Study on the Inhibitory Effects of Three Endophytic Bacillus Strains on Aspergillus flavus in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Condition
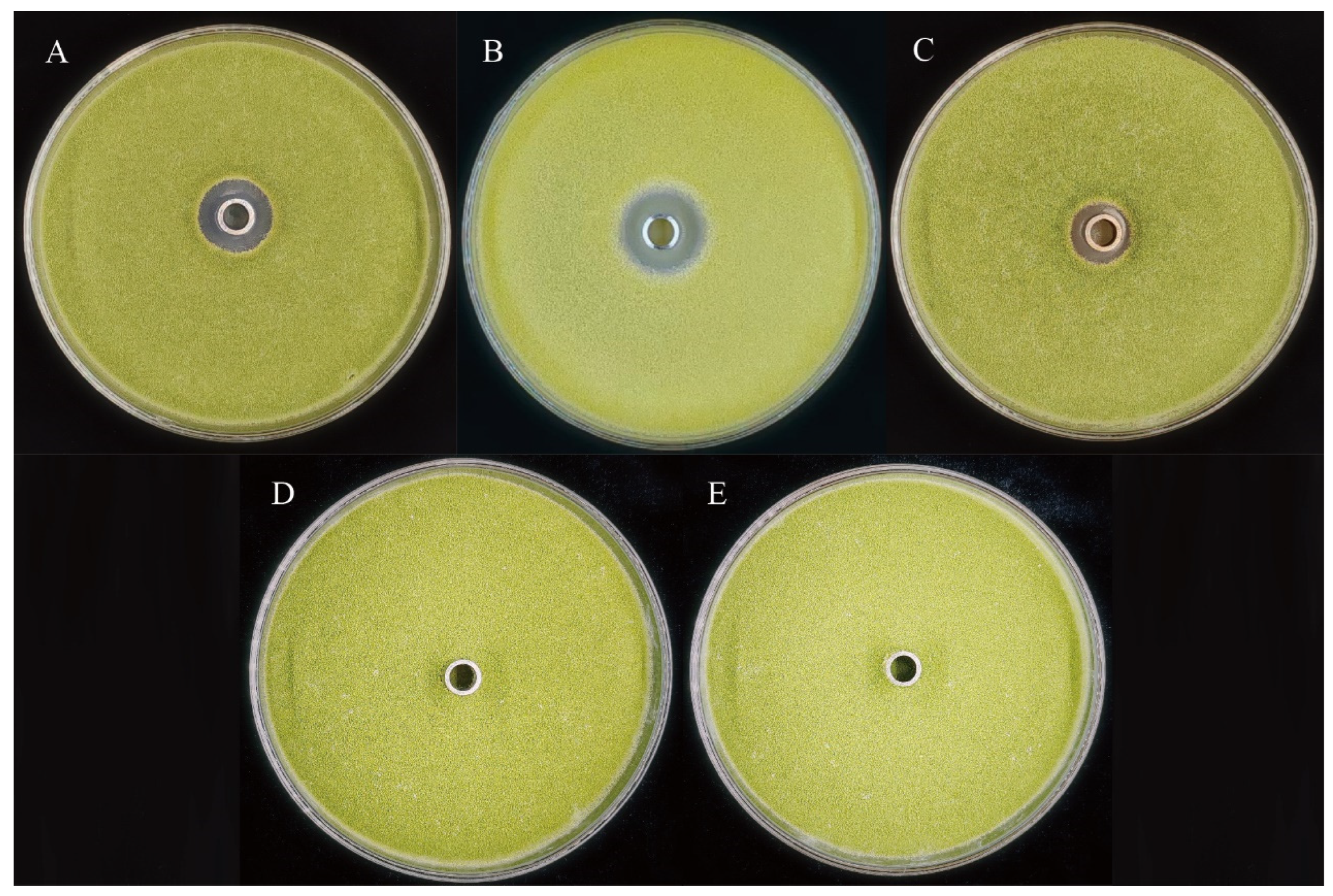
2.2. In Vitro Co-Culture Assay
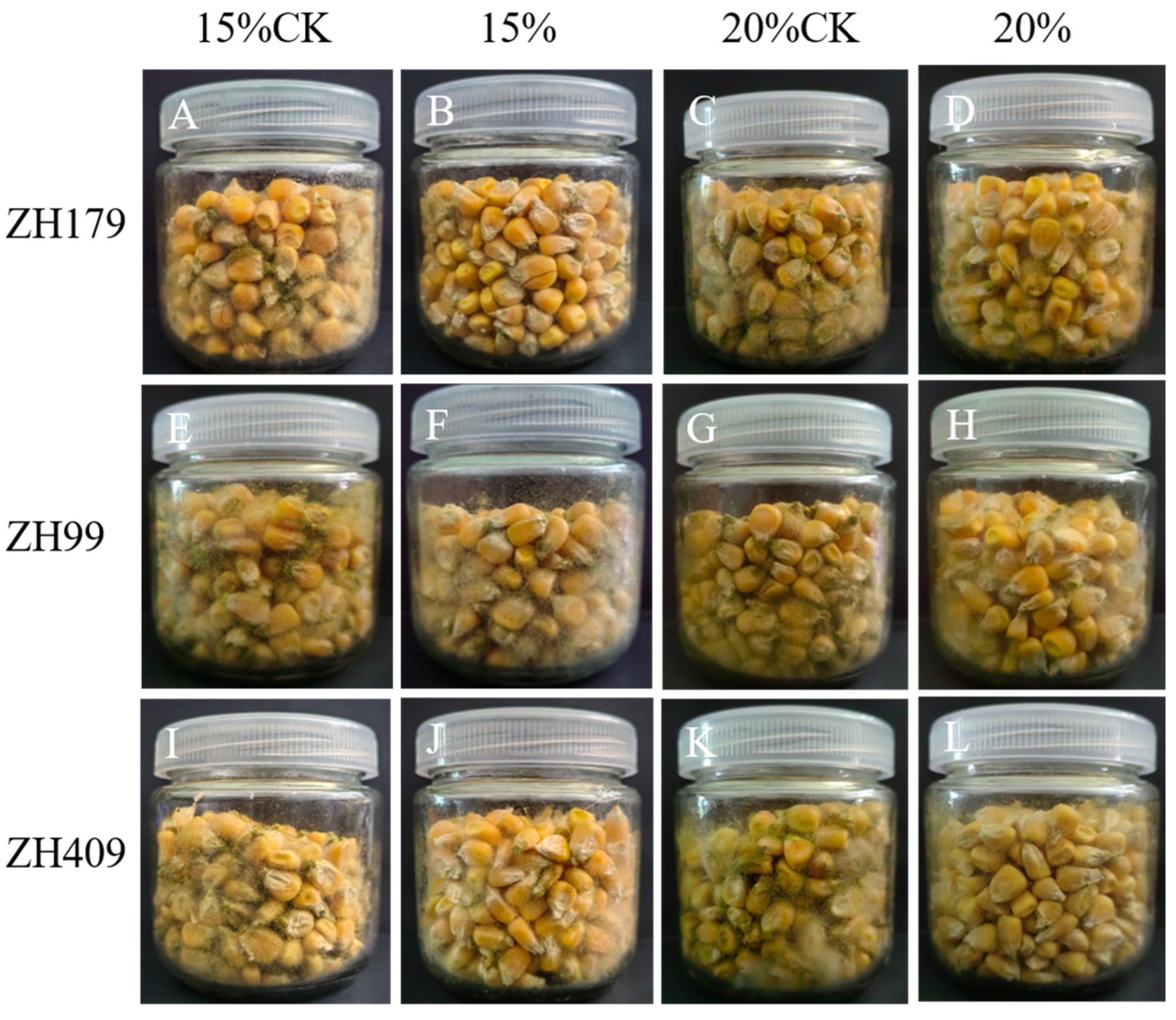
2.3. Determination of Biocontrol Efficacy on Maize Seeds
2.4. Identification of Antagonistic Bacterias
2.5. Detection of Lipopeptide Biosynthesis Genes of the Three Bacteria
2.6. Characterization of the Antifungal Compounds Using LC-MS
2.7. Antagonistic Activity Test of Crude Lipopeptide Extract Against A. flavus
2.8. Statistics
3. Results
3.1. Biocontrol Effect of Three Strains of Bacteria on A. flavus
3.2. Identification Results of Biocontrol Strains
3.3. Test Results of the Lipopeptidase Gene
3.4. LC-MS Test Results of Crude Lipopeptide Extract
3.5. Biocontrol Effect of Crude Lipopeptide Extract of Biocontrol Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited “FAO estimate” of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses 2009, 52, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Franco, A.; Arámbula-Villa, G.; Ramírez-Noguera, P.; Salazar, A.M.; Sordo, M.; Marroquín-Cardona, A.; Figueroa-Cárdenas, J.d.D.; Méndez-Albores, A. Aflatoxin detoxification in tortillas using an infrared radiation thermo-alkaline process: Cytotoxic and genotoxic evaluation. Food Control 2020, 112, 107084. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; da Chagas Oliveira Freire, F.; Erlan Feitosa Maia, F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Popović Milovanović, T.; Iličić, R.; Bagi, F.; Aleksić, G.; Trkulja, N.; Trkulja, V.; Jelušić, A. Biocontrol of seedborne fungi on small-grained cereals using Bacillus halotolerans strain B33. J. Fungi 2025, 11, 144. [Google Scholar] [CrossRef]
