Cerium-Containing N-Acetyl-6-Aminohexanoic Acid Formulation Accelerates Wound Reparation in Diabetic Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Pharmacological Substance and Reference Medications
2.3. Cellular Toxicity Assay
2.4. Laboratory Animals and Models of Experimental Diabetes Mellitus, Pain Management
2.5. Wound Modelling and Experimental Groups
2.6. Morphological Examination
2.7. Immunohistochemistry (IHC)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Tissue Oxidative Stress Assay
2.10. FGFR3 Expression Assay
2.11. Antimicrobial Activity Assay
2.12. Statistical Analysis
3. Results
3.1. LHT-8-17 In Vivo Cell Toxicity
3.2. Efficacy of LHT-8-17 in Healing Linear Wounds
3.3. Efficacy of LHT-8-17 in Healing Non-Infected Planar Wounds
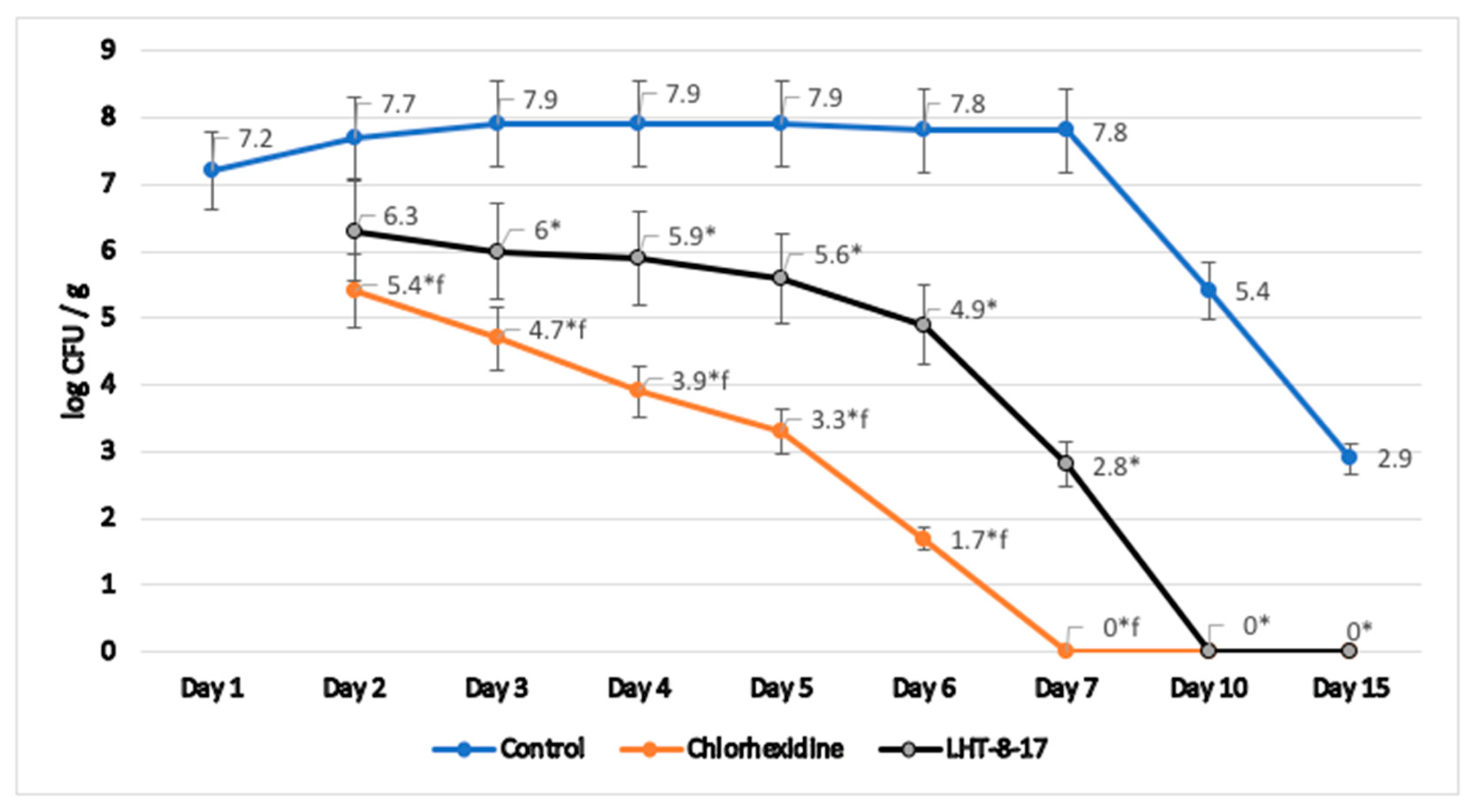
3.4. Antimicrobial Property of LHT-8-17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Day of the Observation | Study Group | Exudation | Presence of Bacterial Colonies | Inflammatory Infiltration | Microcirculation Disorder | Proliferation of Fibroblasts | Neo-Angiogenesis | Prevalence of Granulation Tissue | Maturity of the Granulation Tissue | Fibrous Tissue |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | D-panthenol | 2 | 0 | 3 | 2 | 1 | 3 | 2 | 2 | 0 |
| LHT-8-17 | 1 | 0 | 2 | 1 | 1 | 3 | 3 | 3 | 0 | |
| Control | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | |
| 10 | D-panthenol | 0 | 1 | 0 | 3 | 3 | 3 | 3 | 3 | |
| LHT-8-17 | 0 | 1 | 0 | 3 | 3 | 3 | 3 | 3 | ||
| Control | 2 | 0 | 3 | 3 | 2 | 1 | 2 | 1 | 1 | |
| 15 | D-panthenol | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 4 | 3 |
| LHT-8-17 | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 4 | 3 | |
| Control | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
References
