Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes
Abstract
1. Introduction
2. Results
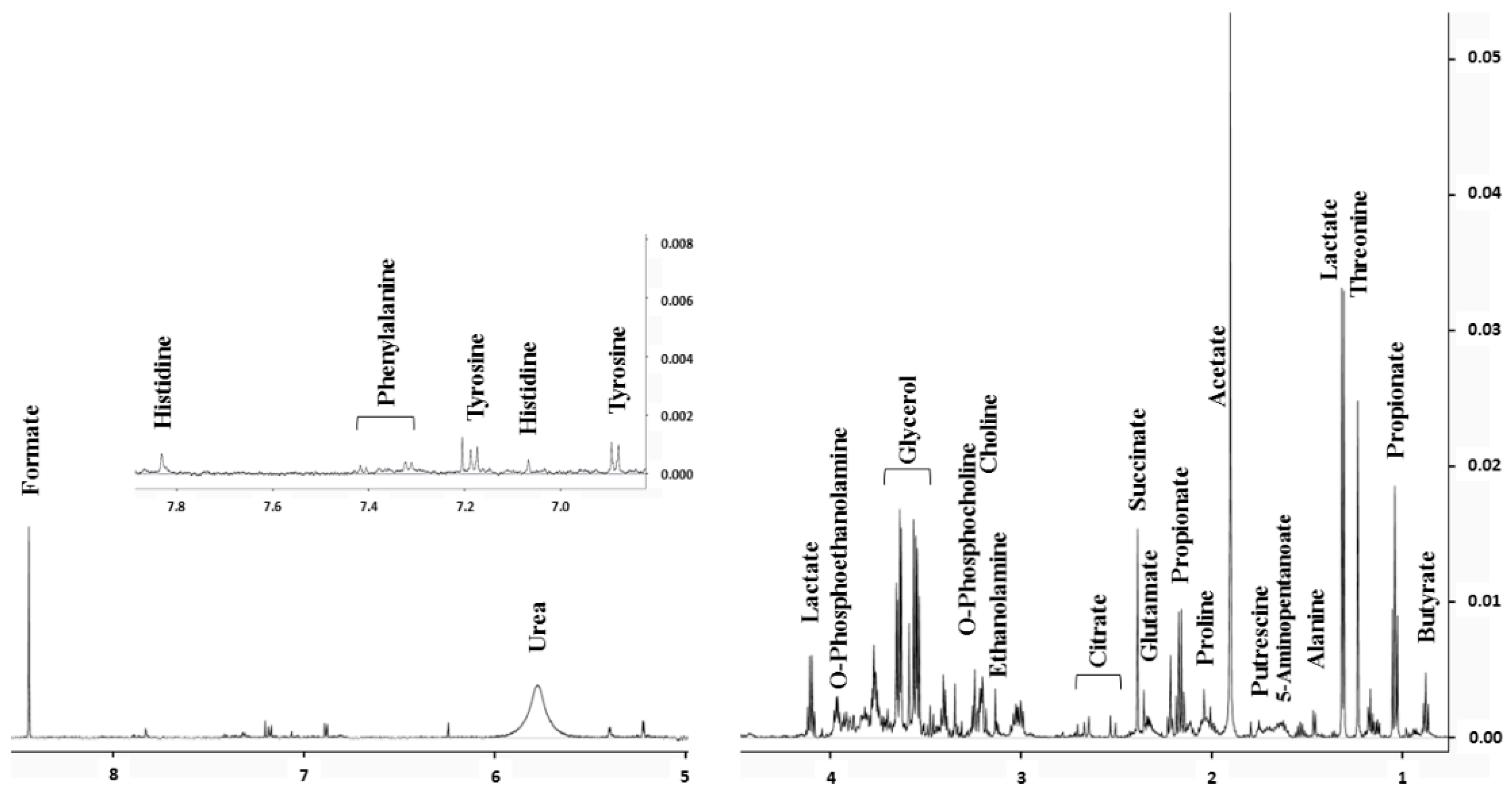
2.1. Saliva Biomarkers Quantified by NMR
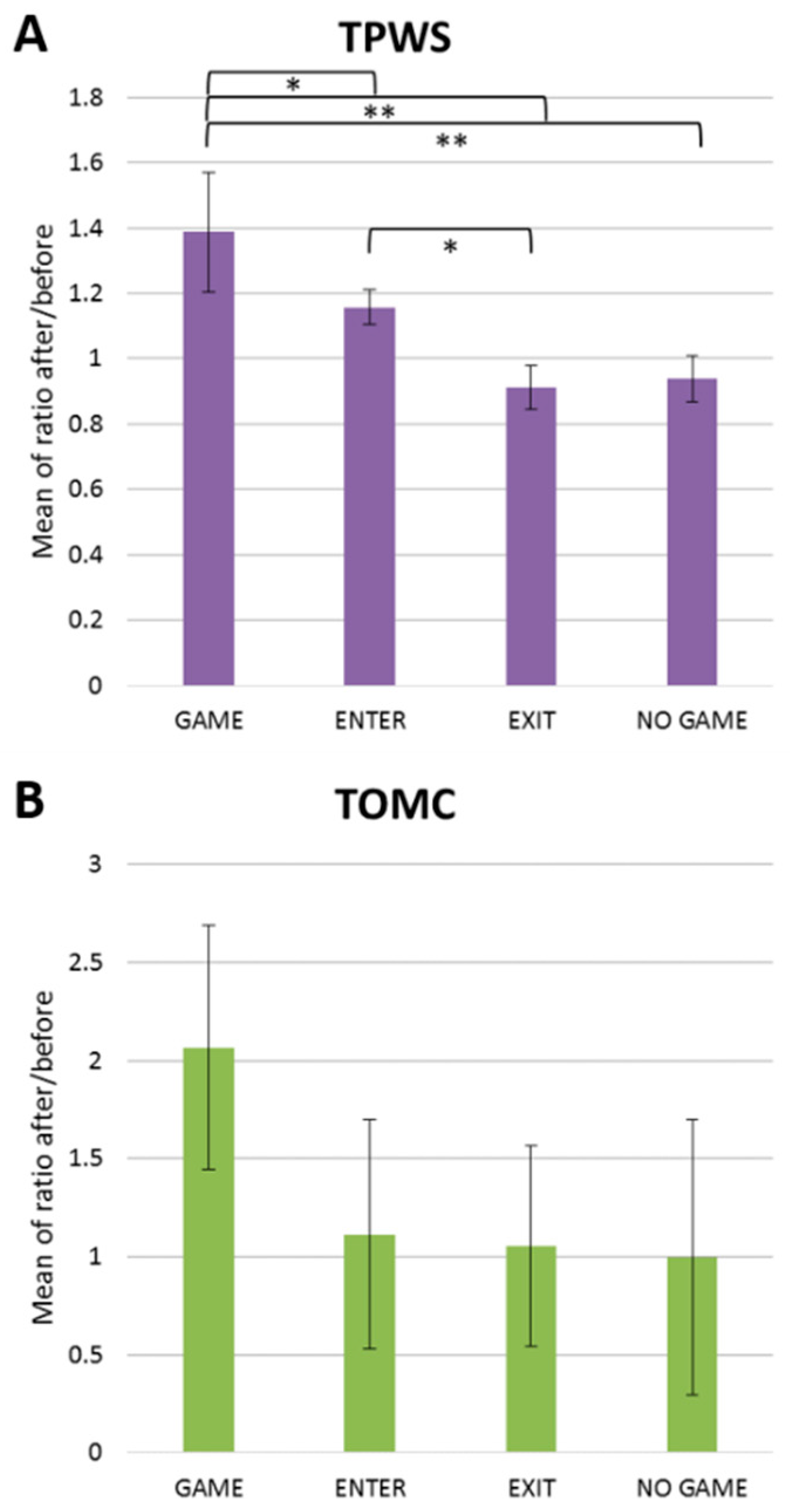
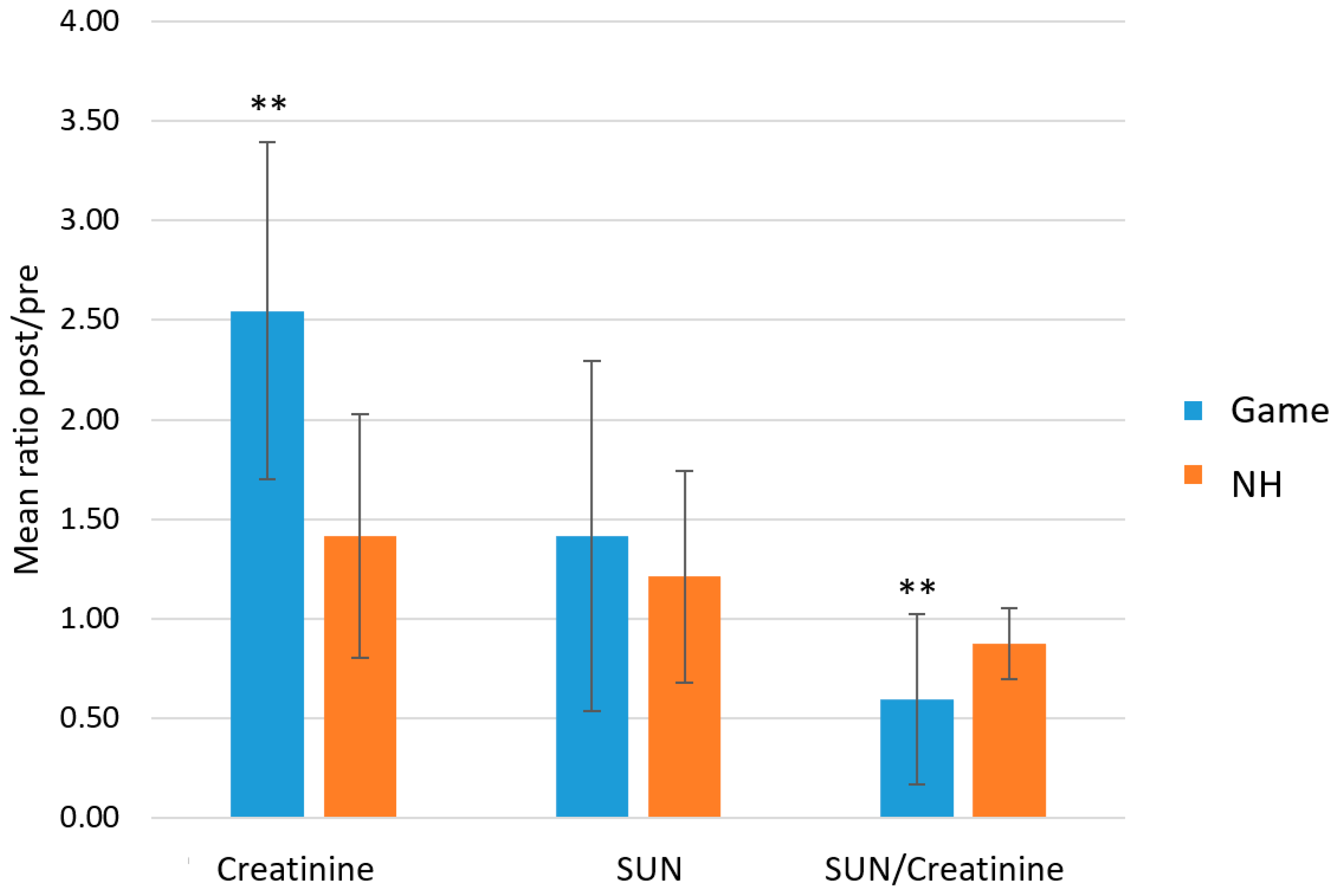
2.2. Total Proteins (TPWS), Total Detected Metabolite Concentration (TOMC) and Urea Nitrogen/Creatinine Ratio as Indexes of Exercise Load and Hydration Status
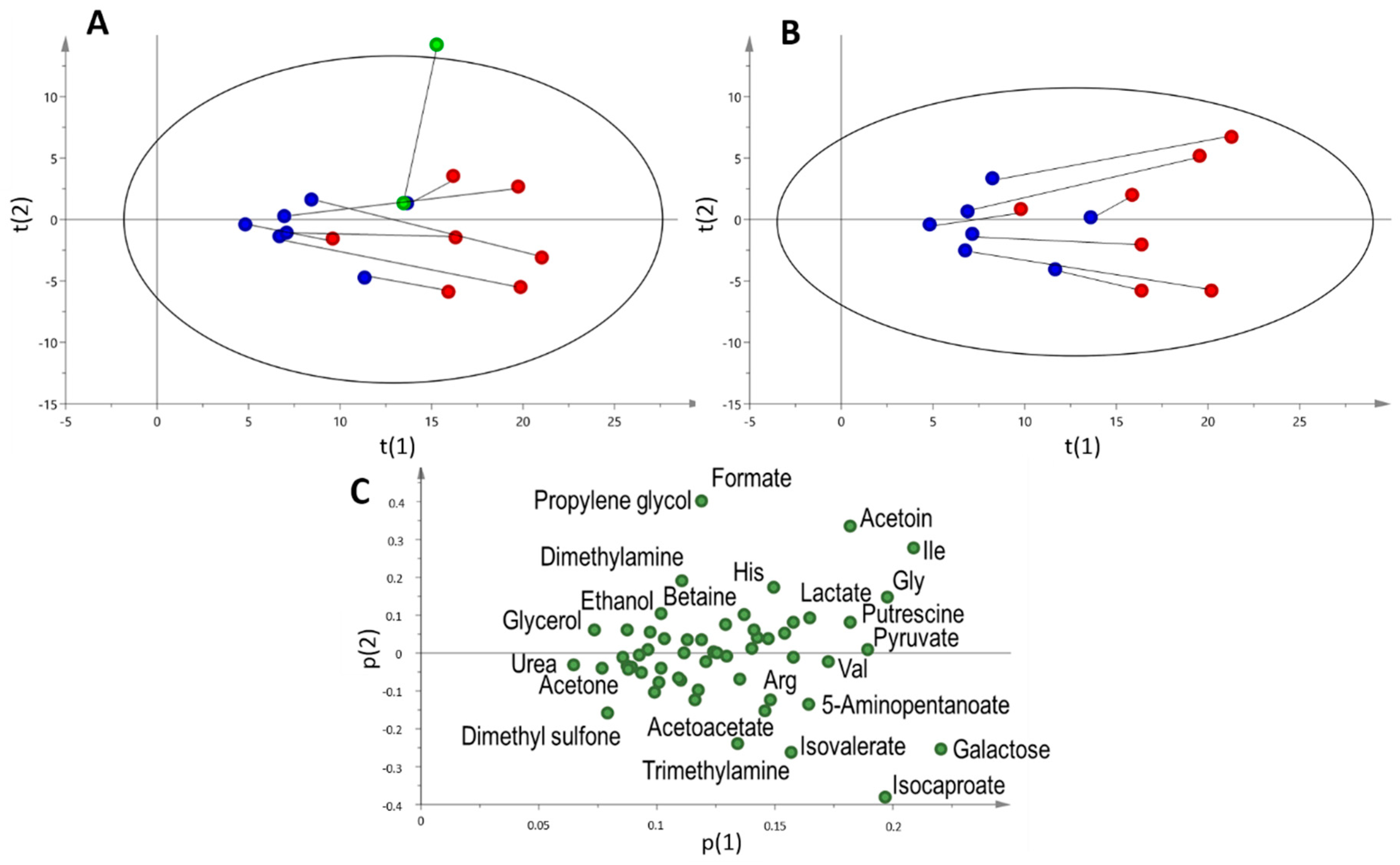
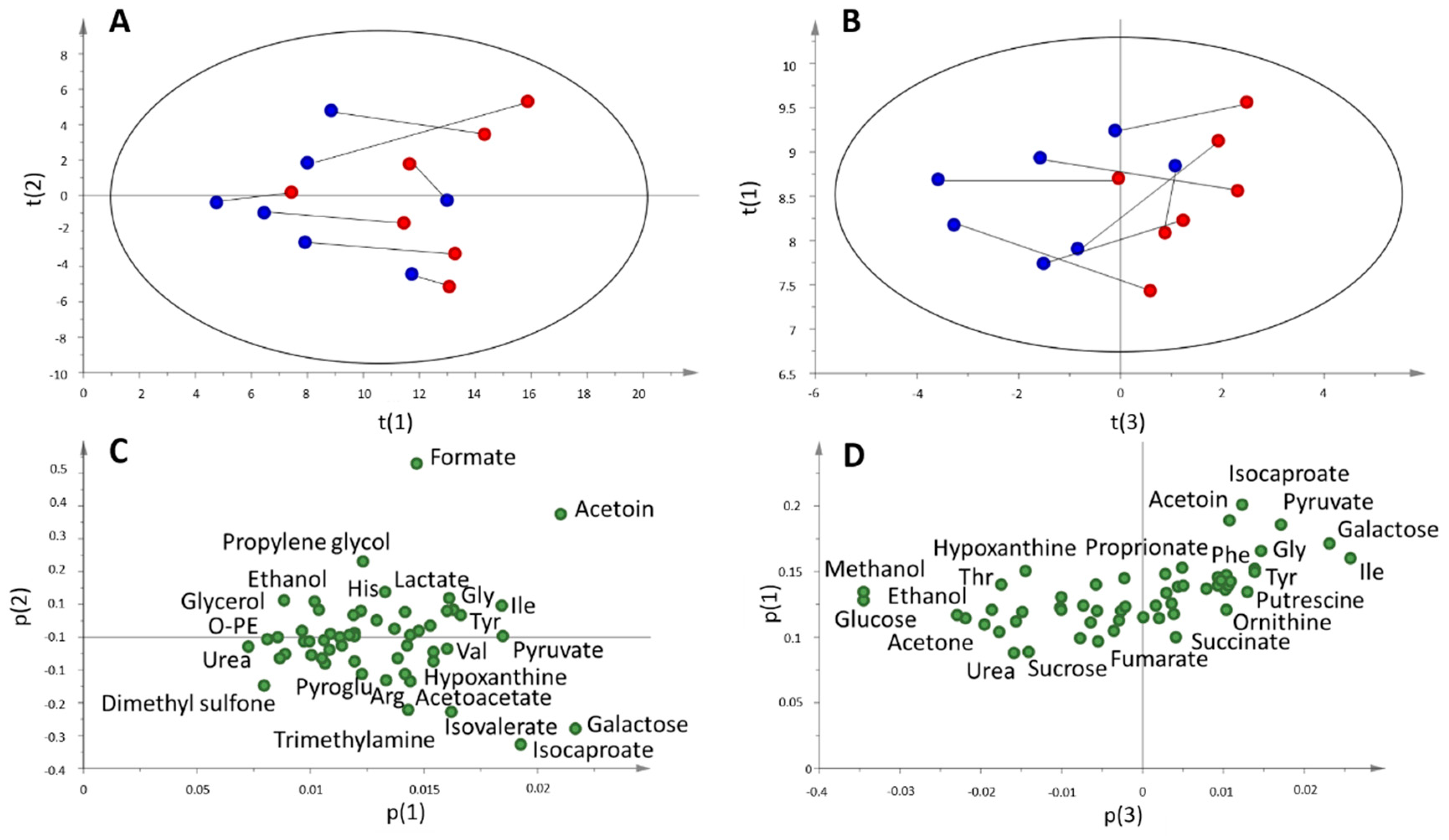
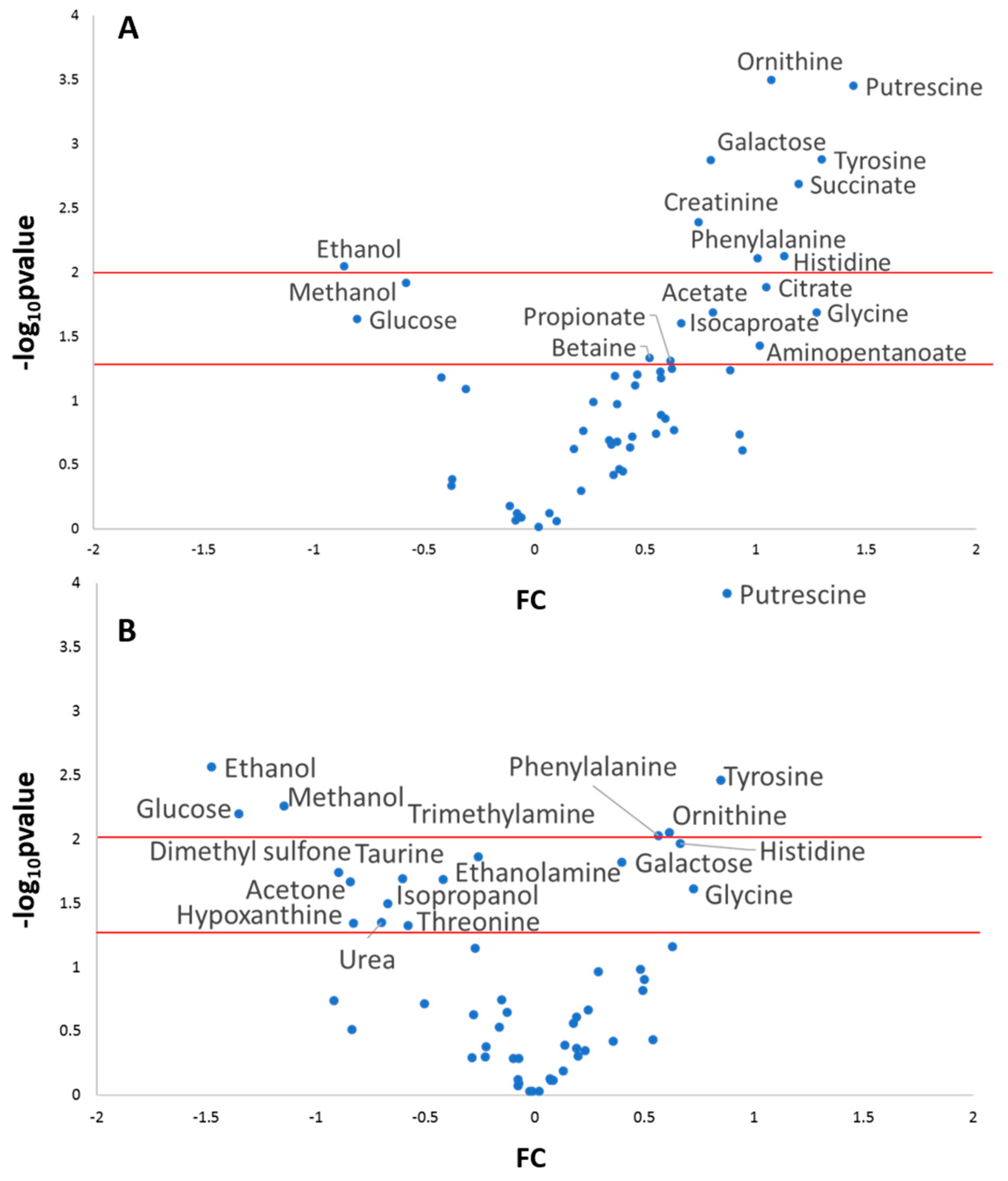
2.3. Multi and Univariate Analysis of the Salivary Metabolome
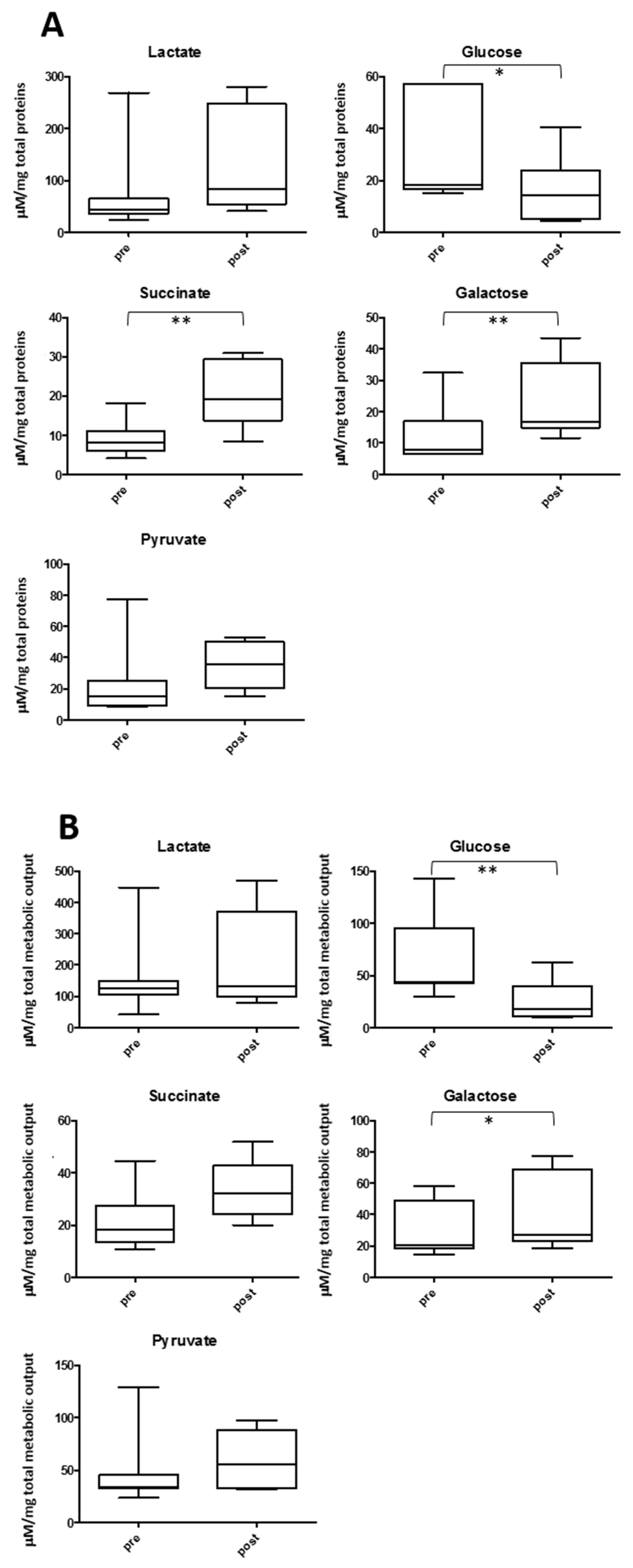
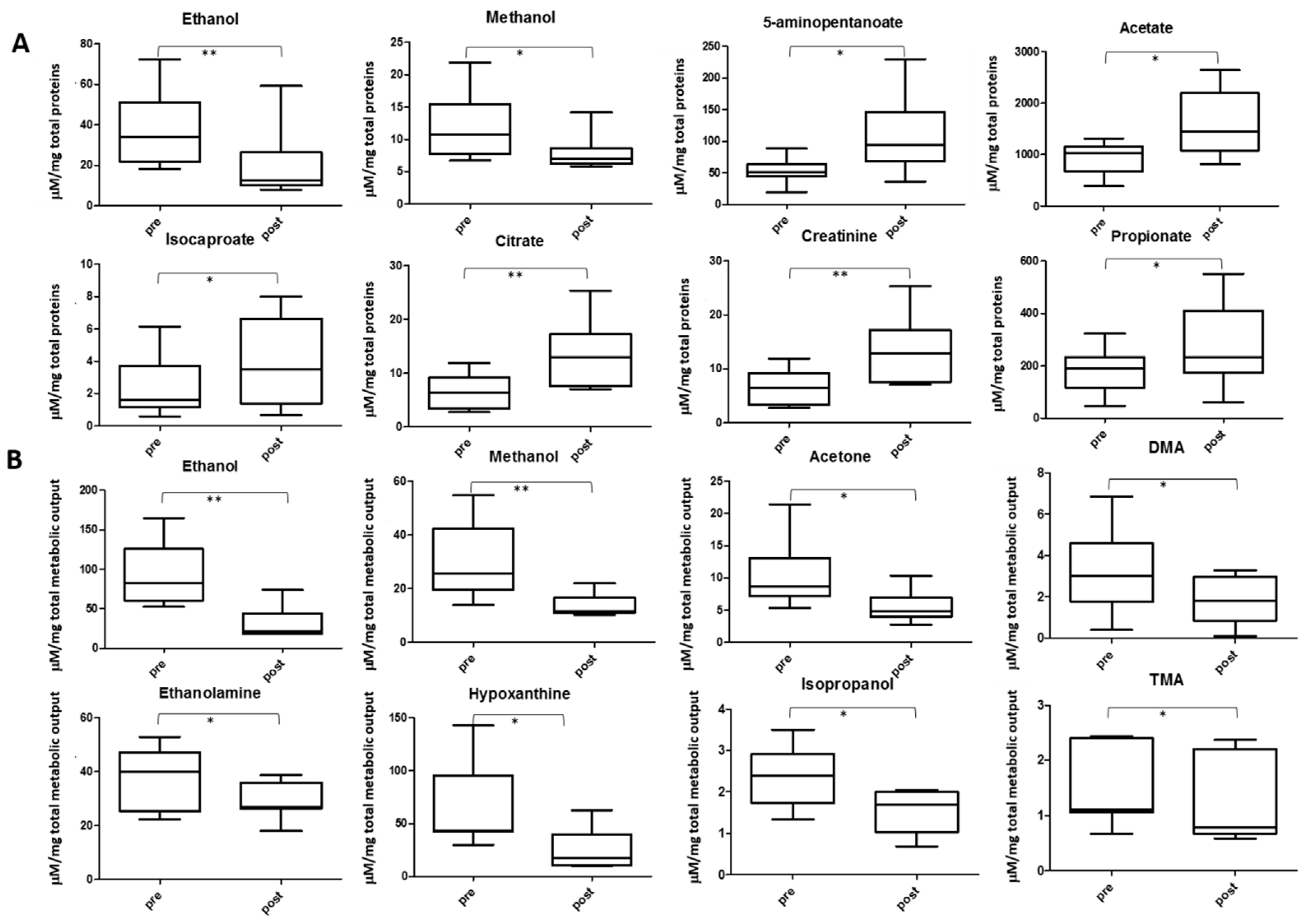
2.4. Salivary Lactate, Pyruvate, Succinate and Energy Related Biomarkers
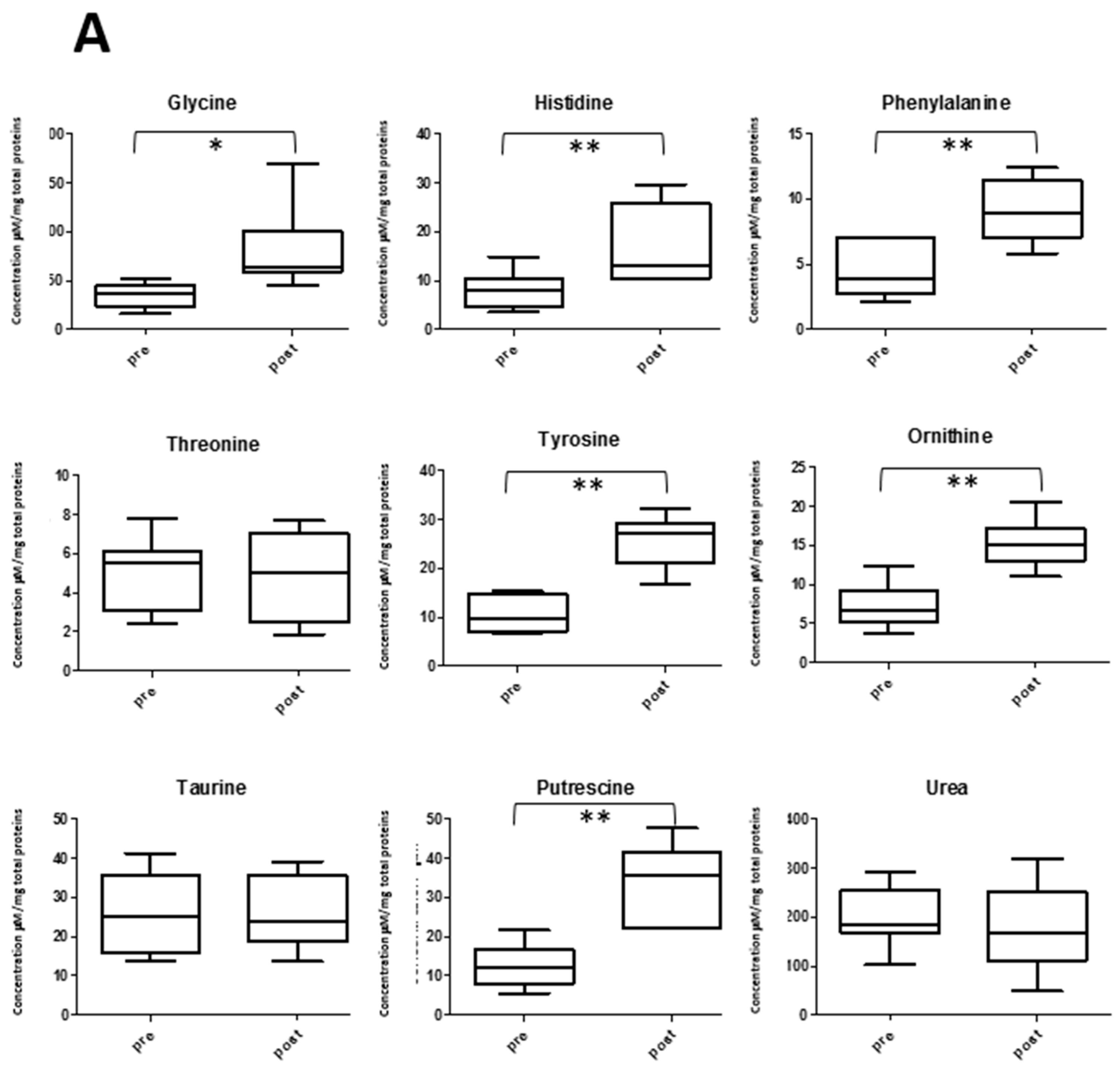
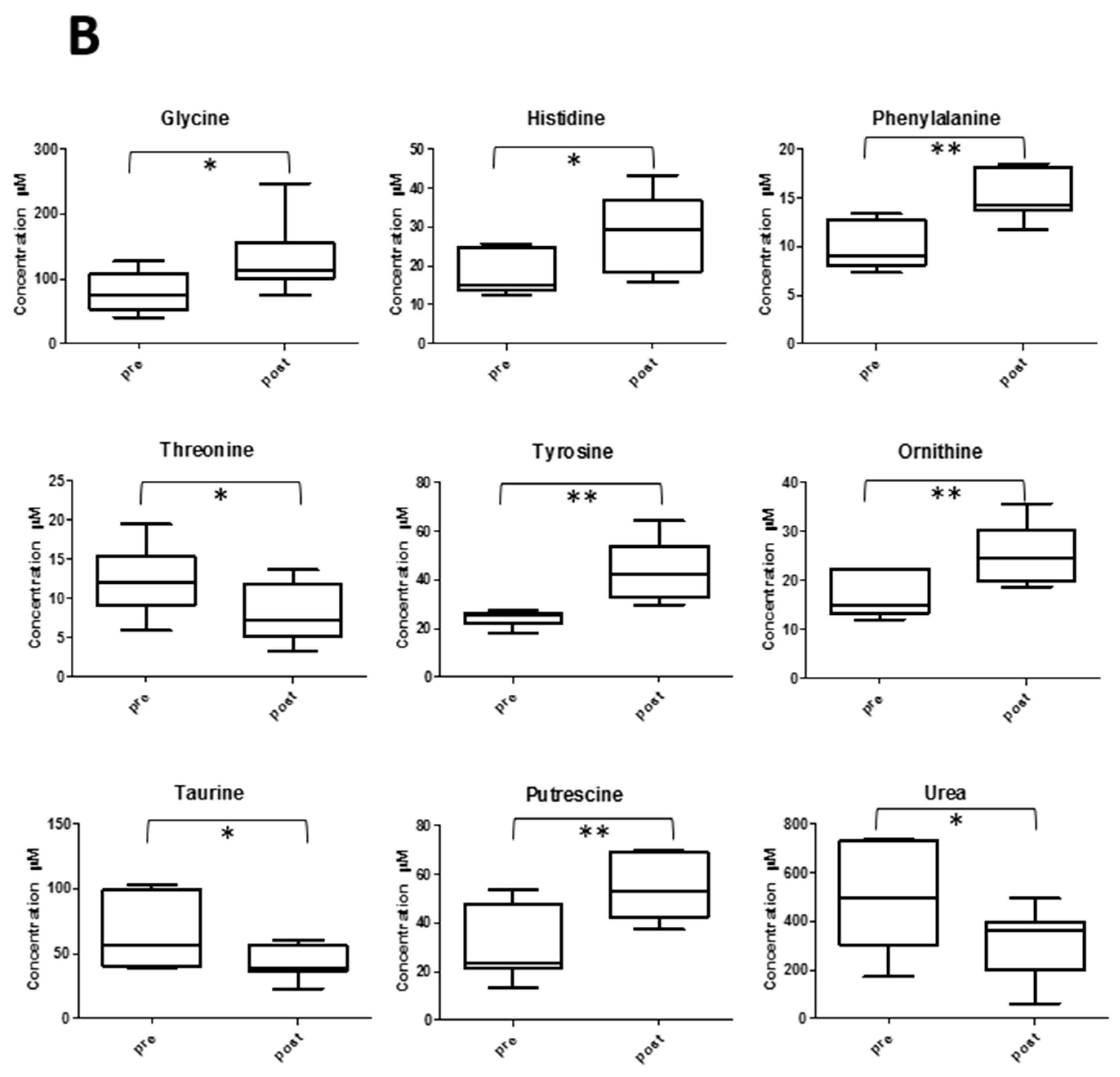
2.5. Amino Acid Profile and Exercise
2.6. Other Metabolite Concentrations Changing after the Soccer Game
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Saliva Sampling
4.3. Total Protein Quantification
4.4. Saliva Protocol Preparation by NMR Spectroscopy
4.5. NMR Spectroscopy Acquisition
4.6. Identification and Quantification of Metabolite
4.7. Multivariate Data Analysis
4.8. Univariate Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Peñailillo, L.; Maya, L.; Niño, G.; Torres, H.; Zbinden-Foncea, H. Salivary hormones and IgA in relation to physical performance in football. J. Sports Sci. 2015, 33, 2080–2087. [Google Scholar] [CrossRef]
