Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Microwave-Assisted Extraction
2.3. Experimental Design
2.4. Extractions Using the Conventional Method
2.5. Quantification of PAC Content of Extracts
2.6. UPLC-ESI-MS Analysis of Catechins in Extracts
2.7. Total Antioxidant Capacity of the Extracts by FRAP Assay
2.8. Inhibitory Effect of Extracts on α-Glucosidase In Vitro
2.9. Cell Culture
2.10. Cytotoxicity of PAC in WRL-68 and HepG2 Cells
2.10.1. MTS Cell Viability/Metabolic Activity Assay
2.10.2. 7-AAD-Stained Flow Cytometry for Cell Viability/Cell Death Evaluation
2.11. Statistical Analysis
3. Results
3.1. Response Surface Modeling
3.1.1. Model Fitting of Parameters Based on Total Monomeric Catechins and Total PAC Content
3.1.2. Optimization of the MAE Operating Conditions
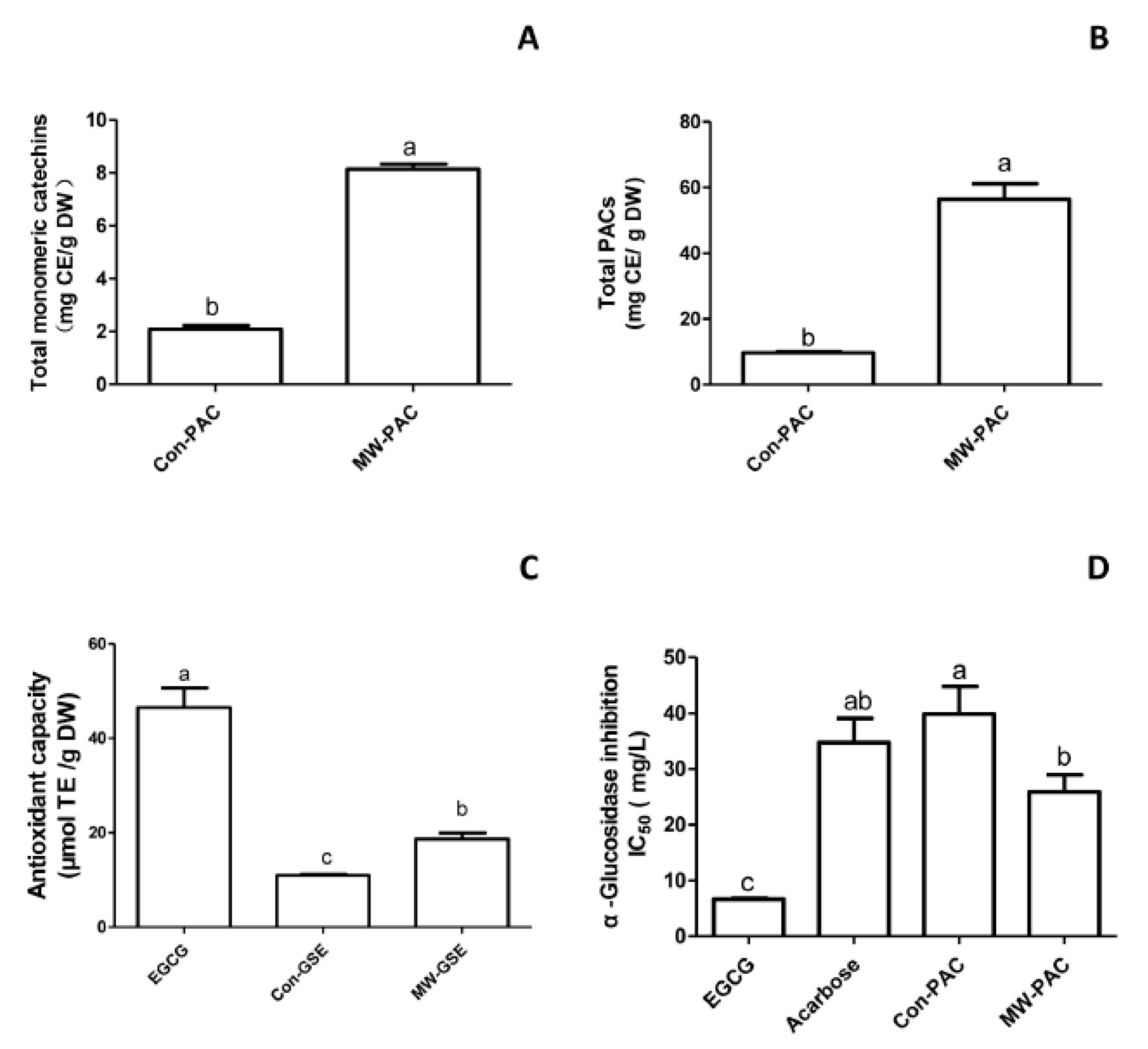
3.2. Comparison with Conventional Extraction
3.2.1. Quantitative Measurement
3.2.2. Total Antioxidant Capacity
3.2.3. α-Glucosidase-Inhibitory Activity
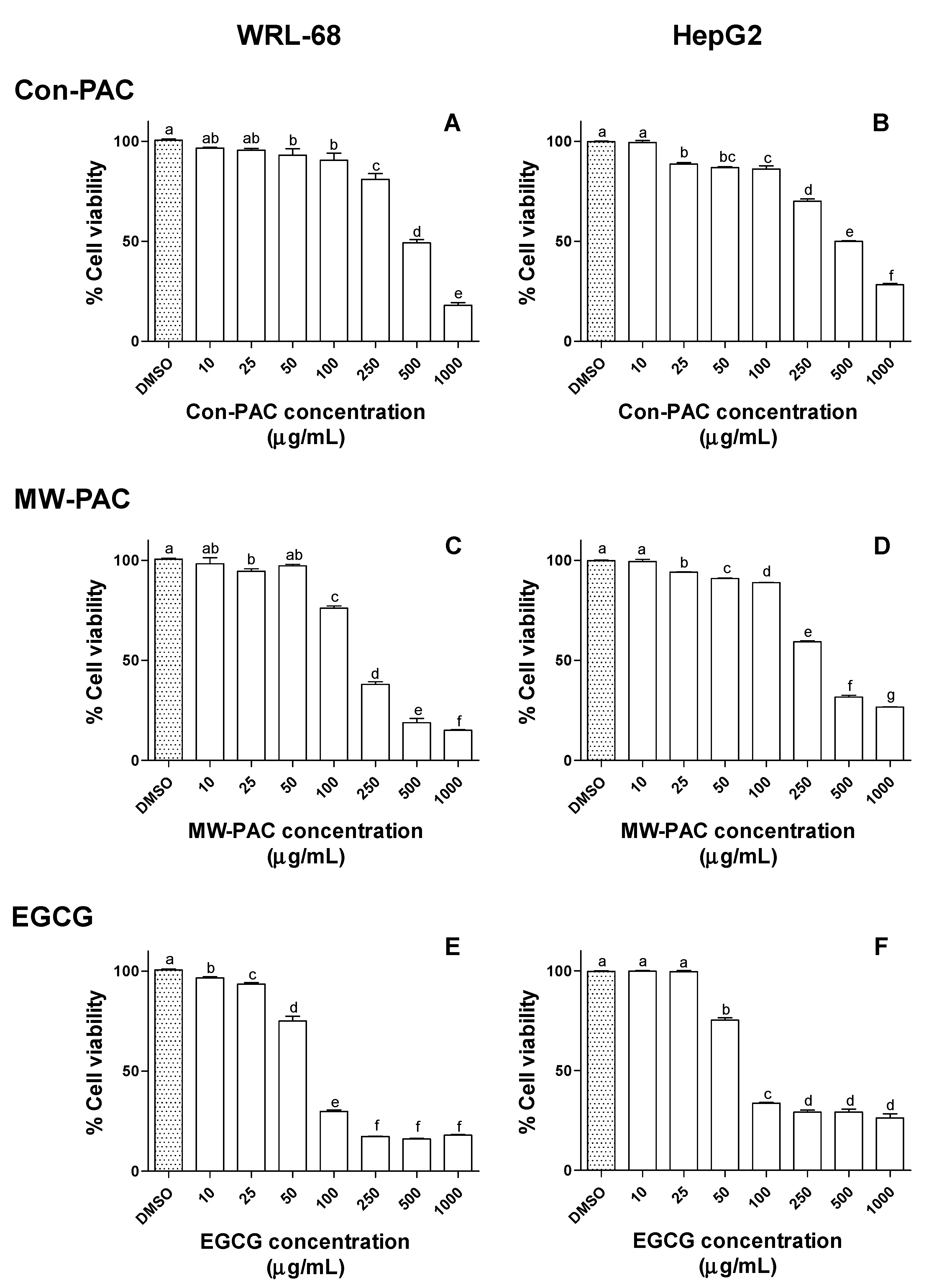
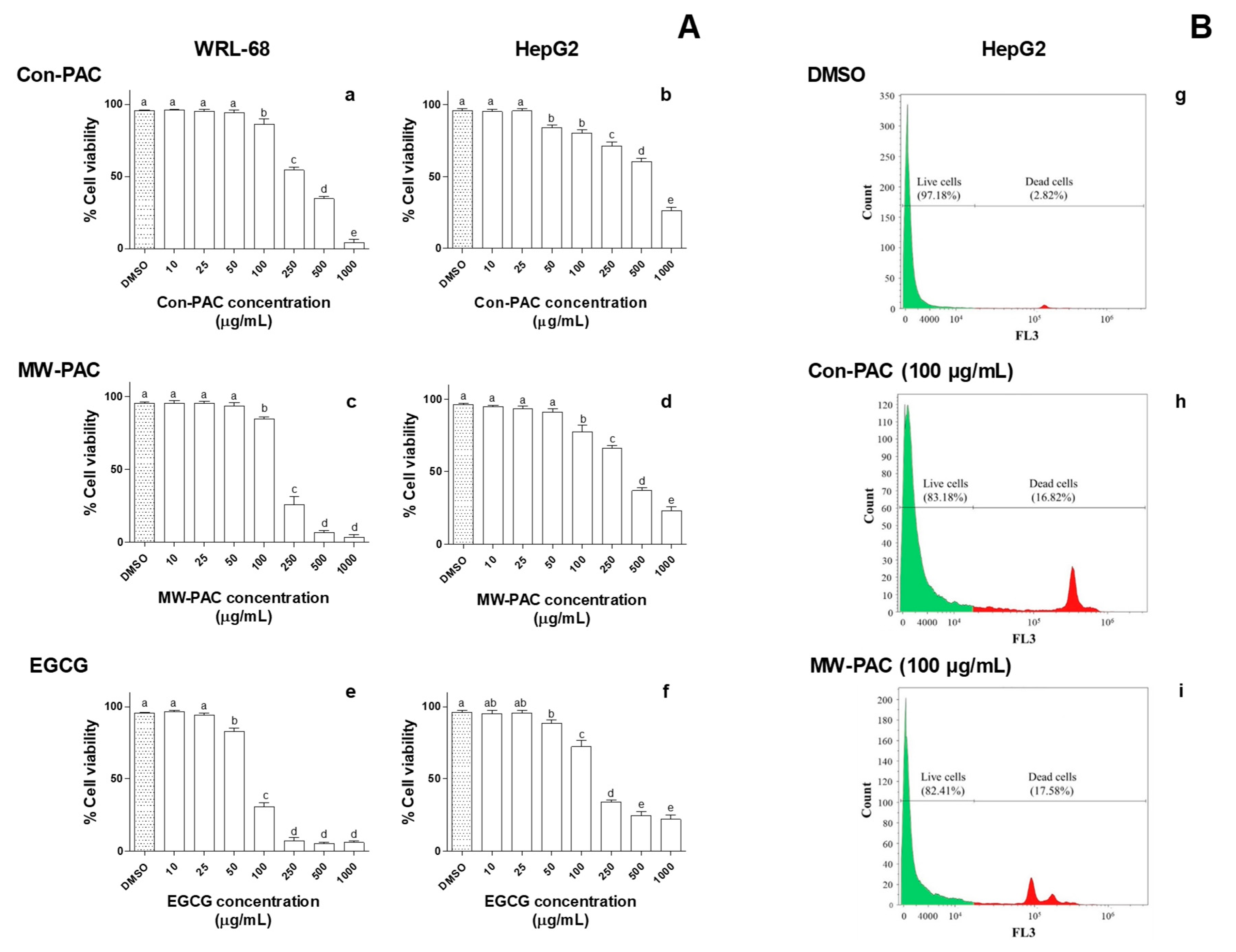
3.2.4. Cytotoxicity of Con-PAC and MW-PAC in WRL-68 and HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Chang, H.P.; Sheen, L.Y.; Lei, Y.P. The protective role of carotenoids and polyphenols in patients with head and neck cancer. J. Chin. Med. Assoc. 2015, 78, 89–95. [Google Scholar] [CrossRef]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and beer compounds: Physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 142–150. [Google Scholar] [CrossRef]