- Clokie, M.; Greenway, A.L.; Harding, K.; Jones, N.J.; Vedhara, K.; Game, F. New horizons in the understanding of the causes and management of diabetic foot disease: Report from the 2017 diabetes UK annual professional conference symposium. Diabet. Med. 2017, 34, 305–315. [Google Scholar] [CrossRef]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-illness studies in chronic ulcers: A systematic review. J. Wound Care 2017, 26, 4–14. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Al Derwish, M.; Ouizi, S.; Youssef, A.M.; Subhani, S.N.; Ibrahim, H.M.; Alamri, B.N. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE 2015, 10, e0124446. [Google Scholar] [CrossRef]
- Maqbool, M.; Dar, M.A.; Gani, I.; Mir, S.A. Animal models in diabetes mellitus: An overview. J. Drug Deliv. Ther. 2019, 9, 472–475. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Red. 2005, 37, 1528–1542. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Reveles, K.; Duhon, B.; Moore, R.; Hand, E.; Howell, C. Epidemiology of methicillin-resistant staphylococcus aureus diabetic foot infections in a large academic hospital: Implications for antimicrobial stewardship. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Unfried, P.; Keppler, B.K. Pharmacological properties of cerium compounds. Rev. Physiol. Biochem. Pharmacol. 2005, 153, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Fromm, K.M. Toxicity and protective effects of cerium oxide nanoparticles (Nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur. J. Inorg. Chem. 2015, 27, 4510–4517. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef]
- Kargar, H.; Ghazavi, H.; Darroudi, M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceramics Int. 2015, 41, 4123–4128. [Google Scholar] [CrossRef]
- Dowding, J.M.; Seal, S.; Self, W.T. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−). Drug Deliv. Transl. Res. 2013, 3, 375–379. [Google Scholar] [CrossRef]