- De Curtis, F.; Caputo, L.; Castoria, R.; Lima, G.; Stea, G.; De Cicco, V. Use of fluorescent amplified fragment length polymorphism (fAFLP) to identify specific molecular markers for the biocontrol agent Aureobasidium pullulans strain LS30. Postharvest Biol. Technol. 2004, 34, 179–186. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Conte, T.; Castoria, R.; Lima, G.; De Curtis, F. Influence of biocontrol and integrated strategies and treatment timing on plum brown rot incidence and fungicide residues in fruits. Agriculture 2022, 12, 1656. [Google Scholar] [CrossRef]
- Ijaz, B. Wall-associated kinases (WAKs): Key players of disease resistance in plants. J. Future Agrisphere 2024, 1, 1–3. [Google Scholar]
- Weaver, M.A.; Abbas, H.K.; Brewer, M.J.; Pruter, L.S.; Little, N.S. Integration of biological control and transgenic insect protection for mitigation of mycotoxins in corn. Crop Prot. 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Ouadhene, M.A.; Callicott, K.A.; Ortega-Beltran, A.; Mehl, H.L.; Cotty, P.J.; Battilani, P. Structure of Aspergillus flavus populations associated with maize in Greece, Spain, and Serbia: Implications for aflatoxin biocontrol on a regional scale. Environ. Microbiol. Rep. 2024, 16, e13249. [Google Scholar] [CrossRef]
- Ouadhene, M.A.; Ortega-Beltran, A.; Sanna, M.; Cotty, P.J.; Battilani, P. Multiple year influences of the aflatoxin biocontrol product AF-X1 on the Aspergillus flavus communities associated with maize production in Italy. Toxins 2023, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Ye, P.; Li, X.; Xu, H.; Lin, F. Anti-aflatoxigenic Burkholderia contaminans BC11-1 exhibits mycotoxin detoxification, phosphate solubilization, and cytokinin production. Microorganisms 2024, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wu, Y.; Jin, J.; Tong, S.; Zhang, L.; Cai, Y. Biocontrol capabilities of Bacillus subtilis E11 against Aspergillus flavus in vitro and for dried red chili (Capsicum annuum L.). Toxins 2023, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Meliani, H.; Makhloufi, A.; Cherif, A.; Mahjoubi, M.; Makhloufi, K. Biocontrol of toxinogenic Aspergillus flavus and Fusarium oxysporum f. sp. albedinis by two rare Saharan actinomycetes strains and LC-ESI/MS-MS profiling of their antimicrobial products. Saudi J. Biol. Sci. 2022, 29, 103288. [Google Scholar] [CrossRef]
