Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury
Abstract
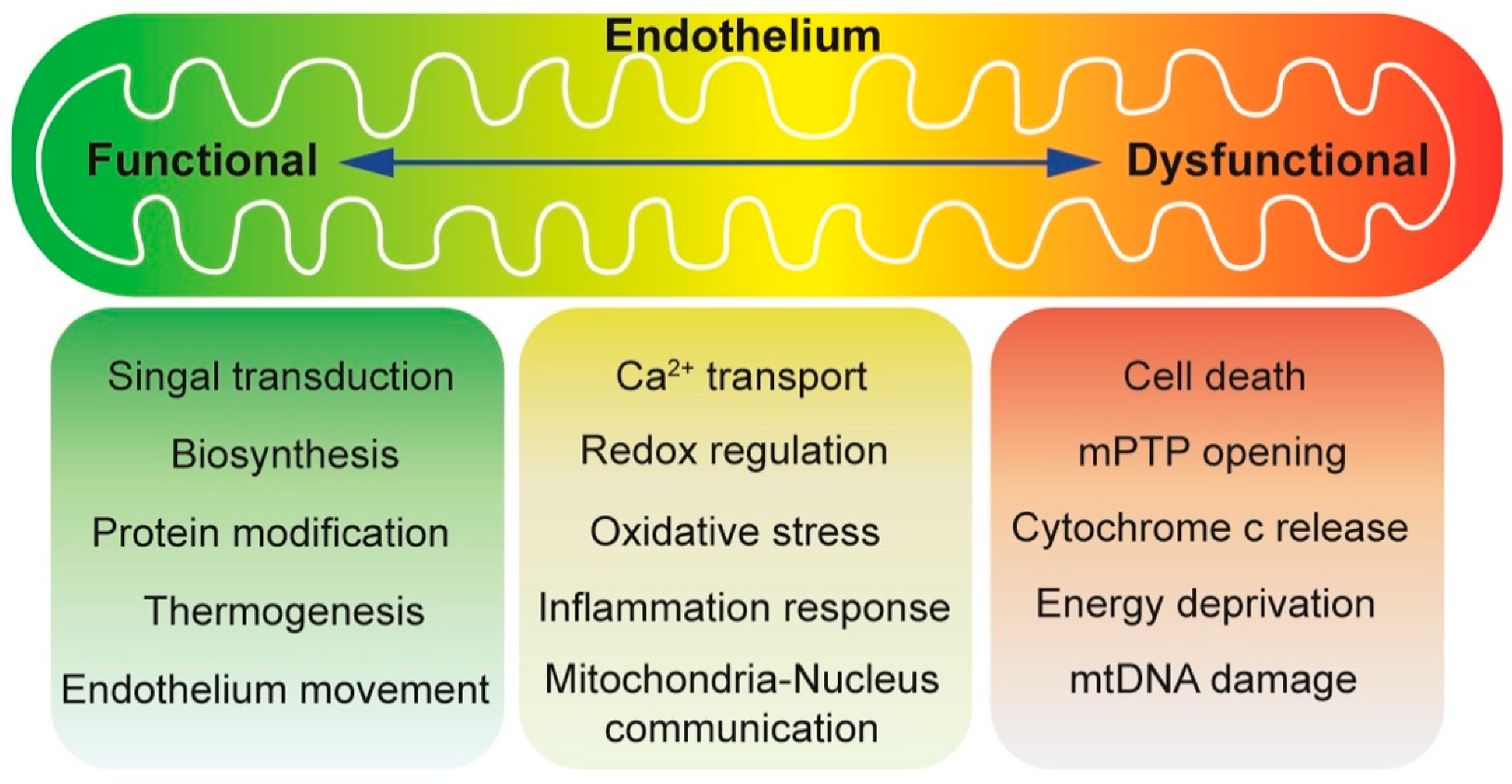
1. Introduction
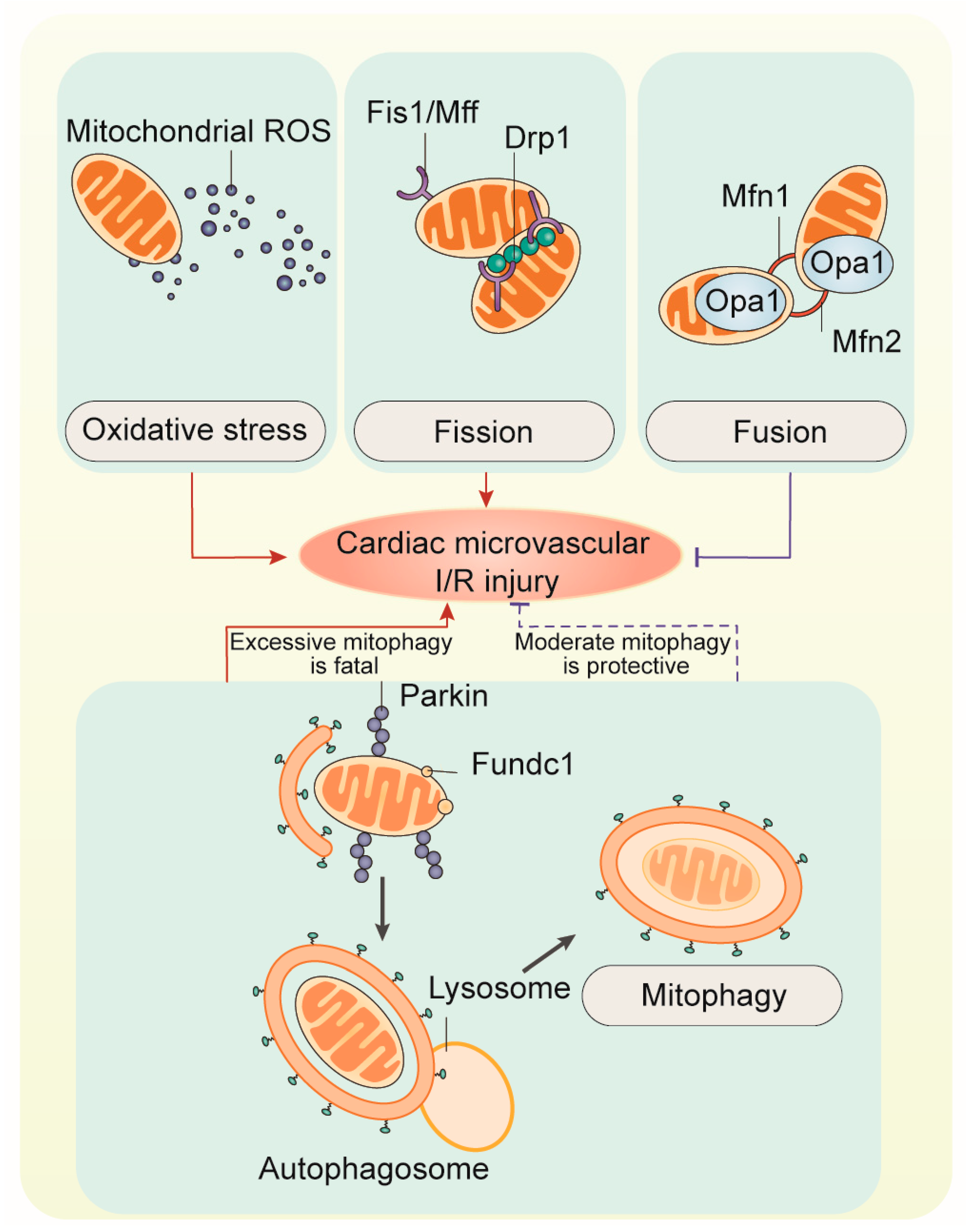
2. Mitochondrial Oxidative Stress
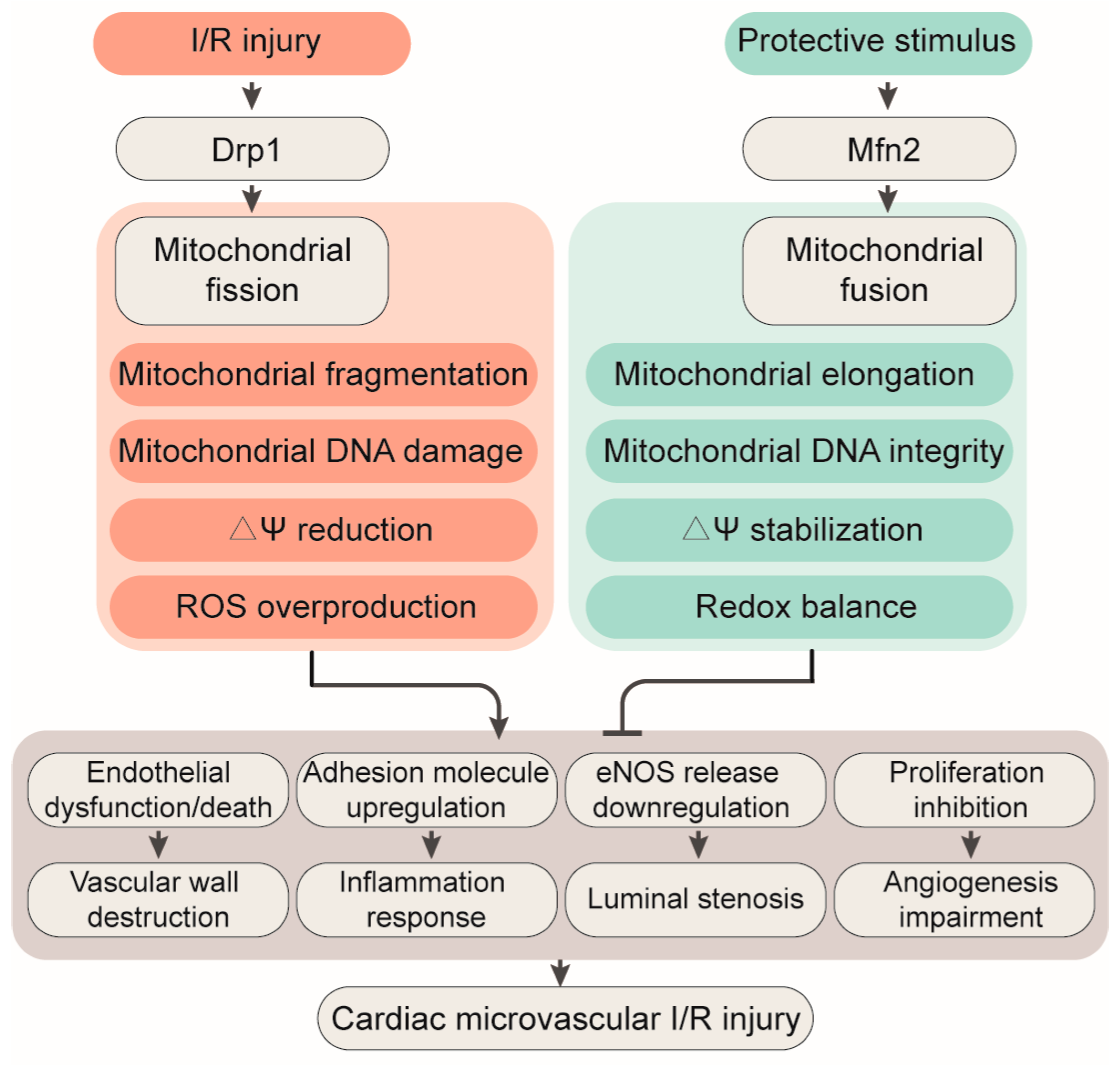
3. Mitochondrial Fission
4. Mitochondrial Fusion
5. Mitophagy
6. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heusch, G. 25 years of remote ischemic conditioning: From laboratory curiosity to clinical outcome. Basic Res. Cardiol. 2018, 113, 15. [Google Scholar] [CrossRef] [PubMed]
- Amanakis, G.; Kleinbongard, P.; Heusch, G.; Skyschally, A. Attenuation of ST-segment elevation after ischemic conditioning maneuvers reflects cardioprotection online. Basic Res. Cardiol. 2019, 114, 22. [Google Scholar] [CrossRef]
- Deussen, A. Mechanisms underlying coronary autoregulation continue to await clarification. Basic Res. Cardiol. 2018, 113, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ao, X.; Chu, X.; Wan, Q.; Wang, Y.; Xiao, D.; Yu, W.; Li, M.; Yu, F.; et al. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 2019, 20, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zou, L.X.; Lin, Q.Y.; Yan, X.; Bi, H.L.; Xie, X.; Wang, S.; Wang, Q.S.; Zhang, Y.L.; Li, H.H. Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload. Redox Biol. 2019, 20, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Tan, B.; Rose, P.; Zhu, D.; Zhu, Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca(2+)/calmodulin-dependent protein kinase II. Redox Biol. 2018, 19, 250–262. [Google Scholar] [CrossRef]
- Hadebe, N.; Cour, M.; Lecour, S. The SAFE pathway for cardioprotection: Is this a promising target? Basic Res. Cardiol. 2018, 113, 9. [Google Scholar] [CrossRef]
- Botker, H.E. The changing face after acute myocardial infarction. Basic Res. Cardiol. 2019, 115, 5. [Google Scholar] [CrossRef]
- Heusch, G. Coronary microvascular obstruction: The new frontier in cardioprotection. Basic Res. Cardiol. 2019, 114, 45. [Google Scholar] [CrossRef]
- Dassanayaka, S.; Brittian, K.R.; Jurkovic, A.; Higgins, L.A.; Audam, T.N.; Long, B.W.; Harrison, L.T.; Militello, G.; Riggs, D.W.; Chitre, M.G.; et al. E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation. Basic Res. Cardiol. 2019, 114, 28. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and endothelial function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; Mouton, A.J.; Ero, O.K.; Ma, Y.; Iyer, R.P.; Flynn, E.R.; Espinoza, I.; Musani, S.K.; Vasan, R.S.; Hall, M.E.; et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res. Cardiol. 2018, 113, 40. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tan, J.L.; Chen, Z.Y.; Huang, G. Cardioprotection of post-ischemic moderate ROS against ischemia/reperfusion via STAT3-induced the inhibition of MCU opening. Basic Res. Cardiol. 2019, 114, 39. [Google Scholar] [CrossRef]
- Bateman, R.M.; Sharpe, M.D.; Ellis, C.G. Bench-to-bedside review: Microvascular dysfunction in sepsis-hemodynamics, oxygen transport, and nitric oxide. Crit. Care 2003, 7, 359–373. [Google Scholar] [CrossRef]
- Wang, J.; Dai, M.; Cao, Q.; Yu, Q.; Luo, Q.; Shu, L.; Zhang, Y.; Bao, M. Carotid baroreceptor stimulation suppresses ventricular fibrillation in canines with chronic heart failure. Basic Res. Cardiol. 2019, 114, 41. [Google Scholar] [CrossRef]
- Zhang, D.X.; Gutterman, D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2023–H2031. [Google Scholar] [CrossRef]
- Bochaton, T.; Claeys, M.J.; Garcia-Dorado, D.; Mewton, N.; Bergerot, C.; Jossan, C.; Amaz, C.; Boussaha, I.; Thibault, H.; Ovize, M. Importance of infarct size versus other variables for clinical outcomes after PPCI in STEMI patients. Basic Res. Cardiol. 2019, 115, 4. [Google Scholar] [CrossRef]
- Botker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 2018, 113, 39. [Google Scholar] [CrossRef]
- Coverstone, E.D.; Bach, R.G.; Chen, L.; Bierut, L.J.; Li, A.Y.; Lenzini, P.A.; O’Neill, H.C.; Spertus, J.A.; Sucharov, C.C.; Stitzel, J.A.; et al. A novel genetic marker of decreased inflammation and improved survival after acute myocardial infarction. Basic Res. Cardiol. 2018, 113, 38. [Google Scholar] [CrossRef]
- Gaspar, A.; Lourenco, A.P.; Pereira, M.A.; Azevedo, P.; Roncon-Albuquerque, R.J.; Marques, J.; Leite-Moreira, A.F. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res. Cardiol. 2018, 113, 14. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, G.; Ben-Aicha, S.; Casani, L.; Badimon, L.; Sabate, M.; Vilahur, G. Molecular pathways involved in the cardioprotective effects of intravenous statin administration during ischemia. Basic Res. Cardiol. 2019, 115, 2. [Google Scholar] [CrossRef]
- Resnic, F.S.; Wainstein, M.; Lee, M.K.; Behrendt, D.; Wainstein, R.V.; Ohno-Machado, L.; Kirshenbaum, J.M.; Rogers, C.D.; Popma, J.J.; Piana, R. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am. Heart J. 2003, 145, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.P.; Yang, X.M.; Crockett, E.S.; Housley, N.; Haq, E.U.; O’Donnell, K.; Cohen, M.V.; Downey, J.M.; Alvarez, D.F. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res. Cardiol. 2018, 113, 32. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schomig, A.; et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- Davidson, S.M.; Arjun, S.; Basalay, M.V.; Bell, R.M.; Bromage, D.I.; Botker, H.E.; Carr, R.D.; Cunningham, J.; Ghosh, A.K.; Heusch, G.; et al. The 10th Biennial Hatter Cardiovascular Institute workshop: Cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res. Cardiol. 2018, 113, 43. [Google Scholar] [CrossRef]
