Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Animal Experiment
2.2.1. Growth Performance and Carcass Evaluation
2.2.2. Biochemical Blood Parameter Estimation
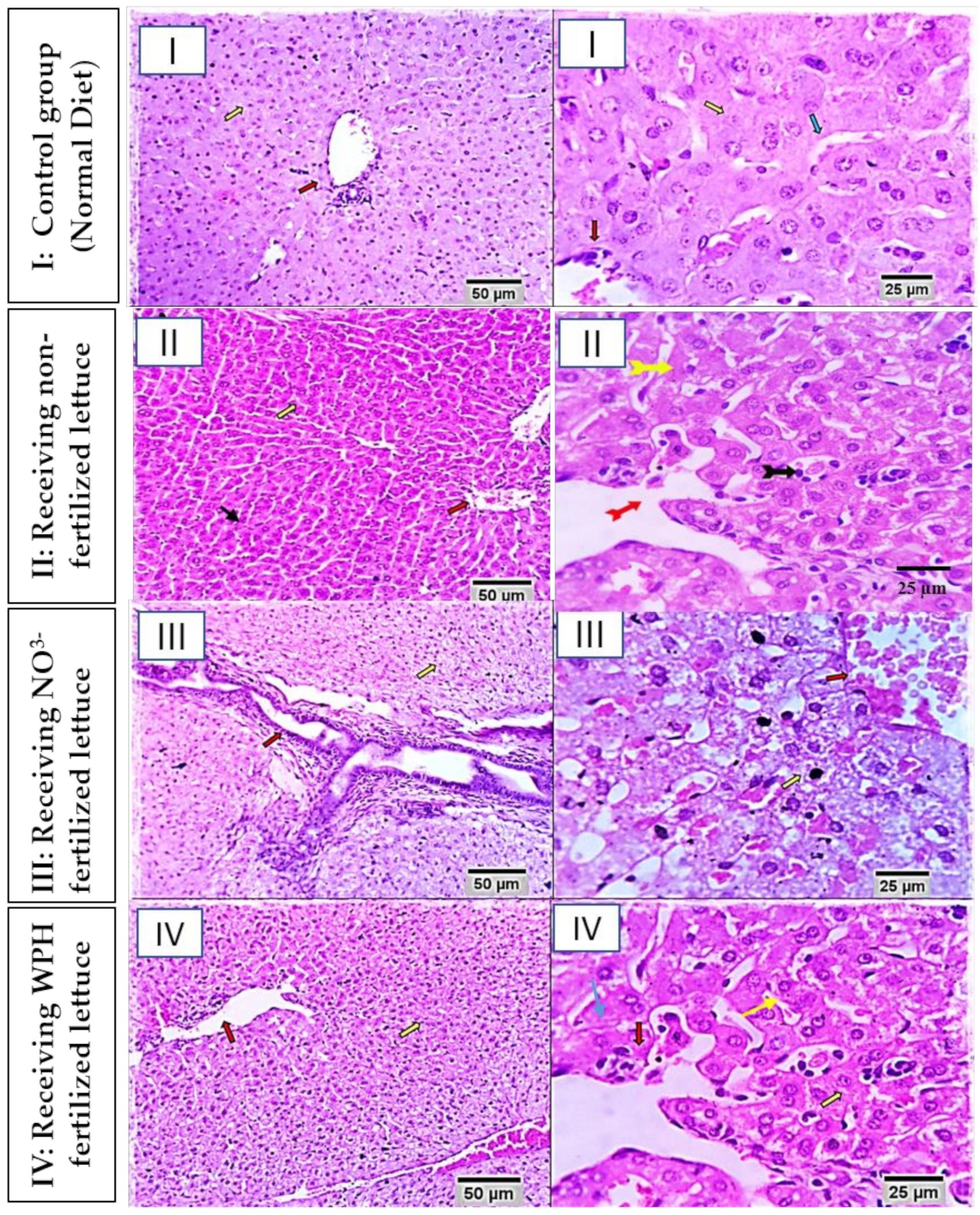
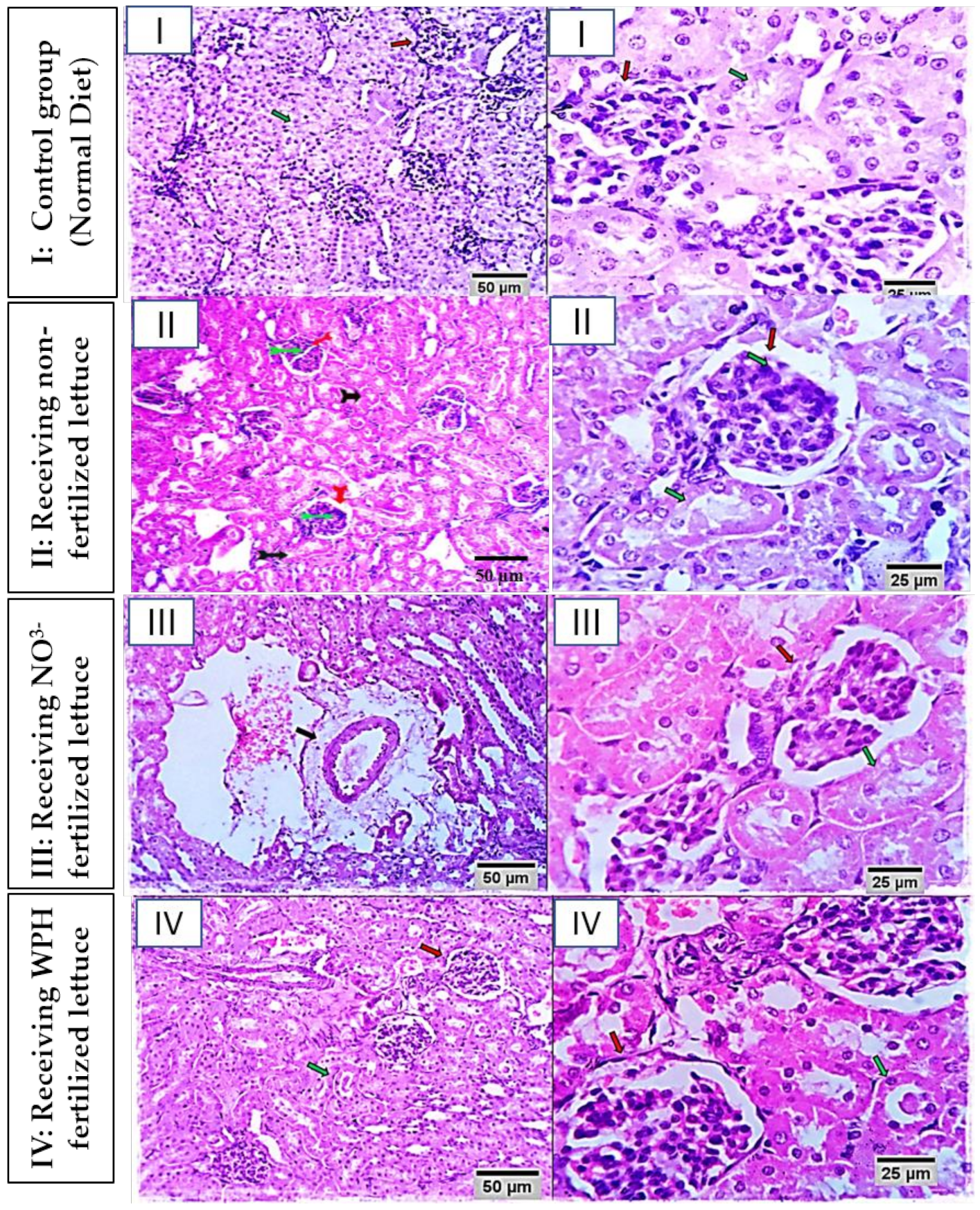
2.2.3. Histopathological Examination
2.2.4. Meat Quality Estimation
2.2.5. Statistical Analysis
3. Results
3.1. Field Experiment
3.2. Animals Experiment
3.2.1. Growth Performance and Carcass Evaluation
3.2.2. Biochemical Blood Parameter Estimation
Liver and Kidney Biomarkers
Hematological Parameters
3.2.3. Histopathological Findings
3.2.4. Meat Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gangolli, S.D.; van den Brandt, P.A.; Feron, V.J.; Janzowsky, C.; Koeman, J.H.; Speijers, G.J.; Spiegelhalder, B.; Walker, R.; Wishnok, J.S. Nitrate, nitrite and N-nitroso compounds. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 292, 1–38. [Google Scholar] [CrossRef]
- Dich, J.; Järvinen, R.; Knekt, P.; Penttilä, P.L. Dietary intakes of nitrate, nitrite and NDMA in the Finnish Mobile Clinic Health Examination Survey. Food Addit. Contam. 1996, 13, 541–552. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Parente, A.; Serio, F. Fertilization strategies for lowering nitrate content in leafy vegetables: Chicory and rocket salad cases. J. Plant Nutr. 1998, 21, 1791–1803. [Google Scholar] [CrossRef]
- Shen, M.Z.; Zhai, B.J.; Dong, H.R.; Li, J.G. Studies on nitrate accumulation in vegetable crops. 1: Evaluation of nitrate and nitrite in different vegetables. Acta Hortic. Sin. 1982, 9, 41–48. [Google Scholar]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Rupa, T.R.; Kalaivanan, D.; Radha, T.K. Nitrogen Management Paradigm in Horticulture Systems in India. In The Indian Nitrogen Assessment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 133–147. [Google Scholar]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Schuffelen, A.; Muller, A.; van Schouwenburg, J.C. Quick-tests for-soil and plant analysis used by small laboratories. NJAS Wagening. J. Life Sci. 1961, 9, 2–16. [Google Scholar]
- Dinauer, R. Methods of Soil Analysis; American Society of Agronomy, Inc. (ASA): Madison, WI, USA, 1982. [Google Scholar]
- Jackson, M. Soil Chemical Analysis; Prentice Hall. Inc.: Englewood Cliffs, NJ, USA, 1958; Volume 498, pp. 183–204. [Google Scholar]
- Holty, J.G.; Potworowski, H.S. Brucine analysis for high nitrate concentrations. Environ. Sci. Technol. 1972, 6, 835–837. [Google Scholar] [CrossRef]
