Pro-Health and Anti-Cancer Activity of Fungal Fractions Isolated from Milk-Supplemented Cultures of Lentinus (Pleurotus) Sajor-caju
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Medium, Growth, Processing, and Preparation of Samples
2.2. Cell Cultures
2.3. Evaluation of Biochemical Properties
2.3.1. Determination of Amino Acids, Proteins, Carbohydrates, and Phenolic Compounds
2.3.2. Determination of Free Radicals
2.3.3. Electrophoretic Assessment of Catalase and Superoxide Dismutase Activity
2.4. Assessment of Anti-Cancer Activity
2.4.1. MTT Assay
2.4.2. NR Uptake Assay
2.4.3. May-Grünwald—Giemsa Staining
2.4.4. Nitric Oxide Secretion Measurement
2.4.5. Apoptosis Evaluation-Hoechst 33342/PI Staining
2.4.6. DAPI/SR101 Staining
2.5. Statistical Analysis
3. Results
3.1. Screening Selection of Samples
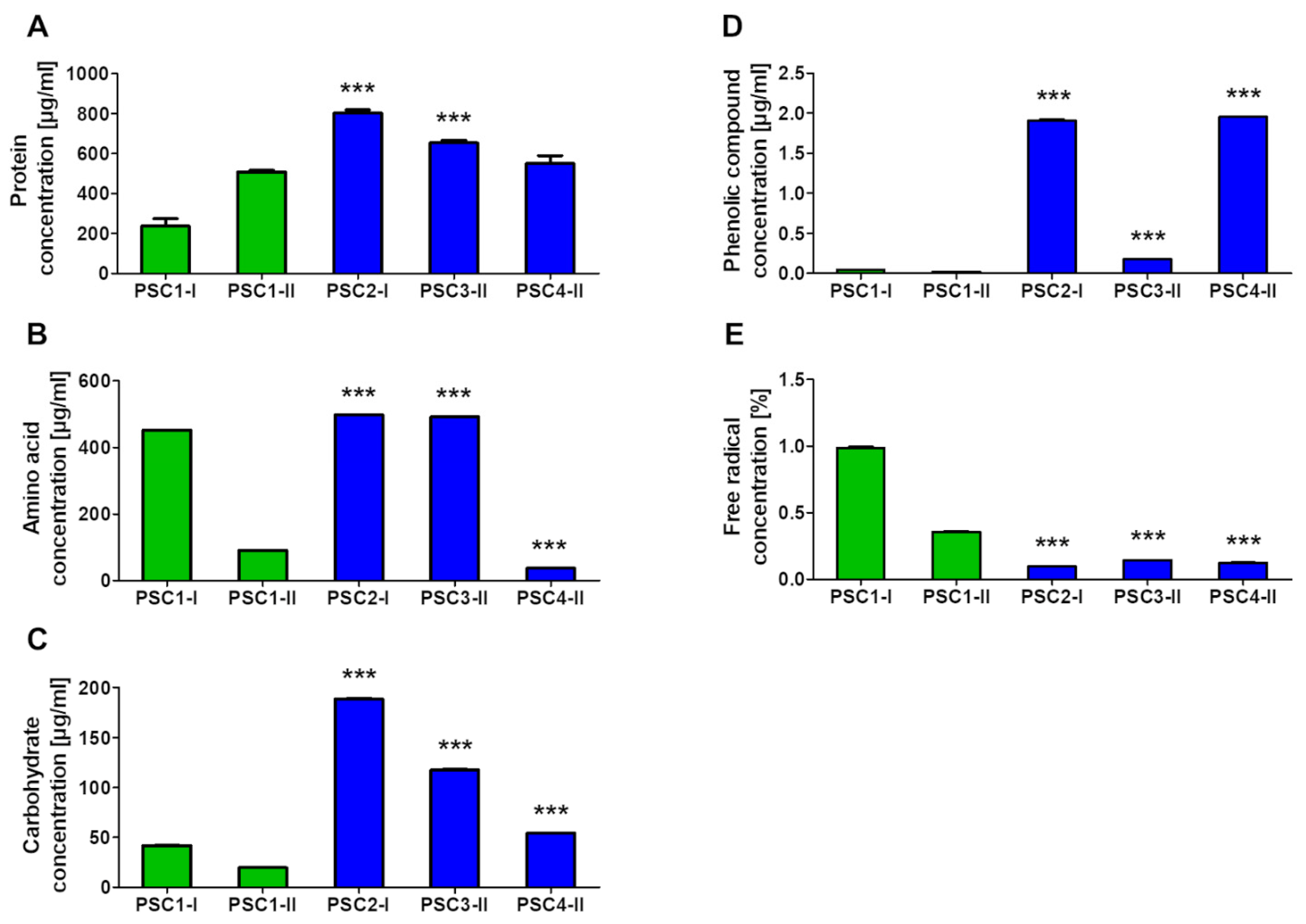
3.2. Biochemical Composition of Extracts
3.2.1. Determination of Proteins, Amino Acids, Carbohydrates, Phenolic Compounds, and Free Radicals
3.2.2. Electrophoretic Analysis of Catalase and SOD Activity
3.3. Evaluation of Anti-Cancer Properties
3.3.1. Cytotoxicity Assessment
3.3.2. Apoptosis and Necrosis Evaluation
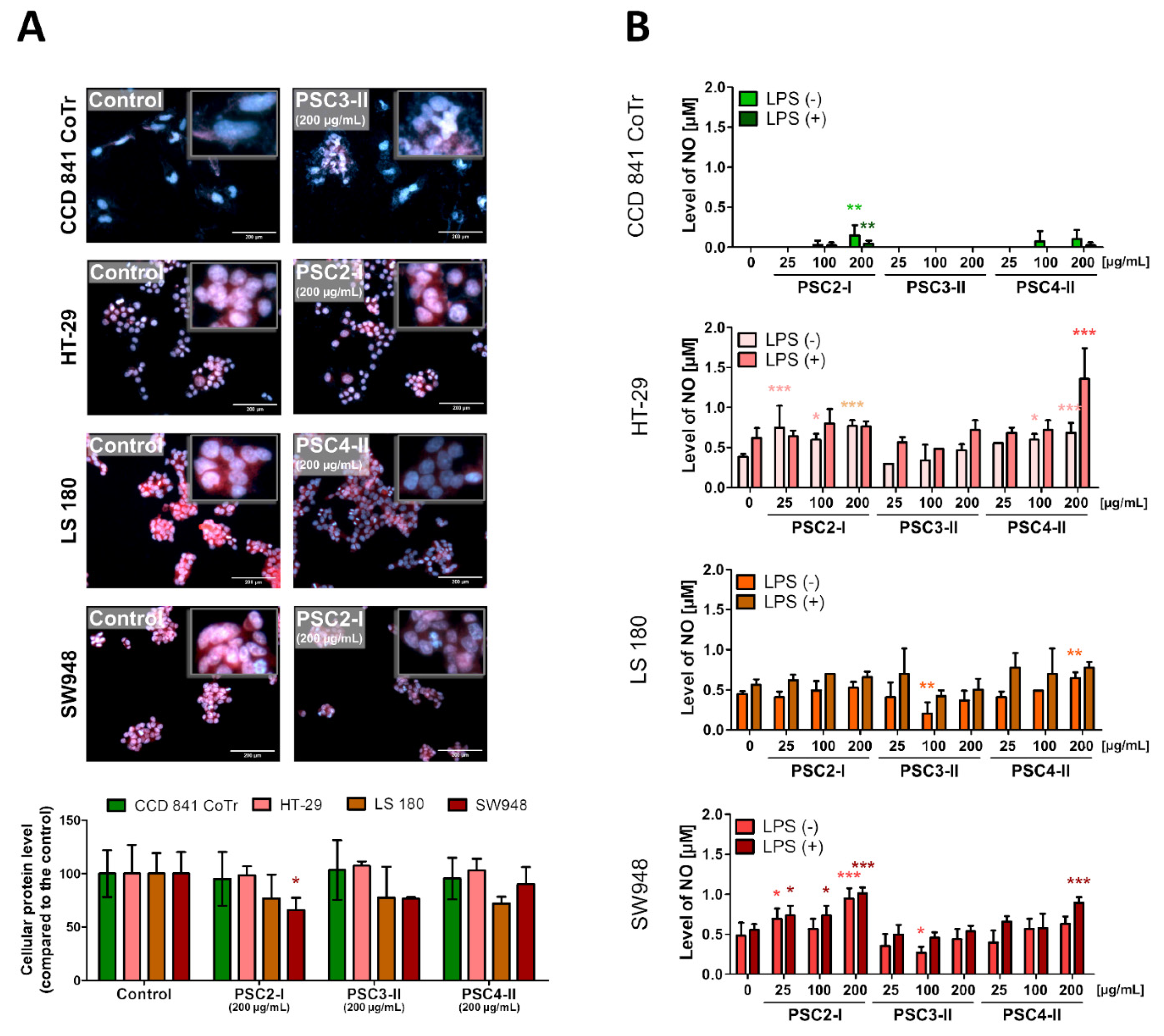
3.3.3. Evaluation of Morphological Changes in Nuclei and the Concentration and Localization of Proteins
3.3.4. NO Secretion Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | amino acids |
| LPS | lipopolysaccharides |
| MGG | May-Grünwald-Giemsa |
| MTT | (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) |
| NBT | nitrotetrazolium blue |
| NO | nitric oxide |
| NR | neutral red dye |
| PBS | phosphate-buffered saline |
| PI | propidium iodide |
| PSC | Lentinus sajor-caju (Pleurotus sajor-caju) |
| PSC2 | I-extract cultured on whole milk only for 2 weeks |
| PSC3 | II-extract cultured on whole milk and Lindeberg-Holm medium 1:1 v/v for 4 weeks |
| PSC4 | II-extract cultured on whole milk and Lindeberg-Holm medium 1:2 v/v for 4 weeks |
| RIPs | ribosome inactivating proteins |
| SOD | superoxide dismutase |
| SR101 | sulforhodamine 101 |
References
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.H.; Wang, Y.P.; Qin, M.H.; Fu, Y.J.; Li, Z.Q.; Zhang, F.S.; Li, J.H. Fiber surface characterization of old newsprint pulp deinked by combining hemicellulase with laccase-mediator system. Bioresour. Technol. 2011, 102, 6536–6540. [Google Scholar] [CrossRef]