- Bhargava, K.; Arya, A.; Gangwar, A.; Singh, S.K.; Roy, M.; Das, M.; Sethy, N. Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK–PKC–CBP signaling cascade. Int. J. Nanomed. 2016, 11, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Škvařil, F.; Grünberger, D. Inhibition of spontaneous splitting of γ-globulin preparations with ɛ-aminocaproic acid. Nature 1962, 196, 481–482. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Nikolova, E.; Kirilov, N. Synergistic inhibition of influenza A virus replication by a plant polyphenol-rich extract and epsilon-aminocaproic acid in vitro and in vivo. Acta Virol. 2010, 54, 137–145. [Google Scholar] [CrossRef]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Derm. 2002, 3, 427–433. [Google Scholar] [CrossRef]
- Dell’Acqua, G.; Schweikert, K. Panthenyl triacetate transformation, stimulation of metabolic pathways, and wound-healing properties in the human skin. J. Cosmet. Sci. 2012, 63, 1–13. [Google Scholar] [PubMed]
- Elder, R.L. Final report on the safety assessment of panthenol and pantothenic acid. J. Am. Col. Toxicol. 1987, 6, 139–162. [Google Scholar] [CrossRef]
- Stozkowska, W.; Piekos, R. Investigation of some topical formulations containing dexpanthenol. Acta Pol. Pharm. Drug Res. 2004, 61, 433–437. [Google Scholar]
- Food and Drug Administration (FDA). 510(K) Report Summary on a Wound Dressing Containing Panthenol. 2003. Available online: https://www.fda.gov/medical-devices/premarket-notification-510k/content-510k (accessed on 17 May 2021).
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yeh, L.T.; Chuang, Y.P.; Chen, S.J.; Chu, C.C.; Sytwu, H.K. Diabetic animal models with infectious diseases: Focus on the dysfunction of immune system. J. Diabetes Metab. 2015, 5. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Klassen-Ross, T.; La Croix-Fralish, M.L.; Glick, S.; Ingrao, J.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Verniers, D.; Coppé, M.C.; Pansart, Y.; Gillardin, J.M. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur. J. Pharm. 2008, 584, 263–271. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef]