- Losada-Echeberria, M.; Herranz-Lopez, M.; Micol, V.; Barrajon-Catalan, E. Polyphenols as promising drugs against main breast cancer signatures. Antioxidants 2017, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The role of natural polyphenols in the prevention and treatment of cervical cancer—An overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in inflammatory bowel disease: A review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef]
- Martin, M.A.; Goya, L.; Ramos, S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem. Toxicol. 2013, 56, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef] [PubMed]
- Parmar, I.; Rupasinghe, H.P.V. Proanthocyanidins in cranberry and grape seeds: Metabolism, bioavailability and biological activity. In Nutraceuticals and Functional Foods: Natural Remedy; Brar, S.K., Kaur, S., Dhillon, G.S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 119–145. [Google Scholar]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, C.A.; Pedrotti, M.F.; Silva, J.S.; Barin, J.S.; Nóbrega, J.A.; Flores, E.M.M. Microwave-assisted digestion methods: Towards greener approaches for plasma-based analytical techniques. J. Anal. At. Spectrom. 2017, 32, 1448–1466. [Google Scholar] [CrossRef]
- Radojković, M.; Moreira, M.M.; Soares, C.; Fátima Barroso, M.; Cvetanović, A.; Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C. Microwave-assisted extraction of phenolic compounds from Morus nigrab leaves: Optimization and characterization of the antioxidant activity and phenolic composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef]
- Dang, Y.-Y.; Zhang, H.; Xiu, Z.-L. Microwave-assisted aqueous two-phase extraction of phenolics from grape (Vitis vinifera) seed. J. Chem. Technol. Biotechnol. 2014, 89, 1576–1581. [Google Scholar] [CrossRef]
- Romero-Diez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodriguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef]
- Dambergs, R.G.; Mercurio, M.D.; Kassara, S.; Cozzolino, D.; Smith, P.A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 2012, 66, 656–664. [Google Scholar] [CrossRef]
- Benzie, I.F.S.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Watanabe, J.; Kawabata, J.; Kurihara, H.; Niki, R. Isolation and identification of α-glucosidase inhibitors from Tochu-cha (Eucommia ulmoides). Biosci. Biotechnol. Biochem. 2014, 61, 177–178. [Google Scholar] [CrossRef]
- Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V. Microbial metabolites of proanthocyanidins reduce chemical carcinogen-induced DNA damage in human lung epithelial and fetal hepatic cells in vitro. Food Chem. Toxicol. 2019, 125, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Majewska, M.; Lewandowska, U. The chemopreventive and anticancer potential against colorectal cancer of polyphenol-rich fruit extracts. Food Rev. Int. 2017, 34, 390–409. [Google Scholar] [CrossRef]
