Validation of an Ultraviolet Light Response Gene Signature for Predicting Prognosis in Patients with Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Resources
2.2. Gene Prioritization
2.3. Ultraviolet Light Response Prognostic Model
2.4. Analysis of Clinicopathological Features in Relation to Risk Groups
2.5. Functional Enrichment
2.6. Gene Set Enrichment Analysis
2.7. Analysis of the Immune Microenvironment Infiltration
2.8. Association of Gene Expression and Clinicopathological Features
2.9. Validation Cohort
2.10. Statistical Analysis
3. Results
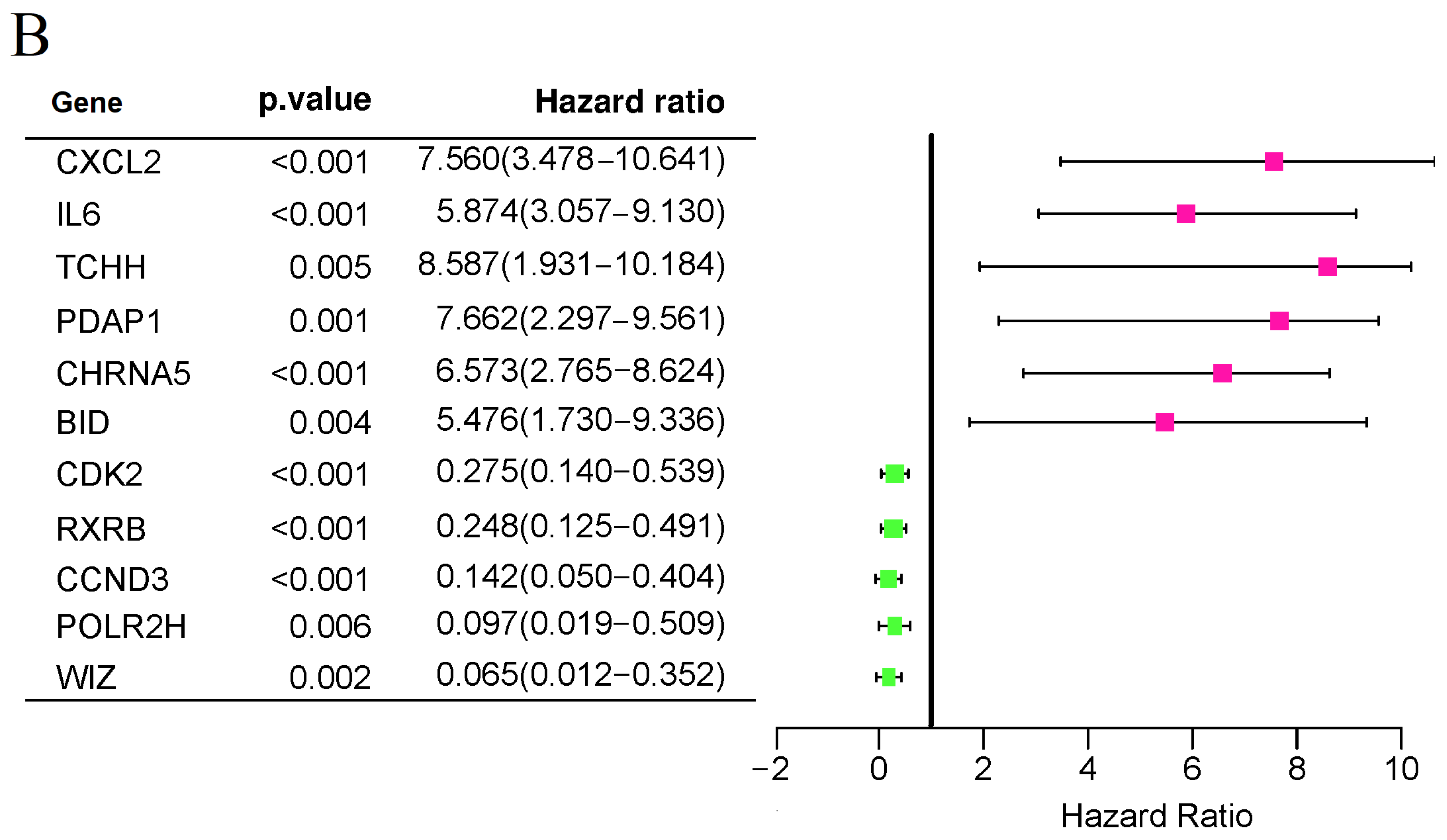
3.1. Identification of UV Response-Related Genes with Prognostic Potential in Uveal Melanoma
3.2. Characteristics of Population
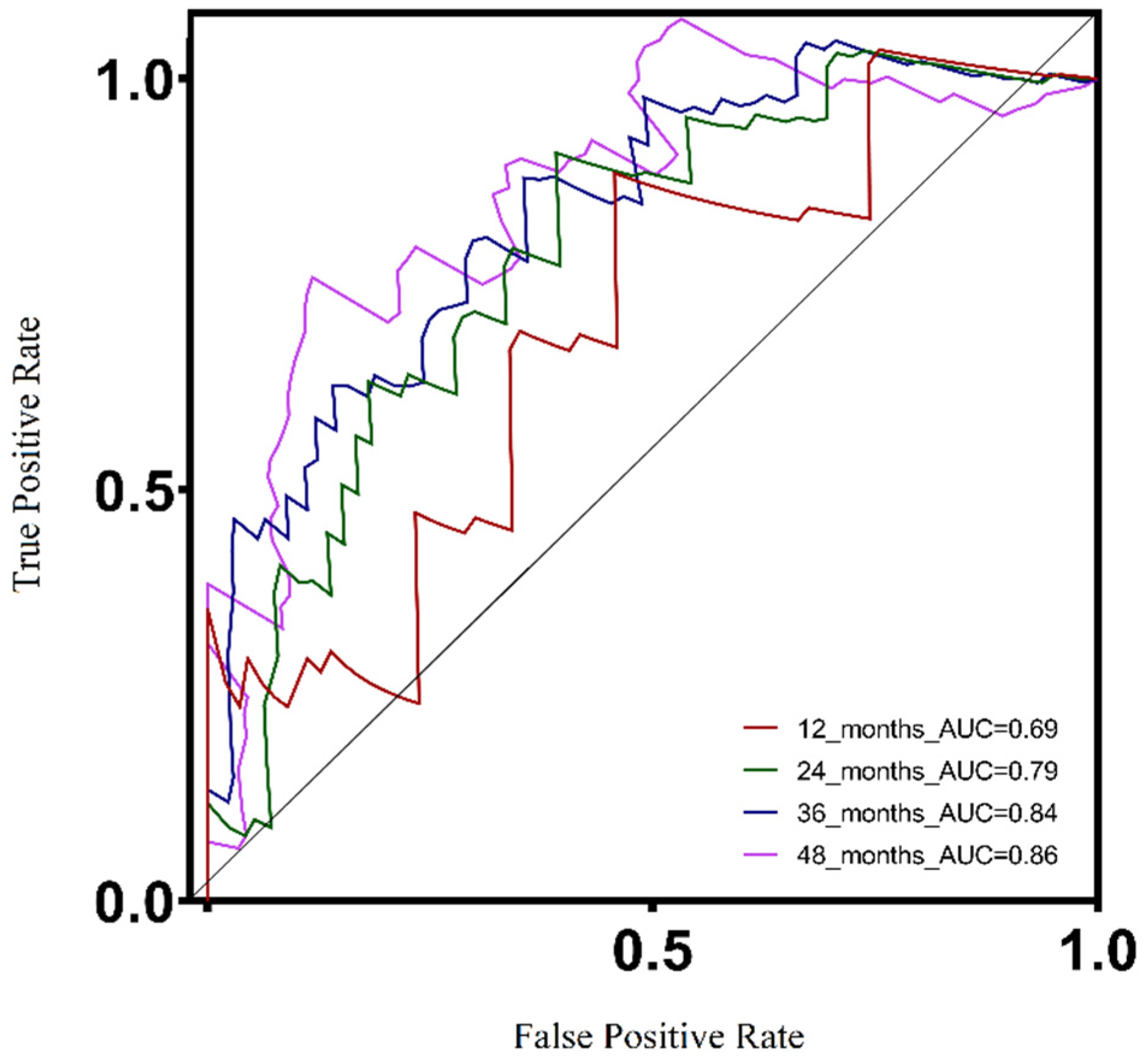
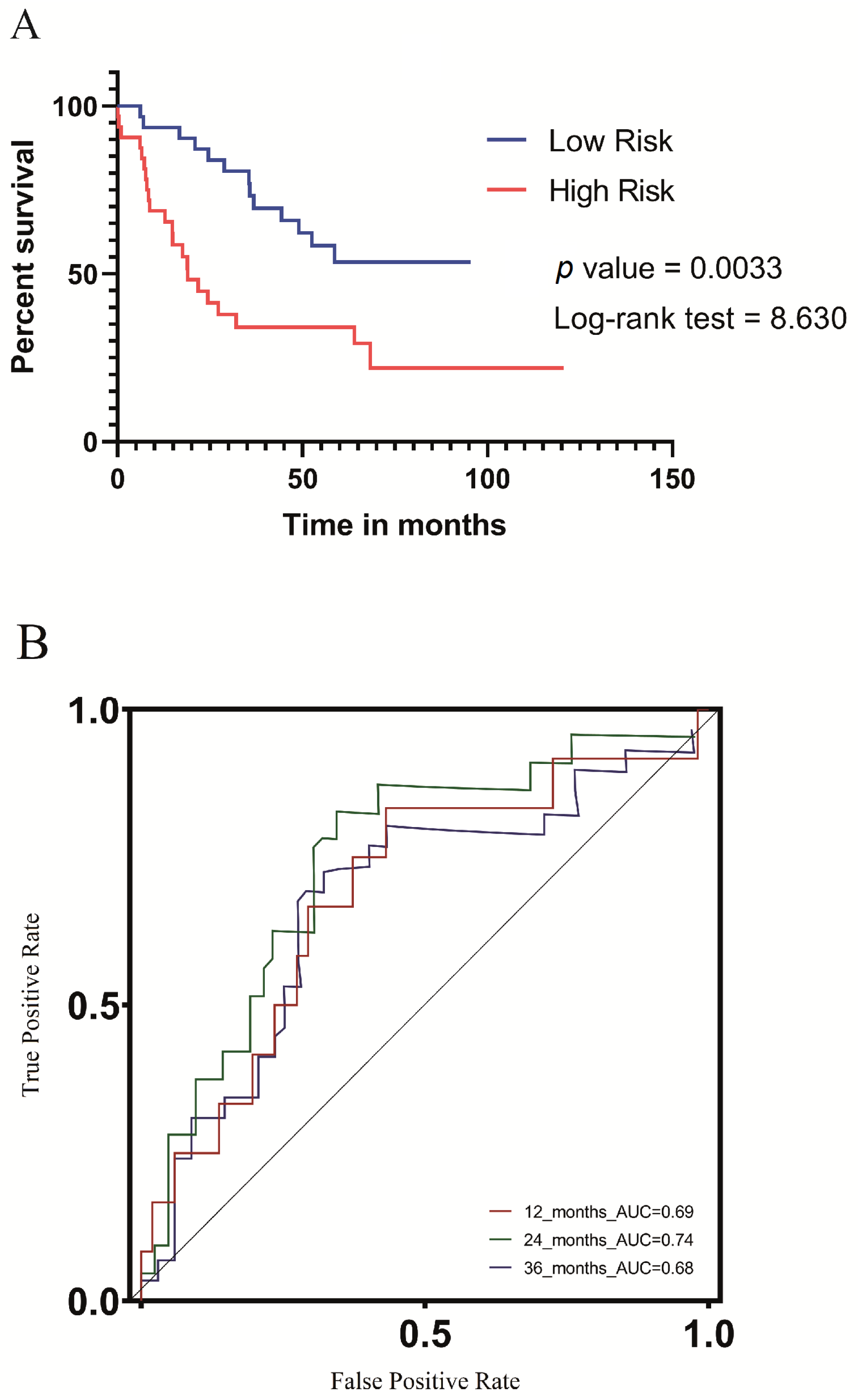
3.3. Survival and Clinicopathological Features Associated with Defined UV Response Risk Scores
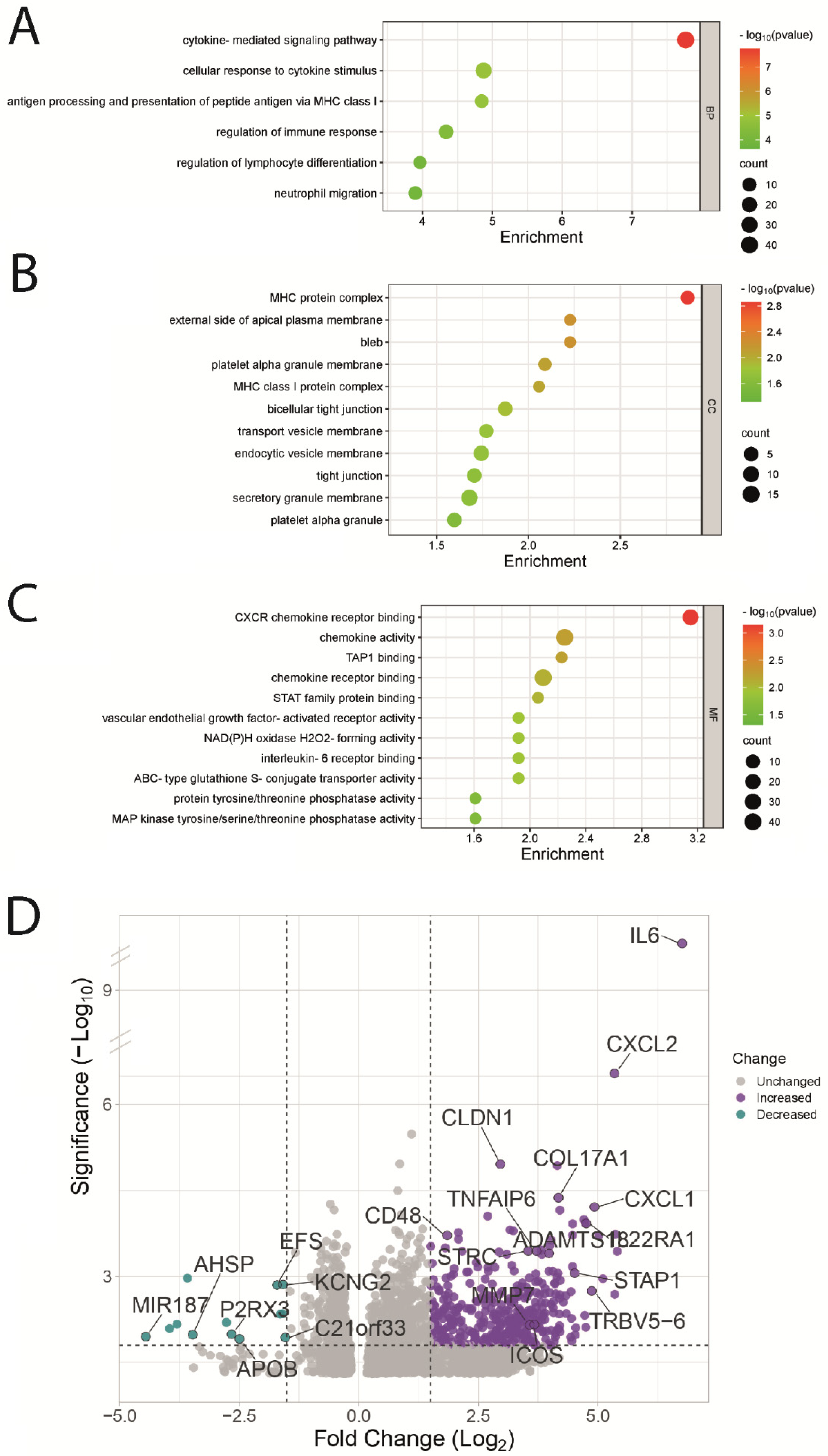
3.4. Functional Enrichment Analysis of UVM Patients by Their Risk Score
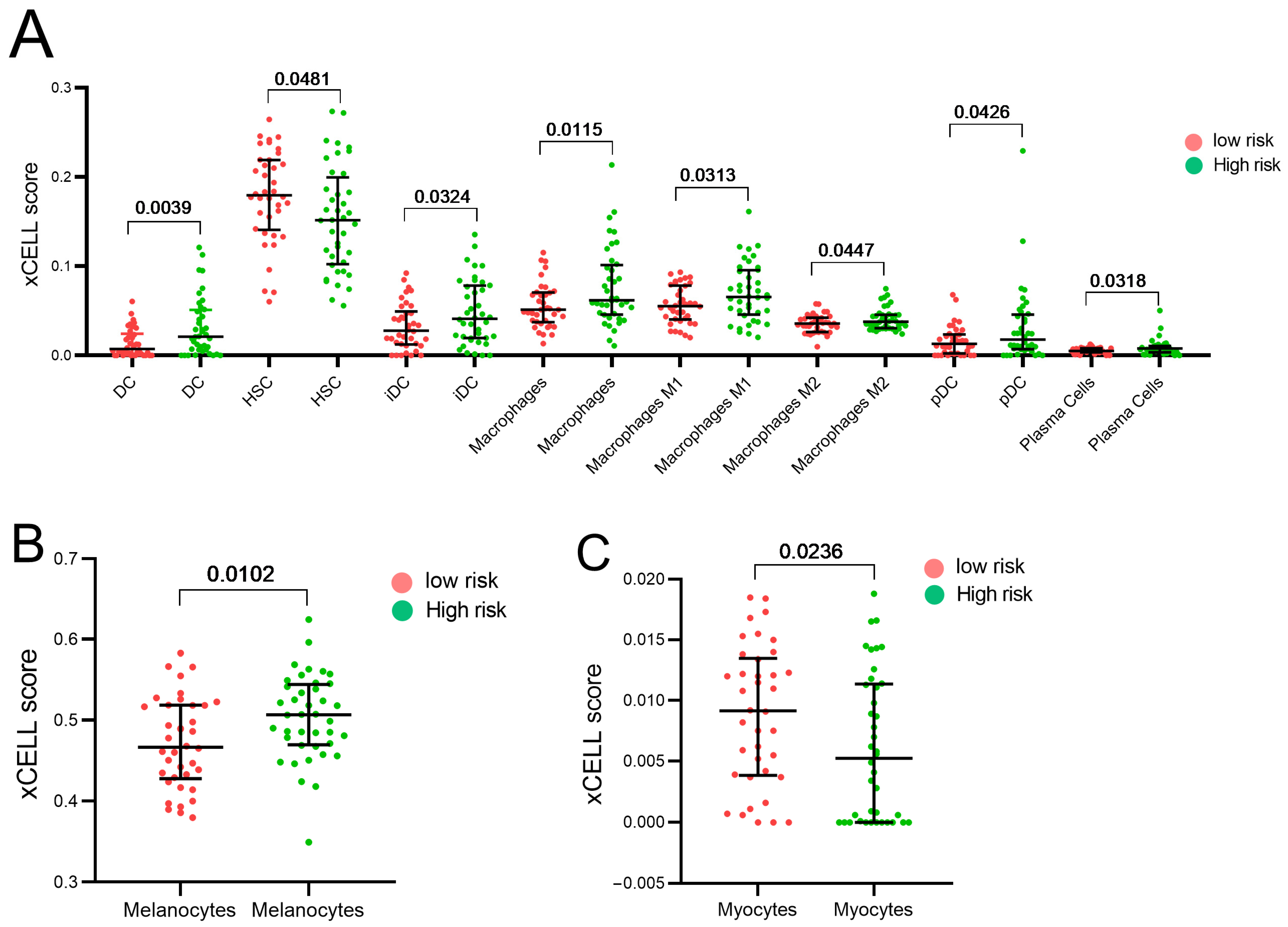
3.5. Estimation of the Stromal and Immune Cells Infiltration Analysis
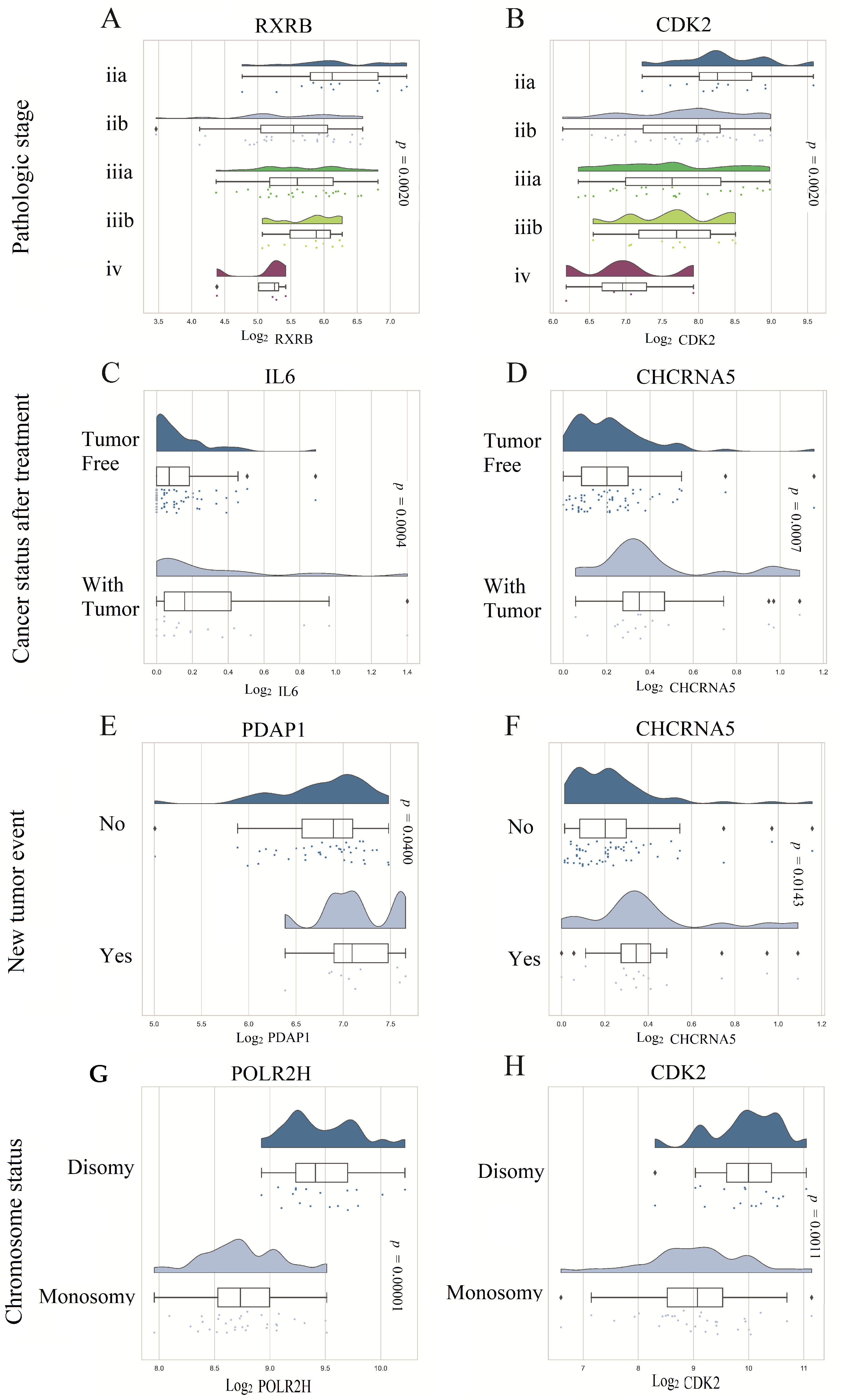
3.6. Prognostic Model Genes Association with Clinicopathological Features of UVM Patients