- Cao, T.; Fan, S.; Zheng, D.; Wang, G.; Yu, Y.; Chen, R.; Song, L.S.; Fan, G.C.; Zhang, Z.; Peng, T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: Role of mitochondrial superoxide anion. Basic Res. Cardiol. 2019, 114, 17. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Ouari, O.; Lopez, M.; Joseph, J.; Zielonka, J.; Dwinell, M.B. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox. Biol. 2018, 14, 316–327. [Google Scholar] [CrossRef]
- Vico, T.A.; Marchini, T.; Ginart, S.; Lorenzetti, M.A.; Adan Arean, J.S.; Calabro, V.; Garces, M.; Ferrero, M.C.; Mazo, T.; D’Annunzio, V.; et al. Mitochondrial bioenergetics links inflammation and cardiac contractility in endotoxemia. Basic Res. Cardiol. 2019, 114, 38. [Google Scholar] [CrossRef]
- Hao, L.; Sun, Q.; Zhong, W.; Zhang, W.; Sun, X.; Zhou, Z. Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: A molecular mechanism of protection against alcoholic liver disease. Redox. Biol. 2018, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M. Endothelial mitochondria and heart disease. Cardiovasc. Res. 2010, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.S.; Ashraf, S.; Lomax, T.M.; Wiseman, J.M.; Hall, M.E.; Gava, F.N.; Hall, J.E.; Hosler, J.P.; Harmancey, R. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res. Cardiol. 2018, 113, 47. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.X. Oxidative stress and diabetic cardiovascular disorders: Roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol. 2010, 88, 241–248. [Google Scholar] [CrossRef]
- Eid, R.A.; Alkhateeb, M.A.; Eleawa, S.; Al-Hashem, F.H.; Al-Shraim, M.; El-Kott, A.F.; Zaki, M.S.A.; Dallak, M.A.; Aldera, H. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res. Cardiol. 2018, 113, 13. [Google Scholar] [CrossRef]
- Groschner, L.N.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Endothelial mitochondria--less respiration, more integration. Pflugers Arch. 2012, 464, 63–76. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Bradley, M.D.; Wagner, B.A.; Buettner, G.R.; Monga, V.; Milhem, M.; Spitz, D.R.; Allen, B.G. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018, 14, 417–422. [Google Scholar] [CrossRef]
- Tai, Y.; Cao, F.; Li, M.; Li, P.; Xu, T.; Wang, X.; Yu, Y.; Gu, B.; Yu, X.; Cai, X.; et al. Enhanced mitochondrial pyruvate transport elicits a robust ROS production to sensitize the antitumor efficacy of interferon-gamma in colon cancer. Redox Biol. 2019, 20, 451–457. [Google Scholar] [CrossRef]
- Eiringhaus, J.; Herting, J.; Schatter, F.; Nikolaev, V.O.; Sprenger, J.; Wang, Y.; Kohn, M.; Zabel, M.; El-Armouche, A.; Hasenfuss, G.; et al. Protein kinase/phosphatase balance mediates the effects of increased late sodium current on ventricular calcium cycling. Basic Res. Cardiol. 2019, 114, 13. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Hu, S.; Chen, Y.; Ren, J. ER-mitochondria microdomains in cardiac ischemia-reperfusion injury: A fresh perspective. Front. Physiol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Q.; Zhu, P.; Ren, J.; Reiter, R.J.; Chen, Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tie, Y.; Fang, Z.; Wu, X.; Yi, T.; Huang, S.; Liang, X.; Qian, Y.; Wang, X.; Pi, R.; et al. Jumonji domain-containing 6 (JMJD6) identified as a potential therapeutic target in ovarian cancer. Signal Transduct.Target. Ther. 2019, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Maafi, F.; Stahli, B.E.; Dang, J.; Nachar, W.; de Oliveira Moraes, A.B.; Kernaleguen, A.E.; Lavoie, V.; Mecteau, M.; Mihalache-Avram, T.; et al. Apolipoprotein A-I proteolysis in aortic valve stenosis: Role of cathepsin S. Basic Res. Cardiol. 2018, 113, 30. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F. A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic Res. Cardiol. 2018, 113, 31. [Google Scholar] [CrossRef] [PubMed]
- Nanadikar, M.S.; Vergel Leon, A.M.; Borowik, S.; Hillemann, A.; Zieseniss, A.; Belousov, V.V.; Bogeski, I.; Rehling, P.; Dudek, J.; Katschinski, D.M. O2 affects mitochondrial functionality ex vivo. Redox Biol. 2019, 22, 101152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Baek, J.I.; Kim, S.H.; Kim, M.A.; Lee, B.; Ryu, N.; Kim, K.H.; Choi, D.G.; Kim, H.M.; Murphy, M.P.; et al. Therapeutic potential of the mitochondria-targeted antioxidant MitoQ in mitochondrial-ROS induced sensorineural hearing loss caused by Idh2 deficiency. Redox Biol. 2019, 20, 544–555. [Google Scholar] [CrossRef]
- Alvarez, S.; Valdez, L.B.; Zaobornyj, T.; Boveris, A. Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem. Biophys. Res. Commun. 2003, 305, 771–775. [Google Scholar] [CrossRef]
- Ekim Kocabey, A.; Kost, L.; Gehlhar, M.; Rodel, G.; Gey, U. Mitochondrial sco proteins are involved in oxidative stress defense. Redox Biol. 2019, 21, 101079. [Google Scholar] [CrossRef]