- Ramos, S.; Martin, M.A.; Goya, L. Effects of cocoa antioxidants in type 2 diabetes mellitus. Antioxidants 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Yammine, S.; Brianceau, S.; Manteau, S.; Turk, M.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M. Extraction and purification of high added value compounds from by-products of the winemaking chain using alternative/nonconventional processes/technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1375–1390. [Google Scholar] [CrossRef]
- Council of Europe. Green tea (Camelliae sinensis non fermentata folia). In European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2018. [Google Scholar]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Huang, D. Starch hydrolase inhibitors from edible plants. Adv. Food Nutr. Res. 2013, 70, 103–136. [Google Scholar]
- Weisburg, J.H.; Weissman, D.B.; Sedaghat, T.; Babich, H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin. Pharmacol. Toxicol. 2004, 95, 191–200. [Google Scholar] [CrossRef]

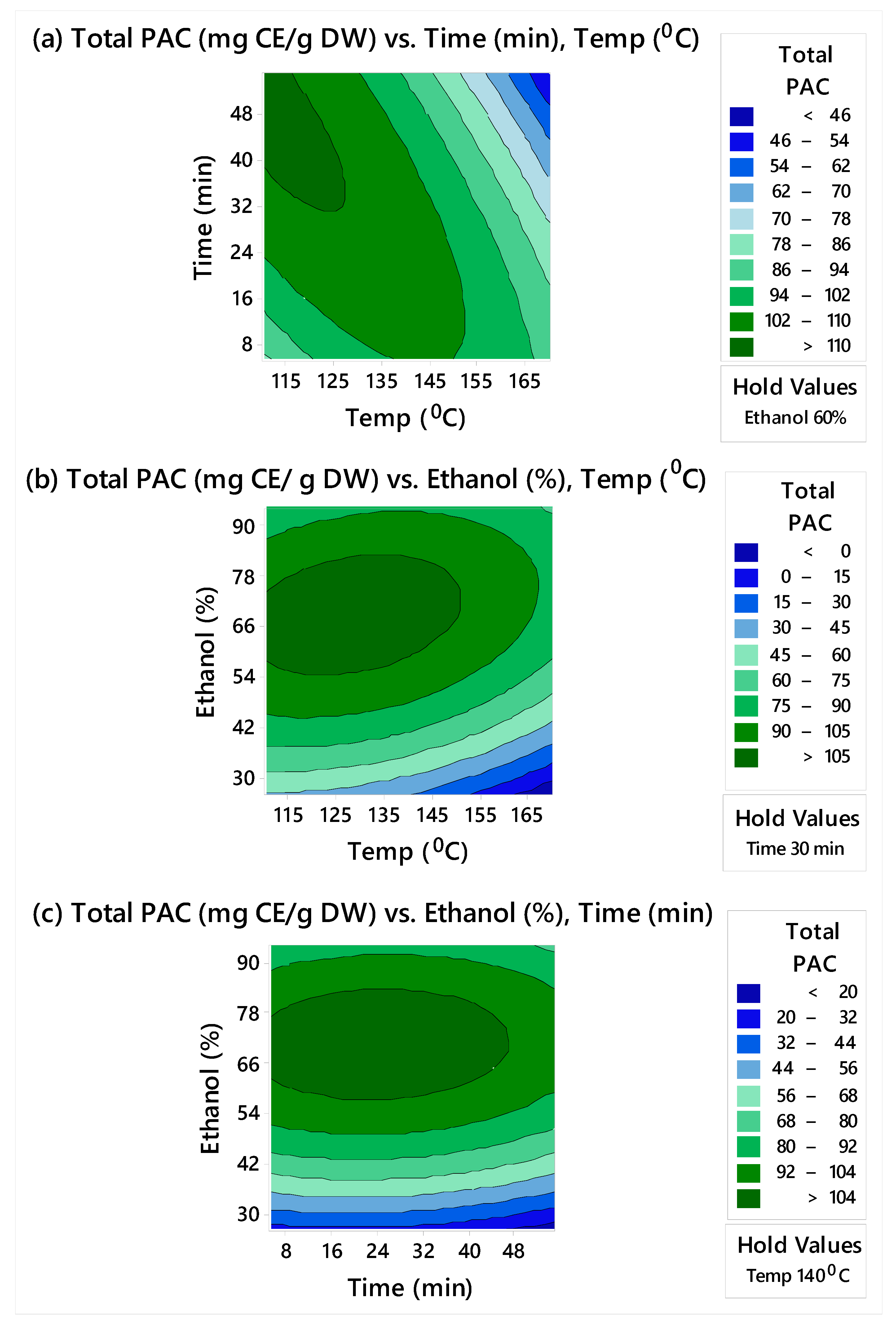

| Run Order | Temperature (°C) | Time (min) | Ethanol Concentration (%) | Total Monomeric Catechins (mg/g DW) | Total PACs (mg CE/g DW) |
|---|---|---|---|---|---|
