Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism
Abstract
1. Introduction
2. Results
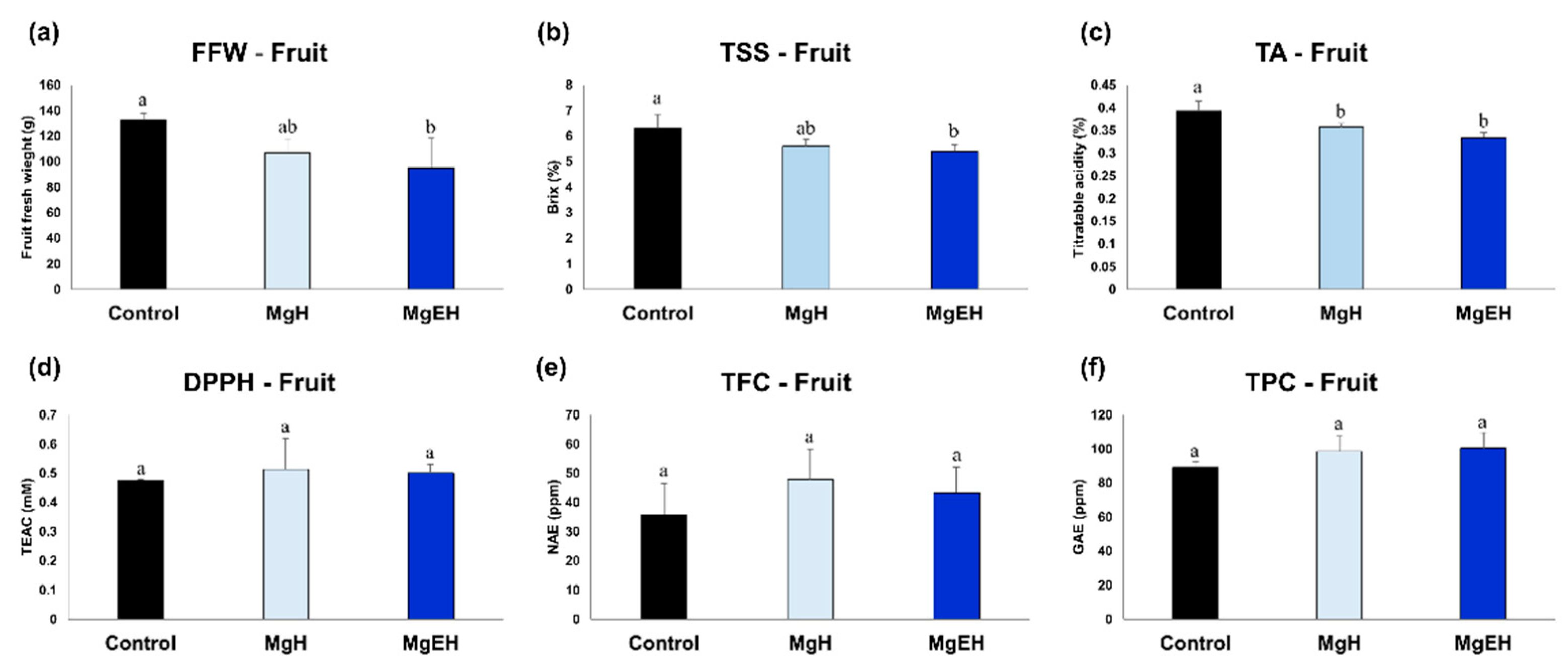
2.1. Fruit Quality Traits and Bio-Activities Induced by Mg Oversupply
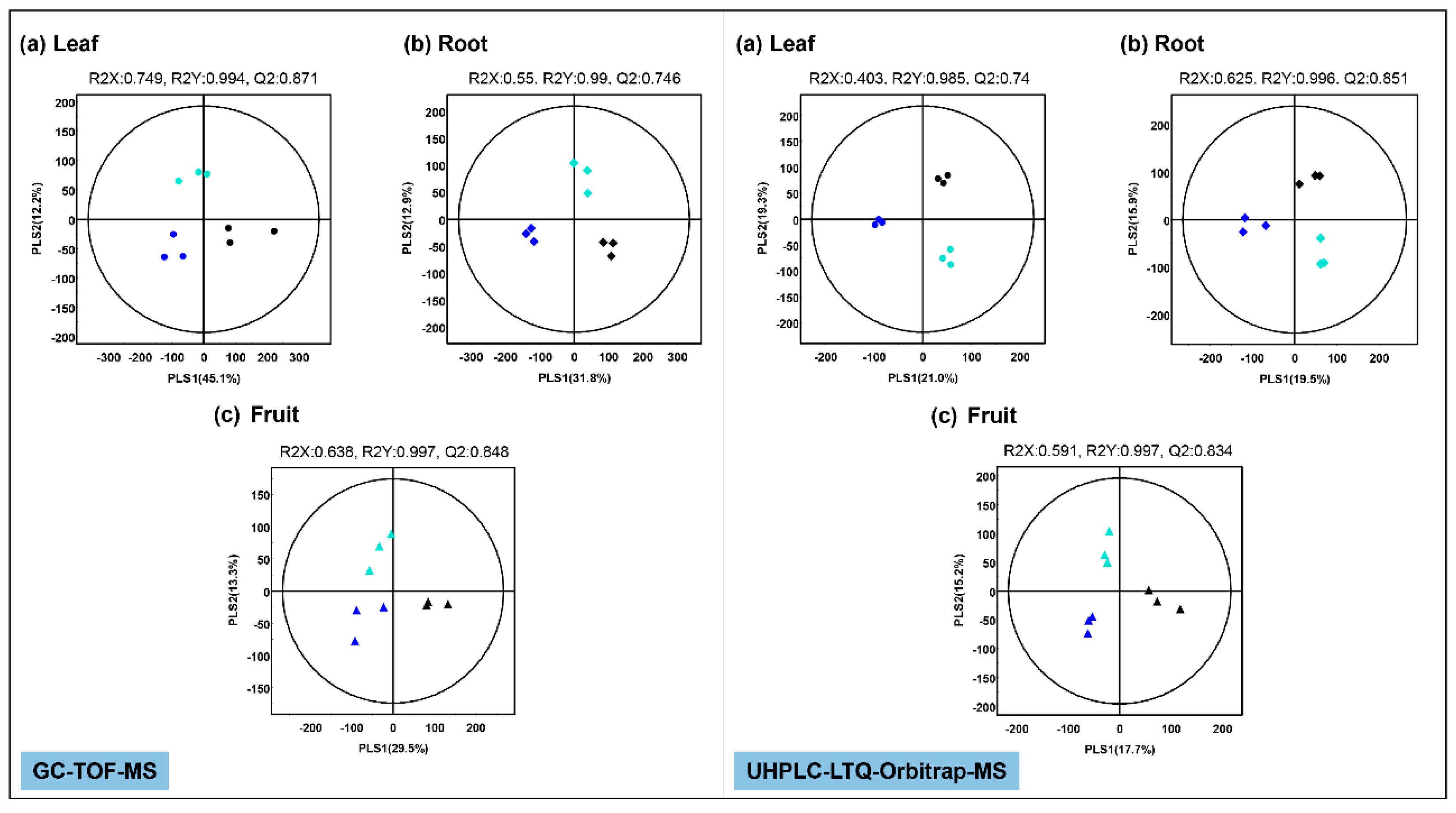
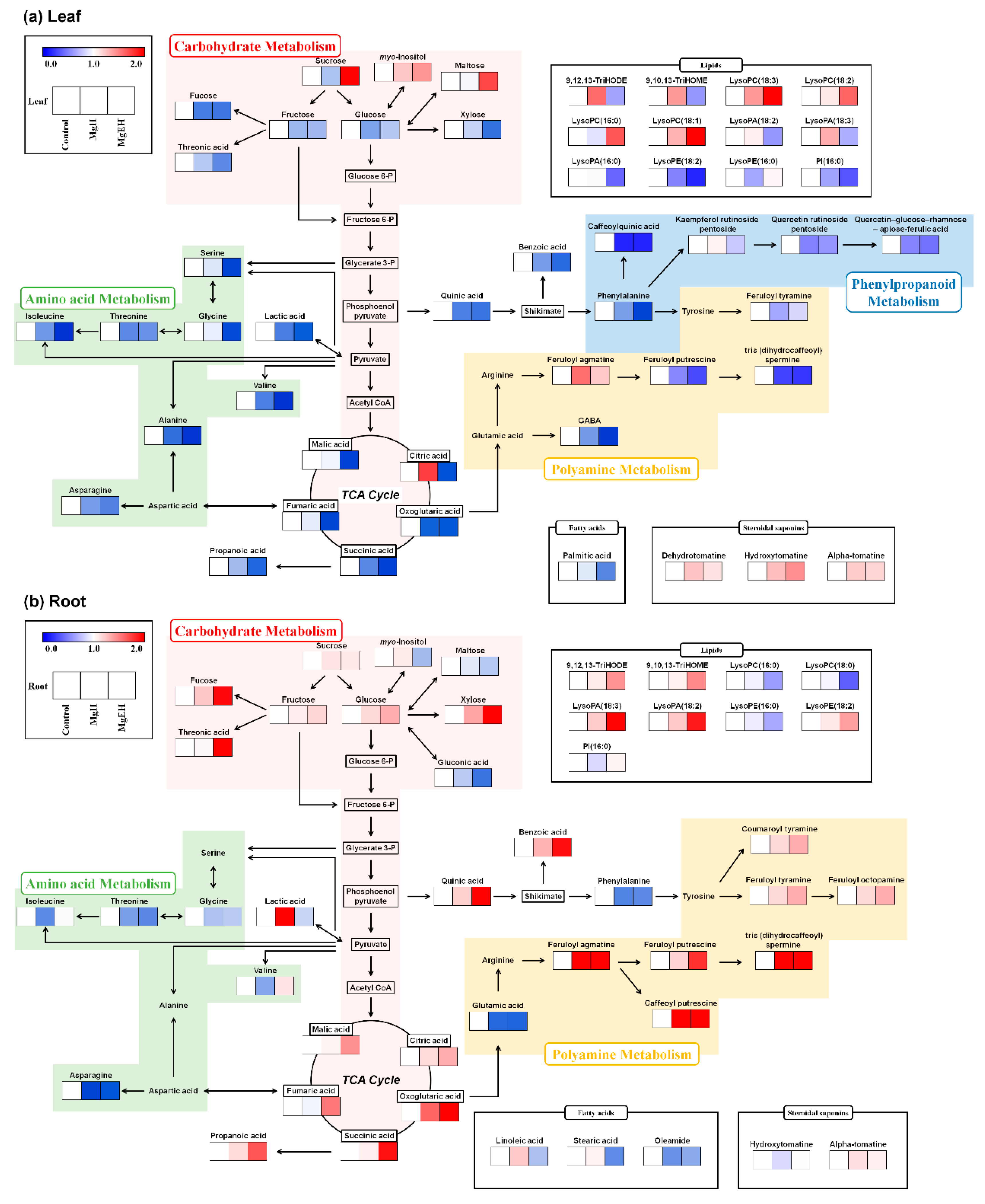
2.2. Effect of Mg Oversupply on Tomato Plant Metabolism
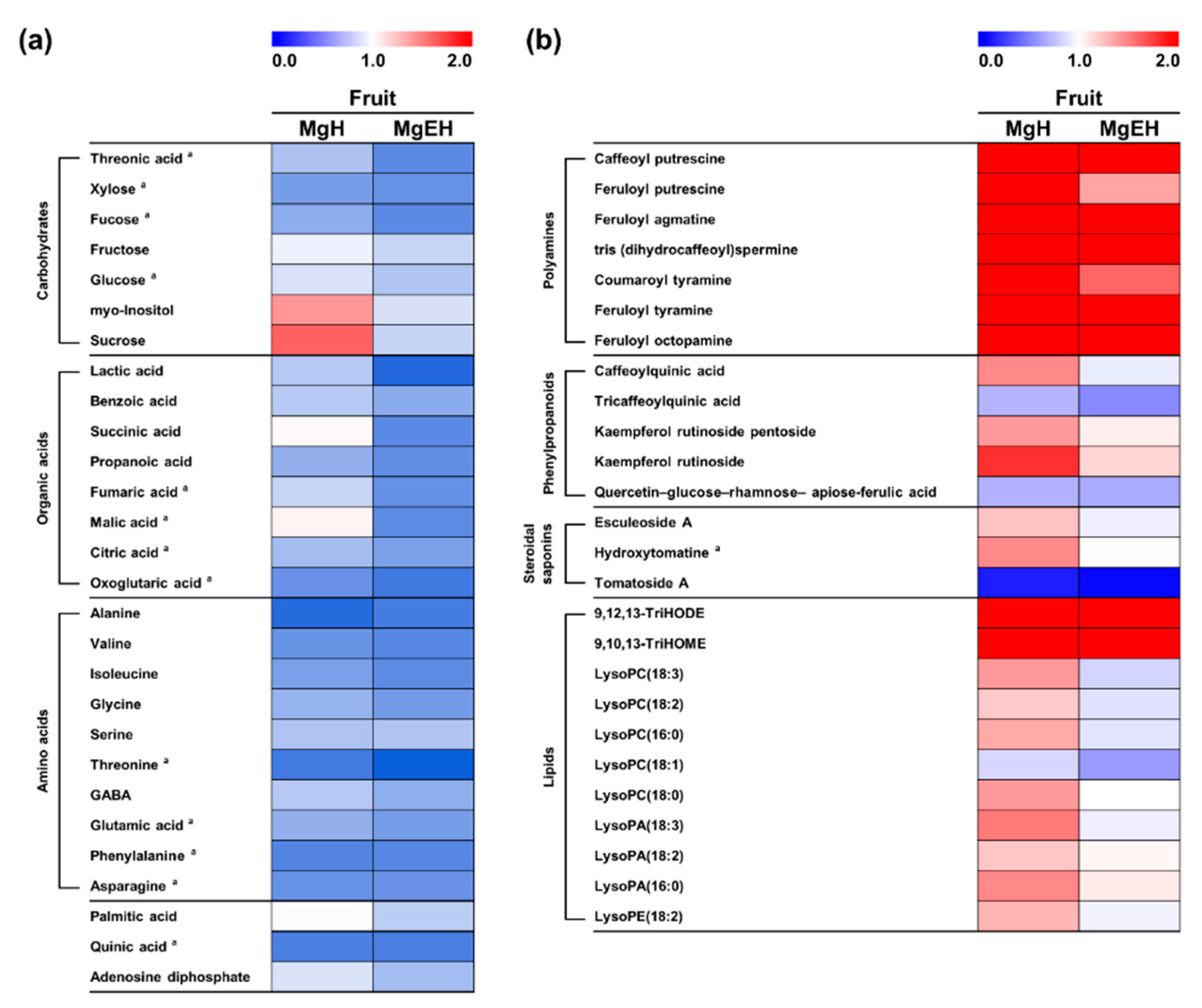
2.3. Effect of Mg Oversupply on Tomato Fruit Metabolites
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant growth, Fruit Harvest, and Preparation
4.3. Sample Extraction
4.4. GC-TOF-MS Analysis
4.5. UHPLC-LTQ-Orbitrap-MS
4.6. Determination of Fruit Quality Traits (Total Soluble Solid Contents and Titratable Acidity) and Bioactivities (Antioxidant Activity and Total Phenolic and Flavonoid Contents)
4.7. Data Processing and Multivariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.F.; Gong, Z.H.; Wu, M.B.; Chan, H.; Yuan, Y.J.; Tang, N.; Zhang, Q.; Miao, M.J.; Chang, W.; Li, Z.; et al. Integrative comparative analyses of metabolite and transcript profiles uncovers complex regulatory network in tomato (Solanum lycopersicum L.) fruit undergoing chilling injury. Sci. Rep. 2019, 9, 4470. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, E.Y. Phytostabilization of salt accumulated soil using plant and biofertilizers: Field application. Int. Biodeterior. Biodegrad. 2017, 124, 188–195. [Google Scholar] [CrossRef]