3.7. Validation of the UV Response Gene Signature in the Context of a Metastatic UVM Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, E.; Croce, M.; Reggiani, F.; Schinzari, G.; Ambrosio, M.; Gangemi, R.; Tortora, G.; Pfeffer, U.; Amaro, A. Uveal Melanoma Metastasis. Cancers 2021, 13, 5684. [Google Scholar] [CrossRef]
- Chua, V.; Lapadula, D.; Randolph, C.; Benovic, J.L.; Wedegaertner, P.B.; Aplin, A.E. Dysregulated GPCR Signaling and Therapeutic Options in Uveal Melanoma. Mol. Cancer Res. MCR 2017, 15, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vidal, C.; Fernandez-Diaz, D.; Fernandez-Marta, B.; Lago-Baameiro, N.; Pardo, M.; Silva, P.; Paniagua, L.; Blanco-Teijeiro, M.J.; Piñeiro, A.; Bande, M. Treatment of Metastatic Uveal Melanoma: Systematic Review. Cancers 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.P.; Lane, A.M.; DeAngelis, M.M.; Mayne, K.; Crabtree, M.; Gragoudas, E.S.; Kim, I.K. Clinical Characteristics of Uveal Melanoma in Patients with Germline BAP1 Mutations. JAMA Ophthalmol. 2015, 133, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yang, J.; Wang, H.; Wei, W. An 11-gene-based prognostic signature for uveal melanoma metastasis based on gene expression and DNA methylation profile. J. Cell. Biochem. 2019, 120, 8630–8639. [Google Scholar] [CrossRef]
- Aaberg, T.M.; Covington, K.R.; Tsai, T.; Shildkrot, Y.; Plasseraud, K.M.; Alsina, K.M.; Oelschlager, K.M.; Monzon, F.A. Gene Expression Profiling in Uveal Melanoma: Five-Year Prospective Outcomes and Meta-Analysis. Ocul. Oncol. Pathol. 2020, 6, 360–367. [Google Scholar] [CrossRef]
