Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients
Abstract
1. Introduction
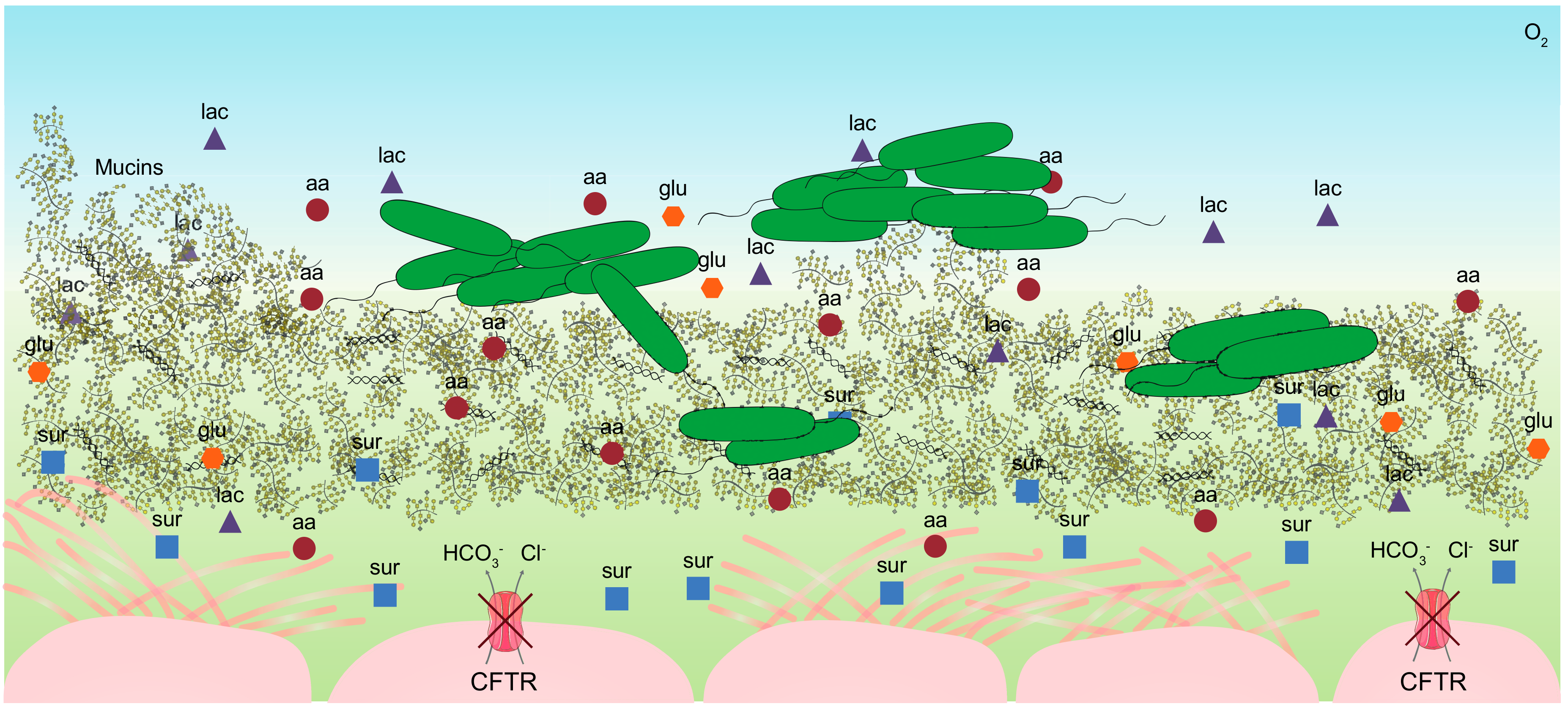
2. The Cystic Fibrosis Mucus
2.1. Individual Complexity of the CF Mucus
2.2. Composition of the CF Sputum
2.3. Mimicking the Nutritional Lung-Like Conditions
3. Diversity of Metabolic Potential over Infection Time
3.1. Heterogeneity of Metabolic Profiles
3.2. Amino Acids Auxotrophies
3.3. Metabolic Rewiring of Clinical Strains
3.4. Mutations on Non-Metabolic Genes Causing Metabolic Changes
4. Pseudomonas aeruginosa In-Situ Metabolic Program
4.1. In Vitro vs. in Situ Expression Profiles
4.2. Metabolic Configuration in the CF Airways
5. Modelling the CF Dynamics in Silico
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.L.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Magrini, N.; Carmeli, Y.; Harbarth, S.; Kahlmeter, G.; Kluytmans, J.; Mendelson, M.; Pulcini, C.; Singh, N.; Theuretzbacher, U. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics; World Health Organization (WHO): Geneva, Switzerland, 2017; Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 1 September 2019).
