Comparative Effects of Turmeric Secondary Metabolites Across Resorptive Bone Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Turmeric Extract Preparation and Analyses
2.2. Animal Procedures
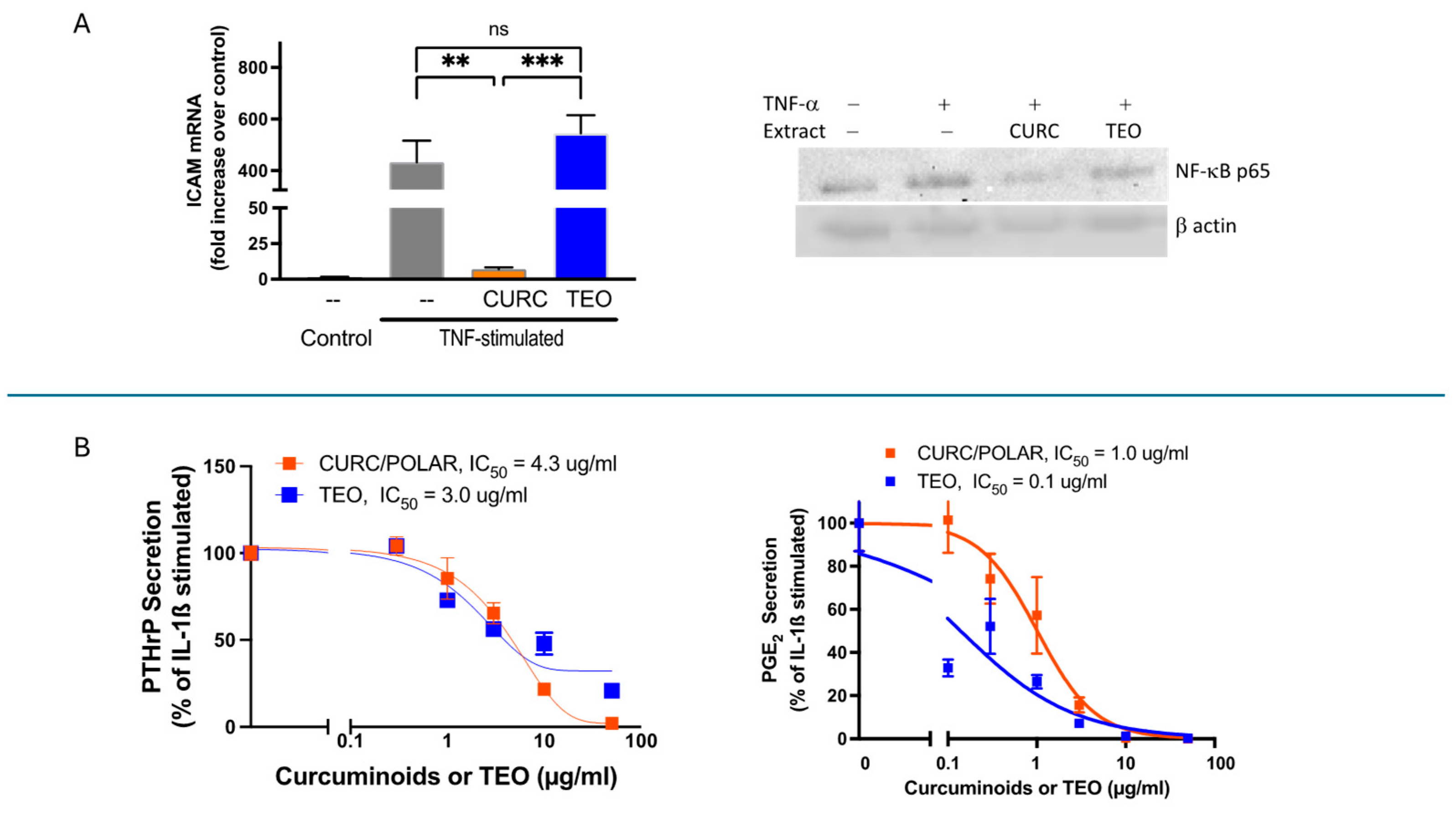
2.3. Cell Culture Methods
2.4. Statistical Analyses
3. Results
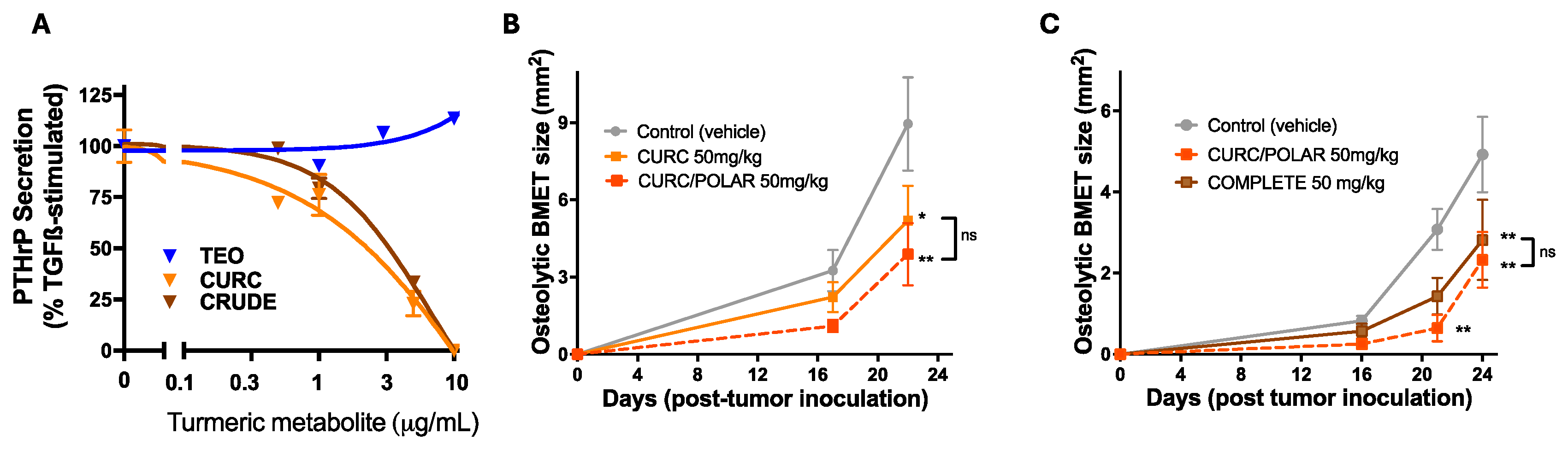
3.1. Characterization of Turmeric Extracts for In Vivo Testing in Bone Resorption Models
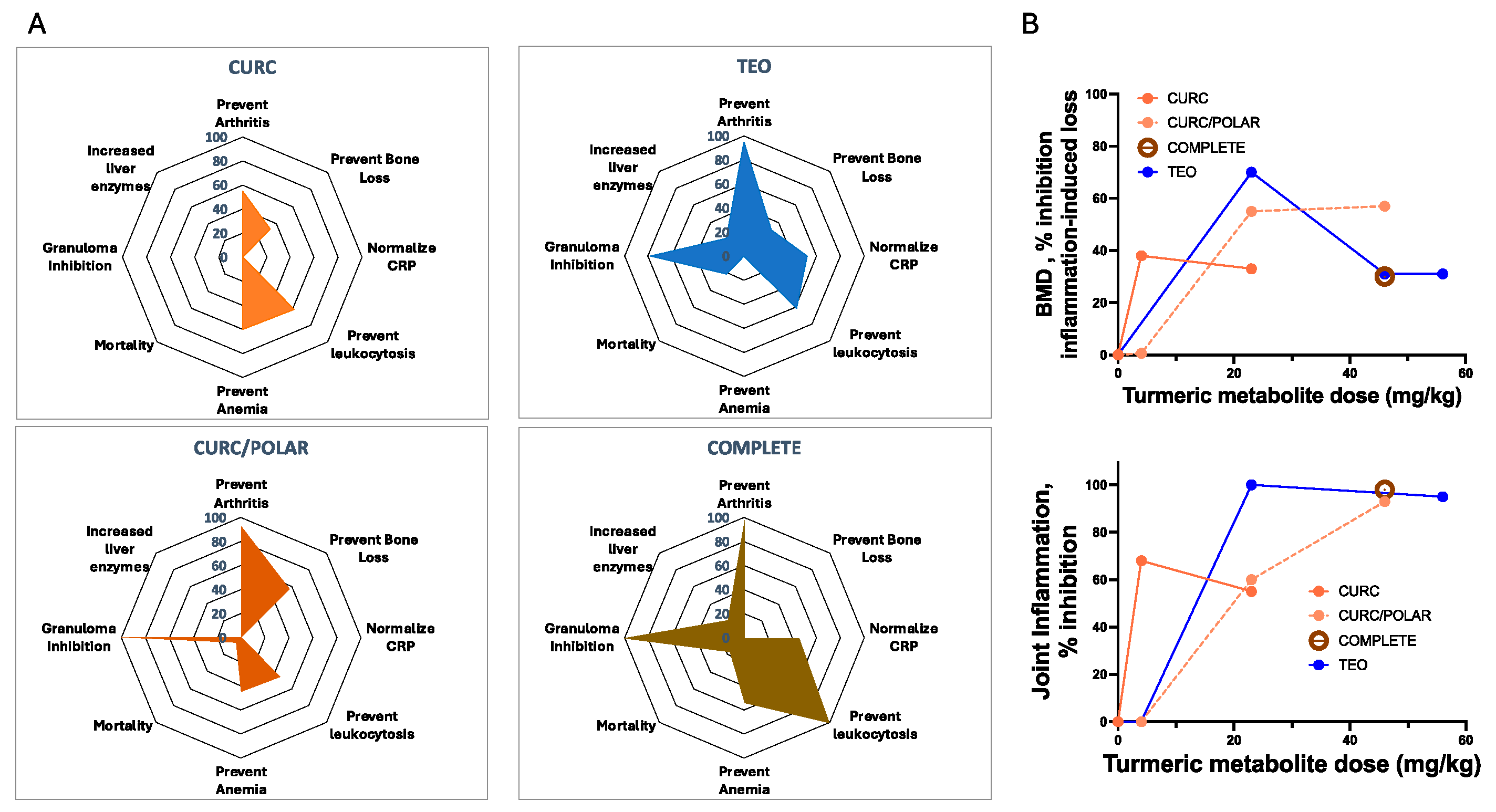
3.2. Turmeric Metabolite Effects in an Inflammatory Rheumatoid Arthritis Model
3.3. Turmeric Metabolite Effects in an Osteolytic Breast Cancer Bone Metastases Model
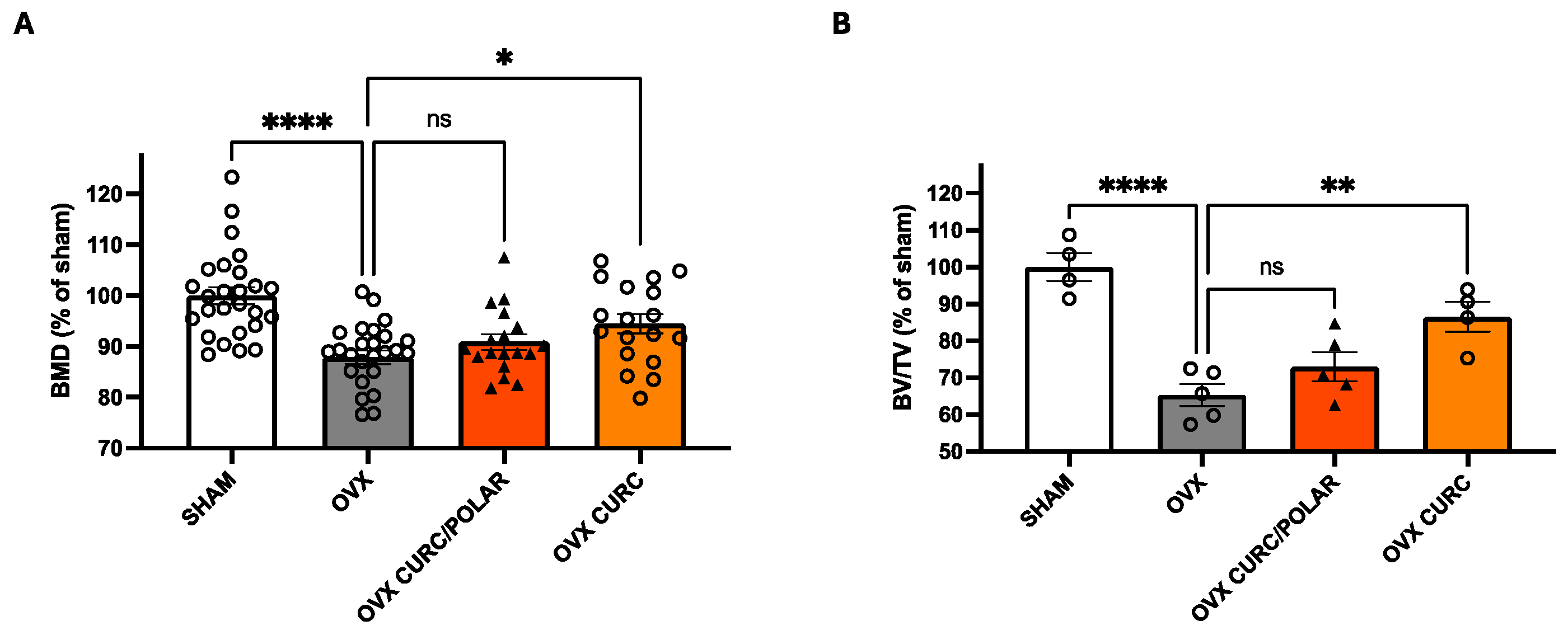
3.4. Turmeric Metabolite Effects in a Post-Menopausal Osteoporosis Model
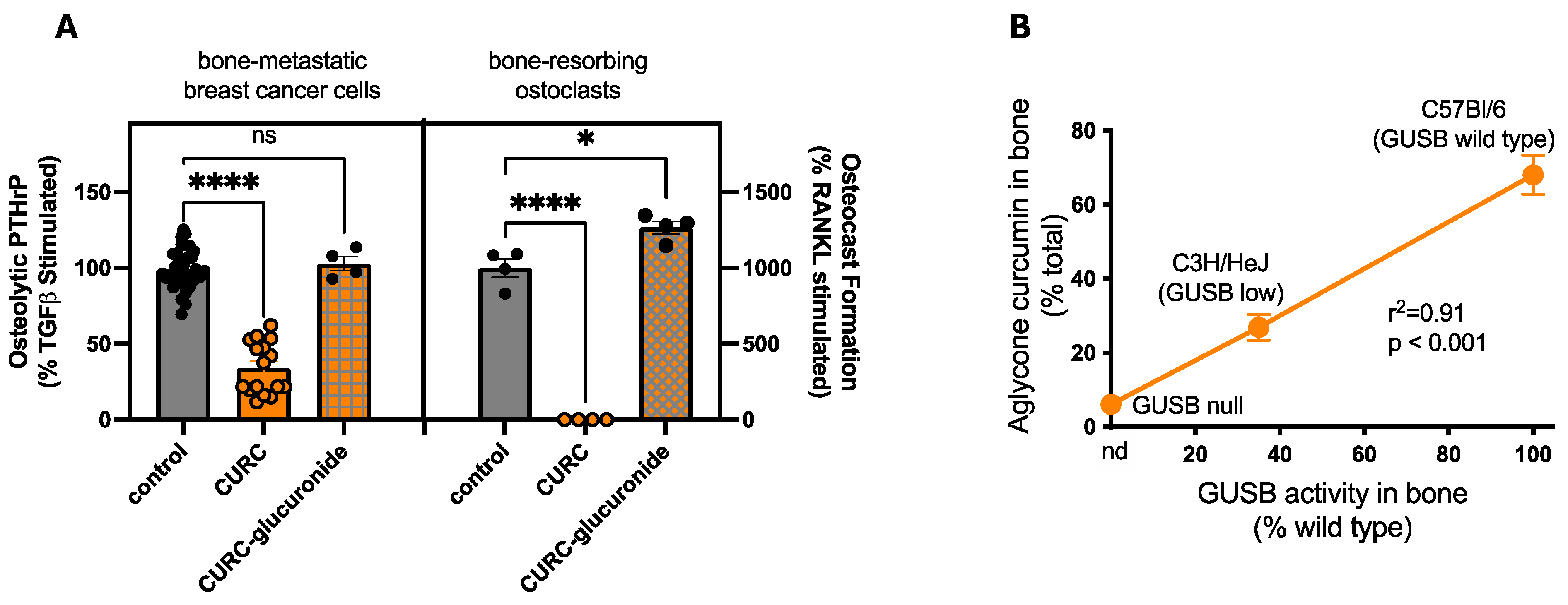
3.5. Disease-Independent, Host Variables Impacting Turmeric Metabolite Bone Protection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velayudhan, K.C.; Dikshit, N.; Nizar, M.A. Ethnobotany of turmeric (Curcuma longa L.). Indian. J. Tradit. Knowl. 2012, 11, 607–614. [Google Scholar]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport. Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef] [PubMed]
- Cozmin, M.; Lungu, I.I.; Gutu, C.; Stefanache, A.; Duceac, L.D.; Șoltuzu, B.D.; Damir, D.; Calin, G.; Bogdan Goroftei, E.R.; Grierosu, C.; et al. Turmeric: From spice to cure. A review of the anti-cancer, radioprotective and anti-inflammatory effects of turmeric sourced compounds. Front. Nutr. 2024, 11, 1399888. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Shojaei-Zarghani, S.; Rafraf, M. Curcumin and rheumatoid arthritis: A systematic review of literature. Int. J. Clin. Pract. 2021, 75, e14280. [Google Scholar] [CrossRef]
- Skiba, M.B.; Luis, P.B.; Alfafara, C.; Billheimer, D.; Schneider, C.; Funk, J.L. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018, 62, e1800143. [Google Scholar] [CrossRef]