- Du, S.T.; Liu, Y.; Zhang, P.; Liu, H.J.; Zhang, X.Q.; Zhang, R.R. Atmospheric application of trace amounts of nitric oxide enhances tolerance to salt stress and improves nutritional quality in spinach (Spinacia oleracea L.). Food Chem. 2015, 173, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R.; May, D.M. Tomato fruit yields and quality under water deficit and salinity. J. Am. Soc. Hortic. Sci. 1991, 116, 215–221. [Google Scholar] [CrossRef]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Saghaiesh, S.P.; Souri, M.K.S.; Moghaddam, M. Effects of different magnesium levels on some morphophysiological characteristics and nutrient elements uptake in Khatouni melons (cucumis melo var. inodorus). J. Plant Nutr. 2019, 42, 27–39. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Liang, W.W.; Huang, J.H.; Li, C.P.; Yang, L.T.; Ye, X.; Lin, D.; Chen, L.S. MicroRNA-mediated responses to long-term magnesium-deficiency in Citrus sinensis roots revealed by Illumina sequencing. BMC Genom. 2017, 18, 657. [Google Scholar] [CrossRef]
- Jin, X.L.; Ma, C.L.; Yang, L.T.; Chen, L.S. Alterations of physiology and gene expression due to long-term magnesium-deficiency differ between leaves and roots of Citrus reticulata. J. Plant Physiol. 2016, 198, 103–115. [Google Scholar] [CrossRef]