- Ehre, C.; Ridley, C.; Thornton, D.J. Cystic fibrosis: An inherited disease affecting mucin-producing organs. Int. J. Biochem. Cell Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Mashburn, L.M.; Singh, P.K.; Whiteley, M. Cystic Fibrosis Sputum Supports Growth and Cues Key Aspects of Pseudomonas aeruginosa Physiology. J. Bacteriol. 2005, 187, 5267–5277. [Google Scholar] [CrossRef]
- Frimmersdorf, E.; Horatzek, S.; Pelnikevich, A.; Wiehlmann, L.; Schomburg, D. How Pseudomonas aeruginosa adapts to various environments: A metabolomic approach. Environ. Microbiol. 2010, 12, 1734–1747. [Google Scholar] [CrossRef]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. Nat. Publ. Group 2012, 10, 841–851. [Google Scholar] [CrossRef]
- La Rosa, R.; Johansen, H.K.; Molin, S. Convergent Metabolic Specialization through Distinct Evolutionary Paths in Pseudomonas aeruginosa. MBio Am. Soc. Microbiol. 2018, 9, e00269–18. [Google Scholar] [CrossRef]
- Muhlebach, M.S.; Sha, W. Lessons learned from metabolomics in cystic fibrosis. Mol. Cell Pediatr. 2015, 2, 9. [Google Scholar] [CrossRef]
- Palmer, K.L.; Aye, L.M.; Whiteley, M. Nutritional Cues Control Pseudomonas aeruginosa Multicellular Behavior in Cystic Fibrosis Sputum. J. Bacteriol. 2007, 189, 8079–8087. [Google Scholar] [CrossRef]
- Quinn, R.A.; Lim, Y.W.; Mak, T.D.; Whiteson, K.; Furlan, M.; Conrad, D.; Rohwer, F.; Dorrestein, P. Metabolomics of pulmonary exacerbations reveals the personalized nature of cystic fibrosis disease. PeerJ 2016, 4, e2174. [Google Scholar] [CrossRef]
- Garg, N.; Wang, M.; Hyde, E.; da Silva, R.R.; Melnik, A.V.; Protsyuk, I.; Bouslimani, A.; Lim, Y.W.; Wong, R.; Humphrey, G.; et al. Three-Dimensional Microbiome and Metabolome Cartography of a Diseased Human Lung. Cell Host Microbe 2017, 22, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Bartell, J.A.; Sommer, L.M.; Haagensen, J.A.J.; Loch, A.; Espinosa, R.; Molin, S.; Johansen, H.K. Evolutionary highways to persistent bacterial infection. Nat. Commun. 2019, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F. Carbon catabolite repression in Pseudomonas: Optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 2010, 34, 658–684. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of microbial metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Abdullah, L.H.; Perlmutt, O.S.; Albert, D.; William Davis, C.; Arnold, R.R.; Yankaskas, J.R.; Gilligan, P.; Neubauer, H.; Randell, S.H.; et al. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect. Immun. 2014, 82, 4729–4745. [Google Scholar] [CrossRef]
- Mallia, P.; Webber, J.; Gill, S.K.; Trujillo-Torralbo, M.B.; Calderazzo, M.A.; Finney, L.; Bakhsoliani, E.; Farne, H.; Singanayagam, A.; Footitt, J.; et al. Role of airway glucose in bacterial infections in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 142, 815–823. [Google Scholar] [CrossRef]
- Bensel, T.; Stotz, M.; Borneff-Lipp, M.; Wollschläger, B.; Wienke, A.; Taccetti, G.; Campana, S.; Meyer, K.C.; Jensen, P.O.; Lechner, U.; et al. Lactate in cystic fibrosis sputum. J. Cyst. Fibros. 2011, 10, 37–44. [Google Scholar] [CrossRef]
- Brandt, T.; Breitenstein, S.; Von Der Hardt, H.; Tümmler, B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax 1995, 50, 880–882. [Google Scholar] [CrossRef]
- Kirchner, K.K.; Wagener, J.S.; Khan, T.Z.; Copenhaver, S.C.; Accurso, F.J. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 1426–1429. [Google Scholar] [CrossRef]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef]