- Hsu, K.Y.; Ho, C.T.; Pan, M.H. The therapeutic potential of curcumin and its related substances in turmeric: From raw material selection to application strategies. J. Food Drug Anal. 2023, 31, 194–211. [Google Scholar] [CrossRef]
- You, H.; Gershon, H.; Goren, F.; Xue, F.; Kantowski, T.; Monheit, L. Analytical strategies to determine the labelling accuracy and economically-motivated adulteration of “natural” dietary supplements in the marketplace: Turmeric case study. Food Chem. 2022, 370, 131007. [Google Scholar] [CrossRef]
- Girme, A.; Saste, G.; Balasubramaniam, A.K.; Pawar, S.; Ghule, C.; Hingorani, L. Assessment of Curcuma longa extract for adulteration with synthetic curcumin by analytical investigations. J. Pharm. Biomed. Anal. 2020, 191, 113603. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Zhang, H.; Timmermann, B.N. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.). J. Agric. Food Chem. 2010, 58, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the Volatile Compositions of Curcuma Species. Foods 2019, 8, 53. [Google Scholar] [CrossRef]
- Kieliszek, M.; Edris, A.; Kot, A.M.; Piwowarek, K. Biological Activity of Some Aromatic Plants and Their Metabolites, with an Emphasis on Health-Promoting Properties. Molecules 2020, 25, 2478. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M. Turmeric Essential Oil Constituents as Potential Drug Candidates: A Comprehensive Overview of Their Individual Bioactivities. Molecules 2024, 29, 4210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Fu, C.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT 2022, 154, 112805. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, Y.; Lin, L.; Gao, T.; Yang, Q.; Fan, Y.; Wang, S.; Fu, C.; Liao, W. Modulating Effects of Turmeric Polysaccharides on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. J. Agric. Food Chem. 2024, 72, 3469–3482. [Google Scholar] [CrossRef]
