Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization
Abstract
1. Introduction
2. Results
2.1. Extraction and Fractionation of Ulvans
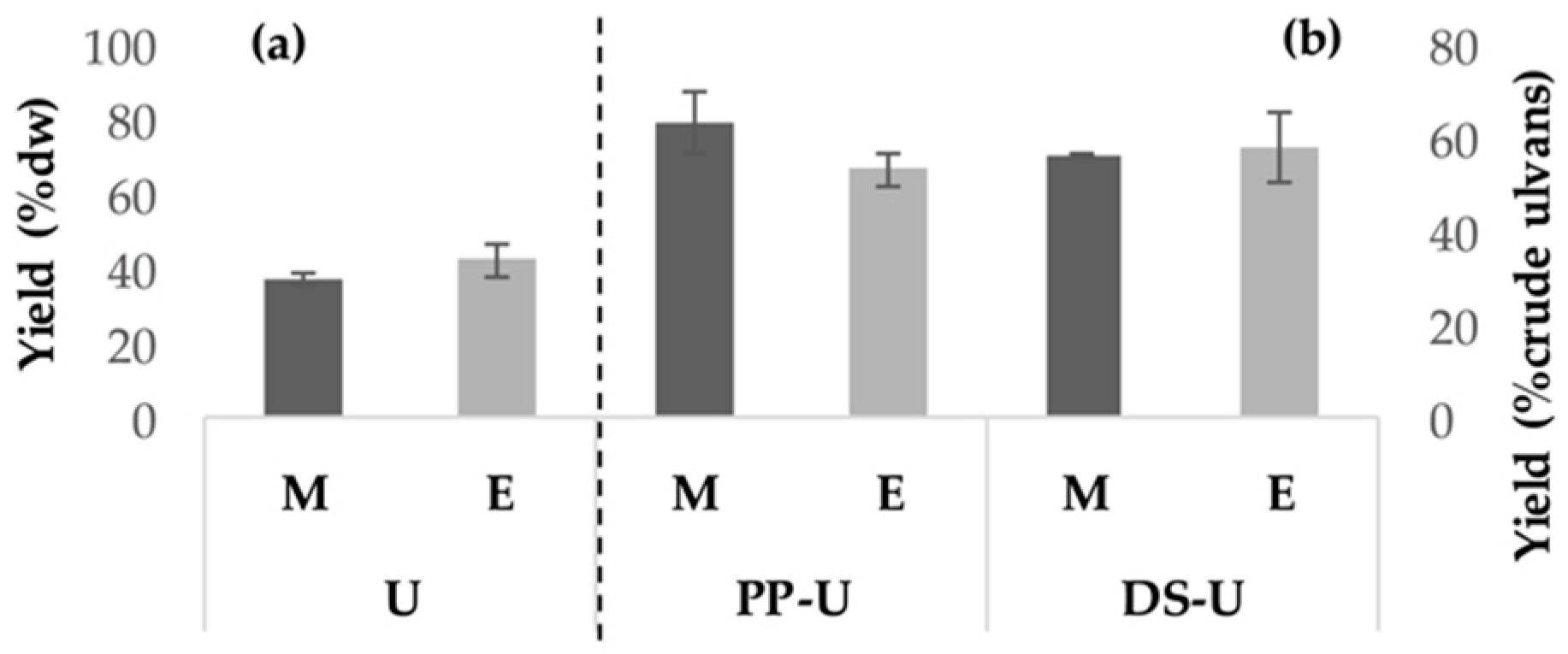
2.1.1. Yields of Extraction and Fractionation
2.1.2. Biochemical Characterization of Ulvans
2.2. Oligosaccharide Production by Ulvans Depolymerization
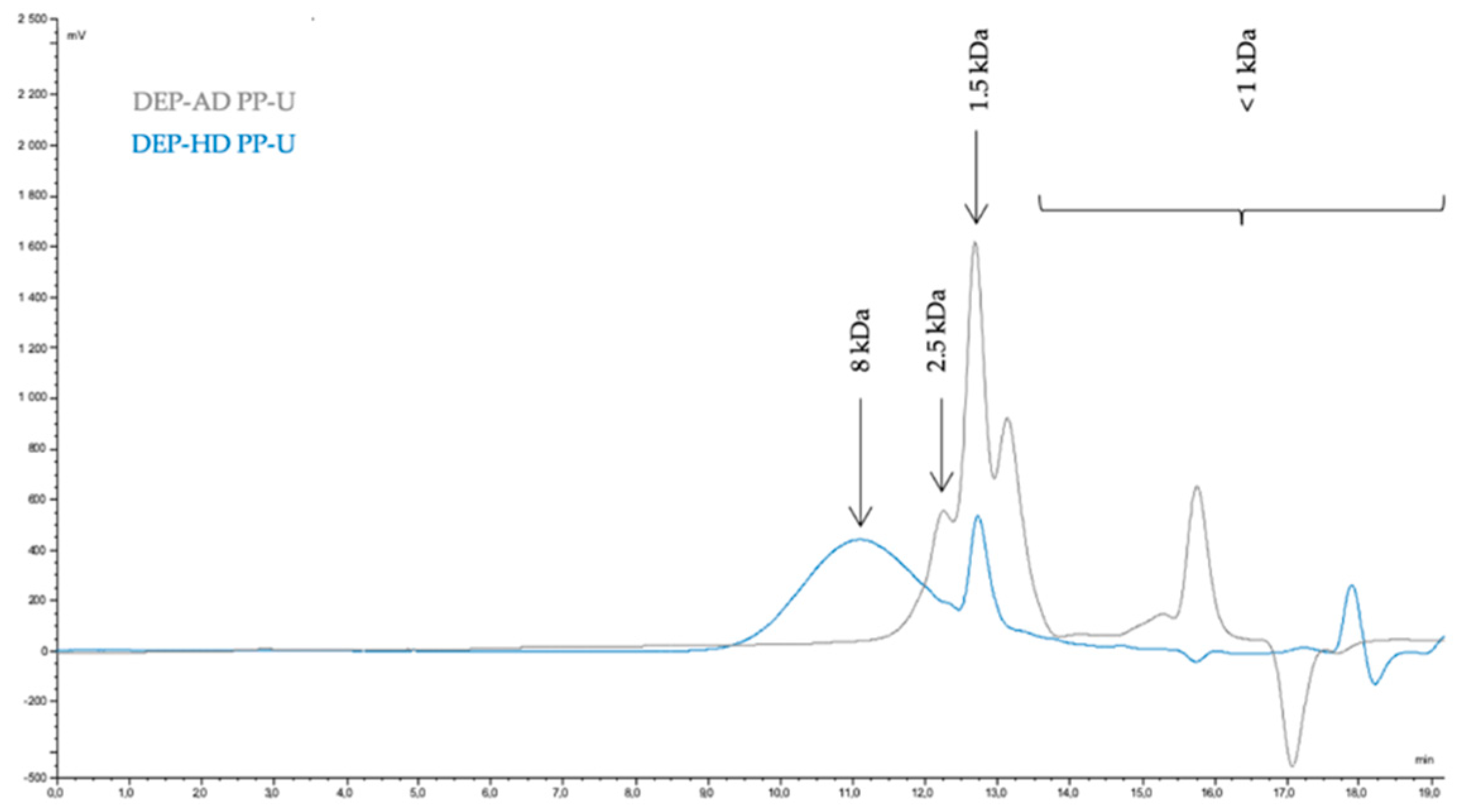
2.2.1. Monitoring of Depolymerization by High Performance Size Exclusion Chromatography (HPSEC)
2.2.2. Yields of Oligosaccharide Production
2.2.3. Biochemical Characterization of Oligosaccharides
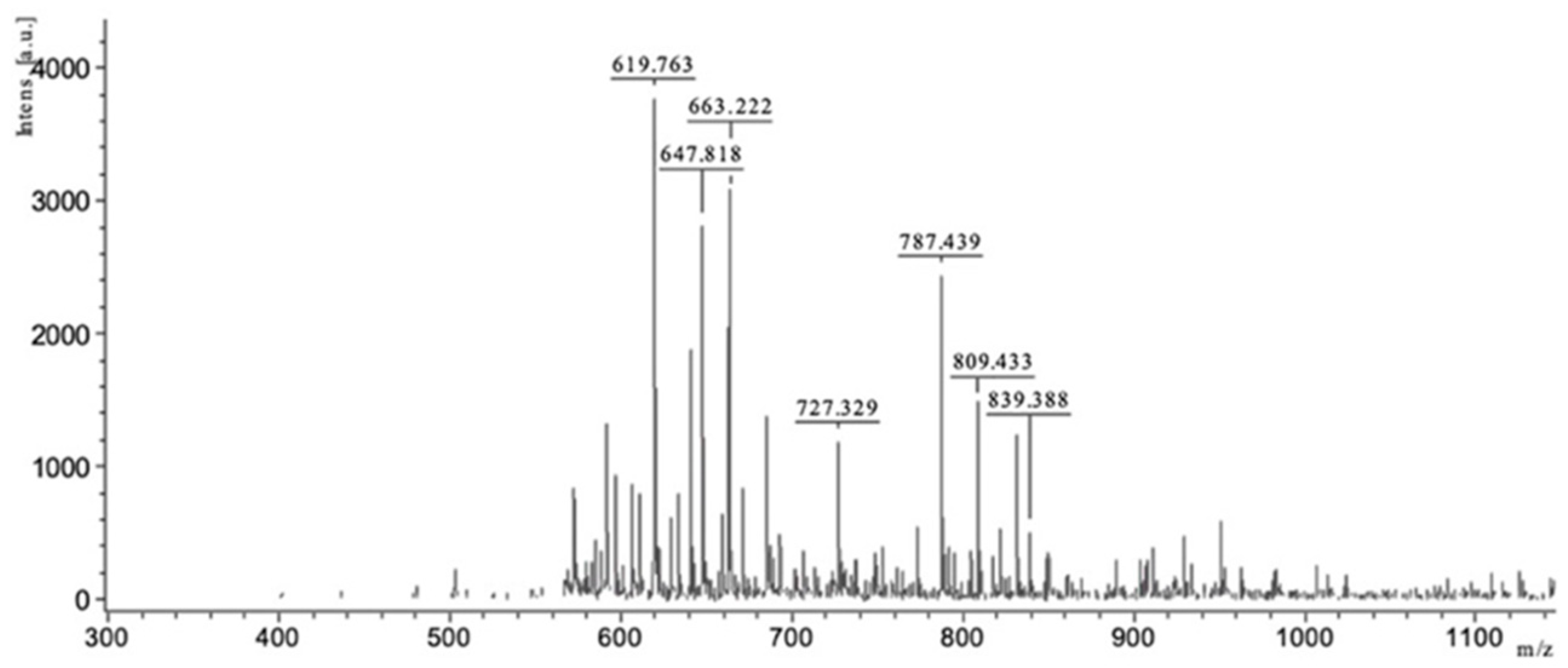
2.2.4. Matrix-Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) Mass Spectrometry
2.3. Biological Activities
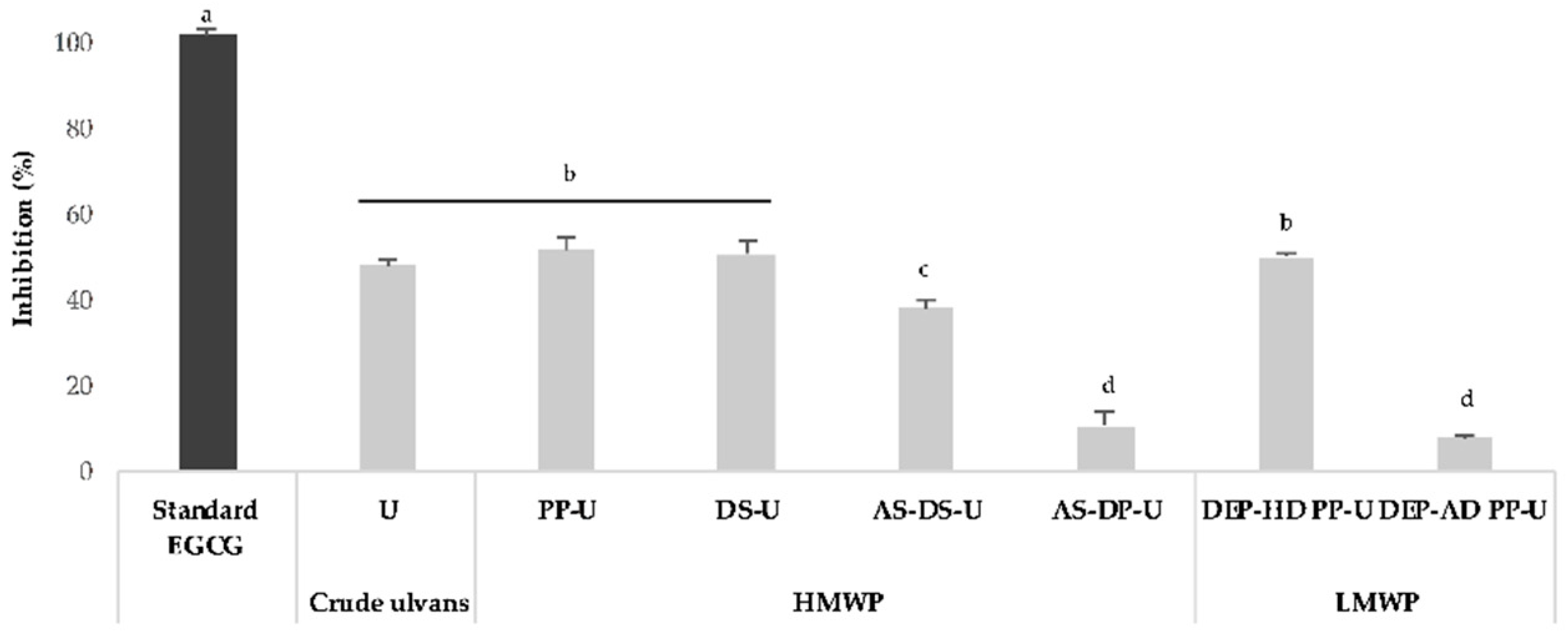
2.3.1. Lipoxygenase Inhibition Assay by Crude Ulvans and HMWP and LMWP Fractions from Ulva sp.
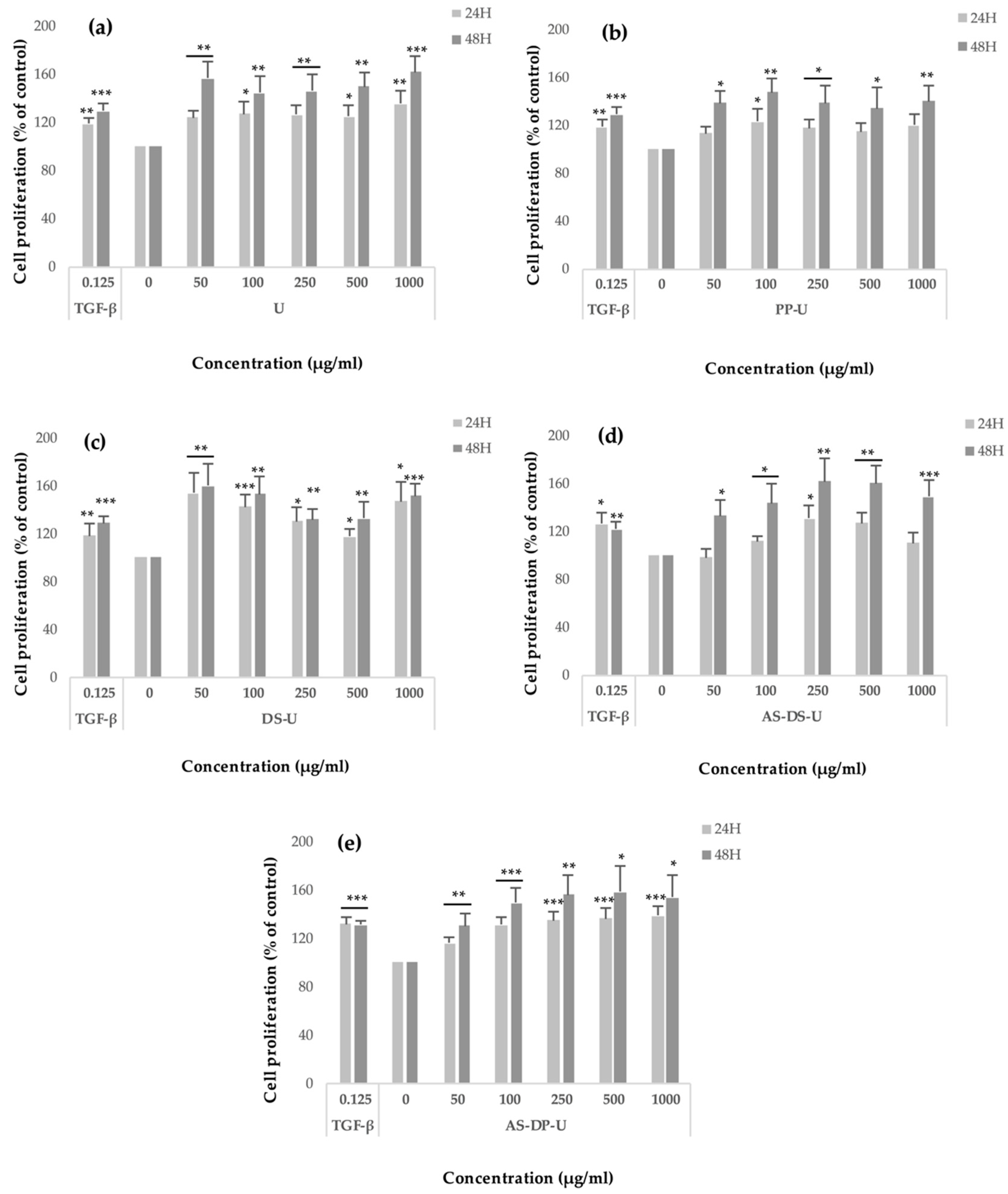
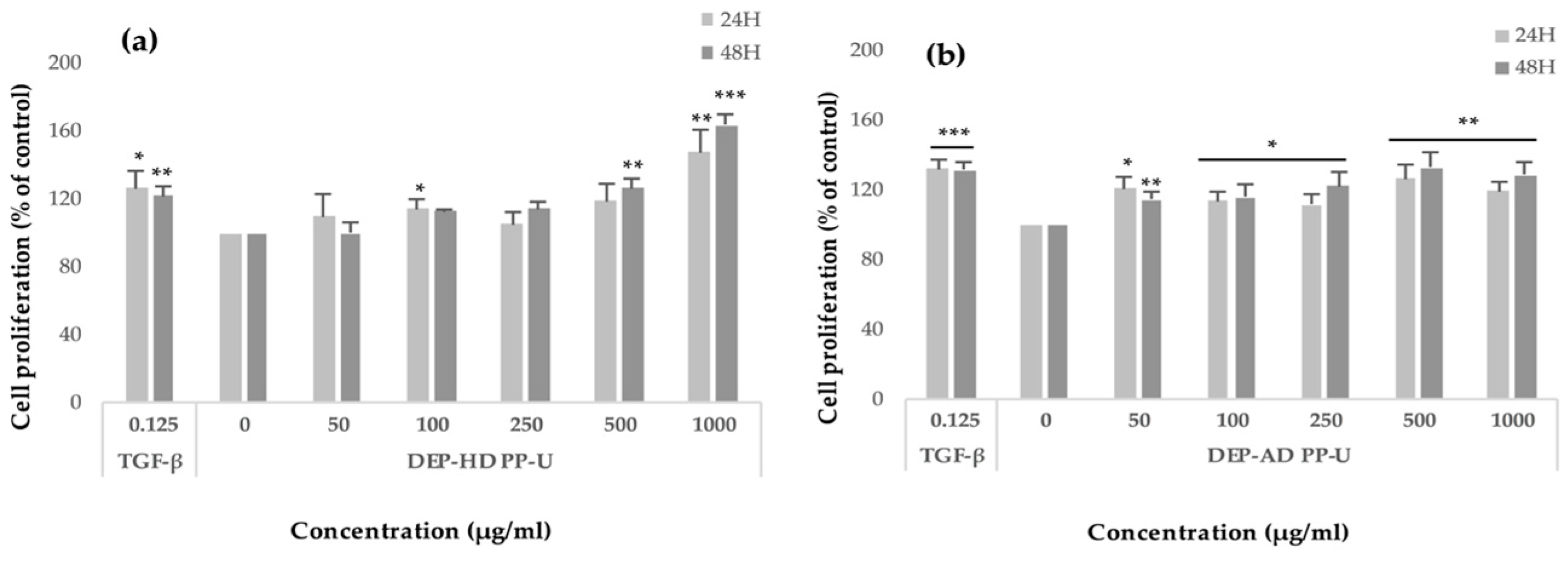
2.3.2. Modulation of Fibroblast Proliferation by Crude Ulvans and HMWP and LMWP Fractions from Ulva sp.
3. Discussion
3.1. Extraction and Fractionation of Ulvans
3.1.1. Ulvan Production
3.1.2. Biochemical Characterization of Ulvans
3.2. Depolymerization of Ulvans for Oligosaccharide Production
3.2.1. Oligosaccharide Production
3.2.2. Biochemical Characterization of Oligosaccharides
3.3. Biological Activities
3.3.1. Anti-Inflammatory Activity by Lipoxygenase Inhibition
3.3.2. Effects of the Ulvans Extracts and Fractions on Fibroblast Proliferation and Viability
4. Materials and Methods
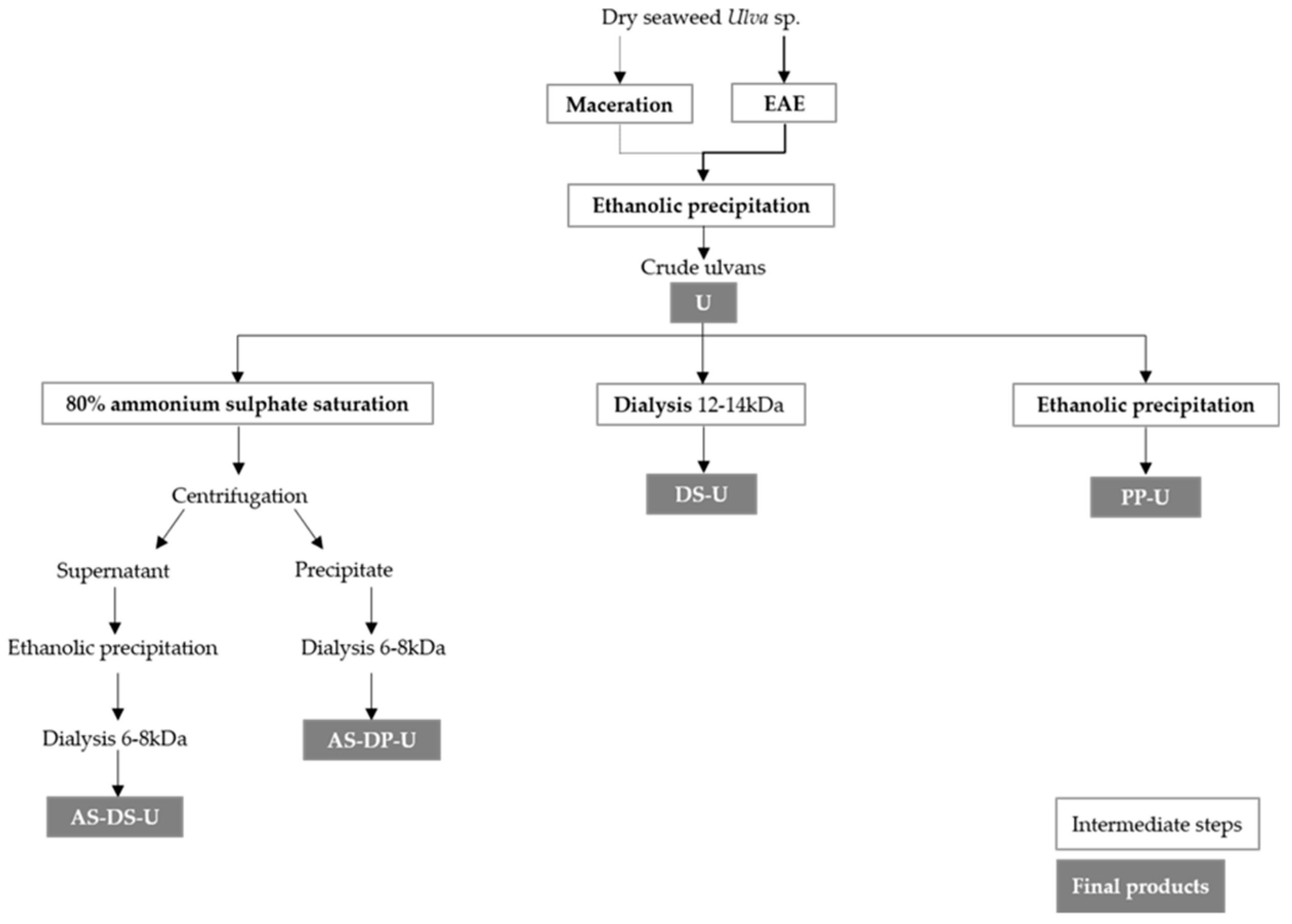
4.1. Extraction and Fractionation Procedure (Figure 6)
4.1.1. Enzyme-Assisted Extraction (EAE) Followed by Polysaccharide Precipitation
4.1.2. Fractionation of Crude Ulvans
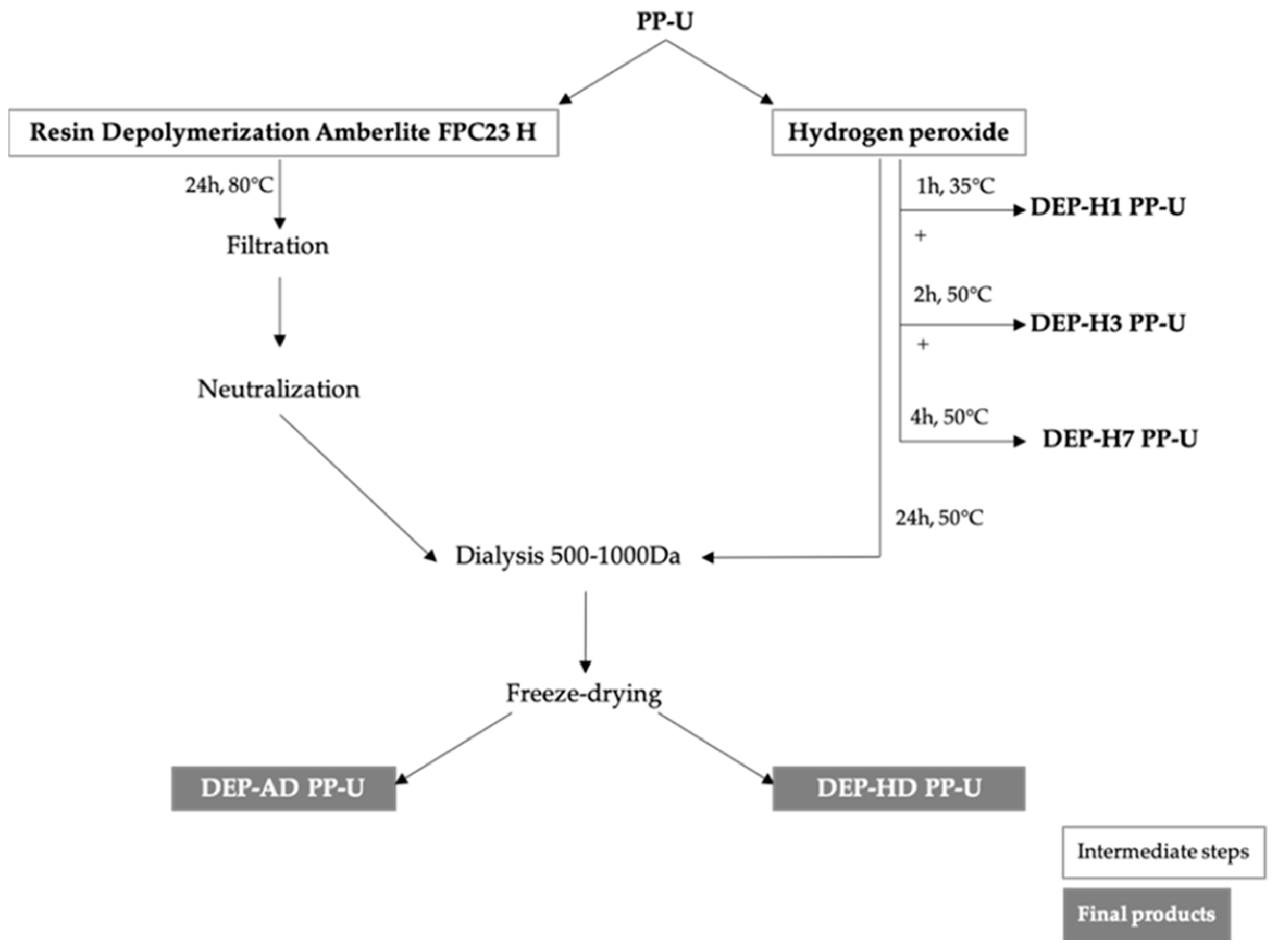
4.2. Production of Oligosaccharides by Ulvan Depolymerization
4.2.1. Hydrogen Peroxide Depolymerization
4.2.2. Depolymerization by Ion-Exchange Resin
4.3. Polysaccharide Molecular Weight (MW) Distribution by HPSEC Analysis
4.4. Biochemical Composition Analysis
4.5. Monosaccharide Composition Determined by High Performance Anion Exchange Chromatography