- Greig, M.P.; Mc Naughton, L.R.; Lovel, R.J. Physiological and mechanical response to soccer specific intermittent activity and steady-state activity. Res. Sports Med. 2006, 14, 29–52. [Google Scholar] [CrossRef]
- Mallo, J.; Navarro, E. Physical load imposed on soccer players during small-sided training games. J. Sports Med. Phys. Fit. 2008, 48, 166–171. [Google Scholar]
- Moreira, A.; Mortatti, A.; Aoki, M.; Arruda, A.; Freitas, C.; Carling, C. Role of free testosterone in interpreting physical performance in elit young Brazilian soccer players. Pediatr. Exerc. Sci. 2013, 28, 467–473. [Google Scholar]
- Francavilla, V.C.; Vitale, F.; Ciaccio, M.; Bongiovanni, T.; Marotta, C.; Caldarella, R.; Todaro, L.; Zarcone, M.; Muratore, R.; Bellia, C.; et al. Use of saliva in alternative to serum sampling to monitor biomarkers modifications in professional soccer players. Front. Physiol. 2018, 9, 1828. [Google Scholar] [CrossRef]
- Haneishi, K.; Fry, A.C.; Moore, C.A.; Schilling, B.K.; Li, Y.; Fry, M.D. Cortisol and stress responses during a game and practice in female collegiate soccer players. J. Strength Cond. Res. 2007, 21, 583–588. [Google Scholar]
- Maya, J.; Marquez, P.; Peñailillo, L.; Contreras-Ferrat, A.; Deldicque, L.; Zbinden-Foncea, H. Salivary biomarker responses to two final matches in women’s professional football. J. Sports Sci. Med. 2016, 15, 365–371. [Google Scholar]
- Gatti, R.; De Palo, E.F. An update: Salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 2011, 21, 157–169. [Google Scholar] [CrossRef]