- Tsai, A.G.; Friesenecker, B.; Mazzoni, M.C.; Kerger, H.; Buerk, D.G.; Johnson, P.C.; Intaglietta, M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc. Natl. Acad. Sci. USA 1998, 95, 6590–6595. [Google Scholar] [CrossRef]
- Tsai, A.G.; Johnson, P.C.; Intaglietta, M. Oxygen gradients in the microcirculation. Physiol. Rev. 2003, 83, 933–963. [Google Scholar] [CrossRef]
- Pung, Y.F.; Sam, W.J.; Hardwick, J.P.; Yin, L.; Ohanyan, V.; Logan, S.; Di Vincenzo, L.; Chilian, W.M. The role of mitochondrial bioenergetics and reactive oxygen species in coronary collateral growth. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1275–H1280. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J. Strategies to detect mitochondrial oxidants. Redox Biol. 2019, 21, 101065. [Google Scholar] [CrossRef] [PubMed]
- Bereiter-Hahn, J.; Voth, M.; Mai, S.; Jendrach, M. Structural implications of mitochondrial dynamics. Biotechnol. J. 2008, 3, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Skyschally, A.; Gent, S.; Pesch, M.; Heusch, G. STAT3 as a common signal of ischemic conditioning: A lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res. Cardiol. 2018, 113, 3. [Google Scholar] [CrossRef]
- Jensen, P.K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim. Biophys. Acta 1966, 122, 157–166. [Google Scholar] [CrossRef]
- Rossello, X.; Yellon, D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2018, 113, 2. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Witting, P.K.; Drummond, G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal 2008, 10, 1713–1765. [Google Scholar] [CrossRef]
- Schulz, R.; Agg, B.; Ferdinandy, P. Survival pathways in cardiac conditioning: Individual data vs. meta-analyses. What do we learn? Basic Res. Cardiol. 2018, 113, 4. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Bice, J.S.; Baxter, G.F. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: A systematic review and meta-analysis. Basic Res. Cardiol. 2018, 113, 6. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013, 18, 634–647. [Google Scholar] [CrossRef]
- Szewczyk, A.; Jarmuszkiewicz, W.; Koziel, A.; Sobieraj, I.; Nobik, W.; Lukasiak, A.; Skup, A.; Bednarczyk, P.; Drabarek, B.; Dymkowska, D.; et al. Mitochondrial mechanisms of endothelial dysfunction. Pharmacol. Rep. 2015, 67, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Brand, D.; Zheng, S.G. Targeting IL-2: An unexpected effect in treating immunological diseases. Signal Transduct. Target. Ther. 2018, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; O’Donnell, V.B.; Wood, J.D.; Broughton, J.P.; Hughes, E.J.; Jones, O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996, 271, H1626–H1634. [Google Scholar] [CrossRef] [PubMed]
- Pichavaram, P.; Mani, A.M.; Singh, N.K.; Rao, G.N. Cholesterol crystals promote endothelial cell and monocyte interactions via H2O2-mediated PP2A inhibition, NFkappaB activation and ICAM1 and VCAM1 expression. Redox Biol. 2019, 24, 101180. [Google Scholar] [CrossRef]
- Li, Y.; Cifuentes-Pagano, E.; DeVallance, E.R.; de Jesus, D.S.; Sahoo, S.; Meijles, D.N.; Koes, D.; Camacho, C.J.; Ross, M.; St Croix, C.; et al. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biol. 2019, 22, 101143. [Google Scholar] [CrossRef]
- Graham, N.A.; Tahmasian, M.; Kohli, B.; Komisopoulou, E.; Zhu, M.; Vivanco, I.; Teitell, M.A.; Wu, H.; Ribas, A.; Lo, R.S.; et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst. Biol. 2012, 8, 589. [Google Scholar] [CrossRef]
- Tang, V.; Fu, S.; Rayner, B.S.; Hawkins, C.L. 8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response. Redox Biol. 2019, 26, 101274. [Google Scholar] [CrossRef]
- Wu, B.; Pan, X.; Chen, X.; Chen, M.; Shi, K.; Xu, J.; Zheng, J.; Niu, T.; Chen, C.; Shuai, X.; et al. Epigenetic drug library screening identified an LSD1 inhibitor to target UTX-deficient cells for differentiation therapy. Signal Transduct. Target. Ther. 2019, 4, 11. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef]
- de Jesus, D.S.; DeVallance, E.; Li, Y.; Falabella, M.; Guimaraes, D.; Shiva, S.; Kaufman, B.A.; Gladwin, M.T.; Pagano, P.J. Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration. Redox Biol. 2019, 22, 101138. [Google Scholar] [CrossRef]
- Butts, B.; Calhoun, D.A.; Denney, T.S., Jr.; Lloyd, S.G.; Gupta, H.; Gaddam, K.K.; Aban, I.; Oparil, S.; Sanders, P.W.; Patel, R.; et al. Plasma xanthine oxidase activity is related to increased sodium and left ventricular hypertrophy in resistant hypertension. Free Radic. Biol. Medi. 2019, 134, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Mergia, E.; Kramer, C.M.; Luckstadt, W.; Yang, J.; Wolff, G.; Panknin, C.; Bracht, T.; Sitek, B.; Pernow, J.; et al. Identification of a soluble guanylate cyclase in RBCs: Preserved activity in patients with coronary artery disease. Redox Biol. 2018, 14, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ribon-Demars, A.; Pialoux, V.; Boreau, A.; Marcouiller, F.; Lariviere, R.; Bairam, A.; Joseph, V. Protective roles of estradiol against vascular oxidative stress in ovariectomized female rats exposed to normoxia or intermittent hypoxia. Acta Physiol. (Oxf.) 2019, 225, e13159. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Bauersachs, J. Of mice and men: Models and mechanisms of diabetic cardiomyopathy. Basic Res. Cardiol. 2018, 114, 2. [Google Scholar] [CrossRef] [PubMed]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Daseke, M.J., 2nd; Valerio, F.M.; Kalusche, W.J.; Ma, Y.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res. Cardiol. 2019, 114, 37. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Vallejo, S.; Sanchez, F.J.; Sandoval, E.; Blanco, E.; Cannata, P.; Peiro, C.; Sanchez-Ferrer, C.F.; Lamas, S. Role of glutathione biosynthesis in endothelial dysfunction and fibrosis. Redox Biol. 2018, 14, 88–99. [Google Scholar] [CrossRef]
