Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dyeing of Cotton Fabric with GSE
2.3. Measurements
3. Results and Discussion
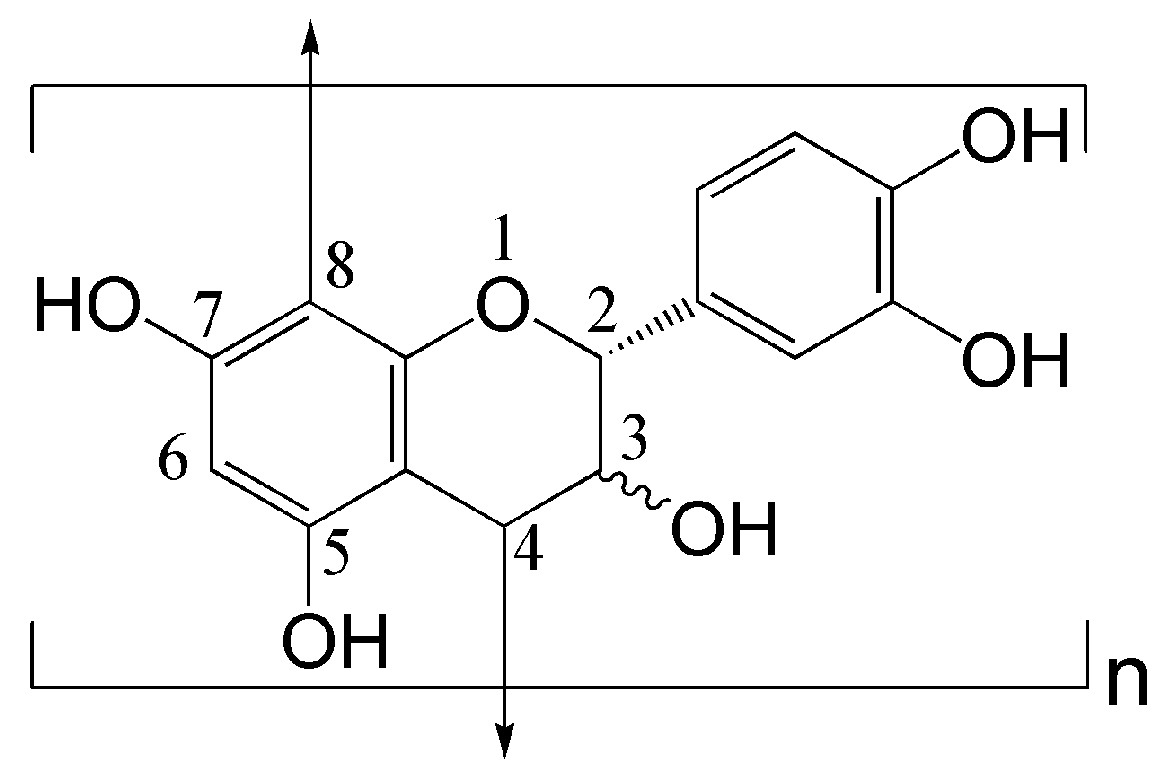
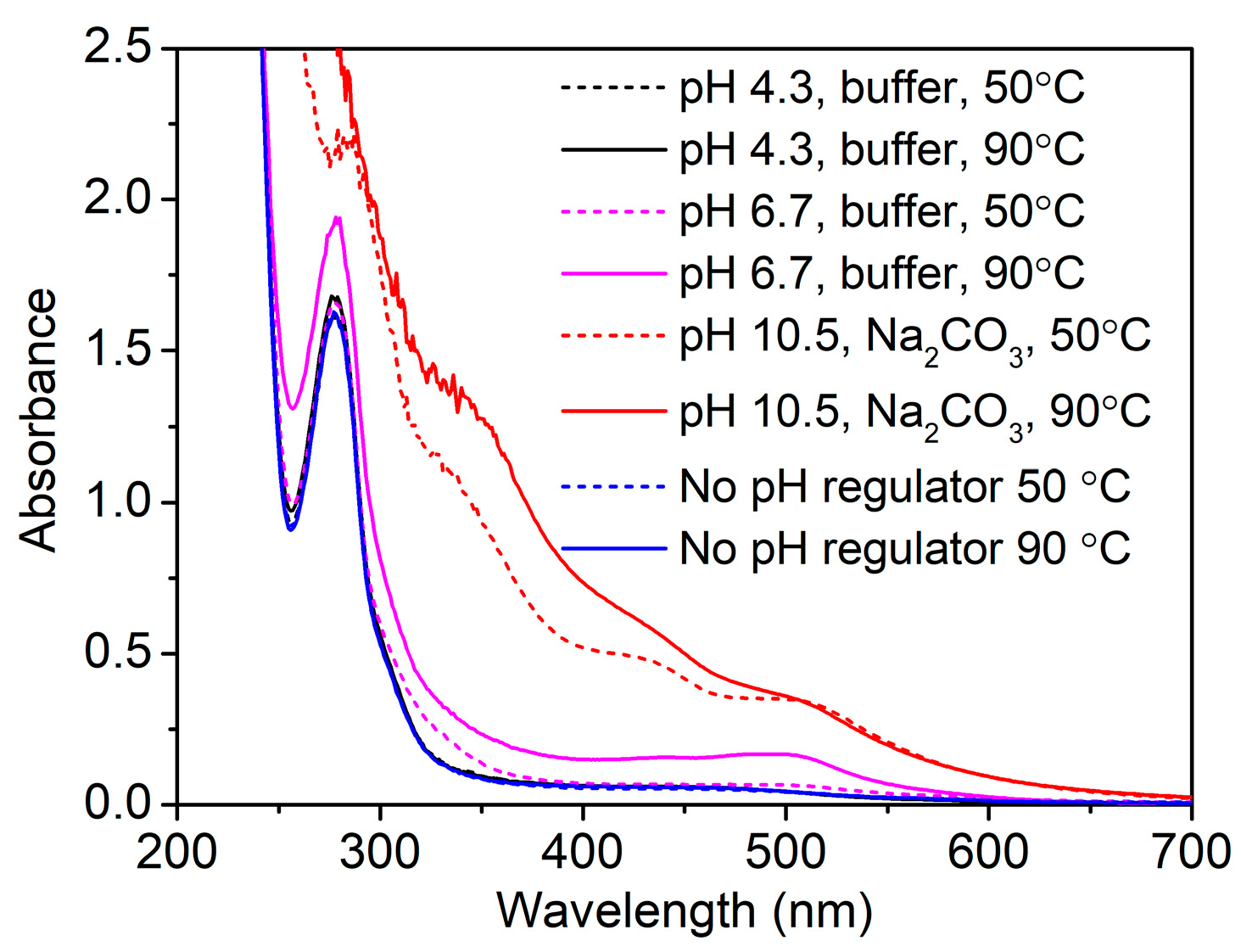
3.1. UV–Vis Absorption Spectroscopic Study of GSE
3.2. Application Conditions of GSE
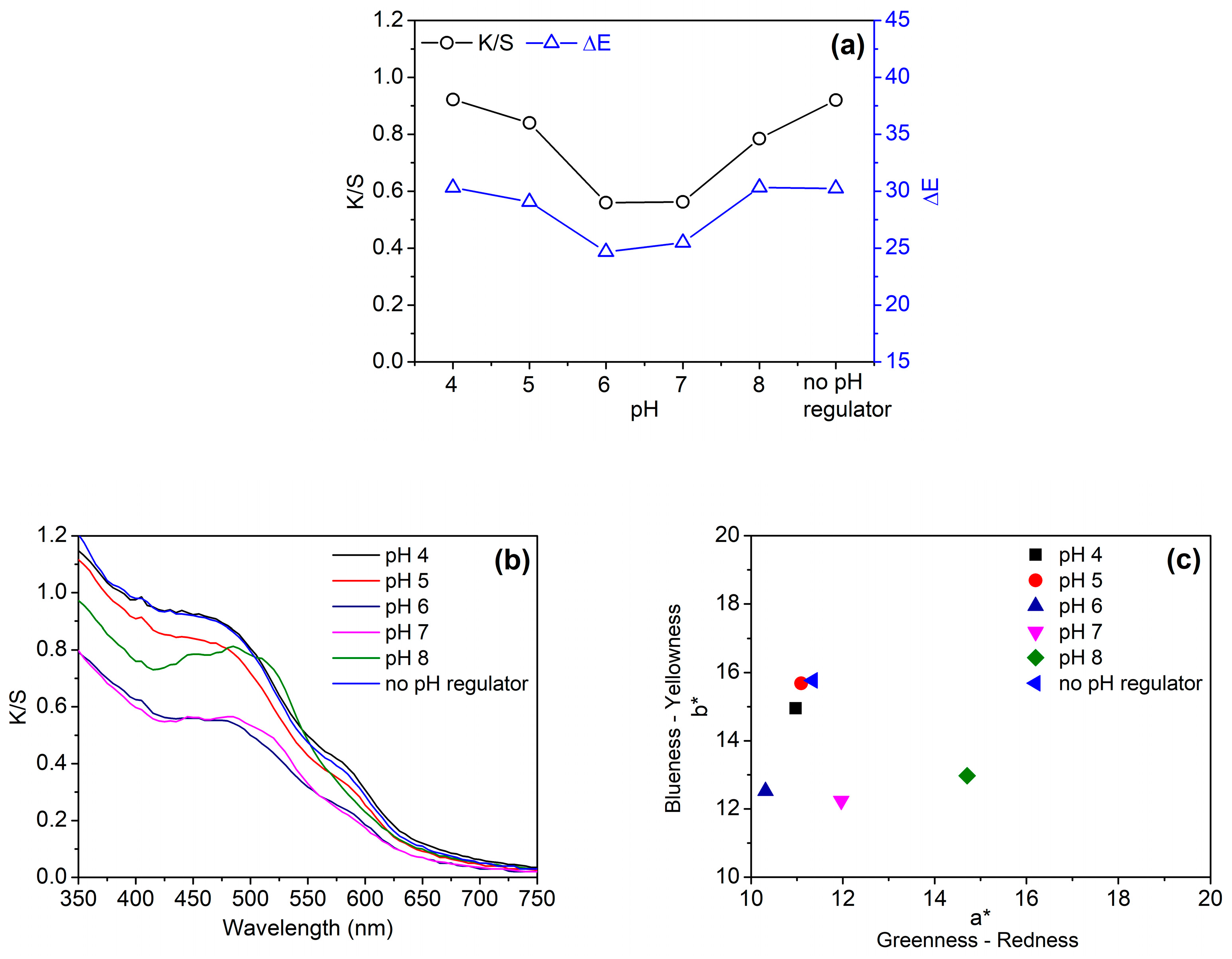
3.2.1. pH
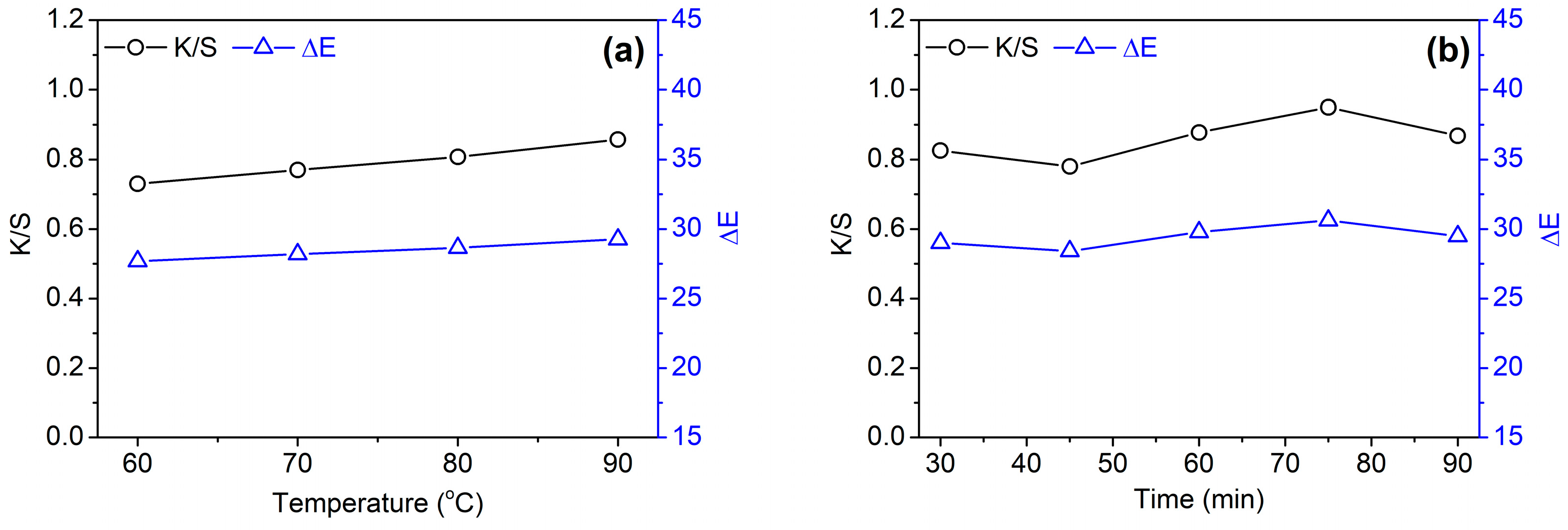
3.2.2. Temperature and Time
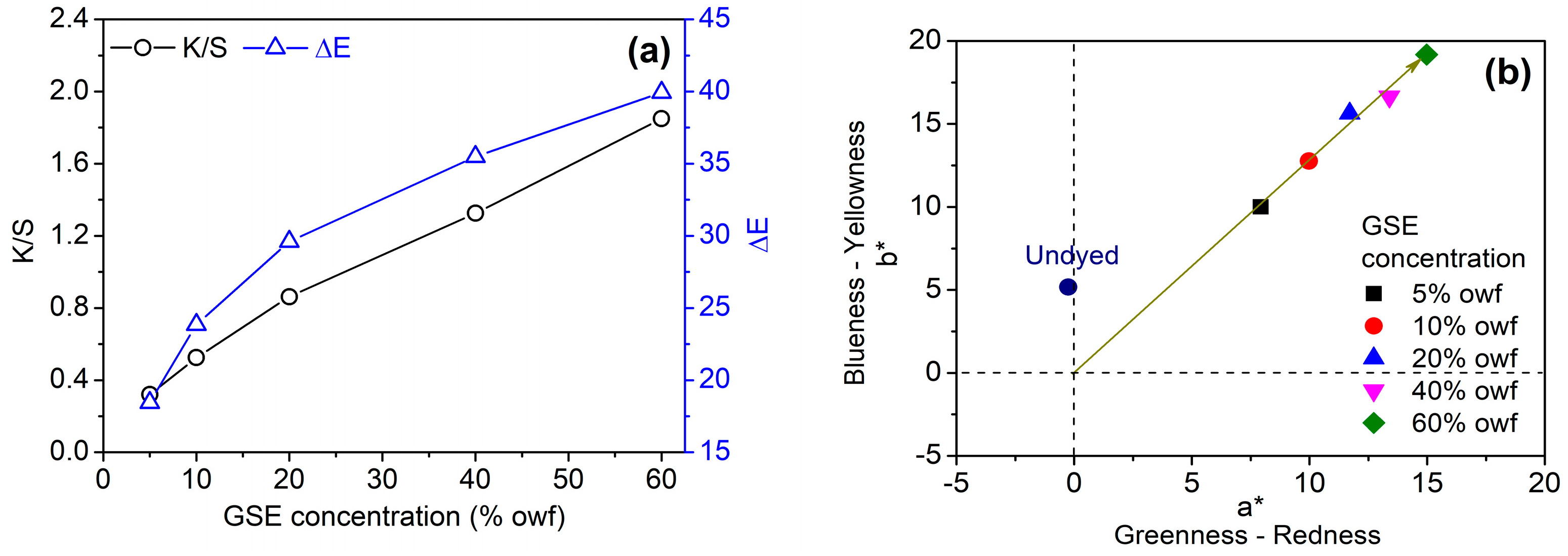
3.2.3. GSE Concentration
3.3. Color Fastness
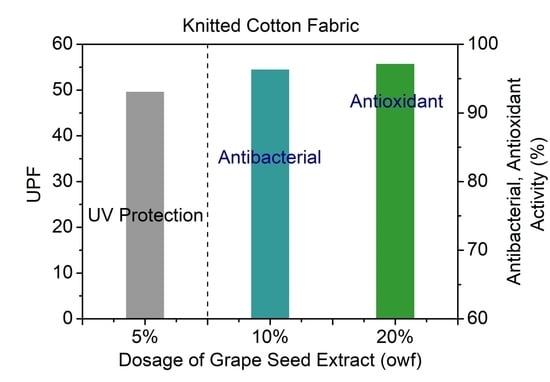
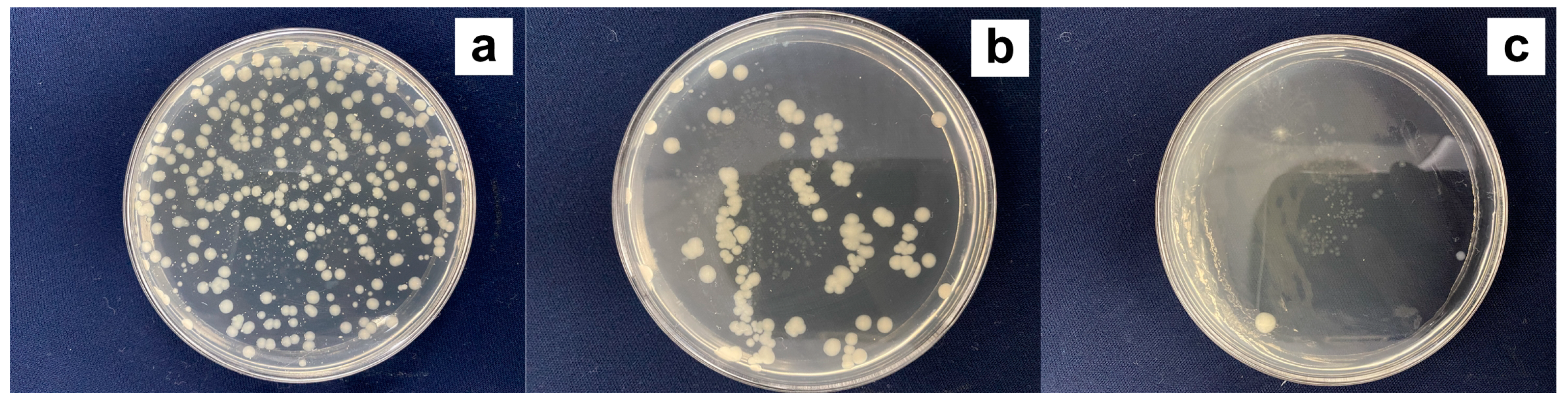
3.4. Antibacterial and Antioxidant Properties
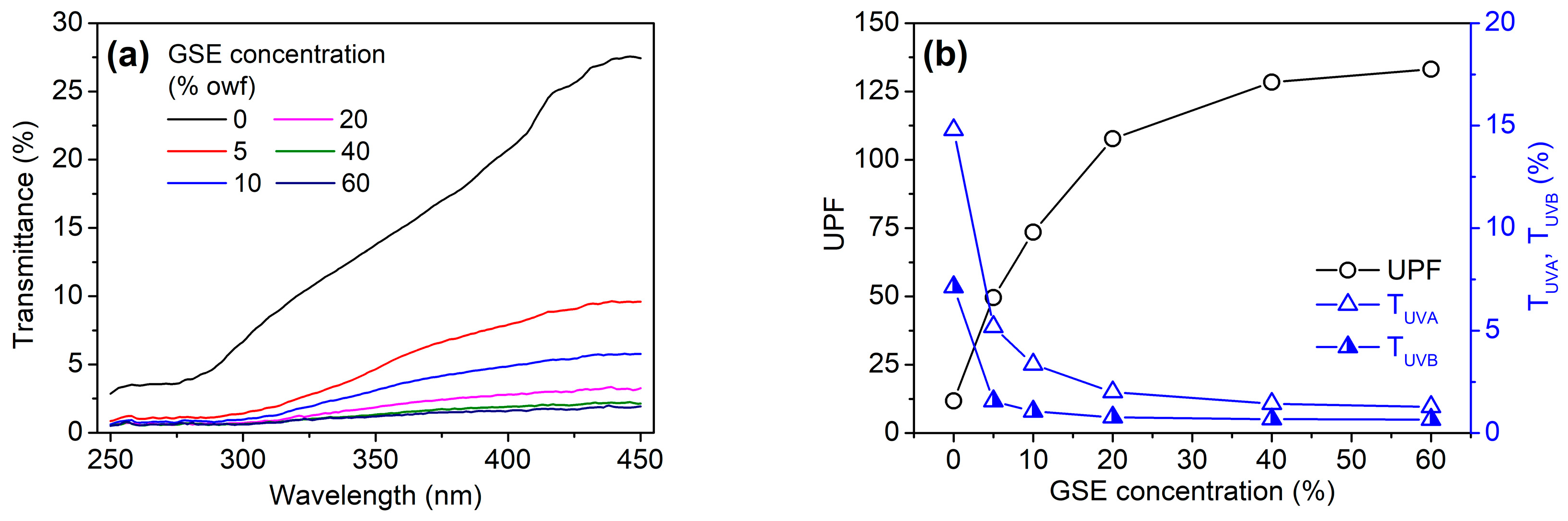
3.5. UV Protection Ability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| a* | Redness-greenness index |
| ABTS | 2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt |
| AS/NZS | Australia/New Zealand standard |
| b* | Yellowness-blueness index |
| C* | Chroma |
| E. coli | Escherichia coli |
| ΔE | Color difference |
| GB/T | Chinese national standard suggested |
| GSE | Grape seed extract |
| ISO | International Organization for Standardization |
| K/S | Apparent color strength |
| L* | Lightness |