- Yi, T.C.; Moochhala, S. Mini-review article—Current opinion on salivary biomarkers as a measurement for stress and fatigue. Open Biomark. J. 2013, 6, 9–14. [Google Scholar] [CrossRef]
- Ligtenberg, A.J.; Brand, H.S.; van den Keijbus, P.A.; Veerman, E.C. The effect of physical exercise on salivary secretion of MUC5B, amylase and lysozyme. Arch. Oral Biol. 2015, 60, 1639–1644. [Google Scholar] [CrossRef]
- Tékus, E.; Kaj, M.; Szabó, E.; Szénási, N.L.; Kerepesi, K.; Figler, M.; Gábriel, R.; Wilhelm, M. Comparison of blood and saliva lactate level after maximum intensity exercise. Acta Biol. Hung. 2012, 63 (Suppl. S1), 89–98. [Google Scholar] [CrossRef]
- Patterson, D.J. Antiarrhythmic mechanisms during exercise. J. Appl. Physiol. 1996, 80, 1853–1862. [Google Scholar] [CrossRef]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Refsum, H.E.; Gjessing, L.R.; Stromme, S.G. Changes in plasma amino acid distribution and urine amino acids excretion during prolonged heavy exercise. Scand. J. Clin. Lab. Investig. 1979, 39, 407–413. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Peg, A.E. Mammalian polyamine metabolism and function. Iubmb Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Cadore, E.; Lhullier, F.; Brentano, M.; Silva, E.; Ambrosini, M.; Spinelli, R.; Silva, R.; Kruel, L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports Sci. 2008, 26, 1067–1072. [Google Scholar] [CrossRef]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 14, 42–434. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C.; Pickler, R.; Grap, M.J.; Elswick, R.K., Jr. Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Pract. 2012, 2012, 246178. [Google Scholar] [CrossRef]