- Henderson, A.G.; Ehre, C.; Button, B.; Abdullah, L.H.; Cai, L.H.; Leigh, M.W.; DeMaria, G.C.; Matsui, H.; Donaldson, S.H.; Davis, C.W.; et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Investig. 2014. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Niccum, D.; Dunitz, J.M.; Hunter, R.C. Evidence and Role for Bacterial Mucin Degradation in Cystic Fibrosis Airway Disease. PLOS Pathog. 2016, 12, e1005846. [Google Scholar] [CrossRef] [PubMed]
- Schick, A.; Kassen, R. Rapid diversification of Pseudomonas aeruginosa in cystic fibrosis lung-like conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 10714–10719. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Diseases of Pulmonary Surfactant Homeostasis. Annu. Rev. Pathol. Mech. Dis. 2015. [Google Scholar] [CrossRef]
- Meyer, K.C.; Sharma, A.; Brown, R.; Weatherly, M.; Moya, F.R.; Lewandoski, J.; Zimmerman, J.J. Function and composition of pulmonary surfactant and surfactant- derived fatty profiles are altered in young adults with cystic fibrosis. Chest 2000. [Google Scholar] [CrossRef]
- Griese, M.; Birrer, P.; Demirsoy, A. Pulmonary surfactant in cystic fibrosis. Eur. Respir. J. 1997. [Google Scholar] [CrossRef]
- Hull, J.; South, M.; Phelan, P.; Grimwood, K. Surfactant composition in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1997. [Google Scholar] [CrossRef][Green Version]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef]
- Grasemann, H.; Ioannidis, I.; Tomkiewicz, R.P.; de Groot, H.; Rubin, B.K.; Ratjen, F. Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child. 1998, 78, 49–53. [Google Scholar] [CrossRef]
- Palmer, K.L.; Brown, S.A.; Whiteley, M. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 2007, 189, 4449–4455. [Google Scholar] [CrossRef] [PubMed]
- Schobert, M.; Tielen, P. Contribution of oxygen-limiting conditions to persistent infection of Pseudomonas aeruginosa. Future Microbiol. 2010, 5, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Fothergill, J.L.; Wright, E.A.; James, C.E.; Mowat, E.; Winstanley, C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J. Vis. Exp. 2012, e3857. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.H.; Wessel, A.K.; Palmer, G.C.; Murray, J.L.; Whiteley, M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 2015, 112, 4110–4115. [Google Scholar] [CrossRef]
- Rainey, P.B.; Travisano, M. Adaptive radiation in a heterogeneous environment. Nature 1998, 394, 69–72. [Google Scholar] [CrossRef]
- Behrends, V.; Ryall, B.; Zlosnik, J.E.A.; Speert, D.P.; Bundy, J.G.; Williams, H.D. Metabolic adaptations of Pseudomonas aeruginosa during cystic fibrosis chronic lung infections. Environ. Microbiol. 2013, 15, 398–408. [Google Scholar] [CrossRef]
- Jørgensen, K.M.; Wassermann, T.; Johansen, H.K.; Christiansen, L.E.; Molin, S.; Høiby, N.; Ciofu, O. Diversity of metabolic profiles of cystic fibrosis Pseudomonas aeruginosa during the early stages of lung infection. Microbiology 2015, 161, 1447–1462. [Google Scholar] [CrossRef]
- Marvig, R.L.; Sommer, L.M.; Molin, S.; Johansen, H.K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 2015, 47, 57–64. [Google Scholar] [CrossRef]
- Thomas, S.R. Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 2000, 55, 795–797. [Google Scholar] [CrossRef]
- Barth, A.L.; Pitt, T.L. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J. Med. Microbiol. 1996, 45, 110–119. [Google Scholar] [CrossRef]