- Lamas, N.J.; Martel, A.; Nahon-Estève, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Mallet, J.D.; Gendron, S.P.; Drigeard Desgarnier, M.C.; Rochette, P.J. Implication of ultraviolet light in the etiology of uveal melanoma: A review. Photochem. Photobiol. 2014, 90, 15–21. [Google Scholar] [CrossRef]
- Dolin, P.J.; Foss, A.J.; Hungerford, J.L. Uveal melanoma: Is solar ultraviolet radiation a risk factor? Ophthalmic Epidemiol. 1994, 1, 27–30. [Google Scholar] [CrossRef]
- Holly, E.A.; Aston, D.A.; Char, D.H.; Kristiansen, J.J.; Ahn, D.K. Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Res. 1990, 50, 5773–5777. [Google Scholar]
- Rigel, D.S. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J. Am. Acad. Dermatol. 2008, 58, S129–S132. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M. Gene set knowledge discovery with enrichr. J. Current Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Laurent, C.; Valet, F.; Planque, N.; Silveri, L.; Maacha, S.; Anezo, O.; Hupe, P.; Plancher, C.; Reyes, C.; Albaud, B.; et al. High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 2011, 71, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Nilsson, L.M.; Mitra, S.; Alsén, S.; Shelke, G.V.; Sah, V.R.; Forsberg, E.M.V.; Stierner, U.; All-Eriksson, C.; Einarsdottir, B.; et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat. Commun. 2020, 11, 1894. [Google Scholar] [CrossRef]