- Bernhart, E.; Kogelnik, N.; Prasch, J.; Gottschalk, B.; Goeritzer, M.; Depaoli, M.R.; Reicher, H.; Nusshold, C.; Plastira, I.; Hammer, A.; et al. 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018, 15, 441–451. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, F.; Guo, Q.; Li, M.; Wang, L.; Zhang, Z.; Jiang, S.; Jin, H.; Chen, A.; Tan, S.; et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef]
- Ter Horst, E.N.; Krijnen, P.A.J.; Hakimzadeh, N.; Robbers, L.; Hirsch, A.; Nijveldt, R.; Lommerse, I.; Fontijn, R.D.; Meinster, E.; Delewi, R.; et al. Elevated monocyte-specific type I interferon signalling correlates positively with cardiac healing in myocardial infarct patients but interferon alpha application deteriorates myocardial healing in rats. Basic Res. Cardiol. 2018, 114, 1. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Liu, N.; Chen, Y. Ribosomal protein L10 in mitochondria serves as a regulator for ROS level in pancreatic cancer cells. Redox Biol. 2018, 19, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.R.; Yousefzadeh, M.J.; Rozgaja, T.A.; Wang, J.; Li, X.; Tilstra, J.S.; Feldman, C.H.; Gregg, S.Q.; Johnson, C.H.; Skoda, E.M.; et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol. 2018, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Tymko, M.M.; Tremblay, J.C.; Bailey, D.M.; Green, D.J.; Ainslie, P.N. The impact of hypoxaemia on vascular function in lowlanders and high altitude indigenous populations. J. Physiol. 2019, 597, 5759–5776. [Google Scholar] [CrossRef] [PubMed]
- Sultan, C.S.; Saackel, A.; Stank, A.; Fleming, T.; Fedorova, M.; Hoffmann, R.; Wade, R.C.; Hecker, M.; Wagner, A.H. Impact of carbonylation on glutathione peroxidase-1 activity in human hyperglycemic endothelial cells. Redox Biol. 2018, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Burgos, J.I.; Gonano, L.A.; Vila Petroff, M. AMPK-dependent nitric oxide release provides contractile support during hyperosmotic stress. Basic Res. Cardiol. 2018, 113, 7. [Google Scholar] [CrossRef] [PubMed]
- Engineer, A.; Saiyin, T.; Greco, E.R.; Feng, Q. Say NO to ROS: Their roles in embryonic heart development and pathogenesis of congenital heart defects in maternal diabetes. Antioxidants 2019, 8, 436. [Google Scholar] [CrossRef]
- Trieb, M.; Kornej, J.; Knuplez, E.; Hindricks, G.; Thiele, H.; Sommer, P.; Scharnagl, H.; Dagres, N.; Dinov, B.; Bollmann, A.; et al. Atrial fibrillation is associated with alterations in HDL function, metabolism, and particle number. Basic Res. Cardiol. 2019, 114, 27. [Google Scholar] [CrossRef]
- Prasai, P.K.; Shrestha, B.; Orr, A.W.; Pattillo, C.B. Decreases in GSH:GSSG activate vascular endothelial growth factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox Biol. 2018, 19, 22–27. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Sewell, R.D.E.; Rafieian-Kopaei, M. Antioxidants and atherosclerosis: Mechanistic aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef]
- Munoz, M.; Martinez, M.P.; Lopez-Oliva, M.E.; Rodriguez, C.; Corbacho, C.; Carballido, J.; Garcia-Sacristan, A.; Hernandez, M.; Rivera, L.; Saenz-Medina, J.; et al. Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol. 2018, 19, 92–104. [Google Scholar] [CrossRef]
- Yellon, D.M.; He, Z.; Khambata, R.; Ahluwalia, A.; Davidson, S.M. The GTN patch: A simple and effective new approach to cardioprotection? Basic Res. Cardiol. 2018, 113, 20. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Sule, Z.; et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019, 24, 101192. [Google Scholar] [CrossRef] [PubMed]
- Santin, Y.; Fazal, L.; Sainte-Marie, Y.; Sicard, P.; Maggiorani, D.; Tortosa, F.; Yucel, Y.Y.; Teyssedre, L.; Rouquette, J.; Marcellin, M.; et al. Mitochondrial 4-HNE derived from MAO-A promotes mitoCa(2+) overload in chronic postischemic cardiac remodeling. Cell Death Differ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Wu, R.; Shang, M.; Sato, T.; Chen, C.; Shapiro, J.S.; Liu, T.; Thakur, A.; Sawicki, K.T.; Prasad, S.V.; et al. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol. Med. 2016, 8, 247–267. [Google Scholar] [CrossRef]
- Trindade, F.; Vitorino, R.; Leite-Moreira, A.; Falcao-Pires, I. Pericardial fluid: An underrated molecular library of heart conditions and a potential vehicle for cardiac therapy. Basic Res. Cardiol. 2019, 114, 10. [Google Scholar] [CrossRef]