- Ike, S.O.; Nubila, T.; Ukaejiofo, E.O.; Nubila, I.N.; Shu, E.N.; Ezema, I. Comparison of haematological parameters determined by the Sysmex KX-2IN automated haematology analyzer and the manual counts. BMC Clin. Pathol. 2010, 10, 3. [Google Scholar] [CrossRef]
- Elokil, A.A.; Imbabi, T.A.; Mohamed, H.I.; Abouelezz, K.F.; Ahmed-Farid, O.; Shishay, G.; Sabike, I.I.; Liu, H. Zinc and copper with new triazine hydrazone ligand: Two novel organic complexes enhanced expression of peptide growth factors and cytokine genes in weaned V-Line rabbit. Animals 2019, 9, 1134. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Silva, D.R.; Torres Filho, R.A.; Cazedey, H.P.; Fontes, P.R.; Ramos, A.L.; Ramos, E.M. Comparison of Warner-Bratzler shear force values between round and square cross-section cores from cooked beef and pork Longissimus muscle. Meat Sci. 2015, 103, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sabike, I.I.; Fujikawa, H.; Edris, A.M. The growth kinetics of Salmonella Enteritidis in raw ground beef. BioControl Sci. 2015, 20, 185–192. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. The influence of drip irrigation or subirrigation on zucchini squash grown in closed-loop substrate culture with high and low nutrient solution concentrations. HortScience 2009, 44, 306–311. [Google Scholar] [CrossRef]
- Meier-Ploeger, A. Organic Farming, Food Quality and Human Health. In Organic Farming for a New Millenium-Status and Future Challenges, Proceedings of the NJF Seminar 369, Alnarp, Sweden 15–17 June 2005; Nordic Association of Agricultural Scientists: Stockholm, Sweden, 2005. [Google Scholar]
- Shahein, M.; Afifi, M.; Algharib, A. Study the effects of humic substances on growth, chemical constituents, yield and quality of two lettuce cultivars (cv. s. Dark Green and Big Bell). J. Mater. Environ. Sci. 2015, 6, 473–486. [Google Scholar]
- Williams, C.M. Nutritional quality of organic food: Shades of grey or shades of green? Proc. Nutr. Soc. 2002, 61, 19–24. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A. Nitrite and nitrate in fresh meats: A contribution to the estimation of admissible maximum limits to introduce in directive 95/2/EC. Int. J. Food Sci. Technol. 2012, 47, 1852–1858. [Google Scholar] [CrossRef]
- Rocha, B.S.; Correia, M.G.; Pereira, A.; Silva, G.J.; Barbosa, R.M.; Laranjinha, J. Gut microbiome is rescued by dietary nitrate during dysbiosis: The impact on epithelial fence function and inflammatory pathways during antibiotic therapy. Free Radic. Biol. Med. 2015, 86, S8. [Google Scholar] [CrossRef]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology 2010, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Salehi, M. The protective effect of garlic oil on hepatotoxicity induced by acetaminophen in mice and comparison with N-acetylcysteine. Saudi Med. J. 2001, 22, 1080–1084. [Google Scholar]