4.6. Matrix-Assisted Laser Desorption Ionisation-Time of Flight (MALDI-TOF) Mass Spectrometry
4.7. Biological Activities
4.7.1. Lipoxygenase Inhibition Assay
4.7.2. Cell Culture
4.7.3. WST-1 Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Charlier, R.; Morand, P.; Finkl, C.; Thys, A. Green Tides on the Brittany Coasts. Env. Res. Eng. Manag. 2007, 3, 52–59. [Google Scholar]
- Briand, X. Seaweed harvesting in Europe. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 259–308. ISBN 0-471-92947-6. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research-Sea Plants; Bourgougnon, N., Ed.; Academic Press: London, UK, 2014; Volume 71, pp. 349–382. ISBN 978-0-12-408062-1. [Google Scholar]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed application in cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK, 2016; pp. 423–441. ISBN 9780128027721. [Google Scholar]
- Pimentel, F.; Alves, R.; Rodrigues, F.; PP Oliveira, M. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Jegou, D.; Buleon, A. Chemical characteristics of insoluble glucans from the cell wall of the marine green alga Ulva lactuca (L.) Thuret. Carbohydr. Res. 1994, 262, 115–125. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr. Res. 1995, 274, 251–261. [Google Scholar]
- Lahaye, M.; Robic, A. Structure and functional properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic recovery of metabolites from seaweeds: Potential applications. In Advances in Botanical Research-Sea Plants; Bourgougnon, N., Ed.; Academic Press: London, UK, 2014; Volume 71, pp. 281–323. ISBN 978-0-12-408062-1. [Google Scholar]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N.; Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; et al. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Shao, P.; Chen, M.; Pei, Y.; Sun, P. In vitro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int. J. Biol. Macromol. 2013, 59, 295–300. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Ahmadi Ashtiani, H.R.; Jaffary, F.; Jahangiri, F.; Nikkhah, N.; Mahmoudbeyk, M.; Fard, M.; Ansari, Z.; Zare, S. Dermal Fibroblast Cells: Biology and Function in Skin Regeneration. J. Ski. Stem Cell 2017, 4, e69080. [Google Scholar] [CrossRef]