- Grose, R.; Werner, S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 2004, 28, 147–166. [Google Scholar] [CrossRef]
- Boyce, J.M. MRSA patients: Proven methods to treat colonization and infection. J. Hosp. Infect. 2001, 48, S9–S14. [Google Scholar] [CrossRef]
- Combined Working Party of the British Society for Antimicrobial Chemotherapy and the Hospital Infection Society. Guidelines on the control of methicillin-resistant Staphylococcus aureus in the community: Report of a combined working party of the British Society for Antimicrobial Chemotherapy and the Hospital Infection Society. J. Hosp. Infect. 1995, 31, 1–12. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Pekshev, A.V.; Vagapov, A.B.; Butenko, A.V.; Fayzullin, A.L.; Rudenko, T.G.; Sharapov, N.A.; Serejnikova, N.B.; Vasilets, V.N. Dose-dependent effect of plasma-chemical NO-containing gas flow on wound healing. An experimental study. Clin. Plasma Med. 2020, 19, 100101. [Google Scholar] [CrossRef]
- Dzhatdoeva, A.A.; Polimova, A.M.; Proskurina, E.V.; Vladimirov, Y.A. Tissue chemiluminescence as a method of evaluation of superoxide radical producing ability of mitochondria. Bull. Rus. State Med. Univ. 2016, 1, 49–55. [Google Scholar] [CrossRef]
- Stepanenko, I.S.; Yamashkin, S.A.; Durib, A.K.; Muhsen, S.S. Antimicrobial activity of a new group of compounds in vivo in a model of experimental surgical wound infection. Ann. Topical Med. Public Health 2021, 24, 270–283. [Google Scholar] [CrossRef]
- McRipley, R.J.; Whitney, R.R. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob. Agents Chemother. 1976, 10, 38–44. [Google Scholar] [CrossRef]