| 1 | 110 (−1.68) | 30 (0) | 60 (0) | 11.18 | 108.22 |
| 2 | 120 (−1) | 15 (−1) | 40 (−1) | 8.11 | 72.12 |
| 3 | 120 (−1) | 15 (−1) | 80 (+1) | 11.72 | 99.92 |
| 4 | 120 (−1) | 45 (+1) | 40 (−1) | 6.75 | 83.98 |
| 5 | 120 (−1) | 45 (+1) | 80 (+1) | 11.65 | 104.18 |
| 6 | 140 (0) | 30 (0) | 60 (0) | 11.03 | 106.87 |
| 7 | 140 (0) | 30 (0) | 60 (0) | 9.31 | 107.03 |
| 8 | 140 (0) | 30 (0) | 60 (0) | 9.71 | 106.83 |
| 9 | 140 (0) | 30 (0) | 60 (0) | 9.26 | 107.81 |
| 10 | 140 (0) | 30 (0) | 60 (0) | 8.83 | 103.46 |
| 11 | 140 (0) | 30 (0) | 60 (0) | 9.05 | 107.58 |
| 12 | 140 (0) | 30 (0) | 26.4 (−1.68) | 3.07 | 24.44 |
| 13 | 140 (0) | 30 (0) | 93.6 (+1.68) | 10.64 | 85.48 |
| 14 | 140 (0) | 5 (−1.68) | 60 (0) | 10.58 | 97.90 |
| 15 | 140 (0) | 55 (+1.68) | 60 (0) | 9.85 | 90.28 |
| 16 | 160 (+1) | 15 (−1) | 40 (−1) | 8.52 | 66.58 |
| 17 | 160 (+1) | 15 (−1) | 80 (+1) | 13.84 | 104.64 |
| 18 | 160 (+1) | 45 (+1) | 40 (−1) | 7.00 | 38.27 |
| 19 | 160 (+1) | 45 (+1) | 80 (+1) | 15.05 | 90.81 |
| 20 | 170 (+1.68) | 30 (0) | 60 (0) | 10.41 | 66.39 |
| Source of Variation | Total Monomeric Catechins | Total PACs |
|---|---|---|
| Temp | 0.179 | 0.001 |
| Time | 0.439 | 0.099 |
| EthConc | 0.001 | 0.001 |
| Temp × Temp | 0.020 | 0.005 |
| Time × Time | 0.126 | 0.075 |
| EthConc × EthConc | 0.015 | 0.001 |
| Temp × Time | 0.698 | 0.005 |
| Temp × EthConc | 0.112 | 0.026 |
| Time × EthConc | 0.181 | 0.683 |