- Williams, I.R. Fibroblasts. In Encyclopedia of Immunology; Delves, P., Roitt, I., Eds.; Academic Press: London, UK, 1998; pp. 905–909. ISBN 9780122267659. [Google Scholar]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Derm. 2014, 7, 301–311. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Casas, M.P.; González, H.D. Enzyme-Assisted Aqueous Extraction Processes. In Water Extraction of Bioactive Compounds; González, H.D., Muñoz, M.J.G., Eds.; Elsevier: London, UK, 2017; pp. 333–368. ISBN 9780128093801. [Google Scholar]
- Lahaye, M.; Axelos, M.A.V. Gelling properties of water-soluble polysaccharides from proliferating marine green seaweeds (Ulva spp.). Carbohydr. Polym. 1993, 22, 261–265. [Google Scholar] [CrossRef]
- Hardouin, K. Production D’extraits Aqueux à Partir d’Ulva sp., au Moyen de Procédés D’hydrolyse Enzymatique: Caractérisation, Valorisation et Perspectives de Développement. Ph.D. Thesis, Université de Bretagne Sud in Marine Biology–Ecole Doctorale des Sciences de la Mer, Lorient, France, 2015. [Google Scholar]
- Lahaye, M.; Alvarez-Cabal Cimadevilla, E.; Kuhlenkamp, R.; Quemener, B.; Lognoné, V.; Dion, P. Chemical composition and 13C NMR spectroscopic characterisation of ulvans from Ulva (Ulvales, Chlorophyta). J. Appl. Phycol. 1999, 11, 1–7. [Google Scholar] [CrossRef]
- Lahaye, M.; Inizan, F.; Vigoureux, J. NMR analysis of the chemical structure of ulvan and of ulvan-boron complex formation. Carbohydr. Polym. 1998, 36, 239–249. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Percival, E.; McDowell, R.H. Chemistry and Enzymology of Marine Algal Polysaccharides; Edition, A.C.I., Ed.; Academic Press: London, UK, 1968; ISBN 0125506503. [Google Scholar]
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar determination in ulvans by a chemical-enzymatic method coupled to high performance anion exchange chromatography. J. Appl. Phycol. 1997, 9, 179–188. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of extraction conditions on the yield and purity of ulvan extracted from Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Borges da Silva, E.A.; Reis, R.L.; Rodrigues, A.E. Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.-F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Selvaraj, K.; Subba Rao, P.V. Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) Doty-An edible seaweed. Food Chem. 2014, 153, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Wong, K.; Cheung, P.C. Nutritional evaluation of some subtropical red and green seaweeds Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. Seaweed Sustain. 2015, 243–269. [Google Scholar]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Limited: Sawston, UK, 2004; pp. 197–211. ISBN 9780081007297. [Google Scholar]
- Pengzhan, Y.; Quanbin, Z.; Hong, Z.; Xizhen, N.; Zhien, L. Preparation of polysaccharides in different molecular weights from Ulva pertusa Kjellm (Chorophyta). Chin. J. Oceanol. Limnol. 2004, 22, 381–385. [Google Scholar] [CrossRef]
- Coste, O.; Malta, E.; López, J.C.; Fernández-Díaz, C. Production of sulfated oligosaccharides from the seaweed Ulva sp. using a new ulvan-degrading enzymatic bacterial crude extract. Algal Res. 2015, 10, 224–231. [Google Scholar] [CrossRef]
- Redouan, E.; Cedric, D.; Emmanuel, P.; Mohamed, E.G.; Bernard, C.; Philippe, M.; Cherkaoui, E.M.; Josiane, C. Improved isolation of glucuronan from algae and the production of glucuronic acid oligosaccharides using a glucuronan lyase. Carbohydr. Res. 2009, 344, 1670–1675. [Google Scholar] [CrossRef]
- Majee, S.; Avlani, D.; Roy Biswas, G. Molecular modification of marine sulfated polysaccharides. In Enzymatic Technologies for Marine Polysaccharides; Trincone, A., Ed.; CRC Press, Taylor and Francis: Abingdon, UK, 2019; p. 395. ISBN 978-1-138-10307-8. [Google Scholar]
- Delattre, C.; Michaud, P.; Courtois, B.; Courtois, J. Oligosaccharides engineering from plants and algae - Applications in Biotechnology and therapeutic. Minerva Biotec. 2005, 17, 107–117. [Google Scholar]
- Kawakishi, S.; Kito, Y.; Namiki, M. Radiation-induced Degradation of d-Glucose in Anaerobic Condition. Agric. Biol. Chem. 1977, 41, 951–957. [Google Scholar]