- MacKinney, A.; Woska, E.; Spasojevic, I.; Batinic-Haberle, I.; Zennadi, R. Disrupting the vicious cycle created by NOX activation in sickle erythrocytes exposed to hypoxia/reoxygenation prevents adhesion and vasoocclusion. Redox Biol. 2019, 25, 101097. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ren, K.; Li, B.; Li, F.; Liang, Z.; Hu, J.; Xu, B.; Zhang, A. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1alpha/VEGF signal pathway. Mol. Cell Biochem. 2019. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, Y.L.; Tan, Y.Z.; Wang, H.J.; Tao, P.; Zhou, P. Enhancement of cardiac lymphangiogenesis by transplantation of CD34(+)VEGFR-3(+) endothelial progenitor cells and sustained release of VEGF-C. Basic Res. Cardiol. 2019, 114, 43. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Rofaeil, R.R.; Ali, D.M.E.; Attya, M.E. Protective effect of dipeptidyl peptidase-4 inhibitors in testicular torsion/detorsion in rats: A possible role of HIF-1alpha and nitric oxide. Naunyn Schmiedebergs Arch. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Abd Al Haleem, E.N.; Ahmed, S.F.; Temraz, A.; El-Tantawy, W.H. Evaluation of the cardioprotective effect of casuarina suberosa extract in rats. Drug Chem. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, I.; Hatfield, K.J.; Gronningsaeter, I.S.; Aasebo, E.; Hernandez-Valladares, M.; Hagen, K.M.; Rye, K.P.; Berven, F.S.; Selheim, F.; Reikvam, H.; et al. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct. Target. Ther. 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, N.; Shedid, S.M. Impact of zinc oxide nanoparticles on thioredoxin-interacting protein and asymmetric dimethylarginine as biochemical indicators of cardiovascular disorders in gamma-irradiated rats. Environ. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Wu, Z.; Ye, F.; Wu, X. Ginkgolide A protects adverse cardiac remodeling through enhancing antioxidation and nitric oxide utilization in mice with pressure overload. Pharmazie 2019, 74, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Helmstadter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kroller-Schon, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide 1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Behairy, S.F.; El-Maraghy, N.N.; Elshazly, S.M. PPAR-gamma agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats. Life Sci. 2019, 239, 117047. [Google Scholar] [CrossRef]
- Yang, J.; Moraga, A.; Xu, J.; Zhao, Y.; Luo, P.; Lao, K.H.; Margariti, A.; Zhao, Q.; Ding, W.; Wang, G.; et al. A histone deacetylase 7-derived peptide promotes vascular regeneration via facilitating 14-3-3gamma phosphorylation. Stem Cell Res. Ther. 2019. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, Y.; Feng, W.; Yu, L.; Li, P.; Cai, B.; Li, C.; Guan, H. Effects and mechanisms of PSS-loaded nanoparticles on coronary microcirculation dysfunction in streptozotocin-induced diabetic cardiomyopathy rats. Biomed. Pharmacother. 2020, 121, 109280. [Google Scholar] [CrossRef]
- Rozengurt, E.; Sinnett-Smith, J.; Eibl, G. Yes-associated protein (YAP) in pancreatic cancer: At the epicenter of a targetable signaling network associated with patient survival. Signal Transduct. Target. Ther. 2018, 3, 11. [Google Scholar] [CrossRef]
- Wu, S.P.; Kao, C.Y.; Wang, L.; Creighton, C.J.; Yang, J.; Donti, T.R.; Harmancey, R.; Vasquez, H.G.; Graham, B.H.; Bellen, H.J.; et al. Increased COUP-TFII expression in adult hearts induces mitochondrial dysfunction resulting in heart failure. Nat. Commun. 2015, 6, 8245. [Google Scholar] [CrossRef]
- Sverdlov, A.L.; Elezaby, A.; Qin, F.; Behring, J.B.; Luptak, I.; Calamaras, T.D.; Siwik, D.A.; Miller, E.J.; Liesa, M.; Shirihai, O.S.; et al. Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Mitochondrial ROS Induces cardiac Inflammation via a pathway through mtDNA damage in a pneumonia-pelated sepsis model. PLoS ONE 2015, 10, e0139416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Wu, W.; Shi, C.; Hu, S.; Yin, T.; Ma, Q.; Han, T.; Zhang, Y.; Tian, F.; et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic. Biol. Med. 2016, 95, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Liepinsh, E.; Murray, A.J.; Lemieux, H.; Dambrova, M.; Tepp, K.; Puurand, M.; Kaambre, T.; Han, W.H.; de Goede, P.; et al. Altered mitochondrial metabolism in the insulin resistant heart. Acta Physiol. (Oxf.) 2019, e13430. [Google Scholar] [CrossRef] [PubMed]
- Shrum, S.; Rusch, N.J.; MacMillan-Crow, L.A. Specific BK channel activator NS11021 protects rat renal proximal tubular cells from cold storage-induced mitochondrial injury in vitro. Biomolecules 2019, 9, 825. [Google Scholar] [CrossRef]
- Breda, C.N.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Camara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Tian, R.; Colucci, W.S.; Arany, Z.; Bachschmid, M.M.; Ballinger, S.W.; Boudina, S.; Bruce, J.E.; Busija, D.W.; Dikalov, S.; Dorn, G.W.; et al. Unlocking the secrets of mitochondria in the cardiovascular system: Path to a cure in heart failure-A report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation 2019, 140, 1205–1216. [Google Scholar] [CrossRef]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of mitochondrial ATP production: Ca(2+) signaling and quality control. Trends Mol. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.E.; Arias-Duran, C.; Avalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol. Aspects Med. 2019. [Google Scholar] [CrossRef]