- Boon, R.J.; Beale, A.S.; Sutherland, R. Efficacy of topical mupirocin against an experimental Staphylococcus aureus surgical wound infection. J. Antimicrob. Chemother. 1985, 16, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Q.M.; Li, Y.; Sun, C.; Kinnunen, T.K.; Fernig, D.G. Fibroblast growth factors as tissue repair and regeneration therapeutics. PeerJ 2016, 4, e1535. [Google Scholar] [CrossRef] [PubMed]
- Butko, Y.; Tkachova, O.; Ulanova, V.; Sahin, Y.; Levashova, O.; Tishakova, T. Immune histochemical study of KI-67 level and ribonucleic acid in the process of healing of burn wounds after treatment with drugs containing dexpanthenol and ceramide. Biointerface Res. Appl. Chem. 2019, 9, 4586–4590. [Google Scholar] [CrossRef]
- Rothe, H.; Fautz, R.; Gerber, E.; Neumann, L.; Rettinger, K.; Schuh, W.; Gronewold, C. Special aspects of cosmetic spray safety evaluations: Principles on inhalation risk assessment. Toxicol. Lett. 2011, 205, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Eid, S.A.; O’Brien, P.D.; Hinder, L.M.; Hayes, J.M.; Mendelson, F.E.; Zhang, H.; Zeng, L.; Kretzler, K.; Narayanan, S.; Abcouwer, S.F.; et al. Differential effects of empagliflozin on microvascular complications in murine models of type 1 and type 2 diabetes. Biology 2020, 9, 347. [Google Scholar] [CrossRef]
- Davis, R.; Castellani, L.; Hosseini, M.; Ben-Zeev, O.; Mao, H.; Weinstein, M.; Jung, D.; Jun, J.; Kim, J.; Lusis, A.; et al. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes 2010, 59, 1616–1625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Zuijlen, P.; Angeles, A.; Kreis, R.; Bos, K.; Middelkoop, E. Scar assessment tools: Implications for current research. Plast. Reconstr. Surg. 2002, 109, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; DeBruler, D.; Blackstone, B.; Coffey, R.; Boyce, S.; Supp, D.; Bailey, J.; Powell, H. Direct comparison of reproducibility