| owf | On the weight of fabric |
| UPF | Ultraviolet protection factor |
| UV | Ultraviolet |
| UVA | Ultraviolet light from 315 nm to 400 nm |
| UVB | Ultraviolet light from 280 nm to 315 nm |
| UV–Vis | Ultraviolet–visible |
References
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Duval, A.; Couture, G.; Caillol, S.; Avérous, L. Biobased and aromatic reversible thermoset networks from condensed tannins via the Diels–Alder reaction. ACS Sustain. Chem. Eng. 2017, 5, 1199–1207. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Blanchart, P.; Dembelé, A.; Dembelé, C.; Pléa, M.; Bergström, L.; Granet, R.; Sol, V.; Gloaguen, V.; Degot, M.; Krausz, P. Mechanism of traditional Bogolan dyeing technique with clay on cotton fabric. Appl. Clay Sci. 2010, 50, 455–460. [Google Scholar] [CrossRef]
- Restivo, A.; Degano, I.; Ribechini, E.; Pérez-Arantegui, J.; Colombini, M.P. Field-emission scanning electron microscopy and energy-dispersive X-ray analysis to understand the role of tannin-based dyes in the degradation of historical wool textiles. Microsc. Microanal. 2014, 20, 1534–1543. [Google Scholar] [CrossRef]