- Tomasi, T.B.; Trudeau, F.B.; Czerwinski, D.; Erredge, S. Immune parameters in athletes before and after strenuous exercise. J. Clin. Immunol. 1982, 2, 173–178. [Google Scholar] [CrossRef]
- Walsh, N.P.; Blannin, A.K.; Clark, A.M.; Cook, L.; Robson, P.J.; Gleeson, M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999, 17, 129–134. [Google Scholar] [CrossRef]
- Rantonen, P.J.; Meurman, J.H. Correlations between total protein, lysozyme, immunoglobulins, amylase, and albumin in stimulated whole saliva during daytime. Acta Odontol. Scand. 2000, 58, 160–165. [Google Scholar] [CrossRef]
- Walsh, N.P.; Laing, S.J.; Oliver, S.J.; Montague, J.C.; Walters, R.; Bilzon, J.L. Saliva parameters as potential indices of hydration status during acute dehydration. Med. Sci. Sports Exerc. 2004, 36, 1535–1542. [Google Scholar] [CrossRef]
- Lloyd, J.E.; Broughton, A.; Selby, C. Salivary creatinine assays as a potential screen for renal disease. Ann. Clin. Biochem. 1996, 33, 428–431. [Google Scholar] [CrossRef]
- Xing, X.; Shi, X.; Zhang, M.; Jin, W.; Ye, J. CE determination of creatinine and uric acid in saliva and urine during exercise. Chromatographia 2008, 67, 985–988. [Google Scholar] [CrossRef]
- Huang, N.J.; Ying, F.L.; Wang, G. Research on tests of the content of urea nitrogen, creatinine and uric acid in saliva and serum of male students at college before and after exercises. J. Beijing Sport Univ. 2006, 29, 939–941. [Google Scholar]