and reliability in quantitative assessments of burn scar properties. Burns 2020, 47, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Broughton, G., II; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Joshi, A.; Carson, W.F.; Schaller, M.; Allen, R.; Mukerjee, S.; Kittan, N.; Feldman, E.L.; Henke, P.K.; Hogaboam, C.; et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015, 64, 1420–1430. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Mudge, B.P.; Harris, C.; Gilmont, R.R.; Adamson, B.S.; Rees, R.S. Role of glutathione redox dysfunction in diabetic wounds. Wound Repair Regen. 2002, 10, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chikezie, P.; Ojiako, O.; Ogbuji, A. Oxidative stress in diabetes mellitus. Int. J. Biol. Chem. 2015, 9, 92–109. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Ruan, Q.; Kong, D.; Chu, Z.; Li, H.; Huang, J.; Huang, X.; et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, R.; Mahmood, A.; Kshatriya, P.; Patel, D.; Srivastava, A. An overview on stem cells in tissue regeneration. Curr. Pharm. Des. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ozeren, M.; Yenilmez, E.; Yulug, E.; Cobanoglu, U.; Arvas, H. Morphologic alterations and immunohistochemical analysis of alpha-fetoprotein and CD34 in chorionic villi of anembryonic pregnancy. Saudi Med. J. 2006, 27, 154–160. [Google Scholar]
- Inan, Z.D.S.; Saraydın, S.U. Investigation of the wound healing effects of chitosan on FGFR3 and VEGF immunlocalization in experimentally diabetic rats. Int. J. Biomed. Mater. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Stepanenko, I.S.; Yamashkin, S.A.; Kostina, Y.A.; Batarsheva, A.A.; Mironov, M.A. A new group of compounds derived from 4-, 5-, 6- and 7-aminoindoles with antimicrobial activity. Res. Results Pharmacol. 2018, 4, 17–26. [Google Scholar] [CrossRef][Green Version]