- Wilson, H.; Carr, C.; Hacke, M. Production and validation of model iron-tannate dyed textiles for use as historic textile substitutes in stabilization treatment studies. Chem. Cent. J. 2012, 6, 44. [Google Scholar] [CrossRef]
- Yang, T.-T.; Guan, J.-P.; Tang, R.-C.; Chen, G. Condensed tannin from Dioscorea cirrhosa tuber as an eco-friendly and durable flame retardant for silk textile. Ind. Crops Prod. 2018, 115, 16–25. [Google Scholar] [CrossRef]
- Gupta, D.; Khare, S.K.; Laha, A. Antimicrobial properties of natural dyes against Gram-negative bacteria. Color Technol. 2004, 120, 167–171. [Google Scholar] [CrossRef]
- Pisitsak, P.; Hutakamol, J.; Thongcharoen, R.; Phokaew, P.; Kanjanawan, K.; Saksaeng, N. Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind. Crops Prod. 2016, 79, 47–56. [Google Scholar] [CrossRef]
- Rather, L.J.; Akhter, S.; Padder, R.A.; Hassan, Q.P.; Hussain, M.; Khan, M.A.; Mohammad, F. Colorful and semi durable antioxidant finish of woolen yarn with tannin rich extract of Acacia nilotica natural dye. Dyes Pigm. 2017, 139, 812–819. [Google Scholar] [CrossRef]
- Singh, R.; Jain, A.; Panwar, S.; Gupta, D.; Khare, S.K. Antimicrobial activity of some natural dyes. Dyes Pigm. 2005, 66, 99–102. [Google Scholar] [CrossRef]
