Chemical Aspects of Gut Metabolism of Flavonoids
Abstract
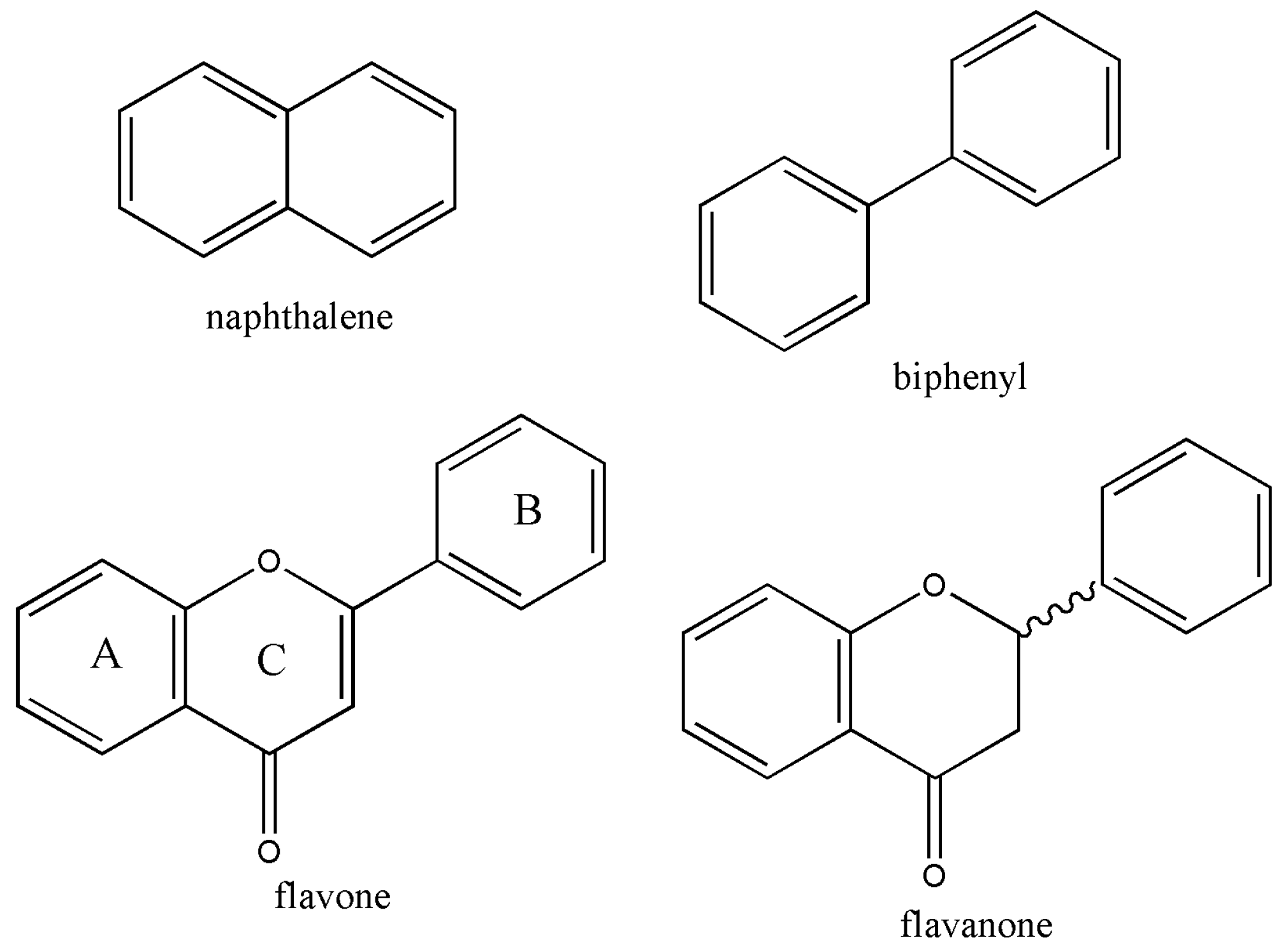
1. Introduction
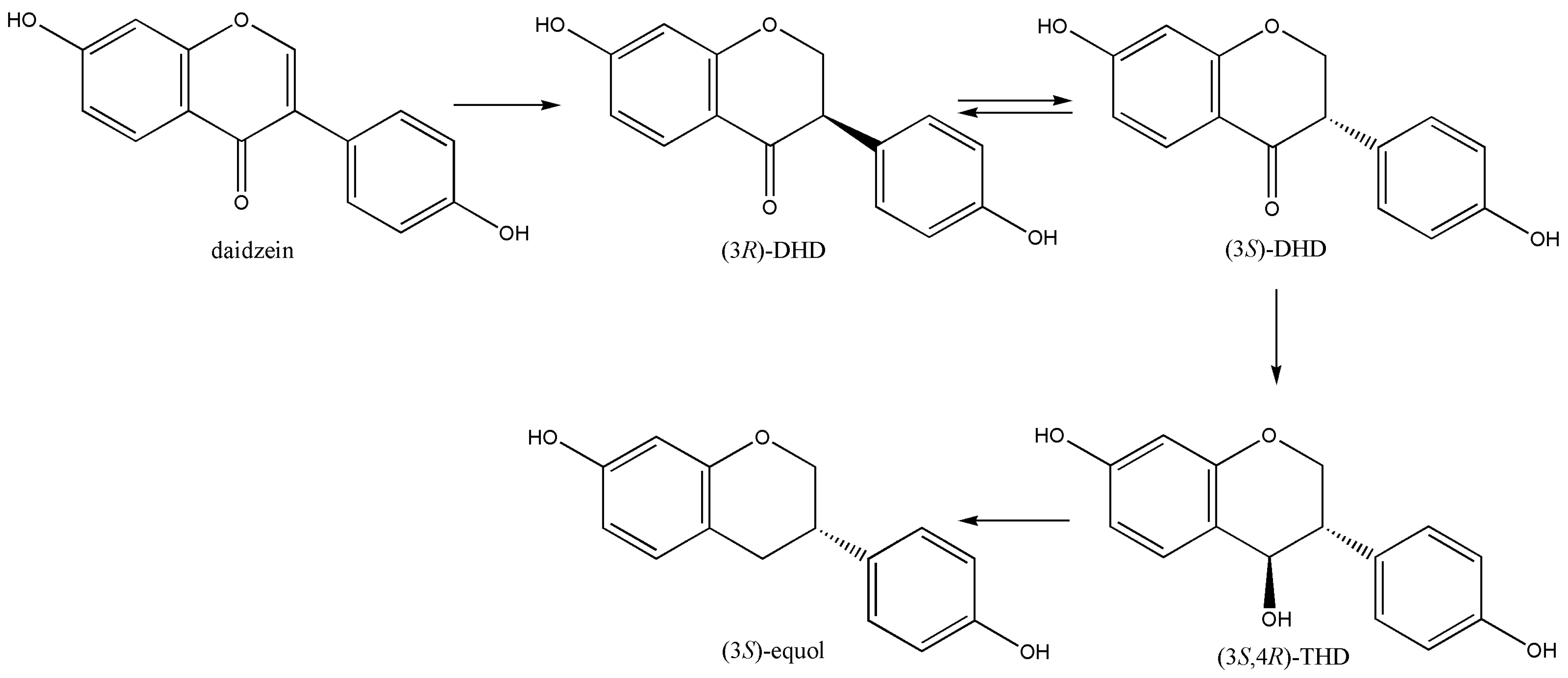
2. S-Equol Biosynthesis
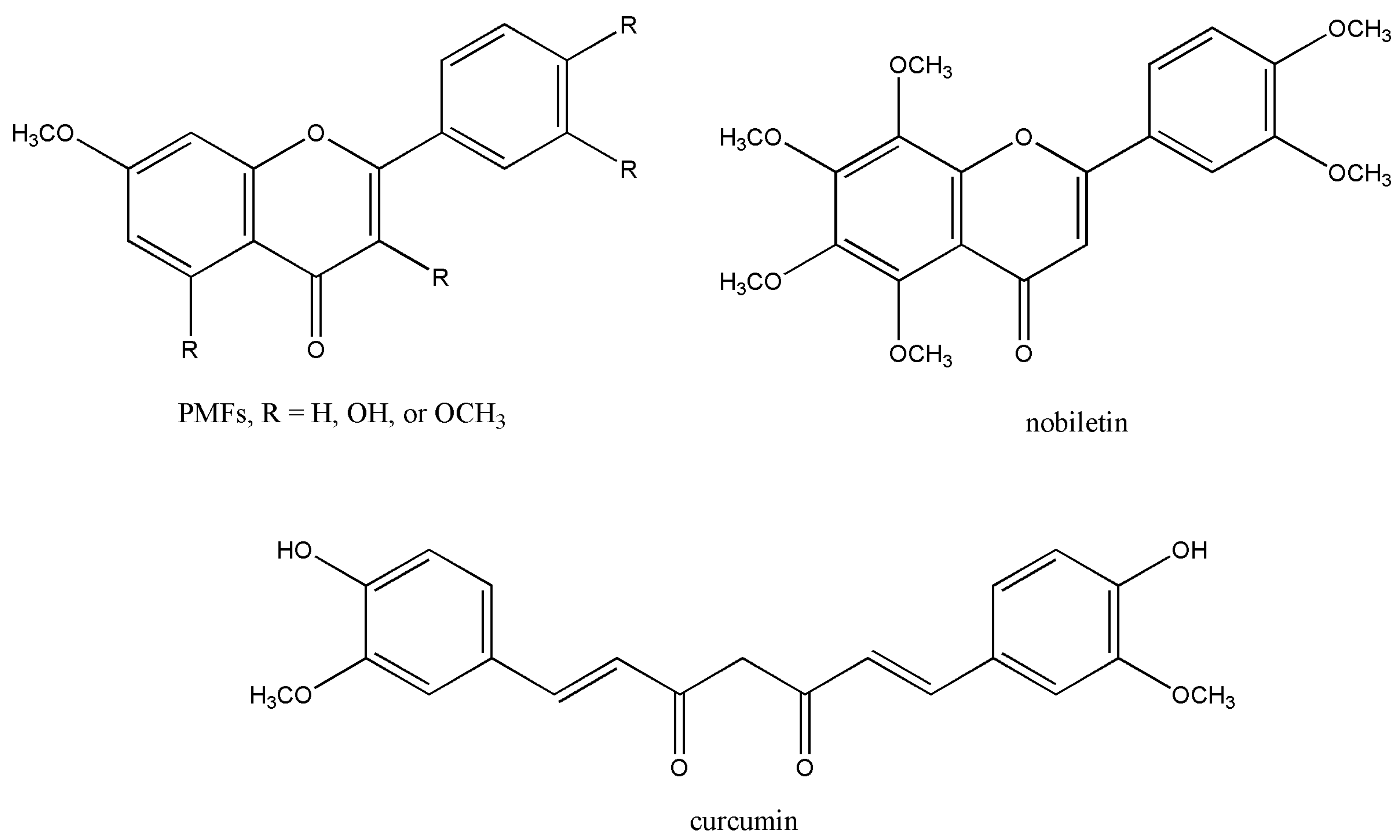
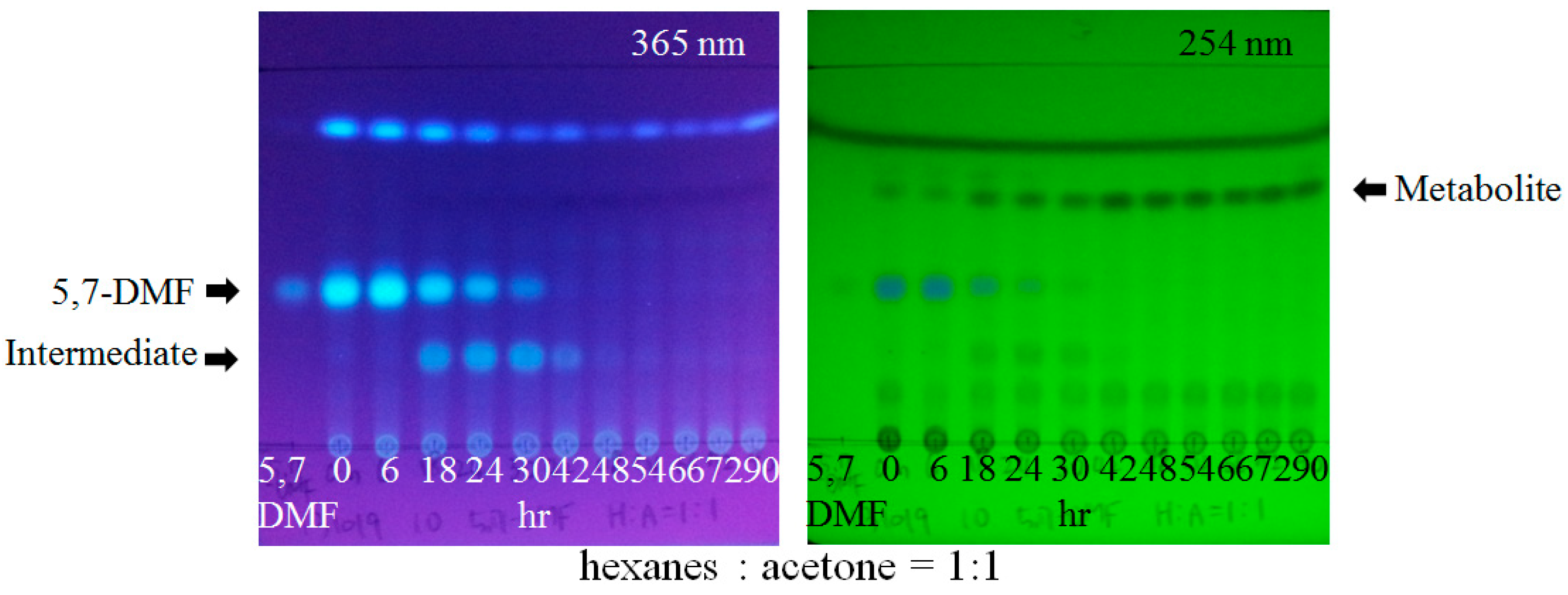
3. Aryl Methyl Ether Cleavage
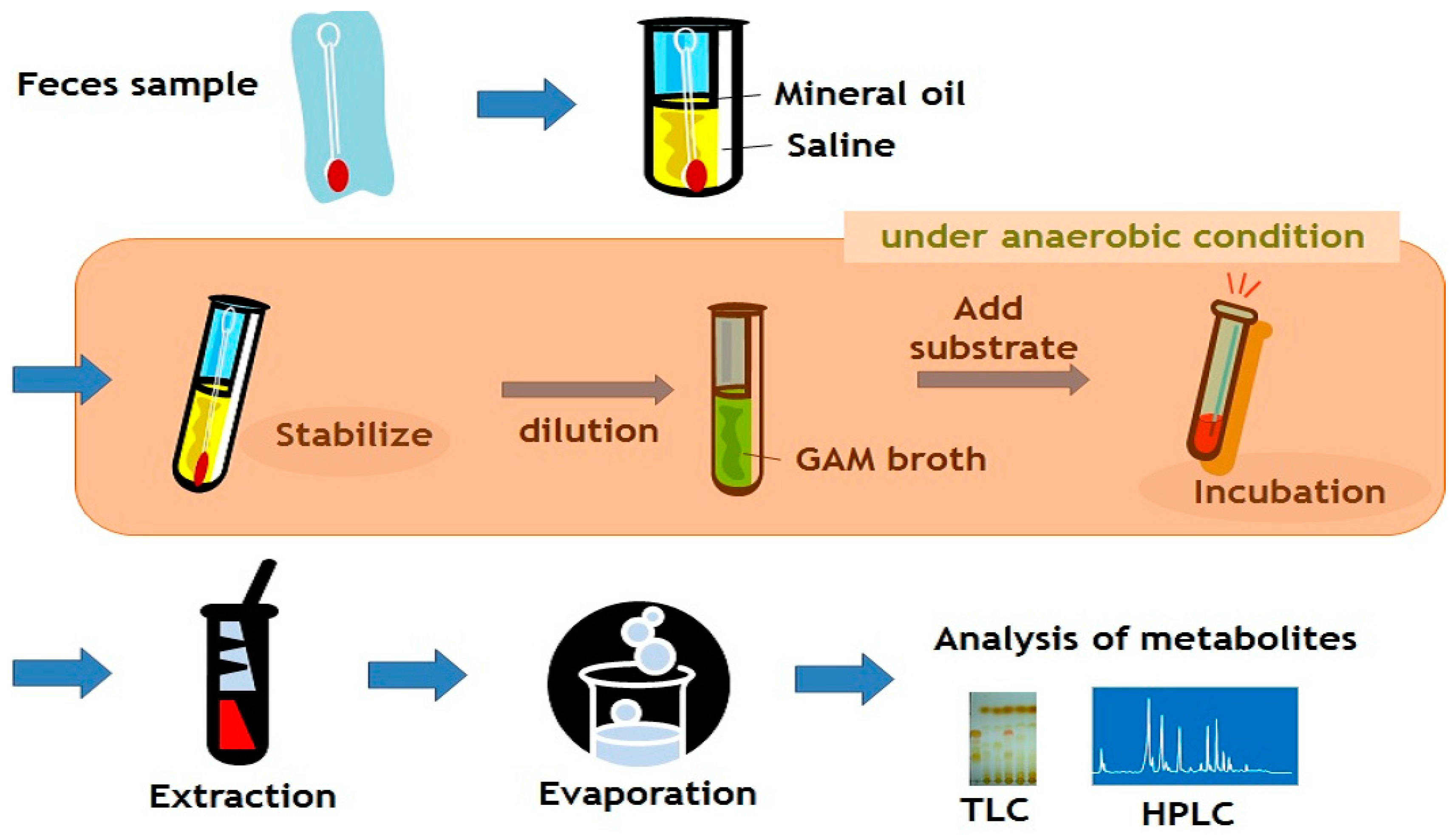
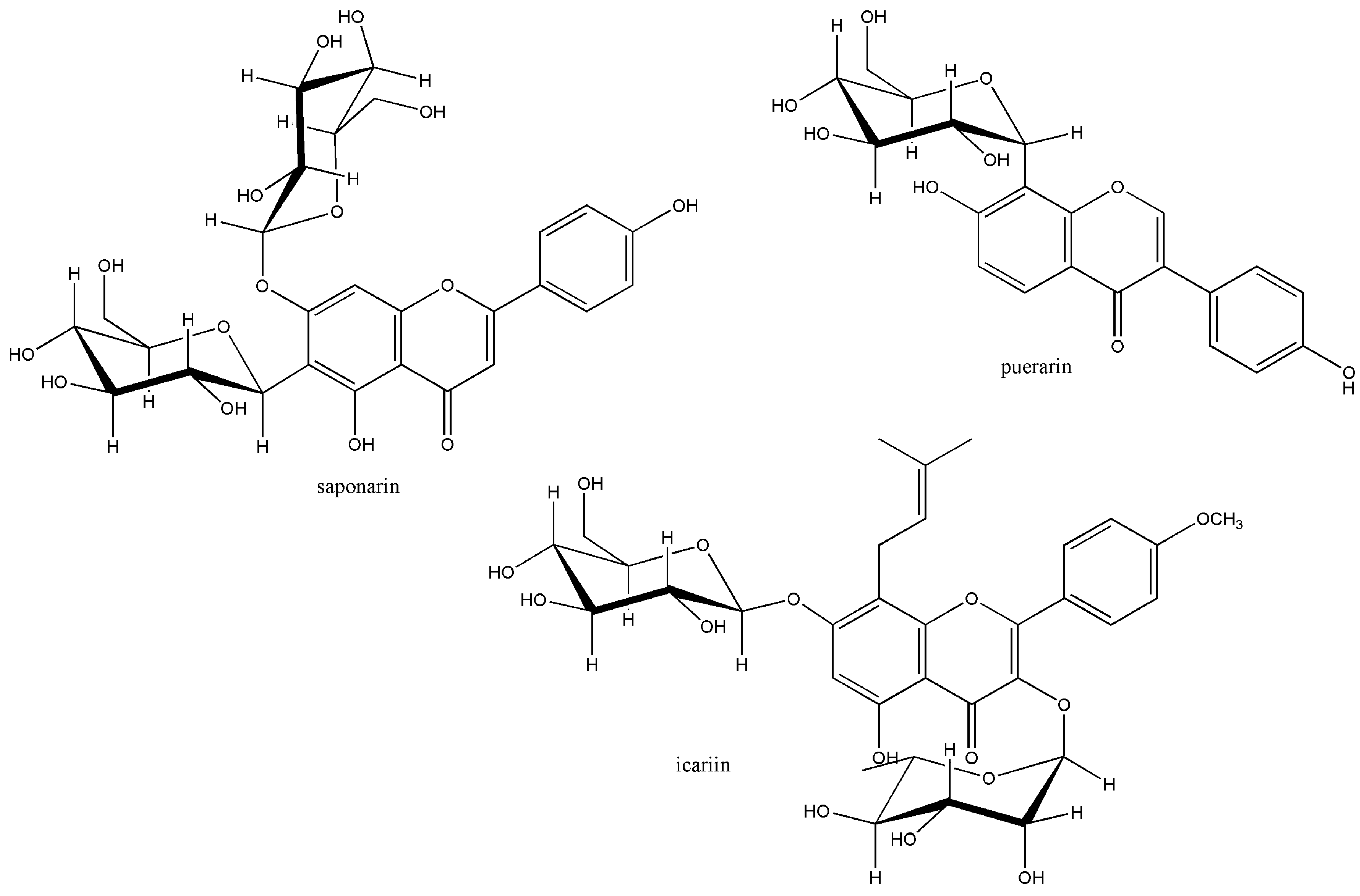
4. Gut Metabolism to be Explored
5. Conclusions
Funding
Conflicts of Interest
References
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P.; Sadowsky, M.J.; Newman, L.M.; Hur, H.G.; Li, S. Metabolism of polyhalogenated compounds by a genetically engineered bacterium. Nature 1994, 368, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-Containing Oxygenases. Chem. Rev. 1996, 96, 2841–2888. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, S.Y.; Jung, J.; Lim, Y.; Ahn, J.H.; Hur, H.G. Epoxide formation on the aromatic B ring of flavanone by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes KF707. Appl. Environ. Microbiol. 2005, 71, 5354–5361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.L.; Hur, H.G.; Lee, J.H.; Kim, K.T.; Kim, S.I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.I.; Han, J.; Wang, X.L.; Song, D.G.; Kim, S.U. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, J.; Kim, S.U. Isoflavone daidzein: Chemistry and bacterial metabolism. J. Appl. Biol. Chem. 2008, 51, 253–261. [Google Scholar] [CrossRef]
- Kim, M.; Han, J. Chiroptical Study and Absolute Configuration of (−)–O—DMA Produced from Daidzein Metabolism. Chirality 2014, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, J. Absolute Configuration of (−)-2-(4-Hydroxyphenyl) propionic acid: Stereochemistry of Soy Isoflavone Metabolism. Bull. Korean Chem. Soc. 2014, 35, 1883–1886. [Google Scholar] [CrossRef]
- Kim, M.; Won, D.; Han, J. Absolute Configuration Determination of Isoflavan-4-ol Stereoisomers. Bioorg. Med. Chem. Lett. 2010, 20, 4337–4341. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Marsh, E.N.G.; Kim, S.U.; Han, J. Conversion of (3S,4R)-Tetrahydrodaidzein