- Hamilton, R.W.; Gardner, L.B.; Penn, A.S.; Goldberg, M. Acute tubular necrosis caused by exercise-induced myoglobinuria. Ann. Intern Med. 1972, 77, 77–82. [Google Scholar] [CrossRef]
- McLellan, T.M.; Cheung, K.S.; Jacobs, I. Incremental test protocol, recovery mode and the individual anaerobic threshold. Int. J. Sports Med. 1991, 12, 190–195. [Google Scholar] [CrossRef]
- Urhausen, A.; Coen, B.; Weiler, B.; Kindermann, W. Individual anaerobic threshold and maximum lactate steady state. Int. J. Sports Med. 1993, 14, 134–139. [Google Scholar] [CrossRef]
- Cicero, D.O.; Di Marino, S.; Dinallo, M.; Pieri, M.; Summa, V.; Desideri, A.; Bernardini, F.; Perondi, S.; D’Ottavio, S. A small sided game session affects salivary metabolite levels in young soccer players. BSI 2016, 5, 55–70. [Google Scholar] [CrossRef]
- Pérez, M.; Lucia, A.; Carvajal, A.; Pardo, J.; Chicharro, J.L. Determination of the maximum steady state of lactate (MLSS) in saliva: An alternative to blood lactate determination. Jpn. J. Physiol. 1999, 49, 395–400. [Google Scholar] [CrossRef][Green Version]
- Deminice, R.; Sicchier, T.; Payão, P.O.; Jordão, A.A. Blood and salivary oxidative stress biomarkers following an acute session of resistance exercise in humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The effects of exercise under hypoxia on cognitive function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Márquez, G.; Martínez Díaz, N.; Cugat, R.; Cerón, J.J.; Jiménez-Reyes, P. Changes in lactate, ferritin, and uric acid in saliva after repeated explosive effort sequences. J. Sports Med. Phys. Fit. 2018, 18. [Google Scholar] [CrossRef]