- Ou, D.; Zou, Y.; Zhang, X.; Jiao, R.; Zhang, D.; Ling, N.; Ye, Y. The potential of antifungal peptides derived from Lactiplantibacillus plantarum WYH for biocontrol of Aspergillus flavus contamination. Int. J. Food Microbiol. 2024, 418, 110727. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Pérez-Través, L.; Briand, L.E.; Querol, A. Bioactive volatiles of brewer’s yeasts: Antifungal action of compounds produced during wort fermentation on Aspergillus sp. Int. J. Food Microbiol. 2024, 417, 110692. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Baker, J.L.; Mahoney, N.E. Isolation of bacterial antagonists of Aspergillus flavus from almonds. Microb. Ecol. 2006, 52, 45–52. [Google Scholar] [CrossRef]
- Al-Saadi, H.A.; Al-Sadi, A.M.; Al-Wahaibi, A.; Al-Raeesi, A.; Al-Kindi, M.; Soundra Pandian, S.B.; Al-Harrasi, M.M.A.; Al-Mahmooli, I.H.; Velazhahan, R. Rice weevil (Sitophilus oryzae L.) gut bacteria inhibit growth of Aspergillus flavus and degrade aflatoxin B1. J. Fungi 2024, 10, 377. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Zhang, Y.; Yang, Z.; Sun, X. Inhibition of non-toxigenic Aspergillus niger FS10 isolated from Chinese fermented soybean on growth and aflatoxin B1 production by Aspergillus flavus. Food Control 2013, 32, 359–365. [Google Scholar] [CrossRef]
- Alshannaq, A.F.; Gibbons, J.G.; Lee, M.-K.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018, 8, 16871. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Glassner, H.; Zchori-Fein, E.; Compant, S.; Sessitsch, A.; Katzir, N.; Portnoy, V.; Yaron, S. Characterization of endophytic bacteria from cucurbit fruits with potential benefits to agriculture in melons (Cucumis melo L.). FEMS Microbiol. Ecol. 2015, 91, fiv074. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Xiang, Y.; Shah, N.; Bilal, H.; Yang, D.; Hu, H.; Li, T.; Ji, X.; Li, H. Unraveling the role of contaminants reshaping the microflora in Zea mays seeds from heavy metal-contaminated and pristine environment. Microb. Ecol. 2024, 87, 133. [Google Scholar] [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Cottyn, B.; Regalado, E.; Lanoot, B.; De Cleene, M.; Mew, T.W.; Swings, J. Bacterial populations associated with rice seed in the tropical environment. Phytopathology 2001, 91, 282–292. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Gerhardt, H.; Sievers-Engler, A.; Jahanshah, G.; Pataj, Z.; Ianni, F.; Gross, H.; Lindner, W.; Lämmerhofer, M. Methods for the comprehensive structural elucidation of constitution and stereochemistry of lipopeptides. J. Chromatogr. A 2016, 1428, 280–291. [Google Scholar] [CrossRef]
- Landy, M.; Warren, G.H. Bacillomycin; an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc. Soc. Exp. Biol. Med. 1948, 67, 539–541. [Google Scholar] [CrossRef]
- Kilian, M.; Steiner, U.; Krebs, B.; Junge, H.; Schmiedeknecht, G.; Hain, R. FZB24® Bacillus subtilis—Mode of action of a microbial agent enhancing plant vitality. Pflanzenschutz-Nachrichten Bayer 2000, 1, 72–93. [Google Scholar]
- Benitez, L.B.; Velho, R.V.; Lisboa, M.P.; Medina, L.F.d.C.; Brandelli, A. Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J. Microbiol. 2010, 48, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, H.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.; Liu, W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Physiol. 2020, 163, 102–107. [Google Scholar] [CrossRef]
- Abdelli, F.; Jardak, M.; Elloumi, J.; Stien, D.; Cherif, S.; Mnif, S.; Aifa, S. Antibacterial, anti-adherent and cytotoxic activities of surfactin(s) from a lipolytic strain Bacillus safensis F4. Biodegradation 2019, 30, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef] [PubMed]
- GB/T 13092–2006; Enumeration of Molds Count in Feeds. National Standard of the People’s Republic of China: Beijing, China, 2006.