- Hoboth, C.; Hoffmann, R.; Eichner, A.; Henke, C.; Schmoldt, S.; Imhof, A.; Heesemann, J.; Hogardt, M. Dynamics of Adaptive Microevolution of Hypermutable Pseudomonas aeruginosa during Chronic Pulmonary Infection in Patients with Cystic Fibrosis. J. Infect. Dis. 2009, 200, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Heizer, E.M.; Raiford, D.W.; Raymer, M.L.; Doom, T.E.; Miller, R.V.; Krane, D.E. Amino acid cost and codon-usage biases in 6 prokaryotic genomes: A whole-genome analysis. Mol. Biol. Evol. 2006, 23, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Waschina, S.; Pande, S.; Bohl, K.; Kaleta, C.; Kost, C. Less is more: Selective advantages can explain the prevalent loss of biosynthetic genes in bacteria. Evolution 2014, 68, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, S.; Marvig, R.L.; Luján, A.M.; Moyano, A.J.; Di Rienzo, J.A.; Krogh Johansen, H.; Molin/, S.; Smania, A.M. Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable Pseudomonas aeruginosa in Long-term Cystic Fibrosis Infections. PLoS Genet. 2014, 10, e1004651. [Google Scholar] [CrossRef]
- Markussen, T.; Marvig, R.L.; Gómez-Lozano, M.; Aanæs, K.; Burleigh, A.E.; Høiby, N.; Johansen, H.K.; Molin, S.; Jelsbak, L. Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa. MBio 2014, 5, e01592–14. [Google Scholar] [CrossRef]
- Behrends, V.; Williams, H.D.; Bundy, J.G. Metabolic Footprinting: Extracellular Metabolomic Analysis. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Springer: New York, NY, USA, 2014; pp. 281–292. [Google Scholar] [CrossRef]
- Mapelli, V.; Olsson, L.; Nielsen, J. Metabolic footprinting in microbiology: Methods and applications in functional genomics and biotechnology. Trends Biotechnol. 2008, 26, 490–497. [Google Scholar] [CrossRef]
- Yang, L.; Jelsbak, L.; Marvig, R.L.; Damkiaer, S.; Workman, C.T.; Rau, M.H.; Hansen, S.K.; Folkesson, A.; Johansen, H.K.; Ciofu, O. Evolutionary dynamics of bacteria in a human host environment. Proc. Natl. Acad Sci. USA 2011, 108, 7481–7486. [Google Scholar] [CrossRef]
- Damkiær, S.; Yang, L.; Molin, S.; Jelsbak, L. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 7766–7771. [Google Scholar] [CrossRef]
- Kostylev, M.; Kim, D.Y.; Smalley, N.E.; Salukhe, I.; Greenberg, E.P.; Dandekar, A.A. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc. Natl. Acad. Sci. USA 2019, 116, 7027–7032. [Google Scholar] [CrossRef]
- Chen, R.; Déziel, E.; Groleau, M.-C.; Schaefer, A.L.; Greenberg, E.P. Social cheating in a Pseudomonas aeruginosa quorum-sensing variant. Proc. Natl. Acad. Sci. USA 2019, 116, 7021–7026. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gerdt, J.P.; McInnis, C.E.; Schell, T.L.; Rossi, F.M.; Blackwell, H.E. Mutational analysis of the quorum-sensing receptor LasR reveals interactions that govern activation and inhibition by nonlactone ligands. Chem. Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; Richardson, A.R.; Houston, L.S.; Kulasekara, H.D.; Martens-Habbena, W.; Klausen, M.; Burns, J.L.; Stahl, D.A.; Hassett, D.J.; Fang, F.C. Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant Pseudomonas aeruginosa within the CF Airway. Monack DM, editor. PLoS Pathog. 2010, 6, e1000712. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, D.A.; Wu, M.; Hoffman, L.R.; Kulasekara, H.D.; Déziel, E.; Smith, E.E.; Nguyen, H.; Ernst, R.K.; Larson Freeman, T.J.; Spencer, D.H. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol. Microbiol. 2007, 64, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.W.; Griffin, J.L.; Welch, M. Quorum Sensing Is Accompanied by Global Metabolic Changes in the Opportunistic Human Pathogen Pseudomonas aeruginosa. O’Toole GA, editor. J. Bacteriol. 2015, 197, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Olivares, J.; Alvarez-Ortega, C.; Alcalde Rico, M.; Martínez, J.L. Metabolic Compensation of Fitness Costs Is a General Outcome for Antibiotic-Resistant Pseudomonas aeruginosa Mutants Overexpressing Efflux Pumps. MBio 2017, 8, e00500–17. [Google Scholar] [CrossRef]