- Clarke, T.C.; Nahin, R.L.; Barnes, P.M.; Stussman, B.J. Use of Complementary Health Approaches for Musculoskeletal Pain Disorders Among Adults: United States, 2012. Natl. Health Stat. Rep. 2016, 1–12. [Google Scholar]
- Dickinson, A.; Blatman, J.; El-Dash, N.; Franco, J.C. Consumer usage and reasons for using dietary supplements: Report of a series of surveys. J. Am. Coll. Nutr. 2014, 33, 176–182. [Google Scholar] [CrossRef]
- Hauer, M.; Rossi, A.M.; Wertheim, B.C.; Kleppel, H.B.; Bea, J.W.; Funk, J.L. Dietary Supplement Use in Women Diagnosed with Breast Cancer. J. Nutr. 2023, 153, 301–311. [Google Scholar] [CrossRef]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Liu, X.; He, J.; Han, L.; Li, J. Overview of mechanisms and novel therapies on rheumatoid arthritis from a cellular perspective. Front. Immunol. 2024, 15, 1461756. [Google Scholar] [CrossRef]
- Pang, L.; Gan, C.; Xu, J.; Jia, Y.; Chai, J.; Huang, R.; Li, A.; Ge, H.; Yu, S.; Cheng, H. Bone Metastasis of Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Cancers 2022, 14, 5727. [Google Scholar] [CrossRef] [PubMed]
- Osteoporosis: Nonclinical Evaluation of Drugs Intended for Treatment Guidance for Industry. Available online: https://www.fda.gov/media/129899/download (accessed on 28 March 2025).
- Kuszak, A.J.; Hopp, D.C.; Williamson, J.S.; Betz, J.M.; Sorkin, B.C. Approaches by the US National Institutes of Health to support rigorous scientific research on dietary supplements and natural products. Drug Test. Anal. 2016, 8, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Oyarzo, J.N.; Frye, J.B.; Chen, G.; Lantz, R.C.; Jolad, S.D.; Sólyom, A.M.; Timmermann, B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006, 69, 351–355. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Yue, G.G.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.; Hon, P.M.; Lee, M.Y.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Nasef, N.A.; Loveday, S.M.; Golding, M.; Martins, R.N.; Shah, T.M.; Clarke, M.; Coad, J.; Moughan, P.J.; Garg, M.L.; Singh, H. Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans. Food Funct. 2019, 10, 4584–4592. [Google Scholar] [CrossRef]