- Chalada, M.; Ramlogan-Steel, C.A.; Dhungel, B.P.; Layton, C.J.; Steel, J.C. The Impact of Ultraviolet Radiation on the Aetiology and Development of Uveal Melanoma. Cancers 2021, 13, 1700. [Google Scholar] [CrossRef]
- Tucker, M.A.; Shields, J.A.; Hartge, P.; Augsburger, J.; Hoover, R.N.; Fraumeni, J.F., Jr. Sunlight exposure as risk factor for intraocular malignant melanoma. N. Engl. J. Med. 1985, 313, 789–792. [Google Scholar] [CrossRef]
- Schmidt-Pokrzywniak, A.; Jöckel, K.H.; Bornfeld, N.; Sauerwein, W.; Stang, A. Positive interaction between light iris color and ultraviolet radiation in relation to the risk of uveal melanoma: A case-control study. Ophthalmology 2009, 116, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef]
- Goh, A.Y.; Ramlogan-Steel, C.A.; Jenkins, K.S.; Steel, J.C.; Layton, C.J. Presence and prevalence of UV related genetic mutations in uveal melanoma: Similarities with cutaneous melanoma. Neoplasma 2020, 67, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.A.; Brooks, K.; Newell, F.; Palmer, J.M.; Wilmott, J.S.; Pritchard, A.L.; Broit, N.; Wood, S.; Carlino, M.S.; Leonard, C.; et al. Whole genome landscapes of uveal melanoma show an ultraviolet radiation signature in iris tumours. Nat. Commun. 2020, 11, 2408. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Yang, X.; Jiang, M.; Wang, Q.; Zhang, L.; Ma, Y.; Shen, Z.; Tian, Z.; Cao, X. Cis-acting lnc-Cxcl2 restrains neutrophil-mediated lung inflammation by inhibiting epithelial cell CXCL2 expression in virus infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2108276118. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, J.; Chen, L.; Hu, Y.; Zhai, L.; Yuan, J.; Suo, L.; Shen, Y.; Ye, R.; Li, J.; et al. Construction and Validation of a Novel Pyroptosis-Related Gene Signature to Predict the Prognosis of Uveal Melanoma. Front. Cell Dev. Biol. 2021, 9, 761350. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, M.; Chen, J.; Xu, N. Autophagy-Related Long Non-coding RNA Signature as Indicators for the Prognosis of Uveal Melanoma. Front. Genet. 2021, 12, 625583. [Google Scholar] [CrossRef]
- Zhao, D.D.; Zhao, X.; Li, W.T. Identification of differentially expressed metastatic genes and their signatures to predict the overall survival of uveal melanoma patients by bioinformatics analysis. Int. J. Ophthalmol. 2020, 13, 1046–1053. [Google Scholar] [CrossRef]
- Gao, G.; Yu, Z.; Zhao, X.; Fu, X.; Liu, S.; Liang, S.; Deng, A. Immune classification and identification of prognostic genes for uveal melanoma based on six immune cell signatures. Sci. Rep. 2021, 11, 22244. [Google Scholar] [CrossRef]