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef]
- Liu, B.; Tao, T.; Wang, J.; Liu, G.; Xiao, R.; Chen, C. Taxonomy of Bacilli; Science Press: Beijing, China, 2016; Volume 2, pp. 135–169. [Google Scholar]
- Chen, D.; Liu, X.; Li, C.; Tian, W.; Shen, Q.; Shen, B. Isolation of Bacillus amyloliquefaciens S20 and its application in control of eggplant bacterial wilt. Environ. Manag. 2014, 137, 120–127. [Google Scholar] [CrossRef]
- Reang, L.; Bhatt, S.; Tomar, R.S.; Joshi, K.; Padhiyar, S.; Vyas, U.M.; Kheni, J.K. Plant growth promoting characteristics of halophilic and halotolerant bacteria isolated from coastal regions of Saurashtra Gujarat. Sci. Rep. 2022, 12, 4699. [Google Scholar] [CrossRef]
- Li, X.; Munir, S.; Xu, Y.; Wang, Y.; He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 2021, 11, 25441–25449. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Borriss, R. More than anticipated—Production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J. Mol. Microbiol. Biotechnol. 2009, 16, 14–24. [Google Scholar] [CrossRef]
- Bie, X.M.; Lu, Z.X.; Lu, F.X. Identification of fengycin homologues from Bacillus subtilis with ESI-MS/CID. J. Microbiol. Methods 2009, 79, 272–278. [Google Scholar] [CrossRef]
- Iwase, N.; Rahman, M.S.; Ano, T. Production of iturin A homologues under different culture conditions. J. Environ. Sci. 2009, 21, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Castro, M.; Principe, A.; Borioli, G.; Fischer, S.; Mori, G.; Jofré, E. The plant-associated Bacillus amyloliquefaciens strains MEP2 18 and ARP2 3 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J. Appl. Microbiol. 2012, 112, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Roongsawang, N.; Thaniyavarn, J.; Thaniyavarn, S.; Kameyama, T.; Haruki, M.; Imanaka, T.; Morikawa, M.; Kanaya, S. Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: Bacillomycin L, plipastatin, and surfactin. Extremophiles 2002, 6, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Shin, B.S.; Choi, S.K.; Kim, C.K.; Park, S.H. Involvement of acetyl phosphate in the in vivo activation of the response regulator ComA in Bacillus subtilis. FEMS Microbiol. Lett. 2001, 195, 179–183. [Google Scholar] [CrossRef]
- Mofid, M.R.; Marahiel, M.A.; Ficner, R.; Reuter, K. Crystallization and preliminary crystallographic studies of Sfp: A phosphopantetheinyl transferase of modular peptide synthetases. Acta Crystallogr. D 1999, 55, 1098–1100. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Blanco Crivelli, X.; Cundon, C.; Bonino, M.P.; Sanin, M.S.; Bentancor, A. The complex and changing genus bacillus: A diverse bacterial powerhouse for many applications. Bacteria 2024, 3, 256–270. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Yang, X.; Mao, Y.; Chen, L.; Guan, X.; Wang, Z.; Huang, T. Structural characteristics, biotechnological production and applications of exopolysaccharides from Bacillus sp.: A comprehensive review. Carbohydr. Polym. 2025, 355, 123363. [Google Scholar] [CrossRef]