- Ong, S.B.; Kwek, X.Y.; Katwadi, K.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Ismail, N.I.; Lin, Y.H.; Yap, E.P.; Lim, S.Y.; Ja, K.; et al. Targeting mitochondrial fission using mdivi-1 in a clinically relevant large animal model of acute myocardial infarction: A pilot study. Int. J. Mol. Sci. 2019, 20, 3972. [Google Scholar] [CrossRef]
- Jhun, B.; Adaniya, S.; Cypress, M.; Yoon, Y. Adrenergic regulation of Drp1-Driven mitochondrial fission in cardiac physio-pathology. Antioxidants 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Wu, X.; Xu, Q.R.; Zhu, R.R.; Xu, H.; Li, Y.Y.; Liu, S.; Huang, H.; Xu, X.; Wan, L.; et al. Notch1 provides myocardial protection by improving mitochondrial quality control. J. Cell Physiol. 2019, 234, 11835–11841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Potus, F.; Wu, D.; Dasgupta, A.; Chen, K.H.; Mewburn, J.; Lima, P.; Archer, S.L. Increased Drp1-Mediated mitochondrial fission promotes proliferation and collagen production by right ventricular fibroblasts in experimental pulmonary arterial hypertension. Front. Physiol. 2018, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef]
- Alam, S.; Abdullah, C.S.; Aishwarya, R.; Miriyala, S.; Panchatcharam, M.; Peretik, J.M.; Orr, A.W.; James, J.; Robbins, J.; Bhuiyan, M.S. Aberrant mitochondrial fission is maladaptive in desmin mutation-induced cardiac proteotoxicity. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Hoque, A.; Sivakumaran, P.; Bond, S.T.; Ling, N.X.Y.; Kong, A.M.; Scott, J.W.; Bandara, N.; Hernandez, D.; Liu, G.S.; Wong, R.C.B.; et al. Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell Death Discov. 2018, 4, 39. [Google Scholar] [CrossRef]
- Jin, Q.; Li, R.; Hu, N.; Xin, T.; Zhu, P.; Hu, S.; Ma, S.; Zhu, H.; Ren, J.; Zhou, H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018, 14, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Hu, S.; Zhu, H.; Toanc, S.; Ren, J. BI1 alleviates cardiac microvascular ischemia-reperfusion injury via modifying mitochondrial fission and inhibiting XO/ROS/F-actin pathways. J. Cell Physiol. 2019, 234, 5056–5069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, S.; Jin, Q.; Shi, C.; Zhang, Y.; Zhu, P.; Ma, Q.; Tian, F.; Chen, Y. Mff-Dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature Ischemia/Reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 Disassociation-Involved mPTP opening. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Zhang, Y.; Zhu, P.J.; Zhou, H.; Chen, Y.D. Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. J. Geriatr. Cardiol. 2017, 14, 57–66. [Google Scholar] [CrossRef]
- Zhu, H.; Jin, Q.; Li, Y.; Ma, Q.; Wang, J.; Li, D.; Zhou, H.; Chen, Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 2018, 23, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Zhu, P.; Zhu, H.; Toan, S.; Hu, S.; Ren, J.; Chen, Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res. Cardiol. 2018, 113, 23. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, C.; Hu, S.; Zhu, H.; Ren, J.; Chen, Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis 2018, 21, 599–615. [Google Scholar] [CrossRef]
- Gumeni, S.; Evangelakou, Z.; Tsakiri, E.N.; Scorrano, L.; Trougakos, I.P. Functional wiring of proteostatic and mitostatic modules ensures transient organismal survival during imbalanced mitochondrial dynamics. Redox Biol. 2019, 24, 101219. [Google Scholar] [CrossRef]
- Marin-Garcia, J.; Akhmedov, A.T. Mitochondrial dynamics and cell death in heart failure. Heart Fail. Rev. 2016, 21, 123–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, J.; Tian, F.; Hu, S.; Chen, Y.; Fu, Z. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 2019, 66, e12542. [Google Scholar] [CrossRef]
- Eisner, V.; Cupo, R.R.; Gao, E.; Csordas, G.; Slovinsky, W.S.; Paillard, M.; Cheng, L.; Ibetti, J.; Chen, S.R.; Chuprun, J.K.; et al. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc. Natl. Acad. Sci. USA 2017, 114, E859–E868. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; Tang, D.; Gao, E.; Li, C.; Wang, K.; Qi, B.; Qiu, J.; Zhao, H.; Chang, P.; et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics 2019, 9, 3687–3706. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.P.; Shao, Q.; Li, P.F.; Sun, Y.; Luo, L.Z.; Yan, X.Q.; Fan, Z.Y.; Hu, J.; Zhao, J.; et al. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic. Biol. Med. 2019, 145, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhuang, J.; Wang, Y.; Zhou, D.; Zhao, D.; Zhu, S.; Pu, J.; Zhang, H.; Yin, M.; Zhao, W.; et al. Propofol ameliorates H9c2 cells apoptosis induced by oxygen glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission. Front. Pharmacol. 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef]
- Yang, F.; Wu, R.; Jiang, Z.; Chen, J.; Nan, J.; Su, S.; Zhang, N.; Wang, C.; Zhao, J.; Ni, C.; et al. Leptin increases mitochondrial OPA1 via GSK3-mediated OMA1 ubiquitination to enhance therapeutic effects of mesenchymal stem cell transplantation. Cell Death Dis. 2018, 9, 556. [Google Scholar] [CrossRef]
- Sprenger, H.G.; Langer, T. The good and the bad of mitochondrial breakups. Trends Cell Biol. 2019, 29, 888–900. [Google Scholar] [CrossRef]