- Medouni-Adrar, S.; Boulekbache-Makhlouf, L.; Cadot, Y.; Medouni-Haroune, L.; Dahmoune, F.; Makhoukhe, A.; Madani, K. Optimization of the recovery of phenolic compounds from Algerian grape by-products. Ind. Crops Prod. 2015, 77, 123–132. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Tounsi, M.S.; Ouerghemmi, I.; Wannes, W.A.; Ksouri, R.; Zemni, H.; Marzouk, B.; Kchouk, M.E. Valorization of three varieties of grape. Ind. Crops Prod. 2009, 30, 292–296. [Google Scholar] [CrossRef]
- Mandic, A.I.; Ðilas, S.M.; Ćetković, G.S.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Polyphenolic composition and antioxidant activities of grape seed extract. Int. J. Food Prop. 2008, 11, 713–726. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Cheynier, V.; Moutounet, M. Interactions of grape seed tannins with salivary proteins. J. Agric. Food Chem. 1999, 47, 42–47. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Antimicrobial wrapping papercoated with a ternary blend of carbohydrates (alginate, carboxymethyl cellulose, carrageenan) and grapefruit seed extract. Carbohyd. Polym. 2018, 196, 92–101. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Baaka, N.; Haddar, W.; Ben Ticha, M.; Amorim, M.T.P.; M’Henni, M.F. Sustainability issues of ultrasonic wool dyeing with grape pomace colorant. Nat. Prod. Res. 2017, 31, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Baaka, N.; Ben Ticha, M.; Haddar, W.; Mhenni, M.F. A challenging approach to improve the low affinity of acrylic fibers to be successfully dyed with a bio-colorant extracted from grape marc. J. Renew. Mater. 2019, 7, 289–300. [Google Scholar] [CrossRef]
- Yang, H.; Park, Y. Optimum dyeing condition of cotton by fermented grape by-products with degraded protein mordant. Text. Coloration Finish. 2015, 27, 202–209. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Rigg, B. Chapter 3 Colorimetry and the CIE system. In Color Physics for Industry, 2nd ed.; McDonald, R., Ed.; Society of Dyers and Colorists: West Yorkshire, UK, 1997; pp. 81–120. [Google Scholar]
- Textiles–Evaluation for Antibacterial Activity–Part 3: Shake Flask Method. GB/T 20944.3–2008, China’s General Administration of Quality Supervision; Inspection and Quarantine and Standardization Administration of China: Beijing, China, 2008.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.-Y.; Cheng, X.-W.; Tang, R.-C.; Qiao, Y.-F. Adsorption, antibacterial and antioxidant properties of tannic acid on silk fiber. Polymers 2019, 11, 970. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemicaltests. J. Cult. Herit. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Effect of pH, temperature, and time on inactivation of tannins and possible implications in detannification studies. J. Agric. Food Chem. 1996, 44, 1291–1295. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- National General Safety Technical Code for Textile Products. GB/T 18401–2010, China’s General Administration of Quality Supervision; Inspection and Quarantine and Standardization Administration of China: Beijing, China, 2010. [Google Scholar]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Shahid-ul-Islam; Mohammad, F. Natural colorants in the presence of anchors so-called mordants as promising coloring and antimicrobial agents for textile materials. ACS Sustain. Chem. Eng. 2015, 3, 2361–2375. [Google Scholar] [CrossRef]
- Zimniewska, M.; Kozłowski, R.; Batog, J. Nanolignin modified linen fabric as a multifunctional product. Mol. Cryst. Liq. Cryst. 2008, 484. [Google Scholar] [CrossRef]
- GöktürkBaydar, N.; Özkan, G.; Yaşar, S. Evaluation of the antiradical and antioxidant potential of grape extracts. Food Control 2007, 18, 1131–1136. [Google Scholar] [CrossRef]
- Gorjanc, M.; Jazbec, K.; Mozetič, M.; Kert, M. UV protective properties of cotton fabric treated with plasma, UV absorber, and reactive dye. Fiber. Polym. 2014, 15, 2095–2104. [Google Scholar] [CrossRef]
- Sahar, A.; Ali, S.; Hussain, T.; Irfan, M.; Eliasson, B.; Iqbal, J. UV absorbers for cellulosic apparels: A computational and experimental study. Spectrochim. Acta A 2018, 188, 355–361. [Google Scholar] [CrossRef]
- Grifoni, D.; Bacci, L.; Di Lonardo, S.; Pinelli, P.; Scardigli, A.; Camilli, F.; Sabatini, F.; Zipoli, G.; Romani, A. UV protective properties of cotton and flax fabrics dyed with multifunctional plant extracts. Dyes Pigm. 2014, 105, 89–96. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid-ul-Islam; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Mishra, P.K.; Wimmer, R. Aerosol assisted self-assembly as a route to synthesize solid and hollowspherical lignin colloids and its utilization in layer by layer deposition. Ultrason. Sonochem. 2017, 35, 45–50. [Google Scholar] [CrossRef]
- Sun Protective Clothing—Evaluation and Classification; Homebush NSW: Wellington, New Zealand, 1996.