- Jose, N.; Ajith, T.A.; Janardhanan, K.K. Antioxidant, Anti-inflammatory, and Antitumor Activities of Culinary-Medicinal Mushroom Pleurotus pufmonanus (Fr.) Quel. (Agaricomycetideae). Int. J. Med. Mushrooms 2002, 4, 7. [Google Scholar] [CrossRef]
- Jaszek, M.; Kos, K.; Matuszewska, A.; Grąz, M.; Stefaniuk, D.; Osińska-Jaroszuk, M.; Prendecka, M.; Jóźwik, E.; Grzywnowicz, K. Effective stimulation of the biotechnological potential of the medicinal white rot fungus: Phellinus pini by menadione-mediated oxidative stress. Appl. Biochem. Biotechnol. 2014, 174, 644–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwulski, M.; Sobierajski, K.; Sas-Golak, I. Nutritive and health-promoting value of mushrooms. Zywnosc. Nauka. Technol. Jakosc/Food Sci. Technol. Qual. 2014, 16–28. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level, 3rd ed.; Voet, D., Voet, J., Pratt, C.W., Wood, E.J., Eds.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.; Van Griensven, L.J. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Skrzypczak, K.; Sławińska, A.; Radzki, W.; Gustaw, W. Lactic Acid Fermentation of Edible Mushrooms: Tradition, Technology, Current State of Research: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Lindeberg, G.; Holm, G. Occurrence of Tyrosinase and Laccase in Fruit Bodies and Mycelia of some Hymenomycetes. Physiol. Plant. 1952, 5, 100–114. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Mouradov, D.; Sloggett, C.; Jorissen, R.N.; Love, C.G.; Li, S.; Burgess, A.W.; Arango, D.; Strausberg, R.L.; Buchanan, D.; Wormald, S.; et al. Colorectal Cancer Cell Lines Are Representative Models of the Main Molecular Subtypes of Primary Cancer. Cancer Res. 2014, 74, 3238–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizerska-Kowalska, M.; Bojarska-Junak, A.; Jakubowicz-Gil, J.; Kandefer-Szerszeń, M. Neutral endopeptidase (NEP) is differentially involved in biological activities and cell signaling of colon cancer cell lines derived from various stages of tumor development. Tumor Biol. 2016, 37, 13355–13368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook, 3rd ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. BioMed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [Green Version]