| Adjusted R2 | 85.2% | 94.2% |
| Factor | Optimum Conditions for Total Monomeric Catechins | Optimum Conditions for Total PACs |
|---|---|---|
| Temperature | 170 °C | 120.3 °C |
| Ethanol concentration | 94% | 67.9% |
| Time | 55 min | 41.4 min |
| Predicted concentration under the optimum condition for each analyte | 18.3 mg/g DW | 113.6 mg CE/g DW |
| Predicted concentration of total monomeric catechins under the optimum condition of total PACs | 10.7 mg/g DW | n/a |
| Predicted concentration of total PACs under the optimum condition of total monomeric catechins | n/a | 43.7 mg CE/g DW |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Thilakarathna, W.P.D.W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules 2020, 10, 243. https://doi.org/10.3390/biom10020243
Chen J, Thilakarathna WPDW, Astatkie T, Rupasinghe HPV. Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules. 2020; 10(2):243. https://doi.org/10.3390/biom10020243
Chicago/Turabian StyleChen, Jing, W. P. D. Wass Thilakarathna, Tessema Astatkie, and H. P. Vasantha Rupasinghe. 2020. "Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction" Biomolecules 10, no. 2: 243. https://doi.org/10.3390/biom10020243
APA StyleChen, J., Thilakarathna, W. P. D. W., Astatkie, T., & Rupasinghe, H. P. V. (2020). Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules, 10(2), 243. https://doi.org/10.3390/biom10020243