- García-Mulero, S.; Alonso, M.H.; Del Carpio, L.P.; Sanz-Pamplona, R.; Piulats, J.M. Additive Role of Immune System Infiltration and Angiogenesis in Uveal Melanoma Progression. Int. J. Mol. Sci. 2021, 22, 2669. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Li, W. A Comprehensive Prognostic and Immunological Analysis of a Six-Gene Signature Associated with Glycolysis and Immune Response in Uveal Melanoma. Front. Immunol. 2021, 12, 738068. [Google Scholar] [CrossRef]
- El-Shabrawi, Y.; Radner, H.; Muellner, K.; Langmann, G.; Hoefler, G. The role of UV-radiation in the development of conjunctival malignant melanoma. Acta Ophthalmol. Scand. 1999, 77, 31–32. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Gregorio, J.; Wang, Y.-H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.-J.J.C.R. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3+ T-regulatory cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S.J.C. Targeting tumor-associated macrophages for cancer treatment. Bioscience 2022, 12, 85. [Google Scholar] [CrossRef]



| Variable | All (n = 80) | Low Risk (n = 40) | High Risk (n = 40) | p Value |
|---|---|---|---|---|
| Age | ||||
| <60 | 36 (43.8%) | 22 | 14 | 0.115 a |
| ≥60 | 44 (56.2%) | 18 | 26 | |
| TNM (Tumor/Node/ Metastasis) | ||||
| T2 | 4 | 4 | 0 | 0.068 b |
| T3 | 36 | 15 | 21 | |
| T4 | 38 | 21 | 17 | |
| Missing data | 2 | 0 | 2 | |
| N0 | 76 | 39 | 37 | 0.304 b |