- Panda, S.K.; Nirvanashetty, S.; Missamma, M.; Jackson-Michel, S. The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation. Medicine 2021, 100, e26601. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- AALAS position statement on the humane care and use of laboratory animals. Comp. Med. 2007, 57, 413.
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Chen, J.; Downey, K.J.; Davee, S.M.; Stafford, G. Blockade of parathyroid hormone-related protein prevents joint destruction and granuloma formation in streptococcal cell wall-induced arthritis. Arthritis Rheum. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Wang, S.X.; Cherian, A.; Dumitriu, M.; Grynpas, M.D.; Carran, J.; Wainman, D.; Anastassiades, T. Disease modifying effects of N-butyryl glucosamine in a streptococcal cell wall induced arthritis model in rats. J. Rheumatol. 2007, 34, 712–720. [Google Scholar]
- Wright, L.E.; Frye, J.B.; Lukefahr, A.L.; Timmermann, B.N.; Mohammad, K.S.; Guise, T.A.; Funk, J.L. Curcuminoids block TGF-β signaling in human breast cancer cells and limit osteolysis in a murine model of breast cancer bone metastasis. J. Nat. Prod. 2013, 76, 316–321. [Google Scholar] [CrossRef]
- Regan, J.N.; Trivedi, T.; Guise, T.A.; Waning, D.L. The Role of TGFbeta in Bone-Muscle Crosstalk. Curr. Osteoporos. Rep. 2017, 15, 18–23. [Google Scholar] [CrossRef]
- Kunihiro, A.G.; Luis, P.B.; Brickey, J.A.; Frye, J.B.; Chow, H.S.; Schneider, C.; Funk, J.L. Beta-Glucuronidase Catalyzes Deconjugation and Activation of Curcumin-Glucuronide in Bone. J. Nat. Prod. 2019, 82, 500–509. [Google Scholar] [CrossRef]