- Einloft, T.C.; Bolzan De Oliveira, P.; Radünz, L.L.; Dionello, R.G. Biocontrol capabilities of three Bacillus isolates towards aflatoxin B1 producer A. flavus in vitro and on maize grains. Food Control 2021, 125, 107978. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Investigation and application of Bacillus licheniformis volatile compounds for the biological control of toxigenic Aspergillus and Penicillium spp. ACS Omega 2019, 4, 17186–17193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Li, H.; Ning, H.; Chen, J.; Chen, Z.; Zhao, H.; Zhao, H. Mechanism Underlying Bacillus subtilis BS-Z15 Metabolite-Induced Prevention of Grain Contamination by Aspergillus flavus. Toxins 2023, 15, 667. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, F.; Pothuvattil, N.S.; Tounsi, S.; Saadaoui, I.; Trigui, M. Synthesis of silver nanoparticles using Bacillus velezensis M3-7 lipopeptides: Enhanced antifungal activity and potential use as a biocontrol agent against Fusarium crown rot disease of wheat seedlings. Int. J. Food Microbiol. 2023, 407, 110420. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Singh, B.P.; Cherepanova, E.A.; Burkhanova, G.F.; Khairullin, R.M. Prospects and applications of lipopeptide-producing bacteria for plant protection (Review). Appl. Biochem. Microbiol. 2020, 56, 15–28. [Google Scholar] [CrossRef]
- Kim, P.I.; Ryu, J.; Kim, Y.H.; Chi, Y.-T. Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010, 20, 138–145. [Google Scholar] [CrossRef]
- Tang, Q.; Bie, X.; Lu, Z.; Lv, F.; Tao, Y.; Qu, X. Effects of fengycin from Bacillus subtilis fmbJ on apoptosis and necrosis in Rhizopus stolonifer. J. Microbiol. 2014, 52, 675–680. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Wang, K.; Brian, K.; Liu, C.; Gu, Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010, 101, 292–297. [Google Scholar] [CrossRef]


| Antimicrobial Lipopeptides | Target Gene | Primers Name | Primers Sequences (5′→3′) | Size/bp |
|---|---|---|---|---|
| Surfactin | srf AA | srfAA-F | GCTTGTTAGGTCATGTGCGCAAG | 722 |
| srfAA-R | CTTTGCTGAGTCAGGAGCACATTCG | |||
| srf AD | srfAD-F | CCTAAGGAAGGAACATCCGAAGC | 606 | |
| srfAD-R | GGACGGGTGATTGAATCATGTGAG | |||
| Fengycin | fenA | fenA-F | GGTTGACTCCCATTATCCTGAGGAAC | 740 |
| fenA-R | GAACACCGATCGGCACATCATCTT | |||
| fenB | fenB-F | CTATAGTTTGTTGACGGCTC | 1600 | |
| fenB-R | CAGCACTGGTTCTTGTCGCA | |||
| Iturin | ituA | ituA-F | ATGTATACCAGTCAATTCC | 1047 |
| ituA-R | GATCCGAAGCTGACAATAG | |||
| ituB | ituB-F | CGATCGGCTGGATTTGATGGTG | 722 | |
| ituB-R | GCTTCATGATGCGGATGCAGAC | |||
| ituD | ituD-F | GCAGGCCATAGCTTAGGCGAATATTC | 361 | |
| ituD-R | AGGCGGATCGTATCATCGAACTG | |||
| Bacillomycin D | bmyA | bmyA-F | AAAGCGGCTCAAGAAGCGAAACCC | 1200 |
| bmyA-R | CGATTCAGCTCATCGACCAGGTAGGC | |||
| bmyB | bmyB-F | CATGCAAATCCTGCATCAAGTCGTG | 816 | |
| bmyB-R | CGCACAATTGATTCAAGCAGAGCTG | |||
| bmyC | bmyC-F | GAAGGACACGGCAGAGAGTC | 875 | |
| bmyC-R | CACTGATGACTGTTCATGCT |
| Strain No. | 15% Water Content | 20% Water Content | ||
|---|---|---|---|---|
| Mean Spore Number (×105 CTU/mL) | Inhibition (%) | Mean Spore Number (×105 CTU/mL) | Inhibition (%) | |
| ZH179 | 3.20 ± 0.20 a | 76.3 | 9.53 ± 1.03 a | 66.0 |
| ZH99 | 4.47 ± 0.32 c | 66.9 | 10.3 ± 1.06 a | 63.2 |
| ZH409 | 5.53 ± 0.57 b | 59.0 | 9.33 ± 0.81 a | 66.7 |
| Test Items | ZH99 | ZH179 | ZH409 |
|---|---|---|---|
| Methyl red | - | - | - |
| Starch hydrolysis | + | + | + |
| V-P | - | - | - |
| Fermentation of sugars or indoleacetic acid | + | + | + |
| indole | + | + | + |
| Gelatin liquefaction | + | + | + |
| Gram stain | + | + | + |
| hydrogen sulfide | - | - | - |
| Utilization of citrate | + | + | + |
| 4% KOH reaction | - | - | - |
| 3% H2O2 reaction | + | + | + |
| Utilization of malonate | - | - | - |
| Urease reaction | + | + | + |
| Strains | Lipopeptide Antibiotic Class | Mass-to-Charge Ratio (m/z) | Calculate the Molecular Weight (Da) | Ionic Type | Relative Content (%) | Literature |