- Leijssen, E.P.; Saris, W.H.; Jeukendrup, A.E.; Wagenmakers, A.J. Oxidation of exogenous (13C) galactose and (13C) glucose during exercise. J. Appl. Physiol. 1995, 79, 720–725. [Google Scholar] [CrossRef]
- Décombaz, J.; Jentjens, R.; Ith, M.; Scheurer, E.; Buehler, T.; Jeukendrup, A.; Boesch, C. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med. Sci. Sports Exerc. 2011, 43, 1964–1971. [Google Scholar]
- Nakamura, Y.; Kodama, H.; Satoh, T.; Adachi, K.; Watanabe, S.; Yokote, Y.; Sakagami, H. Diurnal changes in salivary amino acid concentrations. In Vivo 2010, 24, 837–842. [Google Scholar]
- Yatabe, Y.; Miyakawa, S.; Miyazaki, T.; Matsuzaki, Y.; Ochiai, N. Effects of taurine administration in rat skeletal muscles on exercise. J. Orthop. Sci. 2003, 8, 415–419. [Google Scholar] [CrossRef]
- Monleon, D.; Garcia-Valles, R.; Morales, J.M.; Brioche, T.; Olaso-Gonzalez, G.; Lopez-Grueso, R.; Gomez-Cabrera, M.C.; Viña, J. Metabolomic analysis of long-term spontaneous exercise in mice suggests increased lipolysis and altered glucose metabolism when animals are at rest. J. Appl. Physiol. 2014, 117, 1110–1119. [Google Scholar] [CrossRef]
- Turchanowa, L.; Rogozkin, V.A.; Milovic, V.; Feldkoren, B.I.; Caspary, W.F.; Stein, J. Influence of physical exercise on polyamine synthesis in the rat skeletal muscle. Eur. J. Clin. Investig. 2000, 30, 72–78. [Google Scholar] [CrossRef]