- Romanello, V.; Scalabrin, M.; Albiero, M.; Blaauw, B.; Scorrano, L.; Sandri, M. Inhibition of the Fission Machinery Mitigates OPA1 Impairment in Adult Skeletal Muscles. Cells 2019, 8, 597. [Google Scholar] [CrossRef]
- Yin, W.; Li, R.; Feng, X.; James Kang, Y. The involvement of cytochrome c oxidase in mitochondrial fusion in primary cultures of neonatal rat cardiomyocytes. Cardiovasc. Toxicol. 2018, 18, 365–373. [Google Scholar] [CrossRef]
- Anderson, C.J.; Kahl, A.; Fruitman, H.; Qian, L.; Zhou, P.; Manfredi, G.; Iadecola, C. Prohibitin levels regulate OMA1 activity and turnover in neurons. Cell Death Differ. 2019. [Google Scholar] [CrossRef]
- Schulman, J.J.; Szczesniak, L.M.; Bunker, E.N.; Nelson, H.A.; Roe, M.W.; Wagner, L.E., 2nd; Yule, D.I.; Wojcikiewicz, R.J.H. Bok regulates mitochondrial fusion and morphology. Cell Death Differ. 2019, 26, 2682–2694. [Google Scholar] [CrossRef]
- Ding, M.; Liu, C.; Shi, R.; Yu, M.; Zeng, K.; Kang, J.; Fu, F.; Mi, M. Mitochondrial fusion promoter restores mitochondrial dynamics balance and ameliorates diabetic cardiomyopathy in an Opa1-dependent way. Acta Physiol. (Oxf.) 2019, e13428. [Google Scholar] [CrossRef]
- Hong, Y.; Tak, H.; Kim, C.; Kang, H.; Ji, E.; Ahn, S.; Jung, M.; Kim, H.L.; Lee, J.H.; Kim, W.; et al. RNA binding protein HuD contributes to beta-cell dysfunction by impairing mitochondria dynamics. Cell Death Differ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, D.; Shah, S.Z.A.; Zhang, X.; Lai, M.; Yang, D.; Wu, X.; Guan, Z.; Li, J.; Zhao, H.; et al. OPA1 overexpression ameliorates mitochondrial cristae remodeling, mitochondrial dysfunction, and neuronal apoptosis in prion diseases. Cell Death Dis. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Niu, Y.J.; Zhou, W.; Nie, Z.W.; Shin, K.T.; Cui, X.S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2019, e12627. [Google Scholar] [CrossRef]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Li, X.; Liu, Z.; Han, Y.; Xu, X.; Li, X.; Tang, Y.; Liu, Y.; Yu, T.; et al. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J. Cell Physiol. 2019, 234, 23495–23506. [Google Scholar] [CrossRef]
- Jeremic, N.; Weber, G.J.; Tyagi, S.C. Ablation of toll-like receptor 4 mitigates cardiac mitochondrial dysfunction in hyperhomocysteinemia. Can. J. Physiol. Pharmacol. 2017, 95, 1369–1375. [Google Scholar] [CrossRef]
- Jeremic, N.; Weber, G.J.; Familtseva, A.; Metreveli, N.; Tyagi, S.C. Ablation of Toll-like receptor 4 mitigates central blood pressure response during hyperhomocysteinemia. J. Hypertens. 2017, 35, 2226–2237. [Google Scholar] [CrossRef]
- Scheitlin, C.G.; Nair, D.M.; Crestanello, J.A.; Zweier, J.L.; Alevriadou, B.R. Fluid mechanical forces and endothelial mitochondria: A bioengineering perspective. Cell Mol. Bioeng. 2014, 7, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Scott, B.T.; Dillmann, W.H. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010, 53, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, W.; Fang, Z.; Ding, F.; Zou, F.; Ma, X.; Tao, J.; Guo, J.; Xia, X.; Wang, H.; et al. CircERCC2 ameliorated intervertebral disc degeneration by regulating mitophagy and apoptosis through miR-182-5p/SIRT1 axis. Cell Death Dis. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Hou, Y.; Yu, Z.; Zhao, Z.; Liu, Z. Healthy mitochondria inhibit the metastatic melanoma in lungs. Int. J. Biol. Sci. 2019, 15, 2707–2718. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Wang, L.; Wong, Y.K.; Lee, Y.M.; Zhou, C.; Wu, G.; Zhao, T.; Yang, L.; Lu, L.; et al. Artesunate-induced mitophagy alters cellular redox status. Redox Biol. 2018, 19, 263–273. [Google Scholar] [CrossRef]
- Vistro, W.A.; Zhang, Y.; Bai, X.; Yang, P.; Huang, Y.; Qu, W.; Baloch, A.S.; Wu, R.; Tarique, I.; Chen, Q. In vivo autophagy up-regulation of small intestine enterocytes in chinese soft-shelled turtles during hibernation. Biomolecules 2019, 9, 682. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2020, 28, 101365. [Google Scholar] [CrossRef]
- Vazquez-Calvo, C.; Suhm, T.; Buttner, S.; Ott, M. The basic machineries for mitochondrial protein quality control. Mitochondrion 2019, 50, 121–131. [Google Scholar] [CrossRef]
- Rani, L.; Mondal, A.C. Emerging concepts of mitochondrial dysfunction in Parkinson’s disease progression: Pathogenic and therapeutic implications. Mitochondrion 2019, 50, 25–34. [Google Scholar] [CrossRef]
- Chakraborty, D.; Felzen, V.; Hiebel, C.; Sturner, E.; Perumal, N.; Manicam, C.; Sehn, E.; Grus, F.; Wolfrum, U.; Behl, C. Enhanced autophagic-lysosomal activity and increased BAG3-mediated selective macroautophagy as adaptive response of neuronal cells to chronic oxidative stress. Redox Biol. 2019, 24, 101181. [Google Scholar] [CrossRef]