- Wright, L.E.; Frye, J.B.; Timmermann, B.N.; Funk, J.L. Protection of trabecular bone in ovariectomized rats by turmeric (Curcuma longa L.) is dependent on extract composition. J. Agric. Food Chem. 2010, 58, 9498–9504. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Davis-Gorman, G.; Spera, A.L.; Bernas, M.J.; Witte, M.H.; Weinand, M.E.; Timmermann, B.N.; McDonagh, P.F.; Ritter, L. Curcuminoids limit neutrophil-mediated reperfusion injury in experimental stroke by targeting the endothelium. Microcirculation 2013, 20, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Kuscuoglu, N.; Wilson, J.; McCaffrey, G.; Stafford, G.; Chen, G.; Lantz, R.C.; Jolad, S.D.; et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006, 54, 3452–3464. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Yu, X.; Zheng, H.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Med. 2003, 80, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Silva, M.V.D.; Barros, C.C.O.; Alexandre, P.B.D.; Timóteo, R.P.; Catarino, J.S.; Sales-Campos, H.; Machado, J.R.; Rodrigues, D.B.R.; Oliveira, C.J.; et al. TNF-α blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS ONE 2018, 13, e0194430. [Google Scholar] [CrossRef]
- Vázquez-Del Mercado, M.; Nuñez-Atahualpa, L.; Figueroa-Sánchez, M.; Gómez-Bañuelos, E.; Rocha-Muñoz, A.D.; Martín-Márquez, B.T.; Corona-Sanchez, E.G.; Martínez-García, E.A.; Macias-Reyes, H.; Gonzalez-Lopez, L.; et al. Serum Levels of Anticyclic Citrullinated Peptide Antibodies, Interleukin-6, Tumor Necrosis Factor-α, and C-Reactive Protein Are Associated with Increased Carotid Intima-Media Thickness: A Cross-Sectional Analysis of a Cohort of Rheumatoid Arthritis Patients without Cardiovascular Risk Factors. BioMed Res. Int. 2015, 2015, 342649. [Google Scholar]
- Zhou, H.H.; Tang, Y.L.; Xu, T.H.; Cheng, B. C-reactive protein: Structure, function, regulation, and role in clinical diseases. Front. Immunol. 2024, 15, 1425168. [Google Scholar] [CrossRef]