- Eremin, I.N.; Tocharina, M. Effect of nitrites on the state of thyroid gland in iodine deficiency and different diets. Vopr. Pitan. 1981, 5, 60–62. [Google Scholar]
- Kolpakov, V.; Gordon, D.; Kulik, T.J. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ. Res. 1995, 76, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Osman, A.; El-Hadary, A.; Romeih, E.; Sitohy, M.; Li, L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020, 103, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Salama, A.; Emam, K.; Sitohy, M. Alleviation of carbon tetrachloride-induced hepatocellular damage and oxidative stress in rats by Anabaena oryzae phycocyanin. J. Food Biochem. 2021, 45, 13562. [Google Scholar] [CrossRef]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Metro, F.; Haertle, T.; Chobert, J.M. Antimicrobial activity of ovine whey protein and their peptic hydrolysates. Milchwissenschaft 2004, 59, 653–656. [Google Scholar]
- El-Zahar, K.; Chobert, J.M.; Dalgalarrondo, M.; Sitohy, M.; Haertlé, T. Proteolysis of ewe’s caseins and whey proteins during fermentation of yogurt and storage. Effect of the starters used. J. Food Biochem. 2004, 28, 319–335. [Google Scholar] [CrossRef]
- El-Zahar, K.; Chobert, J.M.; Sitohy, M.; Dalgalarrondo, M.; Haertlé, T. Proteolytic degradation of ewe milk proteins during fermentation of yoghurts and storage. Food/Nahrung 2003, 47, 199–206. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Métro, F.; Haertle, T.; Chobert, J.M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin Exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int. Dairy J. 2005, 15, 17–27. [Google Scholar] [CrossRef]
- Sharma, M.K.; Sharma, H.; Bapna, N. Histopathological changes in the liver of rabbits exposed to high nitrate ingestion in drinking water. J. Clin. Diagn. Res. 2013, 7, 1552. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Sharma, H.; Bapna, N. Histopathological changes in kidneys of rabbit exposed to high nitrate in drinking water. Int. J. Anat. Res. 2014, 2, 413–417. [Google Scholar]