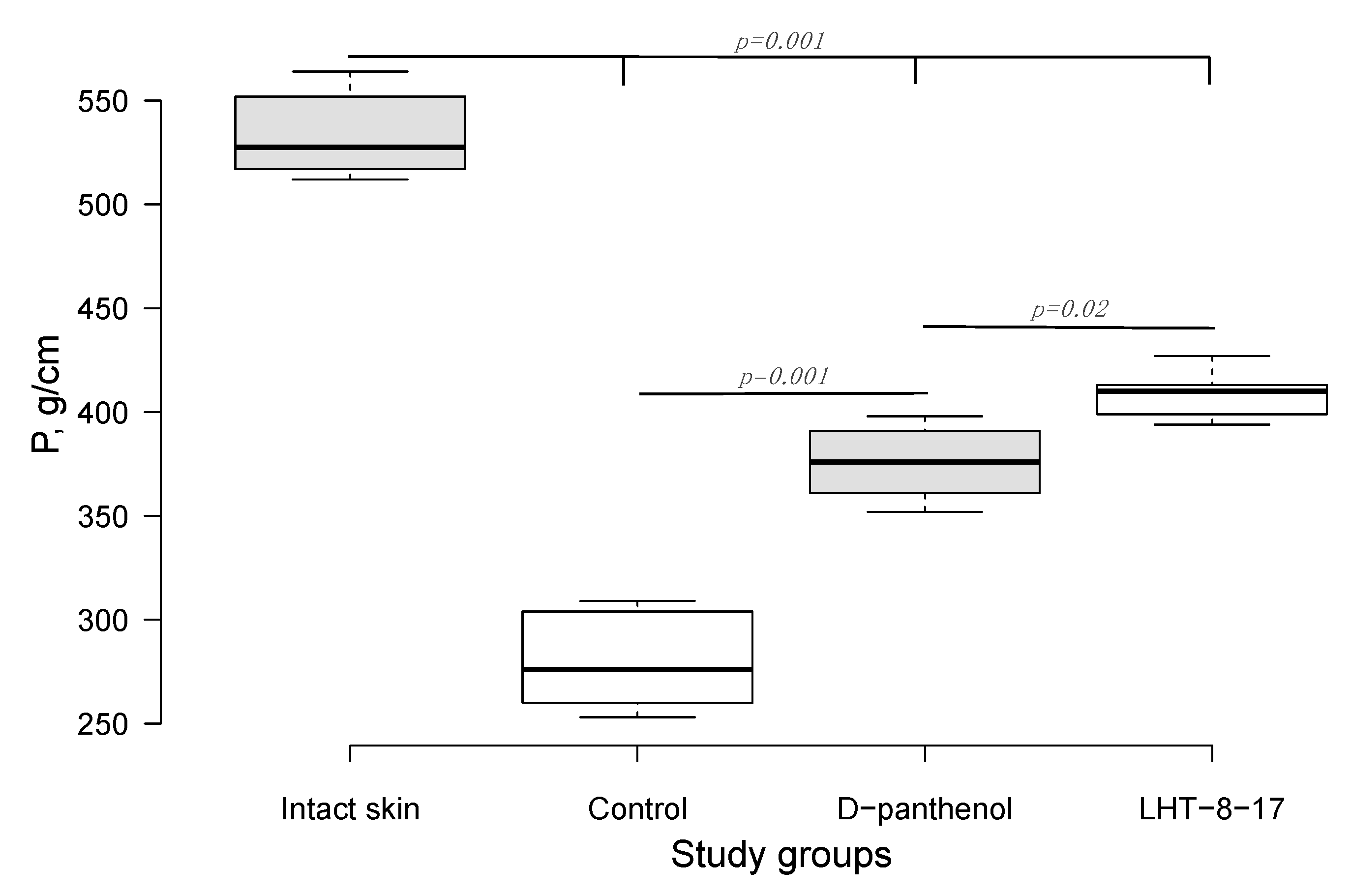

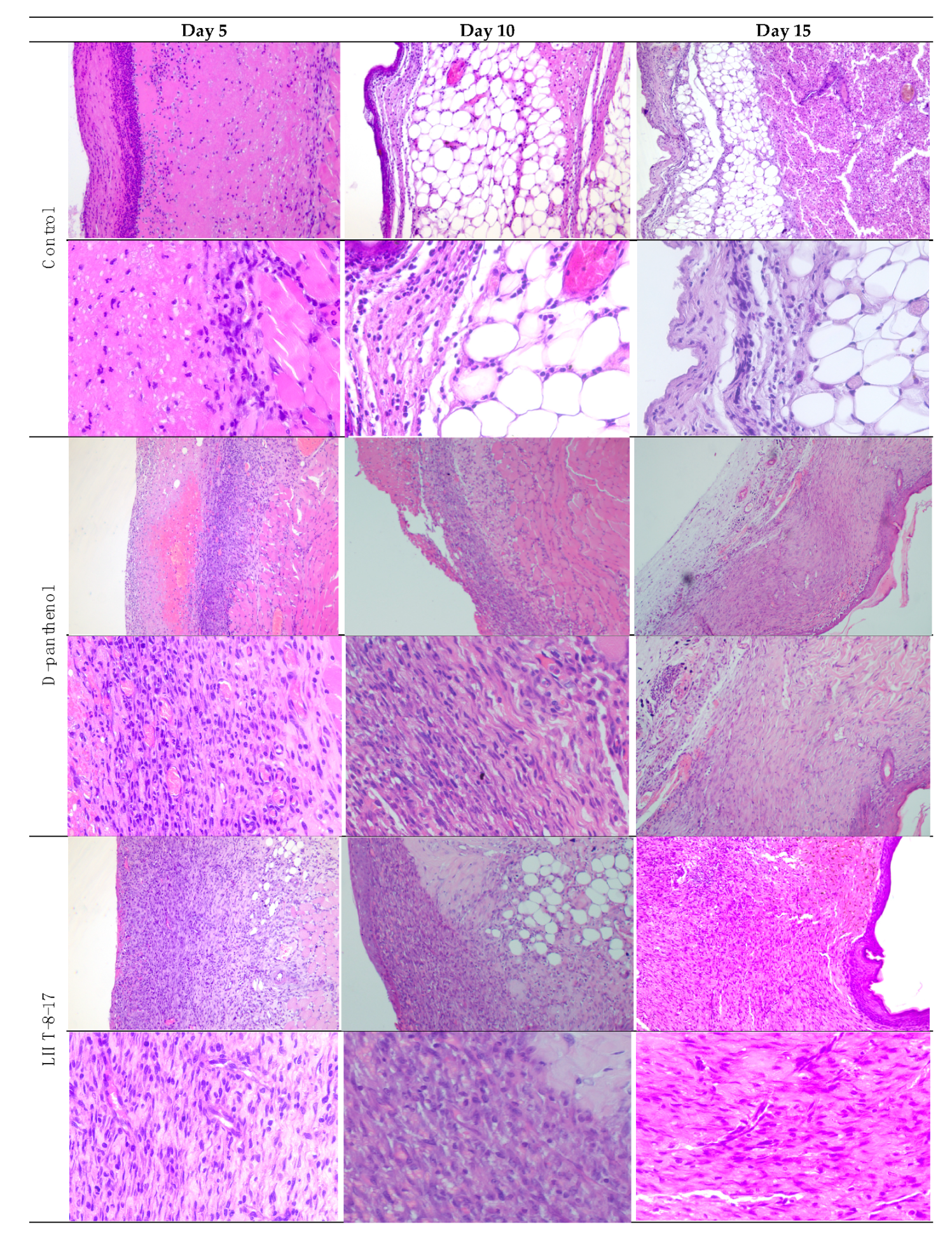
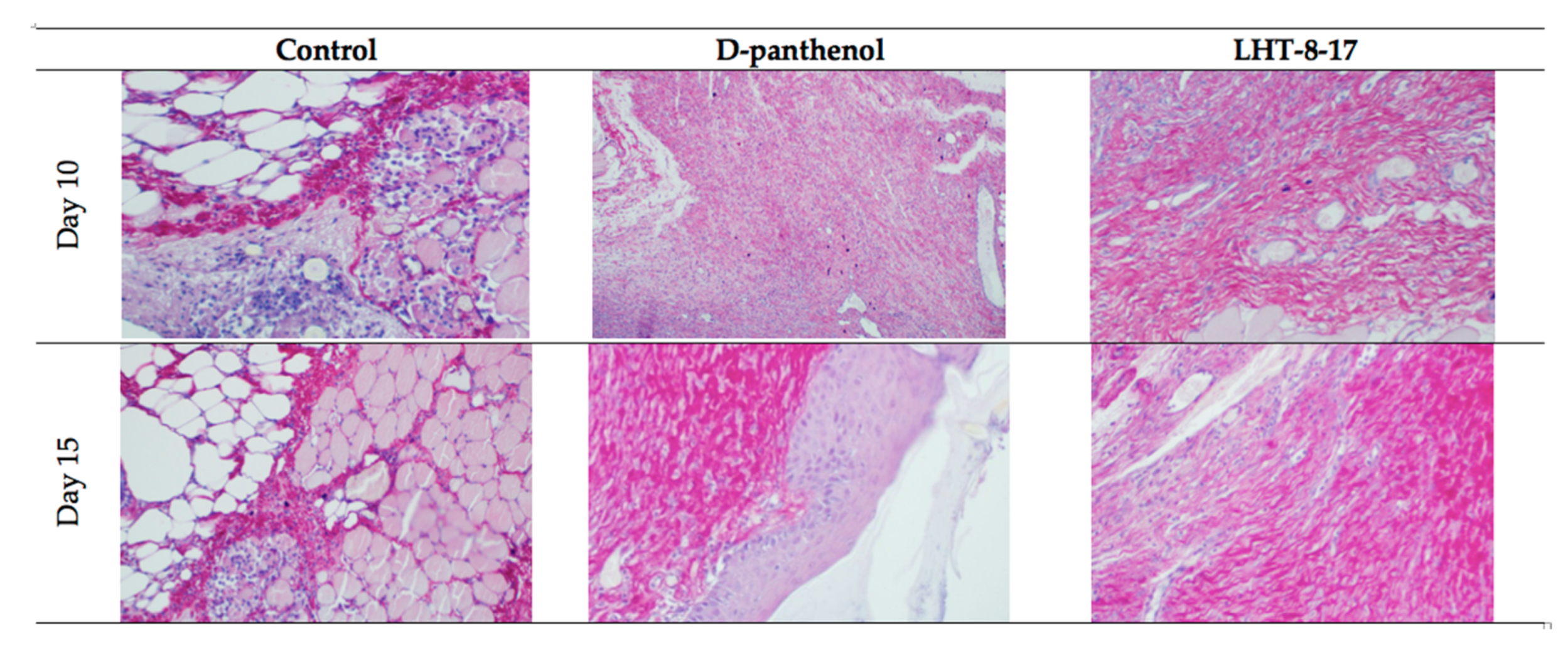
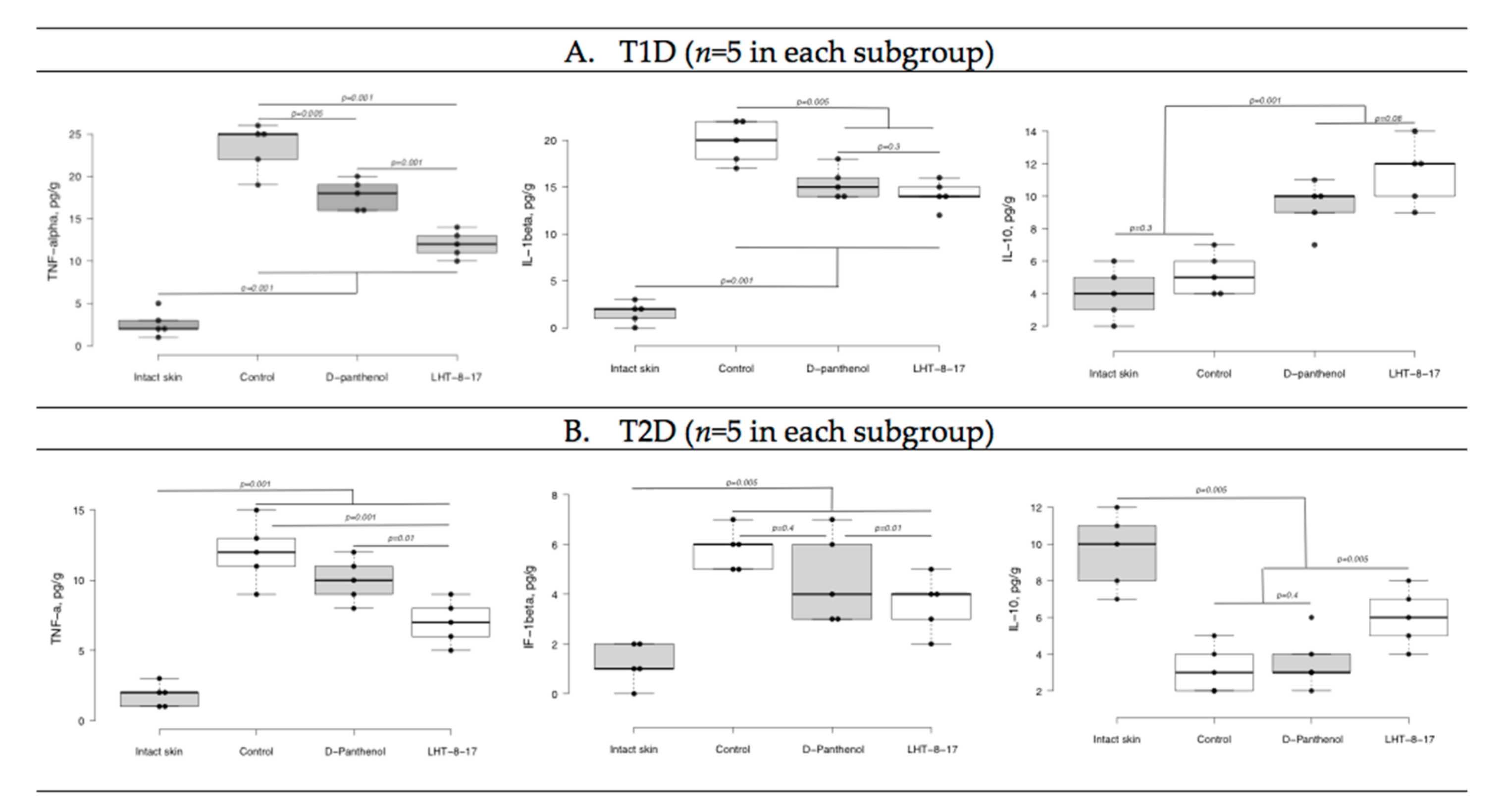
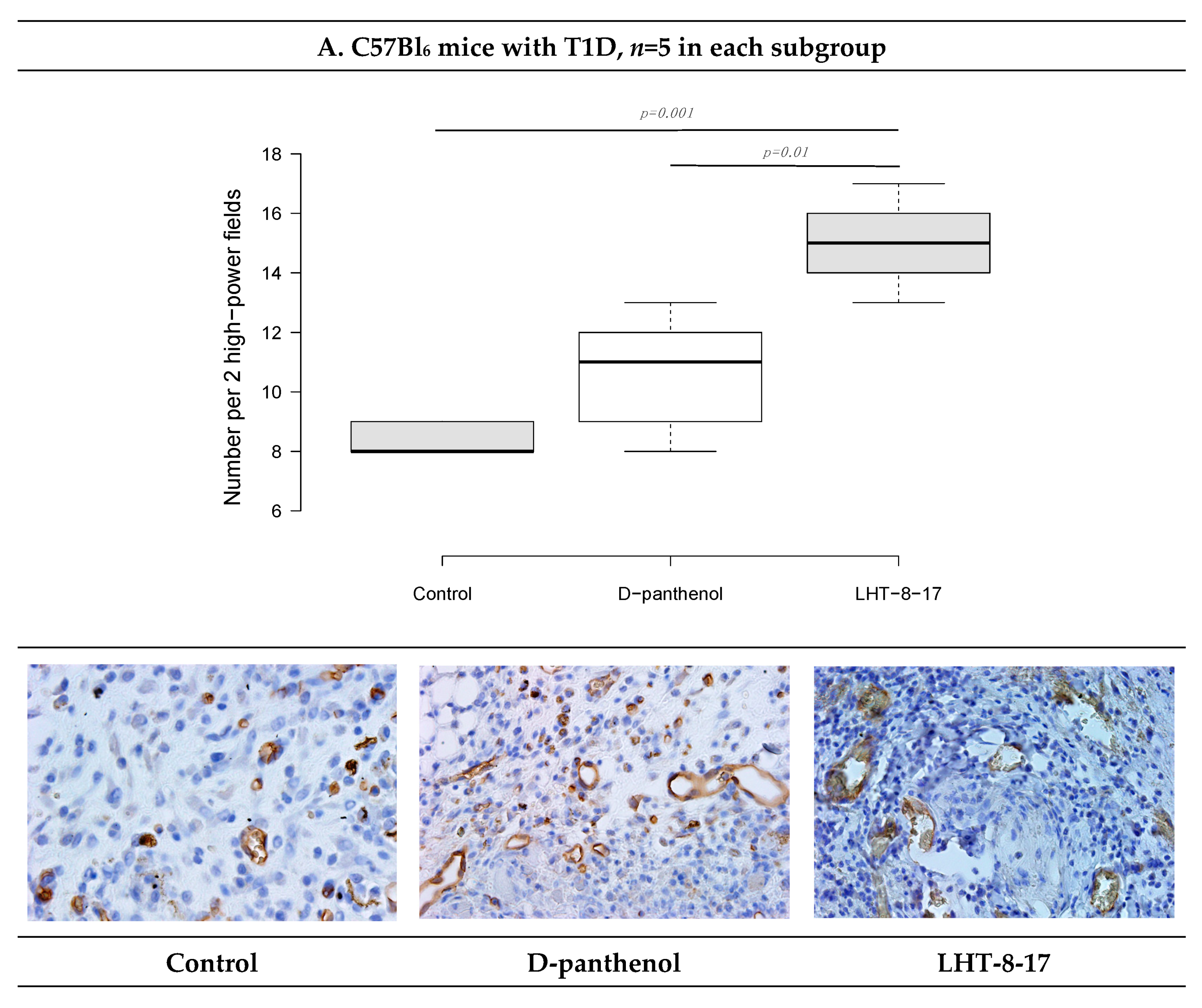
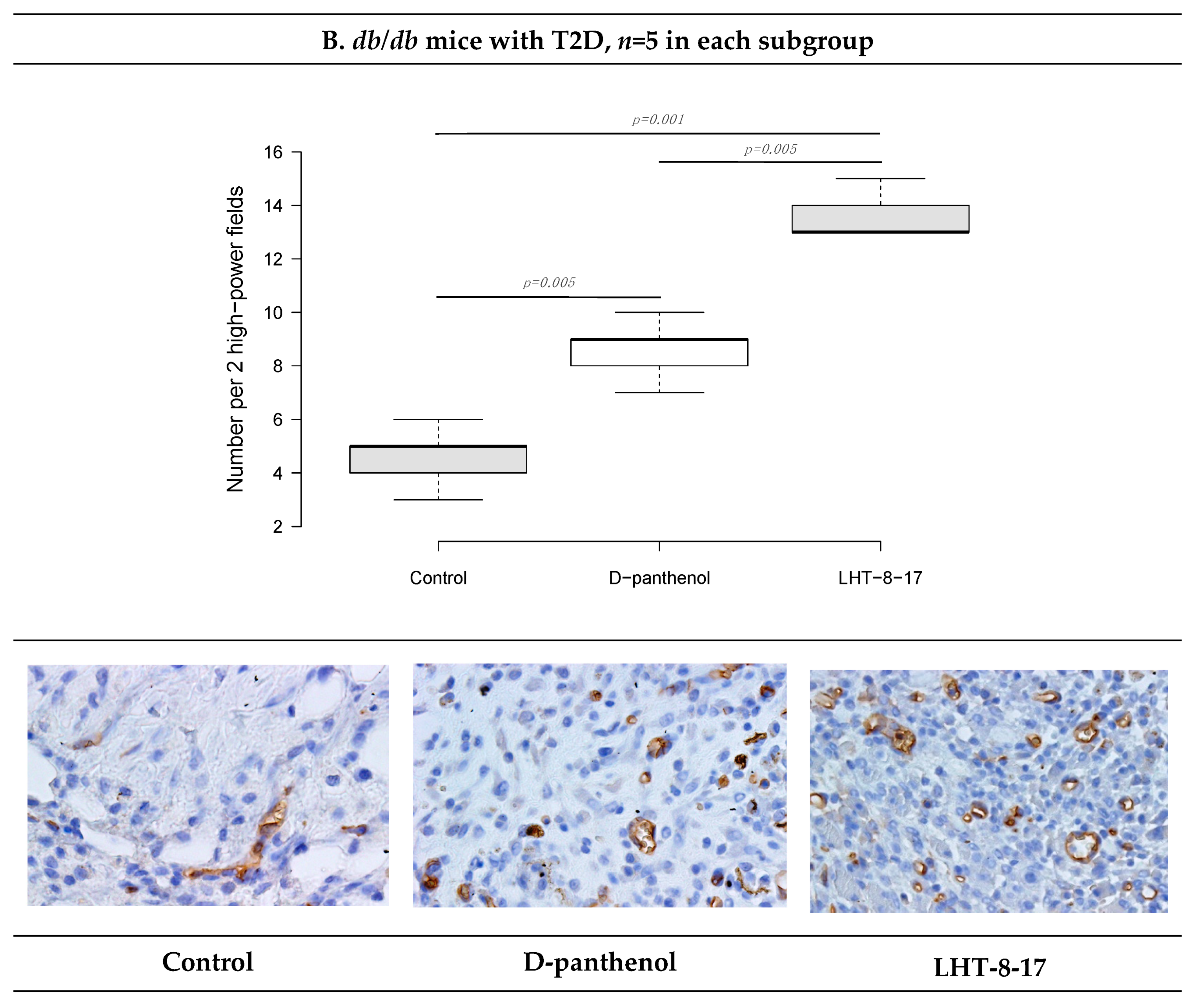

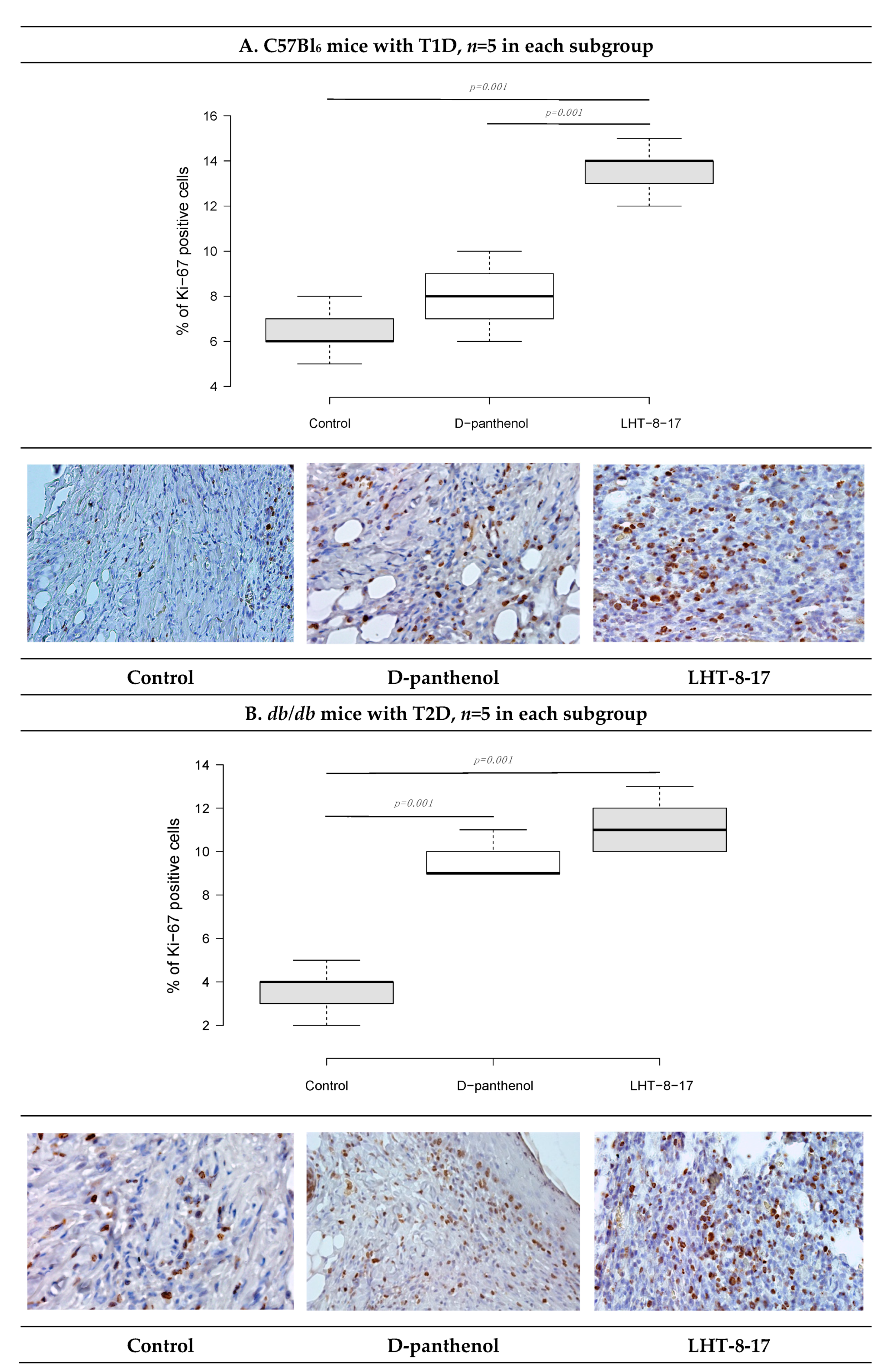
| Study Group | Wound Area, % of Initial Area’s Square | Average Time of Full Wound Healing, Days | Scar Area *, mm2 | |||||
|---|---|---|---|---|---|---|---|---|
| Initial Wound | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |||
| C57Bl6 mice with T1D | ||||||||
| Control | n = 20 100 ± 0 | n = 15 55 ± 2 | n = 10 32 ± 3 | n = 5 9 ± 4 | n = 5 0 ± 0 | n = 5 0 ± 0 | 23.7 ± 1.3 | 56 ± 4 |
| D-Panthenol | n = 20 100 ± 0 | n = 15 25 ± 3 † | n = 10 6 ± 4 † | n = 5 0 ± 0 † | n = 5 0 ± 0 | n = 5 0 ± 0 | 17.8 ± 1.1 † | 39 ± 3 † |
| LHT-8-17 | n = 20 100 ± 0 | n = 15 21 ± 4 † | n = 10 5 ± 3 † | n = 5 0 ± 0 † | n = 5 0 ± 0 | n = 5 0 ± 0 | 16.3 ± 0.7 † | 39 ± 4 † |
| db/db mice with T2D | ||||||||
| Control | n = 20 100 ± 0 | n = 15 70 ± 4 | n = 10 42 ± 5 | n = 5 13 ± 4 | n = 5 7 ± 3 | n = 5 0 ± 0 | 28.5 ± 1.5 | 74 ± 4 |
| D-Panthenol | n = 20 100 ± 0 | n = 15 76 ± 3 | n = 10 35 ± 4 | n = 5 16 ± 3 | n = 5 5 ± 3 | n = 5 0 ± 0 | 27.7 ± 1.1 | 56 ± 3 † |
| LHT-8-17 | n = 20 100 ± 0 | n = 15 33 ± 4 †,‡ | n = 10 21 ± 3 †,‡ | n = 5 5 ± 2 †,‡ | n = 5 0 ± 0 †,‡ | n = 5 0 ± 0 | 22.3 ± 1.0 †,‡ | 43 ± 4 †,‡ |
| Study Group | Fe-Induced Chemiluminescence, ×103 imp/s | |||
|---|---|---|---|---|
| Day 5 | Day 10 | |||
| LOSA | TAC | LOSA | TAC | |
| T1D model (n = 5 in each subgroup) | ||||
| Intact skin | 2.7 ± 0.3 | 3.3 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.3 |
| Control | 8.3 ± 0.4 * | 1.0 ± 0.2 * | 6.7 ± 0.3 * | 1.7 ± 0.2 * |
| D-panthenol | 6.6 ± 0.3 *,# | 1.7 ± 0.4 * | 4.3 ± 0.4 *,# | 1.9 ± 0.3 * |
| LHT-8-17 | 5.2 ± 0.2 *,# | 2.1 ± 0.3 *,# | 4.2 ± 0.3 *,# | 2.7 ± 0.2 #,† |