to (3S)-Equol by THD Reductase: Proposed Mechanism Involving a Radical Intermediate. Biochemistry 2010, 49, 5582–5587. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Matthies, W.E.; Blaut, M.; Barune, A. Identification and Expression of Genes Involved in the Conversion of Daidzein and Genistein by the Equol-Forming Bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 2013, 79, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Wongsrikaew, N.; Woo, H.C.; Vichitphan, K.; Han, J. Supercritical CO2 for Efficient Extraction of Polymethoxyflavones in Kaempferia parviflora. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 1008–1011. [Google Scholar] [CrossRef]
- Kim, M.; Kim, N.; Han, J. Metabolism of Kaempferia parviflora Polymethoxyflavones by Human Intestinal Bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem. 2014, 62, 12377–12383. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Han, J. Demethylation of Polymethoxyflavones by Human Gut Bacterium, Blautia sp. MRG-PMF1. J. Agric. Food Chem. 2017, 65, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.; Han, J. Deglycosylation of Isoflavone C—Glycosides by Newly Isolated Human Intestinal Bacteria. J. Sci. Food Agric. 2015, 95, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef]
- Guo, J.; Nikolic, D.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Identification of Human Hepatic Cytochrome P450 Enzymes Involved in the Metabolism of 8-Prenylnaringenin and Isoxanthohumol from Hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J. Chemical Aspects of Gut Metabolism of Flavonoids. Metabolites 2019, 9, 136. https://doi.org/10.3390/metabo9070136
Han J. Chemical Aspects of Gut Metabolism of Flavonoids. Metabolites. 2019; 9(7):136. https://doi.org/10.3390/metabo9070136
Chicago/Turabian StyleHan, Jaehong. 2019. "Chemical Aspects of Gut Metabolism of Flavonoids" Metabolites 9, no. 7: 136. https://doi.org/10.3390/metabo9070136
APA StyleHan, J. (2019). Chemical Aspects of Gut Metabolism of Flavonoids. Metabolites, 9(7), 136. https://doi.org/10.3390/metabo9070136