- Rodríguez-Estival, J.; Martínez-Haro, M.; Martín-Hernando, M.P.; Mateo, R. Sub-chronic effects of nitrate in drinking water on red-legged partridge (Alectoris rufa): Oxidative stress and T-cell mediated immune function. Environ. Res. 2010, 110, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Atyabi, N.; Yasini, S.P.; Jalali, S.M.; Shaygan, H. Antioxidant effect of different vitamins on methemoglobin production: An in vitro study. Vet. Res. Forum. 2012, 3, 97–101. [Google Scholar]
- Ansari, F.A.; Ali, S.N.; Khan, A.A.; Mahmood, R. Acute oral dose of sodium nitrite causes redox imbalance and DNA damage in rat kidney. J. Cell. Biochem. 2018, 119, 3744–3754. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, H. Evaluation of the haematological responses to high nitrate exposure in rabbits. J. Clin. Diagn. Res. 2012, 6, 145–149. [Google Scholar]
- Coleman, M.D.; Coleman, N.A. Drug-induced methaemoglobinaemia. Drug Saf. 1996, 14, 394–405. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Crow, J.P. Peroxynitrite decreases rabbit tissue factor activity in vitro. Anesth. Analg. 2004, 98, 668–671. [Google Scholar] [CrossRef]
- Gluhchevaa, Y.; Ivanovb, I.; Petrovaa, E.; Pavlovaa, E.; Vladova, I. Sodium nitrite-induced hematological and hemorheological changes in rats. Ser. Biomech. 2012, 27, 53–58. [Google Scholar]
- Chobert, J.M.; El-Zahar, K.; Sitohy, M.; Dalgalarrondo, M.; Métro, F.; Choiset, Y.; Haertlé, T. Angiotensin I-converting-enzyme (ACE)-inhibitory activity of tryptic peptides of ovine $\beta $-lactoglobulin and of milk yoghurts obtained by using different starters. Le Lait 2005, 85, 141–152. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 2020, 111, 107056. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M.A. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT Food Sci. Technol. 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Athira, S.; Mann, B.; Sharma, R.; Kumar, R. Ameliorative potential of whey protein hydrolysate against paracetamol-induced oxidative stress. J. Dairy Sci. 2013, 96, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szymczyk, B.; Arczewska-Włosek, A.; Józefiak, D.; Alloui, M.N. The effects of dietary whey protein concentrate level on performance, selected intestinal tract and blood parameters, and thiobarbituric acid reactive substances in the liver and breast meat of broiler chickens. J. Anim. Feed Sci. 2013, 22, 342–353. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Li, H.; Emmerson, D.A.; Webb Jr, K.E.; Wong, E.A. Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. J. Nutr. 2008, 138, 262–271. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Osman, A.; Goda, H.A.; Sitohy, M. Storage stability of minced beef supplemented with chickpea legumin at 4 °C as a potential substitute for nisin. LWT Food Sci. Technol. 2018, 93, 434–441. [Google Scholar] [CrossRef]