|---|---|---|---|---|---|---|
| ZH179 | Surfactin | 1076.67 | 1075.72 | [M+H]+ | 2.34 | [42] |
| Surfactin | 1062.62 | 1061.70 | [M+H]+ | 0.83 | [42] | |
| Surfactin | 1050.60 | 1049.70 | [M+H]+ | 0.22 | [42] | |
| Surfactin C (C15) | 1058.66 | 1035.70 | [M+Na]+ | 2.59 | [42] | |
| Fengycin | 767.41 | 1532.87 | [M+2H]2+ | 0.92 | [43] | |
| Fengycin | 766.40 | 1530.90 | [M+2H]2+ | 5.40 | [43] | |
| Iturin A2 | 1043.50 | 1044.66 | [M+H]+ | 0.99 | [44] | |
| IturinB (C15) | 1058.61 | 1057.60 | [M+H]+ | 0.43 | [45] | |
| Bacillomycin L (C15) | 525.28 | 1048.50 | [M+2H]2+ | 2.42 | [46] | |
| Bacillomycin L | 1063.56 | 1062.56 | [M+H]+ | 0.93 | [46] | |
| Bacillomycin D | 1067.53 | 1044.55 | [M+Na]+ | 13.83 | [47] | |
| Bacillomycin D | 1081.55 | 1058.56 | [M+Na]+ | 7.53 | [47] | |
| Bacillomycin D | 1095.57 | 1072.58 | [M+Na]+ | 4.69 | [47] | |
| ZH409 | Surfactin | 1062.66 | 1061.70 | [M+H]+ | 0.89 | [42] |
| Surfactin | 1076.68 | 1075.72 | [M+H]+ | 2.40 | [42] | |
| Surfactin | 1065.53 | 1064.70 | [M+H]+ | 3.04 | [42] | |
| Surfactin | 1044.65 | 1021.67 | [M+Na]+ | 3.22 | [42] | |
| Fengycin | 753.45 | 1504.84 | [M+2H]2+ | 1.41 | [43] | |
| Fengycin | 767.41 | 1532.87 | [M+2H]2+ | 11.08 | [43] | |
| IturinB (C15) | 1058.66 | 1057.60 | [M+H]+ | 2.07 | [45] | |
| Iturin A6 | 1072.69 | 1071.50 | [M+H]+ | 0.55 | [48] | |
| Bacillomycin L (C15) | 525.28 | 1048.50 | [M+2H]2+ | 1.94 | [47] | |
| Bacillomycin L | 532.36 | 1062.56 | [M+2H]2+ | 0.23 | [46] | |
| Bacillomycin D | 537.32 | 1072.58 | [M+2H]2+ | 0.05 | [47] | |
| ZH99 | Surfactin | 1062.66 | 1061.70 | [M+H]+ | 1.99 | [42] |
| Surfactin | 1076.68 | 1075.72 | [M+H]+ | 4.30 | [42] | |
| Fengycin | 774.65 | 1546.89 | [M+2H]2+ | 0.07 | [43] | |
| Iturin A2 | 1044.66 | 1043.50 | [M+H]+ | 0.89 | [45] | |
| Iturin B (C15) | 1058.62 | 1057.60 | [M+H]+ | 0.23 | [45] | |
| Iturin A6 | 536.28 | 1071.50 | [M+2H]2+ | 0.22 | [48] | |
| Iturin C | 1054.52 | 1053.51 | [M+H]+ | 7.40 | [45] | |
| Iturin C | 1104.67 | 1081.55 | [M+Na]+ | 1.20 | [45] | |
| Bacillomycin L (C14) | 518.40 | 1034.50 | [M+2H]2+ | 0.26 | [46] | |
| Bacillomycin L (C15) | 1049.60 | 1048.50 | [M+H]+ | 0.66 | [47] | |
| Bacillomycin D | 1067.53 | 1044.55 | [M+Na]+ | 9.65 | [47] | |
| Bacillomycin D | 1095.57 | 1072.58 | [M+Na]+ | 3.53 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Li, M.; Zhang, S.; Yang, Y.; Zhu, F.; Li, X.; Munir, S.; He, P.; He, P.; Wu, Y.; et al. Study on the Inhibitory Effects of Three Endophytic Bacillus Strains on Aspergillus flavus in Maize. Metabolites 2025, 15, 268. https://doi.org/10.3390/metabo15040268
Ma S, Li M, Zhang S, Yang Y, Zhu F, Li X, Munir S, He P, He P, Wu Y, et al. Study on the Inhibitory Effects of Three Endophytic Bacillus Strains on Aspergillus flavus in Maize. Metabolites. 2025; 15(4):268. https://doi.org/10.3390/metabo15040268
Chicago/Turabian StyleMa, Siyu, Min Li, Siqi Zhang, Yin Yang, Fengsha Zhu, Xingyu Li, Shahzad Munir, Pengfei He, Pengbo He, Yixin Wu, and et al. 2025. "Study on the Inhibitory Effects of Three Endophytic Bacillus Strains on Aspergillus flavus in Maize" Metabolites 15, no. 4: 268. https://doi.org/10.3390/metabo15040268
APA StyleMa, S., Li, M., Zhang, S., Yang, Y., Zhu, F., Li, X., Munir, S., He, P., He, P., Wu, Y., He, Y., & Tang, P. (2025). Study on the Inhibitory Effects of Three Endophytic Bacillus Strains on Aspergillus flavus in Maize. Metabolites, 15(4), 268. https://doi.org/10.3390/metabo15040268