- Stathis, C.G.; Carey, M.F.; Hayes, A.; Garnham, A.P.; Snow, R.J. Sprint training reduces urinary purine loss following intense exercise in humans. Appl. Physiol. Nutr. Metab. 2006, 31, 702–708. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
| Outlines | Game Group | Enter Group | Exit Group | No Game Group |
|---|---|---|---|---|
| Players | 8/17 | 3/17 | 3/17 | 3/17 |
| Age (years) | 23 ± 5 | 18 ± 1 | 23 ± 8 | 23 ± 6 |
| Weight (kg) | 61 ± 9 | 55 ± 1 | 59 ± 5 | 67 ± 6 |
| BMI (kg/m2) | 24 ± 2 | 22 ± 2 | 23 ± 3 | 24 ± 3 |
| Metabolite ID | Pre-Samples | Metabolite ID | Pre-Samples |
|---|---|---|---|
| 5-Aminopentanoate | 99 ± 58 | Isocaproate | 4 ± 3 |
| Acetate | 1784 ± 929 | Isoleucine | 3 ± 2 |
| Acetoacetate | 3 ± 3 | Isopropanol | 2 ± 1 |
| Acetoin | 10 ± 7 | Isovalerate | 6 ± 4 |
| Acetone | 9 ± 3 | Lactate | 158 ± 152 |
| Alanine | 25 ± 19 | Leucine | 7 ± 4 |
| Arginine | 13 ± 10 | Methanol | 29 ± 29 |
| Aspartate | 18 ± 9 | O-Phosphocholine | 4 ± 1 |
| Betaine | 2 ± 1 | O-Phosphoethanolamine | 39 ± 21 |
| Butyrate | 42 ± 27 | Ornithine | 15 ± 8 |
| Choline | 5 ± 3 | Phenylalanine | 9 ± 5 |
| Citrate | 18 ± 9 | Proline | 39 ± 29 |
| Creatine | 10 ± 5 | Propionate | 360 ± 229 |
| Creatinine | 3 ± 1 | Propylene glycol | 12 ± 9 |
| Dimethylamine | 2 ± 1 | Putrescine | 25 ± 16 |
| Dimethyl sulfone | 3 ± 2 | Pyroglutamate | 8 ± 10 |
| Ethanol | 135 ± 242 | Pyruvate | 37 ± 42 |
| Ethanolamine | 34 ± 20 | Sarcosine | 2 ± 1 |
| Formate | 188 ± 318 | Serine | 32 ± 18 |
| Fumarate | 0.4 ± 0.2 | Succinate | 23 ± 12 |
| Galactose | 23 ± 24 | Sucrose | 11 ± 13 |
| Glucose | 60 ± 45 | Taurine | 53 ± 24 |