- Yang, N.; Jiang, J.; Xie, H.; Bai, M.; Xu, Q.; Wang, X.; Guan, Y. Metabolomics reveals distinct carbon and nitrogen metabolic responses to magnesium deficiency in leaves and roots of soybean [Glycine max (Linn.) Merr.]. Front. Plant Sci. 2017, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Matsuda, F. Metabolomics for functional genomics, systems biology, and biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.; Zhou, Y.; Xiao, Y.; Jin, M.; Yang, N.; Liu, H.; et al. Genetic determinants of the network of primary metabolism and their relationships to plant performance in a maize recombinant inbred line population. Plant Cell 2015, 27, 1839–1856. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Agricultural Sciences (NIAS). Fertilizer Recommendation for Crop Production, 3rd ed.; NIAS, Rural Development Administration: Jeonju, Korea, 2017; pp. 66–67. [Google Scholar]
- Stingone, C.; Gabrielli, M.; Bandini, M.; Bolzoni, L.; Sandei, L. Evaluation of volatile and non-volatile taste and flavour compounds of some Italian tomato cultivars throughout processing. Acta Hortic. 2016, 1159, 215–222. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Bernardo, L.; Kane, D.; Trevisan, M.; Lucini, L. Zinc excess triggered polyamines accumulation in lettuce root metabolome, as compared to osmotic stress under high salinity. Front. Plant Sci. 2016, 7, 842. [Google Scholar] [CrossRef]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Shelden, M.C.; Dias, D.A.; Jayasinghe, N.S.; Bacic, A.; Roessner, U. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J. Exp. Bot. 2016, 67, 3731–3745. [Google Scholar] [CrossRef]
- León, P.; Sheen, J. Sugar and hormone connections. Trends Plant Sci. 2003, 8, 110–116. [Google Scholar] [CrossRef]
- Ye, Q.; Steudle, E. Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ. 2006, 29, 459–470. [Google Scholar] [CrossRef]
- Peng, W.T.; Zhang, L.D.; Zhou, Z.; Fu, C.; Chen, Z.C.; Liao, H. Magnesium promotes root nodulation through facilitation of carbohydrate allocation in soybean. Physiol. Plant 2018, 163, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.P. Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. New Phytol. 2000, 147, 43–53. [Google Scholar] [CrossRef]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of salt tolerance in Soja based on metabolomics of seedling roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef]