| Varible | Levels | Other Parameters |
|---|---|---|
| pH | 4–8 | GSE 20%owf, 90 °C, 60 min |
| Temperature | 60–90 °C | GSE 20%owf, 60 min |
| Time | 30–90 min | GSE 20%owf, 90 °C |
| GSE concentration | 5–60% owf | 90 °C, 60 min |
| Sample | Washing | Rubbing | |||
|---|---|---|---|---|---|
| Color Change | Staining | Dry | Wet | ||
| Cotton | Wool | ||||
| 10% GSE dyed | 4–5 | 5 | 5 | 4–5 | 4–5 |
| 20% GSE dyed | 3–4 | 4–5 | 5 | 4–5 | 4–5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Yang, Z.-Y.; Tang, R.-C.; Yuan, H.-B. Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric. Biomolecules 2020, 10, 220. https://doi.org/10.3390/biom10020220
Guo L, Yang Z-Y, Tang R-C, Yuan H-B. Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric. Biomolecules. 2020; 10(2):220. https://doi.org/10.3390/biom10020220
Chicago/Turabian StyleGuo, Ling, Zhi-Yi Yang, Ren-Cheng Tang, and Hua-Bin Yuan. 2020. "Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric" Biomolecules 10, no. 2: 220. https://doi.org/10.3390/biom10020220
APA StyleGuo, L., Yang, Z.-Y., Tang, R.-C., & Yuan, H.-B. (2020). Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric. Biomolecules, 10(2), 220. https://doi.org/10.3390/biom10020220