| Glutamate | 58 ± 37 | Threonine | 9 ± 6 |
| Glutamine | 18 ± 14 | Trimethylamine | 1.3 ± 0.9 |
| Glycerol | 267 ± 145 | Tyrosine | 20 ± 9 |
| Glycine | 68 ± 43 | Urea | 561 ± 226 |
| Histidine | 16 ± 9 | Urocanate | 3 ± 2 |
| Hypoxanthine | 4 ± 3 | Valine | 6 ± 4 |
| Dehydration Index | Serum | Saliva | |
|---|---|---|---|
| Literature a | Literature b | This Work c | |
| UN [mg/dL] | 7-20 | 0.22 ± 0.18 | 0.20 ± 0.08 |
| Creatinine [mg/dL] | 0.7-1.2 | 0.06 ± 0.04 | 0.04 ± 0.01 |
| UN/creatinine | 10-20 | 4 ± 2 | 6 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitti, E.; Petrella, G.; Di Marino, S.; Summa, V.; Perrone, M.; D’Ottavio, S.; Bernardini, A.; Cicero, D.O. Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes. Metabolites 2019, 9, 141. https://doi.org/10.3390/metabo9070141
Pitti E, Petrella G, Di Marino S, Summa V, Perrone M, D’Ottavio S, Bernardini A, Cicero DO. Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes. Metabolites. 2019; 9(7):141. https://doi.org/10.3390/metabo9070141
Chicago/Turabian StylePitti, Erica, Greta Petrella, Sara Di Marino, Vincenzo Summa, Marco Perrone, Stefano D’Ottavio, Andrea Bernardini, and Daniel Oscar Cicero. 2019. "Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes" Metabolites 9, no. 7: 141. https://doi.org/10.3390/metabo9070141
APA StylePitti, E., Petrella, G., Di Marino, S., Summa, V., Perrone, M., D’Ottavio, S., Bernardini, A., & Cicero, D. O. (2019). Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes. Metabolites, 9(7), 141. https://doi.org/10.3390/metabo9070141