- Zhang, H.-J.; Mao, W.-J.; Fang, F.; Li, H.-Y.; Sun, H.-H.; Chen, Y.; Qi, X.-H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Sanda Chedea, V.; Jisaka, M. Inhibition of soybean lipoxygenases—Structural and activity models for the lipoxygenase isoenzymes family. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; InTechOpen: London, UK, 2011; pp. 109–130. [Google Scholar]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The Lipoxygenase Pathway. Annu. Rev. Plant. Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, X.; Xu, Z.; Dong, N.; Zou, S.; Shen, X.; Huang, J. Development of a new colorimetric assay for lipoxygenase activity. Anal. Biochem. 2013, 441, 162–168. [Google Scholar] [CrossRef]
- Matsukawa, R.; Dubinsky, Z.; Kishimoto, E.; Masaki, K.; Masuda, Y.; Takeuchi, T.; Chihara, M.; Yamamoto, Y.; Niki, E.; Karube, I. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997, 9, 29–35. [Google Scholar] [CrossRef]
- Mohsin, S.; Muraleedhara Kurup, G. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Biomed. Prev. Nutr. 2011, 1, 294–301. [Google Scholar] [CrossRef]
- Sujina, M.; Soumya, J.; Manjusha, W.A. Assessing the anticancer, anti-inflammatory, antimicrobial and antioxidant potential of bioactive compounds present in marine algae Ulva lactuca. World J. Pharm. Res. 2016, 5, 1482–1500. [Google Scholar]
- Al-Amoudi, O.A.; Mutawie, H.H.; Patel, A.V.; Blunden, G. Chemical composition and antioxidant activities of Jeddah corniche algae, Saudi Arabia. Saudi J. Biol. Sci. 2009, 16, 23–29. [Google Scholar] [CrossRef]
- Kurihara, H.; Kagawa, Y.; Konno, R.; Kim, S.M.; Takahashi, K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorg. Med. Chem. Lett. 2014, 24, 1383–1385. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Kurup, G.M. Alginic acid isolated from Sargassum wightii exhibits anti-inflammatory potential on type II collagen induced arthritis in experimental animals. Int. Immunopharmacol. 2013, 17, 1108–1115. [Google Scholar] [CrossRef]
- Chakraborty, K.; Antony, T. First report of spiro-compounds from marine macroalga Gracilaria salicornia: Prospective natural anti-inflammatory agents attenuate 5-lipoxygenase and cyclooxygenase-2. Nat. Prod. Res. 2019, 1–12. [Google Scholar] [CrossRef]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef]