- Olivares, J.; Álvarez-Ortega, C.; Martinez, J.L. Metabolic Compensation of Fitness Costs Associated with Overexpression of the Multidrug Efflux Pump MexEF-OprN in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3904–3913. [Google Scholar] [CrossRef]
- Rossi, E.; Falcone, M.; Molin, S.; Johansen, H.K. High-resolution in situ transcriptomics of Pseudomonas aeruginosa unveils genotype independent patho-phenotypes in cystic fibrosis lungs. Nat. Commun. 2018, 9, 3459. [Google Scholar] [CrossRef]
- Hornischer, K.; Khaledi, A.; Pohl, S.; Schniederjans, M.; Pezoldt, L.; Casilag, F.; Muthukumarasamy, U.; Bruchmann, S.; Thöming, J.; Kordes, A.; et al. BACTOME—A reference database to explore the sequence- and gene expression-variation landscape of Pseudomonas aeruginosa clinical isolates. Nucleic Acids Res. 2019, 47, D716–D720. [Google Scholar] [CrossRef]
- Klockgether, J.; Cramer, N.; Fischer, S.; Wiehlmann, L.; Tümmler, B. Long-term microevolution of pseudomonas aeruginosa differs between mildly and severely affected cystic fibrosis lungs. Am. J. Respir. Cell Mol. Biol. 2018, 59, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; Rozen, D.E.; Lenski, R.E. Parallel changes in gene expression after 20,000 generations of evolution in Escherichia coli. Proc. Natl. Acad. Sci. USA 2003, 100, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Huse, H.K.; Kwon, T.; Zlosnik, J.E.A.; Speert, D.P.; Marcotte, E.M.; Whiteley, M. Parallel Evolution in Pseudomonas aeruginosa over 39,000 Generations In Vivo. MBio 2010, 1, e00199–10. [Google Scholar] [CrossRef] [PubMed]
- Kordes, A.; Preusse, M.; Willger, S.D.; Braubach, P.; Jonigk, D.; Haverich, A.; Warnecke, G.; Häussler, S. Genetically diverse Pseudomonas aeruginosa populations display similar transcriptomic profiles in a cystic fibrosis explanted lung. Nat. Commun. 2019, 10, 3397. [Google Scholar] [CrossRef]
- Wu, X.; Siehnel, R.J.; Garudathri, J.; Staudinger, B.J.; Hisert, K.B.; Ozer, E.A.; Hauser, A.R.; Eng, J.K.; Manoil, C.; Singh, P.K. In vivo proteome of Pseudomonas aeruginosa in airways of cystic fibrosis patients. J. Proteome Res. 2019, 18, 2601–2612. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Dees, J.L.; Ibberson, C.B.; Huse, H.K.; Mathiesen, I.H.; Kirketerp-Møller, K.; Wolcott, R.D.; Rumbaugh, K.P.; Bjarnsholt, T.; Whiteley, M. Pseudomonas aeruginosa transcriptome during human infection. Proc. Natl. Acad. Sci. USA 2018, 115, E5125–E5134. [Google Scholar] [CrossRef]
- Son, M.S.; Matthews, W.J.; Kang, Y.; Nguyen, D.T.; Hoang, T.T. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect. Immun. 2007, 75, 5313–5324. [Google Scholar] [CrossRef]
- Yang, L.; Haagensen, J.A.J.; Jelsbak, L.; Johansen, H.K.; Sternberg, C.; Hoiby, N.; Molin, S. In Situ Growth Rates and Biofilm Development of Pseudomonas aeruginosa Populations in Chronic Lung Infections. J. Bacteriol. 2008, 190, 2767–2776. [Google Scholar] [CrossRef]
- Schutte, K.M.; Fisher, D.J.; Burdick, M.D.; Mehrad, B.; Mathers, A.J.; Mann, B.J.; Nakamoto, R.K.; Hughes, M.A. Escherichia coli pyruvate dehydrogenase complex is an important component of CXCL10-mediated antimicrobial activity. Infect. Immun. 2015, 84, 320–328. [Google Scholar] [CrossRef]
