. Vis. 2016, 22, 1198. [Google Scholar]
- Petralla, S.; Peña-Altamira, L.E.; Poeta, E.; Massenzio, F.; Virgili, M.; Barile, S.N.; Sbano, L.; Profilo, E.; Corricelli, M.; Danese, A.; et al. Deficiency of Mitochondrial Aspartate-Glutamate Carrier 1 Leads to Oligodendrocyte Precursor Cell Proliferation Defects Both In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 4486. [Google Scholar] [CrossRef]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Vautrin, J. The Synaptomatrix: A Solid Though Dynamic Contact Disconnecting Transmissions from Exocy-totic Events. Neurochem. Int. 2010, 57, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Dani, N.; Broadie, K. Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev. Neurobiol. 2011, 72, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Wasser, C.R.; Masiulis, I.; Durakoglugil, M.S.; Lane-Donovan, C.; Xian, X.; Beffert, U.; Agarwala, A.; Hammer, R.E.; Herz, J. Differential splicing and glycosylation of Apoer2 alters synaptic plasticity and fear learning. Sci. Signal. 2014, 7, ra113. [Google Scholar] [CrossRef]
- Gao, P.; Wang, F.; Huo, J.; Wan, D.; Zhang, J.; Niu, J.; Wu, J.; Yu, B.; Sun, T. ALG13 Deficiency Associated with Increased Seizure Susceptibility and Severity. Neuroscience 2019, 409, 204–221. [Google Scholar] [CrossRef]
- De Ligt, J.; Willemsen, M.H.; Van Bon, B.W.; Kleefstra, T.; Yntema, H.G.; Kroes, T.; Vulto-van Silfhout, A.T.; Koolen, D.A.; De Vries, P.; Gilissen, C.; et al. Diagnostic Exome Sequencing in Persons with Severe Intellectual Disability. N. Engl. J. Med. 2012, 367, 1921–1929. [Google Scholar] [CrossRef]
- Gilissen, C.; Hehir-Kwa, J.Y.; Thung, D.T.; van de Vorst, M.; van Bon, B.W.M.; Willemsen, M.H.; Kwint, M.; Janssen, I.M.; Hoischen, A.; Schenck, A.; et al. Genome sequencing identifies major causes of severe intellectual disability. Nature 2014, 511, 344–347. [Google Scholar] [CrossRef]
- Datta, A.N.; Bahi-Buisson, N.; Bienvenu, T.; Buerki, S.E.; Gardiner, F.; Cross, J.H.; Heron, B.; Kaminska, A.; Korff, C.M.; Lepine, A.; et al. The phenotypic spectrum of X-linked, infantile onset ALG13-related developmental and epileptic encephalopathy. Epilepsia 2021, 62, 325–334. [Google Scholar] [CrossRef]
- Gao, X.-D.; Tachikawa, H.; Sato, T.; Jigami, Y.; Dean, N. Alg14 Recruits Alg13 to the Cytoplasmic Face of the Endoplasmic Reticulum to Form a Novel Bipartite UDP-N-acetylglucosamine Transferase Required for the Second Step of N-Linked Glycosylation. J. Biol. Chem. 2005, 280, 36254–36262. [Google Scholar] [CrossRef]
- Averbeck, N.; Gao, X.-D.; Nishimura, S.-I.; Dean, N. Alg13p, the Catalytic Subunit of the Endoplasmic Reticulum UDP-GlcNAc Glycosyltransferase, Is a Target for Proteasomal Degradation. Mol. Biol. Cell 2008, 19, 2169–2178. [Google Scholar] [CrossRef]
- Gao, X.-D.; Moriyama, S.; Miura, N.; Dean, N.; Nishimura, S.-I. Interaction between the C Termini of Alg13 and Alg14 Mediates Formation of the Active UDP-N-acetylglucosamine Transferase Complex. J. Biol. Chem. 2008, 283, 32534–32541. [Google Scholar] [CrossRef] [PubMed]
- Timal, S.; Hoischen, A.; Lehle, L.; Adamowicz, M.; Huijben, K.; Sykut-Cegielska, J.; Paprocka, J.; Jamroz, E.; van Spronsen, F.J.; Körner, C.; et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum. Mol. Genet. 2012, 21, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Mitusińska, K.; Góra, A.; Bogdańska, A.; Rożdżyńska-Świątkowska, A.; Tylki-Szymańska, A.; Jezela-Stanek, A. Structural Analysis of the Effect of Asn107Ser Mutation on Alg13 Activity and Alg13-Alg14 Complex Formation and Expanding the Phenotypic Variability of ALG13-CDG. Biomolecules 2022, 12, 398. [Google Scholar] [CrossRef]
- Kukuruzinska, M.; Lennon, K. Protein N-Glycosylation: Molecular Genetics and Functional Significance. Crit. Rev. Oral Biol. Med. 1998, 9, 415–448. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Intracellular Functions of N-Linked Glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Dennis, J.W.; Nabi, I.R.; Demetriou, M. Metabolism, Cell Surface Organization, and Disease. Cell 2009, 139, 1229–1241. [Google Scholar] [CrossRef]