| T2D model (n = 5 in each subgroup) | ||||
| Intact skin | 3.1 ± 0.3 | 2.9 ± 0.4 | 3.7 ± 0.3 | 2.4 ± 0.4 |
| Control | 12.7 ± 0.4 * | 0.5 ± 0.1 * | 11.8 ± 0.5 * | 0.7 ± 0.2 * |
| D-panthenol | 9.4 ± 0.3 *,# | 1.3 ± 0.3 *,# | 8.9 ± 0.4 * | 1.5 ± 0.3 # |
| LHT-8-17 | 8.3 ± 0.5 *,# | 1.9 ± 0.2 *,# | 6.3 ± 0.3 *,#,† | 2.2 ± 0.1 #,† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, E.; Pakhomov, D.; Shimanovsky, D.; Kilmyashkina, M.; Mazov, Y.; Demura, T.; Drozdov, V.; Blinov, D.; Deryabina, O.; Samishina, E.; et al. Cerium-Containing N-Acetyl-6-Aminohexanoic Acid Formulation Accelerates Wound Reparation in Diabetic Animals. Biomolecules 2021, 11, 834. https://doi.org/10.3390/biom11060834
Blinova E, Pakhomov D, Shimanovsky D, Kilmyashkina M, Mazov Y, Demura T, Drozdov V, Blinov D, Deryabina O, Samishina E, et al. Cerium-Containing N-Acetyl-6-Aminohexanoic Acid Formulation Accelerates Wound Reparation in Diabetic Animals. Biomolecules. 2021; 11(6):834. https://doi.org/10.3390/biom11060834
Chicago/Turabian StyleBlinova, Ekaterina, Dmitry Pakhomov, Denis Shimanovsky, Marina Kilmyashkina, Yan Mazov, Tatiana Demura, Vladimir Drozdov, Dmitry Blinov, Olga Deryabina, Elena Samishina, and et al. 2021. "Cerium-Containing N-Acetyl-6-Aminohexanoic Acid Formulation Accelerates Wound Reparation in Diabetic Animals" Biomolecules 11, no. 6: 834. https://doi.org/10.3390/biom11060834
APA StyleBlinova, E., Pakhomov, D., Shimanovsky, D., Kilmyashkina, M., Mazov, Y., Demura, T., Drozdov, V., Blinov, D., Deryabina, O., Samishina, E., Butenko, A., Skachilova, S., Sokolov, A., Vasilkina, O., Alkhatatneh, B. A., Vavilova, O., Sukhov, A., Shmatok, D., Sorokvasha, I., ... Lobanova, E. (2021). Cerium-Containing N-Acetyl-6-Aminohexanoic Acid Formulation Accelerates Wound Reparation in Diabetic Animals. Biomolecules, 11(6), 834. https://doi.org/10.3390/biom11060834