- Zhou, Y.; Diao, M.; Chen, X.; Cui, J.; Pang, S.; Li, Y.; Hou, C.; Liu, H.Y. Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Sci. Hortic. 2019, 250, 45–58. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jayaganesh, S. Characterisation of magnesium toxicity, its influence on amino acid synthesis pathway and biochemical parameters of tea. Res. J. Phytochem. 2010, 4, 67–77. [Google Scholar] [CrossRef]
- Adams, S.N.; Ac-Pangan, W.O.; Rossi, L. Effects of Soil Salinity on Citrus Rootstock ‘US-942’Physiology and Anatomy. J. Am. Soc. Hortic. Sci. 2019, 54, 787–792. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C. Effect of salt stress on the growth and fruit quality of tomato plants. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–16. [Google Scholar]
- Kim, E.J.; Park, S.E.; Seo, S.H.; Kweon, O.C.; Son, H.S. A GC–MS based metabolic profiling of fermented tomato by lactic acid bacteria. Appl. Biol. Chem. 2019, 62, 2. [Google Scholar] [CrossRef]
- Kader, A.; Stevens, M.; Albright, M.; Morris, L. Amino acid composition and flavor of fresh market tomatoes as influenced by fruit ripeness when harvested. J. Am. Soc. Hort. Sci. 1978, 103, 541–544. [Google Scholar]
- Sorrequieta, A.; Ferraro, G.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening: A focus on L-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar] [CrossRef]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef]