- Epi4K Consortium; Epilepsy Phenome/Genome Project. De novo mutations in epileptic encephalopathies. Nature 2013, 501, 217–221. [Google Scholar] [CrossRef]
- Bissar-Tadmouri, N.; Donahue, W.L.; Al-Gazali, L.; Nelson, S.F.; Bayrak-Toydemir, P.; Kantarci, S. X chromosome exome sequencing reveals a novel ALG13 mutation in a nonsyndromic intellectual disability family with multiple affected male siblings. Am. J. Med. Genet. Part A 2013, 164, 164–169. [Google Scholar] [CrossRef]
- Esposito, T.; De Stefano, G.; Reccia, M.G.; Di Lorenzo, I.; Napolitano, F.; Scalabrì, F.; Lombardi, A.; Saleem, M.A.; Griffiths, L.R.; Gianfrancesco, F. Dysregulation of the Expression of Asparagine-Linked Glycosylation 13 Short Isoform 2 Affects Nephrin Function by Altering Its N-Linked Glycosylation. Nephron 2017, 136, 143–150. [Google Scholar] [CrossRef]
- Charych, E.I.; Akum, B.F.; Goldberg, J.S.; Jörnsten, R.J.; Rongo, C.; Zheng, J.Q.; Firestein, B.L. Activity-Independent Regulation of Dendrite Patterning by Postsynaptic Density Protein PSD-95. J. Neurosci. 2006, 26, 10164–10176. [Google Scholar] [CrossRef]
- Matsuo, N.; Reijmers, L.; Mayford, M. Spine-Type-Specific Recruitment of Newly Synthesized AMPA Receptors with Learning. Science 2008, 319, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Arikkath, J. Molecular mechanisms of dendrite morphogenesis. Front. Cell Neurosci. 2012, 6, 37943. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Xia, Y.; Wang, C.; Wang, F.; Zhang, C.; Xiao, L.; Zhang, X.; Meng, Y.; Wang, Y.; Ding, J.; et al. Decreased cognitive function of ALG13KO female mice may be related to the decreased plasticity of hippocampal neurons. Neuropeptides 2022, 96, 102290. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T.; Proia, R.L.; Sandhoff, K. Combinatorial Ganglioside Biosynthesis. J. Biol. Chem. 2002, 277, 25859–25862. [Google Scholar] [CrossRef]
- Wang, B. Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Audry, M.; Jeanneau, C.; Imberty, A.; Harduin-Lepers, A.; Delannoy, P.; Breton, C. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology 2010, 21, 716–726. [Google Scholar] [CrossRef]
- Yoo, S.; Motari, M.G.; Susuki, K.; Prendergast, J.; Mountney, A.; Hurtado, A.; Schnaar, R.L. Sialylation regulates brain structure and function. FASEB J. 2015, 29, 3040–3053. [Google Scholar] [CrossRef]
- Rivero, O.; Alhama-Riba, J.; Ku, H.-P.; Fischer, M.; Ortega, G.; Álmos, P.; Diouf, D.; Hove, D.v.D.; Lesch, K.-P. Haploinsufficiency of the Attention-Deficit/Hyperactivity Disorder Risk Gene St3gal3 in Mice Causes Alterations in Cognition and Expression of Genes Involved in Myelination and Sialylation. Front. Genet. 2021, 12, 688488. [Google Scholar] [CrossRef]
- Hu, H.; Eggers, K.; Chen, W.; Garshasbi, M.; Motazacker, M.M.; Wrogemann, K.; Kahrizi, K.; Tzschach, A.; Hosseini, M.; Bahman, I.; et al. ST3GAL3 Mutations Impair the Development of Higher Cognitive Functions. Am. J. Hum. Genet. 2011, 89, 407–414. [Google Scholar] [CrossRef]
- Taniguchi, N.; Honke, K.; Fukuda, M.; Narimatsu, H.; Yamaguchi, Y.; Angata, T. Handbook of Glycosyltransferases and Related Genes, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volumes 1–2. [Google Scholar]
- Edvardson, S.; Baumann, A.; Mühlenhoff, M.; Stephan, O.; Kuss, A.W.; Shaag, A.; He, L.; Zenvirt, S.; Tanzi, R.; Gerardy-Schahn, R.; et al. West syndrome caused by ST3Gal-III deficiency. Epilepsia 2012, 54, e24–e27. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Indellicato, R.; Domenighini, R.; Malagolini, N.; Cereda, A.; Mamoli, D.; Pezzani, L.; Iascone, M.; Dall’olio, F.; Trinchera, M. A novel nonsense and inactivating variant of ST3GAL3 in two infant siblings suffering severe epilepsy and expressing circulating CA19. Glycobiology 2019, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).