- McCoy, J.M.; Wicks, J.R.; Audoly, L.P. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Investig. 2002, 110, 651–658. [Google Scholar] [CrossRef]
- Goldring, S.R.; Gravallese, E.M. Pathogenesis of bone lesions in rheumatoid arthritis. Curr. Rheumatol. Rep. 2002, 4, 226–231. [Google Scholar] [CrossRef]
- Yoshida, T.; Sakamoto, H.; Horiuchi, T.; Yamamoto, S.; Suematsu, A.; Oda, H.; Koshihara, Y. Involvement of prostaglandin E(2) in interleukin-1alpha-induced parathyroid hormone-related peptide production in synovial fibroblasts of patients with rheumatoid arthritis. J. Clin. Endocrinol. Metab. 2001, 86, 3272–3278. [Google Scholar] [CrossRef]
- Elson, A.; Anuj, A.; Barnea-Zohar, M.; Reuven, N. The origins and formation of bone-resorbing osteoclasts. Bone 2022, 164, 116538. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Rhoades, J.; Martin, T.J. Parathyroid hormone-related protein in breast cancer bone metastasis. Vitam. Horm. 2022, 120, 215–230. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef] [PubMed]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Küşümler, A.S.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxidative Med. Cell. Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Pilonis, N.; Ptacek, R.; Kream, R.M. Reciprocal Evolution of Opiate Science from Medical and Cultural Perspectives. Med. Sci. Monit. 2017, 23, 2890–2896. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Sultana, A.; Raj, A.; Emon, N.U.; Richi, F.T.; Sharmin, T.; Moon, M.; Park, M.N.; Kim, B. Papaverine: A Miraculous Alkaloid from Opium and Its Multimedicinal Application. Molecules 2023, 28, 3149. [Google Scholar] [CrossRef]
- Christensen, C.; Rose, M.; Cornett, C.; Allesø, M. Decoding the Postulated Entourage Effect of Medicinal Cannabis: What It Is and What It Isn’t. Biomedicines 2023, 11, 2323. [Google Scholar] [CrossRef]