- Berri, M.; Olivier, M.; Holbert, S.; Dupont, J.; Demais, H.; Le Goff, M.; Collen, N. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res. 2017, 28, 39–47. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In vitro cytotoxicity assessment of ulvan, a polysaccharide extracted from green algae. Phyther. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Robledo, D.; Freile-Pelegrín, Y.; Peña-Hernández, M.A.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Synergistic Effects of Sulfated Polysaccharides from Mexican Seaweeds against Measles Virus. Biomed. Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Ennamany, R.; Saboureau, D.; Mekideche, N.; Creppy, E.E. SECMA 1®, a mitogenic hexapeptide from Ulva algeae modulates the production of proteoglycans and glycosaminoglycans in human foreskin fibroblast. Hum. Exp. Toxicol. 1998, 17, 18–22. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, G.B.; Lee, D.H.; Lee, G.S.; Pyo, H.B. The effect of hydrolyzed Jeju Ulva pertusa on the proliferation and type I collagen synthesis in replicative senescent fibroblasts. J. Soc. Cosmet. Sci. Korea 2013, 39, 177–186. [Google Scholar]
- Cai, C.; Guo, Z.; Yang, Y.; Geng, Z.; Tang, L.; Zhao, M.; Qiu, Y.; Chen, Y.; He, P. Inhibition of hydrogen peroxide induced injuring on human skin fibroblast by Ulva prolifera polysaccharide. Int. J. Biol. Macromol. 2016, 91, 241–247. [Google Scholar] [CrossRef]
- Andrès, E.; Molinari, J.; Péterszegi, G.; Mariko, B.; Ruszova, E.; Velebny, V.; Faury, G.; Robert, L. Pharmacological properties of rhamnose-rich polysaccharides, potential interest in age-dependent alterations of connectives tissues. Pathol. Biol. 2006, 54, 420–425. [Google Scholar] [CrossRef]
- Faury, G.; Ruszova, E.; Molinari, J.; Mariko, B.; Raveaud, S.; Velebny, V.; Robert, L. The α-l-Rhamnose recognizing lectin site of human dermal fibroblasts functions as a signal transducer: Modulation of Ca2+ fluxes and gene expression. Biochim. Biophys. Acta-Gen. Subj. 2008, 1780, 1388–1394. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Moulin, V.; Beaulieu, M.; Turgeon, S.L. Human skin fibroblast response is differentially regulated by galactofucan and low molecular weight galactofucan. Bioact. Carbohydr. Diet. Fibre 2013, 1, 105–110. [Google Scholar] [CrossRef]