- Xiao, W.; Xiong, Z.; Xiong, W.; Yuan, C.; Xiao, H.; Ruan, H.; Song, Z.; Wang, C.; Bao, L.; Cao, Q.; et al. Melatonin/PGC1A/UCP1 promotes tumor slimming and represses tumor progression by initiating autophagy and lipid browning. J. Pineal Res. 2019, 67, e12607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Wang, M.; Zhou, L.; Feng, X.; Yu, L.; Lan, J.; Gao, W.; Zhang, C.; Bu, Y.; et al. CPX targeting DJ-1 triggers ROS-induced cell death and protective autophagy in colorectal cancer. Theranostics 2019, 9, 5577–5594. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, R.; Bartkova, J.; Merchut-Maya, J.M.; Hall, A.; Bouchal, J.; Dyrskjot, L.; Frankel, L.B.; Gorgoulis, V.; Maya-Mendoza, A.; Jaattela, M.; et al. Autophagy role(s) in response to oncogenes and DNA replication stress. Cell Death Differ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Gomez, D.; Mora-Lorca, J.A.; Saenz-Narciso, B.; Naranjo-Galindo, F.J.; Munoz-Lobato, F.; Parrado-Fernandez, C.; Goikolea, J.; Cedazo-Minguez, A.; Link, C.D.; Neri, C.; et al. Loss of glutathione redox homeostasis impairs proteostasis by inhibiting autophagy-dependent protein degradation. Cell Death Differ. 2019, 26, 1545–1565. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Naik, P.P.; Panigrahi, D.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Sethi, G.; Bhutia, S.K. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: It is implication in cancer therapeutics. Cell Mol. Life Sci. 2019, 76, 1641–1652. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Shao, X.; Zhang, M.; Jin, H.; Zhang, Z.; Shen, J.; Zhou, Y.; Zhou, W.; et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019, 26, 101254. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Dorn, G.W., 2nd. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev. 2019, 99, 853–892. [Google Scholar] [CrossRef]
- Yao, L.; Chen, H.; Wu, Q.; Xie, K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int. J. Mol. Med. 2019, 44, 1048–1062. [Google Scholar] [CrossRef]
- Bayne, A.N.; Trempe, J.F. Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond. Cell Mol. Life Sci. 2019, 76, 4589–4611. [Google Scholar] [CrossRef]
- Van Humbeeck, C.; Cornelissen, T.; Vandenberghe, W. Ambra1: A Parkin-binding protein involved in mitophagy. Autophagy 2011, 7, 1555–1556. [Google Scholar] [CrossRef]
- Villa, E.; Marchetti, S.; Ricci, J.E. No Parkin Zone: Mitophagy without Parkin. Trends Cell Biol. 2018, 28, 882–895. [Google Scholar] [CrossRef]
- Naik, P.P.; Birbrair, A.; Bhutia, S.K. Mitophagy-driven metabolic switch reprograms stem cell fate. Cell Mol. Life Sci. 2019, 76, 27–43. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Wang, J.; Toan, S.; Ren, J. DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy. Signal Transduct. Target. Ther. 2019, 4, 56. [Google Scholar] [CrossRef]
- Zhang, J.; Ney, P.A. NIX induces mitochondrial autophagy in reticulocytes. Autophagy 2008, 4, 354–356. [Google Scholar] [CrossRef]
- Sandoval, H.; Thiagarajan, P.; Dasgupta, S.K.; Schumacher, A.; Prchal, J.T.; Chen, M.; Wang, J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008, 454, 232–235. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017, 13, 498–507. [Google Scholar] [CrossRef]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Wang, J.; Zhu, H.; Ren, J.; Chen, Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018, 25, 1080–1093. [Google Scholar] [CrossRef]
- Wei, T.; Huang, G.; Gao, J.; Huang, C.; Sun, M.; Wu, J.; Bu, J.; Shen, W. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Haslip, M.; Dostanic, I.; Huang, Y.; Zhang, Y.; Russell, K.S.; Jurczak, M.J.; Mannam, P.; Giordano, F.; Erzurum, S.C.; Lee, P.J. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1166–1178. [Google Scholar] [CrossRef]
- Abukar, Y.; Ramchandra, R.; Hood, S.G.; McKinley, M.J.; Booth, L.C.; Yao, S.T.; May, C.N. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res. Cardiol. 2018, 113, 35. [Google Scholar] [CrossRef]
- Aluja, D.; Inserte, J.; Penela, P.; Ramos, P.; Ribas, C.; Iniguez, M.A.; Mayor, F., Jr.; Garcia-Dorado, D. Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res. Cardiol. 2019, 114, 21. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, M.; Sanz-Flores, M.; Sanz-Castillo, B.; Salazar-Roa, M.; Partida, D.; Zapatero-Solana, E.; Ali, H.R.; Manchado, E.; Lowe, S.; VanArsdale, T.; et al. Therapeutic relevance of the PP2A-B55 inhibitory kinase MASTL/Greatwall in breast cancer. Cell Death Differ. 2018, 25, 828–840. [Google Scholar] [CrossRef]
- Anderton, H.; Bandala-Sanchez, E.; Simpson, D.S.; Rickard, J.A.; Ng, A.P.; Di Rago, L.; Hall, C.; Vince, J.E.; Silke, J.; Liccardi, G.; et al. RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development. Cell Death Differ. 2019, 26, 877–889. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.; Shi, Y.; Shao, C. The tango of ROS and p53 in tissue stem cells. Cell Death Differ. 2018, 25, 637–639. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L. Serotonylation: A novel histone H3 marker. Signal Transduct. Target. Ther. 2019, 4, 15. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. https://doi.org/10.3390/biom10010085
Zhou H, Toan S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules. 2020; 10(1):85. https://doi.org/10.3390/biom10010085
Chicago/Turabian StyleZhou, Hao, and Sam Toan. 2020. "Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury" Biomolecules 10, no. 1: 85. https://doi.org/10.3390/biom10010085
APA StyleZhou, H., & Toan, S. (2020). Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules, 10(1), 85. https://doi.org/10.3390/biom10010085