- Malarczyk, E. Transformation of Phenolic Acids by Nocardia. Acta Microbiol. Pol. 1989, 38, 45–53. [Google Scholar]
- Jarosz-Wilkołazka, A.; Fink-Boots, M.; Malarczyk, E.; Leonowicz, A. Formaldehyde as a proof and response to various kind of stress in some Basidiomycetes. Acta Biol. Hung. 1998, 49, 393–403. [Google Scholar] [CrossRef]
- Malarczyk, E.; Wilkołazka, A. Affection of laccase but not superoxide dismutase activity in cadmium enriched cultures of some white-rot fungi. In Abst. Intern. Cong. Stress of Life. Stress and Adaptation from Molecules to Man; Springer: Budapest, Hungary, 1997; p. 176. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The Potential Role of Nitric Oxide in Halting Cancer Progression Through Chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Xiang, J.; Su, L.; Tang, X. The regulation of nitric oxide in tumor progression and therapy. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [Green Version]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T.; et al. Mushrooms and Health Summit Proceedings1–3. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef] [PubMed]
- Pięt, M.; Zając, A.; Paduch, R.; Jaszek, M.; Frant, M.; Stefaniuk, D.; Matuszewska, A.; Grzywnowicz, K. Chemopreventive activity of bioactive fungal fractions isolated from milk-supplemented cultures of Cerrena unicolor and Pycnoporus sanguineus on colon cancer cells. 3 Biotech 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Sheu, G.-T.; Lin, Y.-W.; Yeh, C.-S.; Huang, Y.-H.; Lai, Y.-C.; Chang, J.-G.; Ko, J.-L. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Bioactives from mushroom and their application. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 23–57. ISBN 9783319516394. [Google Scholar]
- Ivanova, T.S.; Krupodorova, T.A.; Barshteyn, V.Y.; Artamonova, A.B.; Shlyakhovenko, V.A. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66. [Google Scholar]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Ekmekcioglu, C.; Feyertag, J.; Marktl, W. Cinnamic acid inhibits proliferation and modulates brush border membrane enzyme activities in Caco-2 cells. Cancer Lett. 1998, 128, 137–144. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Calhelha, R.C.; Esteves, A.P.; Martins, A.; Queiroz, M.J.R.P. Cytotoxicity of Coprinopsis atramentaria extract, organic acids and their synthesized methylated and glucuronate derivatives. Food Res. Int. 2014, 55, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Vaz, J.A.; Almeida, G.M.; Ferreira, I.C.F.R.; Martins, A.; Vasconcelos, M.H. Clitocybe alexandri extract induces cell cycle arrest and apoptosis in a lung cancer cell line: Identification of phenolic acids with cytotoxic potential. Food Chem. 2012, 132, 482–486. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J.A.P.; Dillon, A.J.P.; Roesch-Ely, M. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr. Res. 2013, 33, 76–84. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Abreu, R.M.V.; Bonetto, N.; Scariot, F.J.; Dillon, A.J.P.; Echeverrigaray, S.; Barros, L.; Ferreira, I.C.F.R.; Henriques, J.A.P.; Roesch-Ely, M. Apoptosis induction by Pleurotus sajor-caju (Fr.) Singer extracts on colorectal cancer cell lines. Food Chem. Toxicol. 2018, 112, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Seedevi, P.; Ganesan, A.R.; Mohan, K.; Raguraman, V.; Sivakumar, M.; Sivasankar, P.; Loganathan, S.; Rajamalar, P.; Vairamani, S.; Shanmugam, A. Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju. RSC Adv. 2019, 9, 20472–20482. [Google Scholar] [CrossRef] [Green Version]
- Finimundy, T.C.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Prieto, M.A.; Abreu, R.M.V.; Dillon, A.J.P.; Henriques, J.A.P.; Roesch-Ely, M.; Ferreira, I.C.F.R. Multifunctions of Pleurotus sajor-caju (Fr.) Singer: A highly nutritious food and a source for bioactive compounds. Food Chem. 2018, 245, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Ellah, N.H.A.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.S.; Hetta, H.F.; Singh, M.P. Pleurotus sajor-caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, W.-D.; Deng, R.; Zhang, H.; Tang, J.; Wu, K.-W.; Li, D.-D.; Feng, G.-K.; Lan, W.-J.; Li, H.-J.; et al. Hirsutanol A, a novel sesquiterpene compound from fungus Chondrostereum sp., induces apoptosis and inhibits tumor growth through mitochondrial-independent ROS production: Hirsutanol A inhibits tumor growth through ROS production. J. Transl. Med. 2013, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Han, J.; Li, B.; Huang, L.; Ma, K.; Chen, Q.; Liu, X.; Bao, L.; Liu, H. Identification of a new cyathane diterpene that induces mitochondrial and autophagy-dependent apoptosis and shows a potent in vivo anti-colorectal cancer activity. Eur. J. Med. Chem. 2016, 111, 183–192. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Ng, T.B. Purification and characterization of a lectin with antiproliferative activity toward cancer cells from the dried fruit bodies of Lactarius flavidulus. Carbohydr. Res. 2011, 346, 2576–2581. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem. Biophys. Res. Commun. 2004, 314, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Sun, J.; Wang, H.X.; Ng, T.B. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, G.; Ng, T.B.; Wang, H. A Novel Lectin with Antiproliferative and HIV-1 Reverse Transcriptase Inhibitory Activities from Dried Fruiting Bodies of the Monkey Head Mushroom Hericium erinaceum. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickok, J.R.; Thomas, D.D. Nitric Oxide and Cancer Therapy: The Emperor has NO Clothes. Curr. Pharm. Des. 2010, 16, 381–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, A.; Pięt, M.; Stefaniuk, D.; Chojnacki, M.; Jakubowicz-Gil, J.; Paduch, R.; Matuszewska, A.; Jaszek, M. Pro-Health and Anti-Cancer Activity of Fungal Fractions Isolated from Milk-Supplemented Cultures of Lentinus (Pleurotus) Sajor-caju. Biomolecules 2021, 11, 1089. https://doi.org/10.3390/biom11081089
Zając A, Pięt M, Stefaniuk D, Chojnacki M, Jakubowicz-Gil J, Paduch R, Matuszewska A, Jaszek M. Pro-Health and Anti-Cancer Activity of Fungal Fractions Isolated from Milk-Supplemented Cultures of Lentinus (Pleurotus) Sajor-caju. Biomolecules. 2021; 11(8):1089. https://doi.org/10.3390/biom11081089
Chicago/Turabian StyleZając, Adrian, Mateusz Pięt, Dawid Stefaniuk, Michał Chojnacki, Joanna Jakubowicz-Gil, Roman Paduch, Anna Matuszewska, and Magdalena Jaszek. 2021. "Pro-Health and Anti-Cancer Activity of Fungal Fractions Isolated from Milk-Supplemented Cultures of Lentinus (Pleurotus) Sajor-caju" Biomolecules 11, no. 8: 1089. https://doi.org/10.3390/biom11081089
APA StyleZając, A., Pięt, M., Stefaniuk, D., Chojnacki, M., Jakubowicz-Gil, J., Paduch, R., Matuszewska, A., & Jaszek, M. (2021). Pro-Health and Anti-Cancer Activity of Fungal Fractions Isolated from Milk-Supplemented Cultures of Lentinus (Pleurotus) Sajor-caju. Biomolecules, 11(8), 1089. https://doi.org/10.3390/biom11081089