- Wingfield, P.T. Protein precipitation using ammonium sulfate. Curr. Protoc. Protein Sci. 2016, 84, A.3F.1–A.3F.9. [Google Scholar]
- Mulloy, B.; Hogwood, J. Chromatographic molecular weight measurements for heparin, Its fragments and fractions, and other glycosaminoglycans. In Methods in Molecular Biology (Clifton, N.J.); Balagurunathan, K., Nakato, H., Desai, U.R., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1229, pp. 105–118. ISBN 978-1-4939-1714-3. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Jaques, L.B.; Ballieux, R.E.; Dietrich, C.P.; Kavanagh, L.W. A microelectrophoresis method for heparin. Can. J. Physiol. Pharm. 1968, 46, 351–360. [Google Scholar] [CrossRef]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr. Polym. 2006, 66, 408–416. [Google Scholar] [CrossRef]
- Chen, S.-K.; Hsu, C.-H.; Tsai, M.-L.; Chen, R.-H.; Drummen, G. Inhibition of Oxidative Stress by Low-Molecular-Weight Polysaccharides with Various Functional Groups in Skin Fibroblasts. Int. J. Mol. Sci. 2013, 14, 19399–19415. [Google Scholar] [CrossRef]
- Lahrsen, E.; Schoenfeld, A.-K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef]
- Morya, V.K.; Kim, J.; Kim, E.-K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
| Mineral Matter | Carbohydrates | Uronic Acids | Sulfate Groups | Proteins | |
|---|---|---|---|---|---|
| Crude Ulvans | |||||
| U | 35.2 ± 0.2 a | 23.5 ± 0.5 c | 18.5 ± 0.6 c | 29.9 ± 0.5 c | 10.5 ± 0.4 b,c |
| HMWPs | |||||
| PP-U | 26.8 ± 1.0 b | 30.7 ± 0.2 b | 23.7 ± 0.7 b | 41.9 ± 0.5 b | 11.2 ± 0.2 b,c |
| DS-U | 13.4 ± 0.8 c | 37.4 ± 0.2 a | 37.0 ± 0.7 a | 49.1 ± 1.0 a | 12.8 ± 0.2 a,b |
| AS-DS-U | - | 35.1 ± 0.4 a,b | 36.0 ± 0.8 a | 49.4 ± 0.5 a | 8.9 ± 0.5 c |
| AS-DP-U | - | 31.5 ± 0.6 b | 13.3 ± 0.4 c | 23.9 ± 0.5 d | 16.4 ± 0.2 a |
| Rhamnose | Galactose | Glucose | Xylose | Glucuronic Acid | |
|---|---|---|---|---|---|
| Crude Ulvans | |||||
| U | 50.4 ± 6.0 a | 1.6 ± 0.1 b | 14.0 ± 1.2 a | 3.2 ± 0.2 a,b | 10.6 ± 0.9 a |