- Jung, E.S.; Park, H.M.; Lee, K.E.; Shin, J.H.; Mun, S.; Kim, J.K.; Lee, S.J.; Liu, K.H.; Hwang, J.K.; Lee, C.H. A metabolomics approach shows that catechin-enriched green tea attenuates ultraviolet B-induced skin metabolite alterations in mice. Metabolomics 2015, 11, 861–871. [Google Scholar] [CrossRef]
- Suh, D.H.; Jung, E.S.; Lee, G.M.; Lee, C.H. Distinguishing six edible berries based on metabolic pathway and bioactivity correlations by non-targeted metabolite profiling. Front. Plant Sci. 2018, 9, 1462. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites 2019, 9, 231. https://doi.org/10.3390/metabo9100231
Kwon MC, Kim YX, Lee S, Jung ES, Singh D, Sung J, Lee CH. Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites. 2019; 9(10):231. https://doi.org/10.3390/metabo9100231
Chicago/Turabian StyleKwon, Min Cheol, Yangmin X. Kim, Seulbi Lee, Eun Sung Jung, Digar Singh, Jwakyung Sung, and Choong Hwan Lee. 2019. "Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism" Metabolites 9, no. 10: 231. https://doi.org/10.3390/metabo9100231
APA StyleKwon, M. C., Kim, Y. X., Lee, S., Jung, E. S., Singh, D., Sung, J., & Lee, C. H. (2019). Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites, 9(10), 231. https://doi.org/10.3390/metabo9100231