- Kunihiro, A.G.; Brickey, J.A.; Frye, J.B.; Cheng, J.N.; Luis, P.B.; Schneider, C.; Funk, J.L. Curcumin Inhibition of TGFβ signaling in bone metastatic breast cancer cells and the possible role of oxidative metabolites. J. Nutr. Biochem. 2021, 99, 108842. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Bordy, R.; Totoson, P.; Prati, C.; Marie, C.; Wendling, D.; Demougeot, C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat. Rev. Rheumatol. 2018, 14, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Crowson, C.S.; Liao, K.P.; Davis, J.M., 3rd; Solomon, D.H.; Matteson, E.L.; Knutson, K.L.; Hlatky, M.A.; Gabriel, S.E. Rheumatoid arthritis and cardiovascular disease. Am. Heart J. 2013, 166, 622–628.e621. [Google Scholar] [CrossRef]
- Pope, J.E.; Choy, E.H. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [PubMed]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhöfer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, e2100613. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef]
- Ortutay, Z.; Polgar, A.; Gomor, B.; Geher, P.; Lakatos, T.; Glant, T.T.; Gay, R.E.; Gay, S.; Pallinger, E.; Farkas, C.; et al. Synovial fluid exoglycosidases are predictors of rheumatoid arthritis and are effective in cartilage glycosaminoglycan depletion. Arthritis Rheum. 2003, 48, 2163–2172. [Google Scholar] [CrossRef]
- Bramwell, K.K.; Ma, Y.; Weis, J.H.; Chen, X.; Zachary, J.F.; Teuscher, C.; Weis, J.J. Lysosomal beta-glucuronidase regulates Lyme and rheumatoid arthritis severity. J. Clin. Investig. 2014, 124, 311–320. [Google Scholar] [CrossRef]
- Bucchireddigari, B.; BR; Frye, J.B.; Nicholas, J.S.; Chen, Z.; Bea, J.W.; Funk, J.L. Mechanistic determinants of frailty in breast cancer survivors. In Proceedings of the American Soc of Preventive Oncology, Tucson, AZ, USA, 29 March–31 May 2021; 2021. [Google Scholar]
- Gao, R.C.; Wu, Z.G.; Wu, Z.Z.; Hao, M.; Wu, G.C. Frailty in rheumatoid arthritis: A systematic review and meta-analysis. Jt. Bone Spine 2022, 89, 105343. [Google Scholar] [CrossRef]