- Osman, A.; Abdel-Shafi, S.; Al-Mohammadi, A.R.; Kamal, N.; Enan, G.; Sitohy, M. Catfish Glycoprotein, a Highly Powerful Safe Preservative of Minced Beef Stored at 4 °C for 15 Days. Foods 2020, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, S533–S537. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
| Soil Characteristics | Values |
|---|---|
| Soil particles distribution | |
| Sand,% | 40.5 |
| Silt,% | 35.2 |
| Clay,% | 24.3 |
| Textural class | Clay Loam |
| Field capacity (FC),% | 22.9 |
| CaCO3, (g kg−1) | 15.1 |
| Organic matter, (g kg−1) | 3.6 |
| pH * | 7.92 |
| EC, (dSm−1) ** | 1.45 |
| Available N, (mg kg−1 soil) | 62.5 |
| Available P, (mg kg−1 soil) | 12.6 |
| Available K, (mg kg−1 soil) | 194 |
| No. | Groups | Main Diet | Supplement | Diet Nitrate from Lettuce (mg/kg bw/day) |
|---|---|---|---|---|
| I | Negative control (healthy animals) | Basal diet | — | NA |
| II | Non-fertilized lettuce | Basal diet+ | 10 mL lettuce juice (1.16 mg NO3) | 2.5 |
| III | Nitrate-fertilized lettuce | Basal diet+ | 10 mL lettuce juice (5.08 mg NO3) | 11.16 |
| IV | WPH-fertilized lettuce | Basal diet+ | 10 mL lettuce juice (1.76 mg NO3) | 3.86 |
| Ingredients, g/kg | Content |
|---|---|
| Alfalfa hay | 330 |
| Yellow corn | 110 |
| Soybean meal | 96.9 |
| Barely grain | 90 |
| Wheat bran | 300 |
| Wheat straw | 50 |
| Di-calcium phosphate | 12.5 |
| L-Lysine HCl | 1.8 |
| DL-Methionine | 2.3 |
| Sodium chloride | 5 |
| Vitamin/mineral premix 1 | 1.5 |
| Total | 1000.0 |
| Calculated analysis (g/ kg, as-fed) | |
| Dry matter (DM), g/kg | 914.41 |
| Digestible energy (MJ/kg) | 10.37 |
| Crude protein (CP), g/kg | 181.79 |
| Crude fiber (CF), g/kg | 135.36 |
| Neutral detergent fiber (NDF), g/kg | 348.88 |
| Acid detergent fiber (ADF), g/kg | 178.15 |
| Ether extract (EE), g/kg | 30.23 |
| Ash, g/kg | 65.30 |
| Ca, g/kg | 10.74 |
| Available Phosphorus, g/kg | 5.94 |
| Methionine, g/kg | 4.34 |
| Lysine, g/kg | 9.08 |
| Fertilization Treatments | N-NO3 mg kg−1 Soil | Plant Height (cm) | Fresh Weight (g plant−1) | Dry Weight (g plant−1) | Fresh Yield (Mg ha−1) | N-NO3 mg kg−1 Plant |
|---|---|---|---|---|---|---|
| Control | 27.47 ± 0.90 | 25.37 ± 0.49 | 473.6 ± 1.83 | 38.03 ± 0.52 | 31.42 ± 0.25 | 1343 ± 4.27 |
| NH4NO3 * | 61.53 ± 0.68 | 37.17 ± 0.61 | 962.3 ± 2.81 | 64.73 ± 0.76 | 43.55 ± 0.60 | 5858 ± 2.50 |
| WPH ** | 30.13 ± 0.56 | 34.7 ± 0.38 | 877.6 ± 1.04 | 56.23 ± 0.44 | 41.57 ± 0.31 | 2028 ± 3.58 |