| NX | 4 | 1 | 3 | |
| M0 | 73 | 38 | 35 | 0.482 b |
| M1 | 3 | 1 | 2 | |
| MX | 4 | 1 | 3 | |
| Pathologic stage | ||||
| IIA | 4 | 3 | 1 | 0.376 b |
| IIB | 32 | 16 | 16 | |
| IIIA | 27 | 15 | 12 | |
| IIIB | 10 | 5 | 5 | |
| IIIC | 3 | 0 | 3 | |
| IV | 4 | 1 | 3 | |
| Cytogenetic abnormality | ||||
| Chr 1 loss | 3 | 2 | 1 | 0.042 b |
| Chr 1 loss Chr 3 loss | 2 | 0 | 2 | |
| Chr 1 loss Chr 3 loss Chr 8q gain | 4 | 0 | 4 | |
| Chr 3 loss | 2 | 1 | 1 | |
| Chr 3 loss Chr 6p gain | 1 | 1 | 0 | |
| Chr 3 loss Chr 6p gain Chr 8q gain | 5 | 3 | 2 | |
| Chr 3 loss Chr 8q gain | 18 | 8 | 10 | |
| Chr 6p gain | 11 | 9 | 2 | |
| Chr 6p gain Chr 8q gain | 5 | 5 | 0 | |
| Chr 8q gain | 1 | 1 | 0 | |
| Missing data | 28 | 10 | 18 | |
| Gender | ||||
| Female | 35 | 19 | 16 | 0.490 b |
| Male | 45 | 21 | 24 | |
| Histological type | ||||
| Epithelioid Cell | 13 | 5 | 8 | 0.322 b |
| Epithelioid Cell Spindle Cell | 21 | 9 | 12 | |
| Spindle Cell | 30 | 19 | 11 | |
| Spindle Cell Epithelioid Cell | 16 | 7 | 9 | |
| Cancer status | ||||
| Tumor free | 54 | 31 | 23 | 0.050 a |
| With tumor | 26 | 9 | 17 | |
| Tumor basal diameter count | ||||
| From 7 to 16 | 27 | 10 | 17 | 0.174 a |
| ≥16 | 52 | 30 | 22 | |
| Missing data | 1 | 0 | 1 | |
| Tumor tissue site | ||||
| Choroid | 56 | 29 | 27 | 0.818 a |
| Choroid Ciliary body | 22 | 10 | 12 | |