| HMWPs | |||||
| PP-U | 42.4 ± 0.3 a | 1.3 ± 0.1 b | 5.7 ± 0.7 b,c | 2.7 ± 0.2 b | 11.6 ± 1.9 a |
| DS-U | 50.2 ± 2.2 a | 1.6 ± 0.0 b | 3.3 ± 0.2 c | 2.8 ± 0.2 a,b | 11.9 ± 0.3 a |
| AS-DS-U | 59.0 a | 1.3 b | 4.0 b,c | 3.0 a,b | 11.2 a |
| AS-DP-U | 29.1 a | 6.2 a | 9.0 b | 4.3 a | 3.4 b |
| Mn (kDa) | Mw (kDa) | I | |
|---|---|---|---|
| PP-U | 1651 | 2290 | 1.4 |
| DEP-H1 PP-U | 1480 | 2154 | 1.5 |
| DEP-H3 PP-U | 1343 | 1943 | 1.5 |
| DEP-H7 PP-U | 128 | 510 | 4.0 |
| DEP-HD PP-U | 7.0 | 8.7 | 1.2 |
| Carbohydrates | Uronic Acids | Sulfate Groups | Proteins | |
|---|---|---|---|---|
| DEP-AD PP-U | 30.4 ± 0.2 a | 30.8 ± 1.0 a | nd | 12.8 ± 1.0 a |
| DEP-HD PP-U | 24.4 ± 0.4 b | 21.6 ± 0.4 b | 6.6 ± 0.1 | 16.8 ± 0.4 a |
| Rhamnose | Galactose | Glucose | Xylose | Glucuronic Acid | |
|---|---|---|---|---|---|
| DEP-AD PP-U | 55.4 ± 2.3 a | 1.7 ± 0.1 a | 7.0 ± 0.4 a | 2.6 ± 0.2 a | 11.0 ± 0.7 a |
| DEP-HD PP-U | 44.9 a | 1.0 b | 4.9 a | 2.1 a | 7.5 b |
| Fractions | Temperature (°C) | Time (h) | Dialysis (500–1000 Da) |
|---|---|---|---|
| DEP-H1 PP-U | 35 | 1 | No |
| DEP-H3 PP-U | Step 1: 35 Step 2: 50 | 1 2 | No |
| DEP-H7 PP-U | Step 1: 35 Step 2: 50 | 1 6 | No |
| DEP-HD PP-U | 50 | 24 | Yes |
| Chromatographic Columns | |
|---|---|
| PP-U | G6000PWXL G4000PWXL G3000PWXL |
| DEP-H1 PP-U | |
| DEP-H3 PP-U | |
| DEP-H7 PP-U | |
| DEP-HD PP-U | G3000PWXL |
| DEP-AD PP-U |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. https://doi.org/10.3390/metabo9090182
Fournière M, Latire T, Lang M, Terme N, Bourgougnon N, Bedoux G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites. 2019; 9(9):182. https://doi.org/10.3390/metabo9090182
Chicago/Turabian StyleFournière, Mathilde, Thomas Latire, Marie Lang, Nolwenn Terme, Nathalie Bourgougnon, and Gilles Bedoux. 2019. "Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization" Metabolites 9, no. 9: 182. https://doi.org/10.3390/metabo9090182
APA StyleFournière, M., Latire, T., Lang, M., Terme, N., Bourgougnon, N., & Bedoux, G. (2019). Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites, 9(9), 182. https://doi.org/10.3390/metabo9090182