| p-value | 0.0104 | 0.0052 | 0.0021 | 0.0021 | 0.0021 | 0.0021 |
| Growth Parameters | Lettuce Juice Supplementation | p-Value | Sig. | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| BW4 (g) | 455.0 ± 2.9 | 456.7 ± 4.4 | 455.0 ± 2.9 | 458.3 ± 4.4 | 0.9183 | NS |
| BW8 (g) | 843.3 ± 8.8 b | 982.7 ± 8.2 a | 983.3 ± 8.8 a | 1046 ± 32.1 a | 0.0017 | ** |
| BW12 (g) | 1253 ± 8.8 b | 1599 ± 74.6 a | 1543 ± 66.9 a | 1635 ± 41.9 a | 0.0046 | ** |
| ADG4–8 (g/d) | 18.87 ± 0.4 a | 18.79 ± 0.4 a | 13.87 ± 0.3 b | 21.00 ± 1.3 a | 0.0035 | ** |
| ADG8–12 (g/d) | 20.00 ± 2.1 a | 22.01 ± 2.6 a | 14.64 ± 0.4 b | 21.02 ± 0.4 a | 0.0461 | * |
| ADG4–12 (g/d) | 19.43 ± 1.2 a | 20.40 ± 1.3 a | 14.26 ± 0.1 b | 21.01 ± 0.8 a | 0.0043 | ** |
| Parameters (g) | Lettuce Juice Supplementation | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||||||
| Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | ||
| Carcass (%) | 40.7 | 43.8 | 46.1 | 47.0 | 47.3 | 50.4 | 47.3 | 47.6 | 48.2 | 48.1 | 48.2 | 48.5 | 0.0296 |
| Hind legs (%) | 15.6 | 15.7 | 16.1 | 15.2 | 15.7 | 18.4 | 18.3 | 19.2 | 19.5 | 18.2 | 18.5 | 18.9 | 0.0398 |
| Saddle (%) | 8.7 | 9.1 | 9.3 | 9.2 | 10.1 | 10.7 | 11.9 | 12.3 | 13.4 | 9.6 | 10.3 | 10.9 | 0.0030 |
| Fore legs (%) | 6.1 | 6.4 | 6.9 | 7.0 | 7.2 | 8.1 | 7.6 | 8.1 | 9.1 | 7.3 | 7.8 | 7.8 | 0.0398 |
| Thoracic neck (%) | 6.0 | 6.4 | 6.4 | 5.9 | 6.8 | 8.0 | 8.1 | 8.4 | 9.4 | 6.3 | 6.6 | 7.8 | 0.0418 |
| Liver index | 3.0 | 3.2 | 3.3 | 3.2 | 3.5 | 4.5 | 4.2 | 4.3 | 4.4 | 3.5 | 3.6 | 3.7 | 0.0702 |
| Kidney index | 0.5 | 0.6 | 0.7 | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 | 0.6 | 0.3541 |
| Heart index | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.3 | 0.3 | 0.4 | 0.3692 |
| Lung index | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.5 | 0.5 | 0.5 | 0.0381 |
| Spleen index | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5514 |
| Parameters | Lettuce Juice Supplementation | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||||||
| Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | ||
| AST (U/L) | 20.0 | 20.0 | 25.0 | 23 | 24 | 28 | 45 | 50 | 65 | 16 | 19 | 20 | 0.0014 |
| ALT (U/L) | 30.0 | 35.0 | 38.0 | 30 | 32 | 36 | 68 | 70 | 75 | 30 | 30 | 34 | 0.0294 |
| ALP (U/L) | 82.2 | 86.2 | 87.8 | 80.3 | 84.1 | 85.2 | 120.7 | 124.28 | 130.2 | 80.1 | 81.2 | 83.1 | 0.0109 |
| Total BL * (mg/dL) | 0.9 | 1.0 | 1.1 | 0.82 | 0.97 | 1.05 | 1.88 | 2.03 | 2.44 | 0.83 | 0.9 | 1.02 | 0.0454 |
| Direct BL (mg/dL) | 0.2 | 0.3 | 0.3 | 0.19 | 0.25 | 0.27 | 0.68 | 0.7 | 0.78 | 0.19 | 0.2 | 0.26 | 0.0450 |