- Lundgren, B.R.; Villegas-Peñaranda, L.R.; Harris, J.R.; Mottern, A.M.; Dunn, D.M.; Boddy, C.N.; Nomura, C.T. Genetic analysis of the assimilation of C5-Dicarboxylic acids in pseudomonas aeruginosa PAO1. J. Bacteriol. 2014, 196, 2543–2551. [Google Scholar] [CrossRef]
- Zheng, L.; Cardaci, S.; Jerby, L.; Mackenzie, E.D.; Sciacovelli, M.; Johnson, T.I.; Gaude, E.; King, A.; Leach, J.D.G.; Edrada-Ebel, R.; et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 2015, 6, 6001. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Lear, G.; Singhal, N. Metabolic network modeling of microbial interactions in natural and engineered environmental systems. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using genome-scale models to predict biological capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Bartell, J.A.; Blazier, A.S.; Yen, P.; Thøgersen, J.C.; Jelsbak, L.; Goldberg, J.B.; Papin, J.A. Reconstruction of the metabolic network of Pseudomonas aeruginosa to interrogate virulence factor synthesis. Nat. Commun. 2017, 8, 14631. [Google Scholar] [CrossRef]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent Advances in Anti-virulence Therapeutic Strategies With a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 1–24. [Google Scholar] [CrossRef]
- Henson, M.A.; Orazi, G.; Phalak, P.; O’Toole, G.A. Metabolic Modeling of Cystic Fibrosis Airway Communities Predicts Mechanisms of Pathogen Dominance. mSystems 2019, 4, 1–20. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Andersen, S.B.; Marvig, R.L.; Molin, S.; Krogh Johansen, H.; Griffin, A.S. Long-term social dynamics drive loss of function in pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 10756–10761. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. Nat. Publ. Group 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Eng, R.H.; Padberg, F.T.; Smith, S.M.; Tan, E.N.; Cherubin, C.E. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 1991, 35, 1824–1828. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci. Signal. 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muir, M.E.; van Heeswyck, R.S.; Wallace, B.J. Effect of growth rate on streptomycin accumulation by Escherichia coli and Bacillus megaterium. J. Gen. Microbiol. 1984, 130, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Oktyabrsky, O.N. Relationship between Escherichia coli growth rate and bacterial susceptibility to ciprofloxacin. FEMS Microbiol. Lett. 2018, 365, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koeva, M.; Gutu, A.D.; Hebert, W.; Wager, J.D.; Yonker, L.M.; O’Toole, G.A.; Ausubel, F.M.; Moskowitz, S.M.; Joseph-McCarthy, D. An Antipersister Strategy for Treatment of Chronic Pseudomonas aeruginosa Infections. Antimicrob. Agents Chemother. 2017, 61, e00987–17. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, R.; Johansen, H.K.; Molin, S. Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients. Metabolites 2019, 9, 234. https://doi.org/10.3390/metabo9100234
La Rosa R, Johansen HK, Molin S. Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients. Metabolites. 2019; 9(10):234. https://doi.org/10.3390/metabo9100234
Chicago/Turabian StyleLa Rosa, Ruggero, Helle Krogh Johansen, and Søren Molin. 2019. "Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients" Metabolites 9, no. 10: 234. https://doi.org/10.3390/metabo9100234
APA StyleLa Rosa, R., Johansen, H. K., & Molin, S. (2019). Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients. Metabolites, 9(10), 234. https://doi.org/10.3390/metabo9100234