- Wennberg, A.M.; Matthews, A.; Talbäck, M.; Ebeling, M.; Ek, S.; Feychting, M.; Modig, K. Frailty Among Breast Cancer Survivors: Evidence From Swedish Population Data. Am. J. Epidemiol. 2023, 192, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
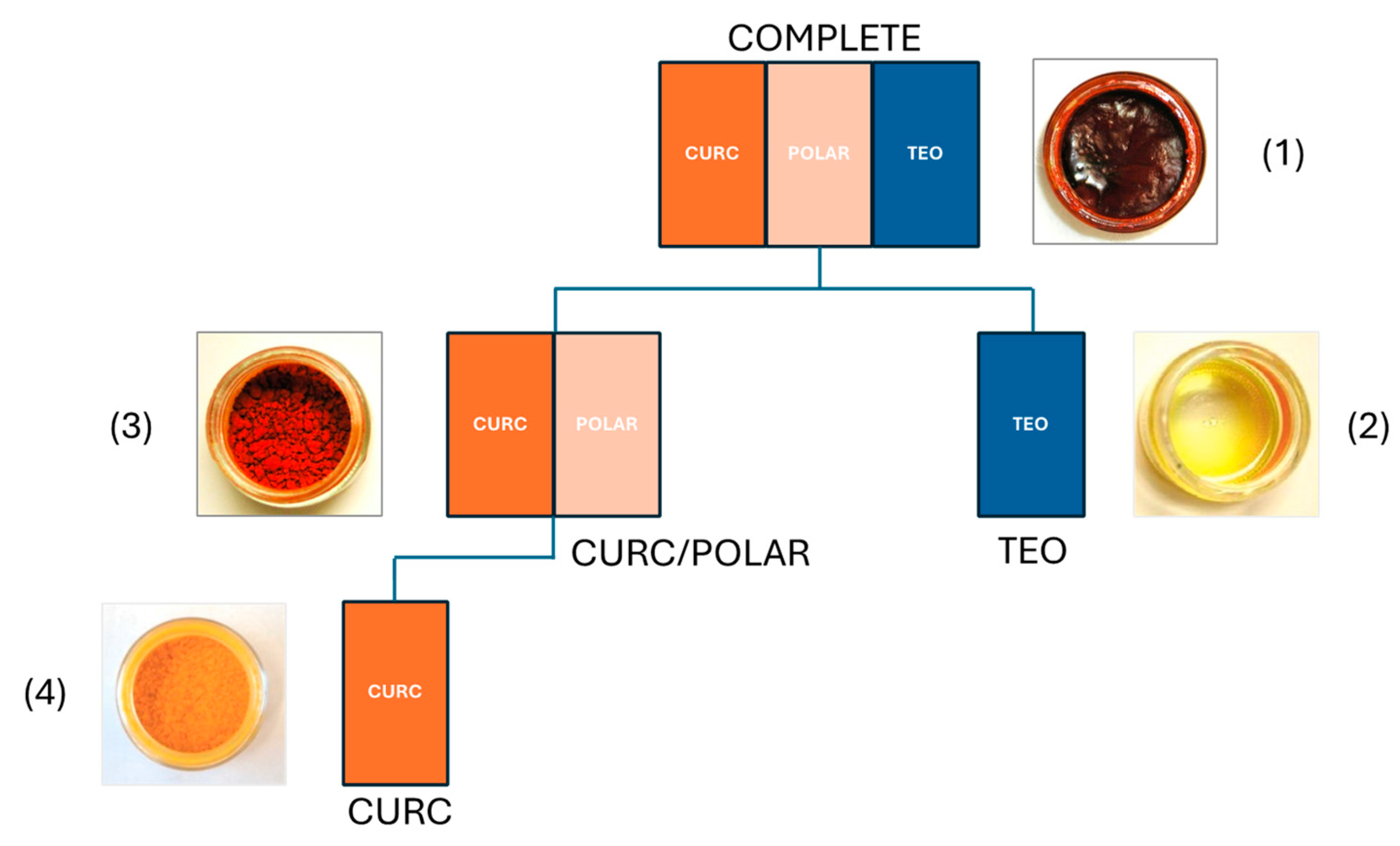
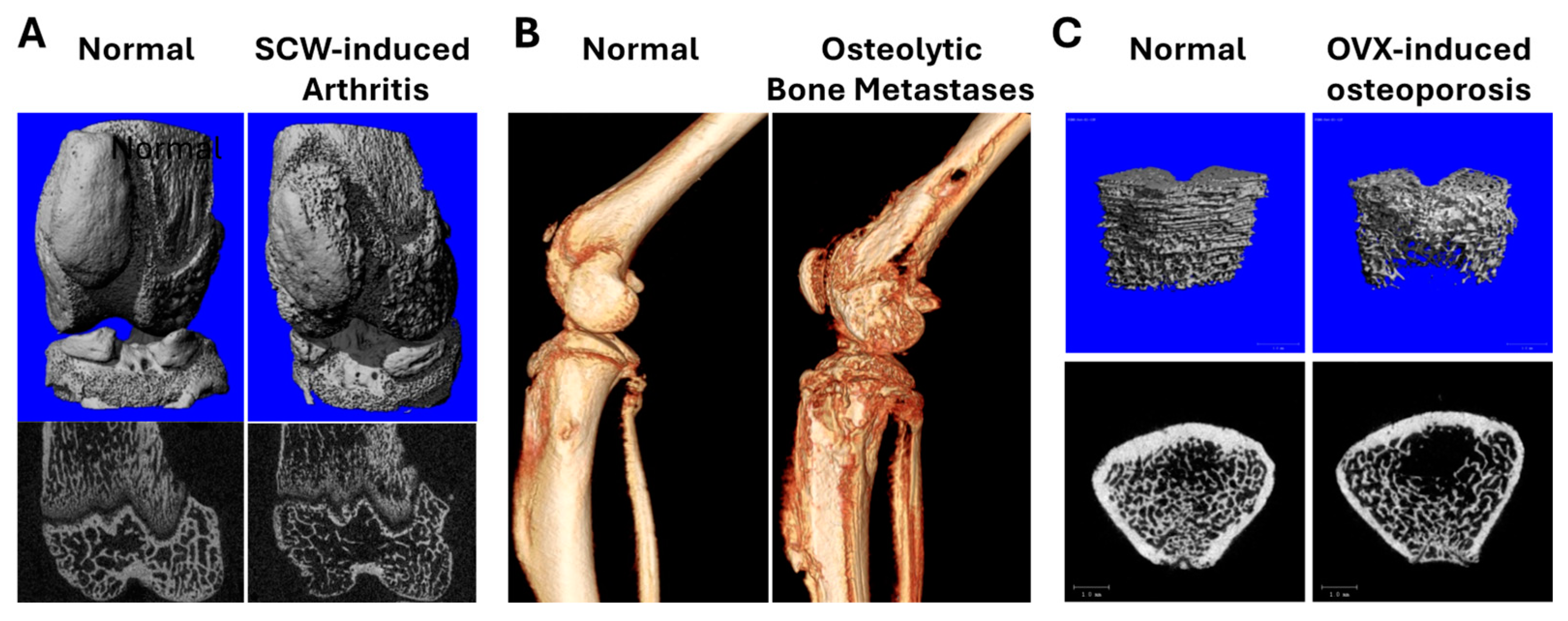

| Disease Being Modeled | In Vivo Model | Animal | In Vivo Turmeric Treatments | |||
|---|---|---|---|---|---|---|
| CURC | CURC/POLAR | TEO | COMPLETE | |||
| Arthritis, rheumatoid | SCW-induced arthritis | rat, female | X | X | X | X |
| Bone tumor, osteolytic breast cancer | Bone-disseminated human breast cancer cells | mouse, female | X | X | X | |
| Osteoporosis, post-menopausal | OVX-induced bone loss | rat, female | X | X | ||
| (bone GUSB levels, variable) | various mouse strains | mouse, female/male | X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, L.E.; Frye, J.B.; Kunihiro, A.G.; Timmermann, B.N.; Funk, J.L. Comparative Effects of Turmeric Secondary Metabolites Across Resorptive Bone Diseases. Metabolites 2025, 15, 266. https://doi.org/10.3390/metabo15040266
Wright LE, Frye JB, Kunihiro AG, Timmermann BN, Funk JL. Comparative Effects of Turmeric Secondary Metabolites Across Resorptive Bone Diseases. Metabolites. 2025; 15(4):266. https://doi.org/10.3390/metabo15040266
Chicago/Turabian StyleWright, Laura E., Jennifer B. Frye, Andrew G. Kunihiro, Barbara N. Timmermann, and Janet L. Funk. 2025. "Comparative Effects of Turmeric Secondary Metabolites Across Resorptive Bone Diseases" Metabolites 15, no. 4: 266. https://doi.org/10.3390/metabo15040266
APA StyleWright, L. E., Frye, J. B., Kunihiro, A. G., Timmermann, B. N., & Funk, J. L. (2025). Comparative Effects of Turmeric Secondary Metabolites Across Resorptive Bone Diseases. Metabolites, 15(4), 266. https://doi.org/10.3390/metabo15040266