| Indirect BL (mg/dL) | 0.7 | 0.7 | 0.8 | 0.63 | 0.72 | 0.78 | 1.2 | 1.33 | 1.66 | 0.64 | 0.7 | 0.76 | 0.0454 |
| Parameters | Lettuce Juice Supplementation | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||||||
| Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | ||
| Total protein (g/dL) | 6.9 | 7.3 | 8.0 | 6.92 | 7.66 | 8.2 | 4.92 | 5.03 | 5.11 | 7.85 | 8.2 | 8.31 | 0.0114 |
| Albumin (g/dL) | 3.7 | 3.9 | 4.1 | 3.72 | 3.95 | 4.25 | 2.03 | 2.23 | 2.48 | 4.01 | 4.26 | 4.3 | 0.0088 |
| Globulin (g/dL) | 3.2 | 3.4 | 3.9 | 3.2 | 3.71 | 3.95 | 2.63 | 2.8 | 2.89 | 3.84 | 3.94 | 4.01 | 0.0181 |
| Creatinine (mg/dL) | 0.8 | 1.0 | 1.3 | 0.75 | 0.88 | 0.92 | 0.73 | 0.78 | 0.84 | 2.66 | 3.56 | 4.02 | 0.0109 |
| Urea (mg/dL) | 25.1 | 35.8 | 40.2 | 30.11 | 35.12 | 43.12 | 50.88 | 64.46 | 71.46 | 22.57 | 28.18 | 30.77 | 0.0181 |
| Uric acid (mg/dL) | 3.5 | 4.4 | 5.5 | 3.31 | 4.42 | 6.5 | 8.92 | 9.93 | 10.22 | 3.22 | 4.62 | 4.93 | 0.0674 |
| Hematological Parameters | lettuce Juice Supplementation | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||||||
| Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | Q1 | Med | Q3 | ||
| RBcs (× 106/uL) | 4.7 | 4.7 | 4.8 | 5.21 | 5.32 | 5.44 | 3.88 | 4 | 4.19 | 5.64 | 5.8 | 5.92 | <0.001 |
| Hb (g/dL) | 10.4 | 10.5 | 11.1 | 10.8 | 11.24 | 12.65 | 8.33 | 9 | 9.11 | 12.8 | 13.24 | 15.25 | <0.001 |
| HCT (%) | 32.6 | 35.7 | 40.1 | 31.1 | 35.49 | 40.11 | 24.08 | 26.14 | 29.7 | 35.9 | 39.18 | 42.16 | 0.0296 |
| MCV(fL) | 69.8 | 74.1 | 85.1 | 59.69 | 66.54 | 73.71 | 61.85 | 65.25 | 70.88 | 63.65 | 67.41 | 71.11 | 0.2849 |
| MCH(Pg) | 21.6 | 22.5 | 23.5 | 20.73 | 21.13 | 23.25 | 21.46 | 21.48 | 22.77 | 22.07 | 23.47 | 25.76 | 0.2638 |
| MCHC(g/dL) | 27.6 | 29.1 | 32.2 | 31.54 | 31.67 | 34.74 | 30.3 | 34.59 | 34.85 | 32.67 | 36.17 | 36.88 | 0.0989 |
| RDW (%) | 13.5 | 14.2 | 18.7 | 11.6 | 12.5 | 14.8 | 17.9 | 18.9 | 19.6 | 11.3 | 13.5 | 16.6 | 0.0603 |
| PLT (×103/uL) | 388.0 | 400.0 | 410.0 | 409 | 415 | 420 | 300 | 310 | 329 | 405 | 420 | 425 | 0.0093 |
| WBCs (×103/uL) | 0.94 | 0.94 | 1.3 | 1.48 | 1.59 | 1.71 | 0.34 | 0.46 | 0.65 | 2.05 | 2.21 | 2.33 | 0.0109 |
| Parameters | Lettuce Juice Supplementation | p-Value | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| pHu | 5.73 ± 0.08 | 5.65 ± 0.06 | 5.61 ± 0.05 | 5.64 ± 0.03 | 0.278 |
| WHC | 74.19 ± 2.53 | 79.05 ± 13.16 | 76.37 ± 1.05 | 81.67 ± 7.53 | 0.677 |
| Drip loss (48 h) | 5.50 ± 0.60 | 5.51 ± 1.26 | 8.90 ± 4.69 | 4.19 ± 0.58 | 0.194 |