| Choroid Ciliary body Iris | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco, C.A.; Mejía-García, A.; Ramírez, M.; González, J.; Castro-Vega, L.; Kreider, R.B.; Serrano, S.; Combita, A.L.; Bonilla, D.A. Validation of an Ultraviolet Light Response Gene Signature for Predicting Prognosis in Patients with Uveal Melanoma. Biomolecules 2023, 13, 1148. https://doi.org/10.3390/biom13071148
Orozco CA, Mejía-García A, Ramírez M, González J, Castro-Vega L, Kreider RB, Serrano S, Combita AL, Bonilla DA. Validation of an Ultraviolet Light Response Gene Signature for Predicting Prognosis in Patients with Uveal Melanoma. Biomolecules. 2023; 13(7):1148. https://doi.org/10.3390/biom13071148
Chicago/Turabian StyleOrozco, Carlos A., Alejandro Mejía-García, Marcela Ramírez, Johanna González, Luis Castro-Vega, Richard B. Kreider, Silvia Serrano, Alba Lucia Combita, and Diego A. Bonilla. 2023. "Validation of an Ultraviolet Light Response Gene Signature for Predicting Prognosis in Patients with Uveal Melanoma" Biomolecules 13, no. 7: 1148. https://doi.org/10.3390/biom13071148
APA StyleOrozco, C. A., Mejía-García, A., Ramírez, M., González, J., Castro-Vega, L., Kreider, R. B., Serrano, S., Combita, A. L., & Bonilla, D. A. (2023). Validation of an Ultraviolet Light Response Gene Signature for Predicting Prognosis in Patients with Uveal Melanoma. Biomolecules, 13(7), 1148. https://doi.org/10.3390/biom13071148