| Cooking loss | 13.31 ± 1.36 | 10.33 ± 2.86 | 14.72 ± 3.48 | 9.75 ± 4.06 | 0.241 |
| WBSF | 4.93 ± 0.47 | 3.30 ± 1.21 | 4.42 ± 0.59 | 3.87 ± 1.21 | 0.005 |
| L * | 58.85 ± 1.88 | 62.69 ± 4.33 | 60.16 ± 1.74 | 63.06 ± 1.08 | 0.097 |
| a * | 10.59 ± 1.59 | 10.44 ± 2.00 | 9.77 ± 0.27 | 9.58 ± 0.95 | 0.673 |
| b * | 9.97 ± 0.95 | 7.57 ± 0.54 | 7.16 ± 0.35 | 8.38 ± 0.52 | 0.0002 |
| Chroma | 14.56 ± 1.62 | 12.91 ± 1.91 | 12.12 ± 0.24 | 12.75 ± 0.59 | 0.101 |
| Hue | 43.42 ± 3.59 | 36.30 ± 3.34 | 36.24 ± 1.80 | 41.29 ± 4.11 | 0.021 |
| Lettuce Juice Supplementation | p-Value | ||||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| Storage Time (day) | Aerobic Plate Count (Log CFU/ g Meat) | ||||
| 1 | 5.79 ± 0.07 | 4.50 ± 0.29 | 3.92 ± 0.04 | 4.72 ± 0.10 | 0.0255 |
| 3 | 5.70 ± 0.003 | 4.48 ± 0.00 | 3.80 ± 0.09 | 4.75 ± 0.06 | 0.0024 |
| 5 | 5.72 ± 0.16 | 4.22 ± 0.25 | 3.40 ± 0.10 | 4.55 ± 0.22 | 0.0112 |
| 7 | 6.84 ± 0.07 | 4.93 ± 0.008 | 3.74 ± 0.007 | 5.80 ± 0.007 | 0.0042 |
| 10 | 7.69 ± 0.11 | 6.25 ± 0.03 | 4.47 ± 0.04 | 5.65 ± 0.24 | 0.0042 |
| pH | |||||
| 1 | 5.66 ± 0.02 | 5.60 ± 0.007 | 5.61 ± 0.00 | 5.62 ± 0.007 | 0.0059 |
| 3 | 6.00 ± 0.02 | 5.79 ± 0.02 | 5.73 ± 0.07 | 5.83 ± 0.03 | 0.0157 |
| 5 | 5.87 ± 0.03 | 5.76 ± 0.01 | 5.63 ± 0.01 | 5.71 ± 0.007 | 0.0005 |
| 7 | 5.97 ± 0.01 | 5.86 ± 0.007 | 5.74 ± 0.01 | 5.85 ± 0.007 | 0.0112 |
| 10 | 5.9 ± 0.01 | 6.04 ± 0.14 | 5.90 ± 0.09 | 6.02 ± 0.19 | 0.7687 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.; Imbabi, T.A.; El-Hadary, A.; Sabeq, I.I.; Edris, S.N.; Merwad, A.-R.; Azab, E.; Gobouri, A.A.; Mohammadein, A.; Sitohy, M. Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate. Biomolecules 2021, 11, 835. https://doi.org/10.3390/biom11060835
Osman A, Imbabi TA, El-Hadary A, Sabeq II, Edris SN, Merwad A-R, Azab E, Gobouri AA, Mohammadein A, Sitohy M. Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate. Biomolecules. 2021; 11(6):835. https://doi.org/10.3390/biom11060835
Chicago/Turabian StyleOsman, Ali, Tharwat A. Imbabi, Abdalla El-Hadary, Islam Ibrahim Sabeq, Shimaa N. Edris, Abdel-Rahaman Merwad, Ehab Azab, Adil A. Gobouri, Amaal Mohammadein, and Mahmoud Sitohy. 2021. "Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate" Biomolecules 11, no. 6: 835. https://doi.org/10.3390/biom11060835
APA StyleOsman, A., Imbabi, T. A., El-Hadary, A., Sabeq, I. I., Edris, S. N., Merwad, A.-R., Azab, E., Gobouri, A. A., Mohammadein, A., & Sitohy, M. (2021). Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate. Biomolecules, 11(6), 835. https://doi.org/10.3390/biom11060835









