Optimization of Tunisian Myrtus communis L. Essential Oil Extraction by Complete Factorial Experimental Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Relative Water Content
2.3. Extraction Methods of Myrtle Essential Oil
2.4. Multi-Level Factorial Experimental Design
2.5. Determination of Essential Oil Yield
2.6. Antioxidant Activity
2.7. Chemical Composition
2.8. Statistical Analysis
3. Results
3.1. Relative Water Content
3.2. Essential Oil Yield
3.2.1. Analysis of Factors’ Effects on Yield
3.2.2. Effect of Two-Factor Interactions on Yield
3.3. Antioxidant Activity
3.3.1. Analysis of the Effects of Factors on Antioxidant Activity
3.3.2. Effect of Two-Factor Interactions on the Antioxidant Activity
3.4. Chemical Composition of Myrtle Essential Oil
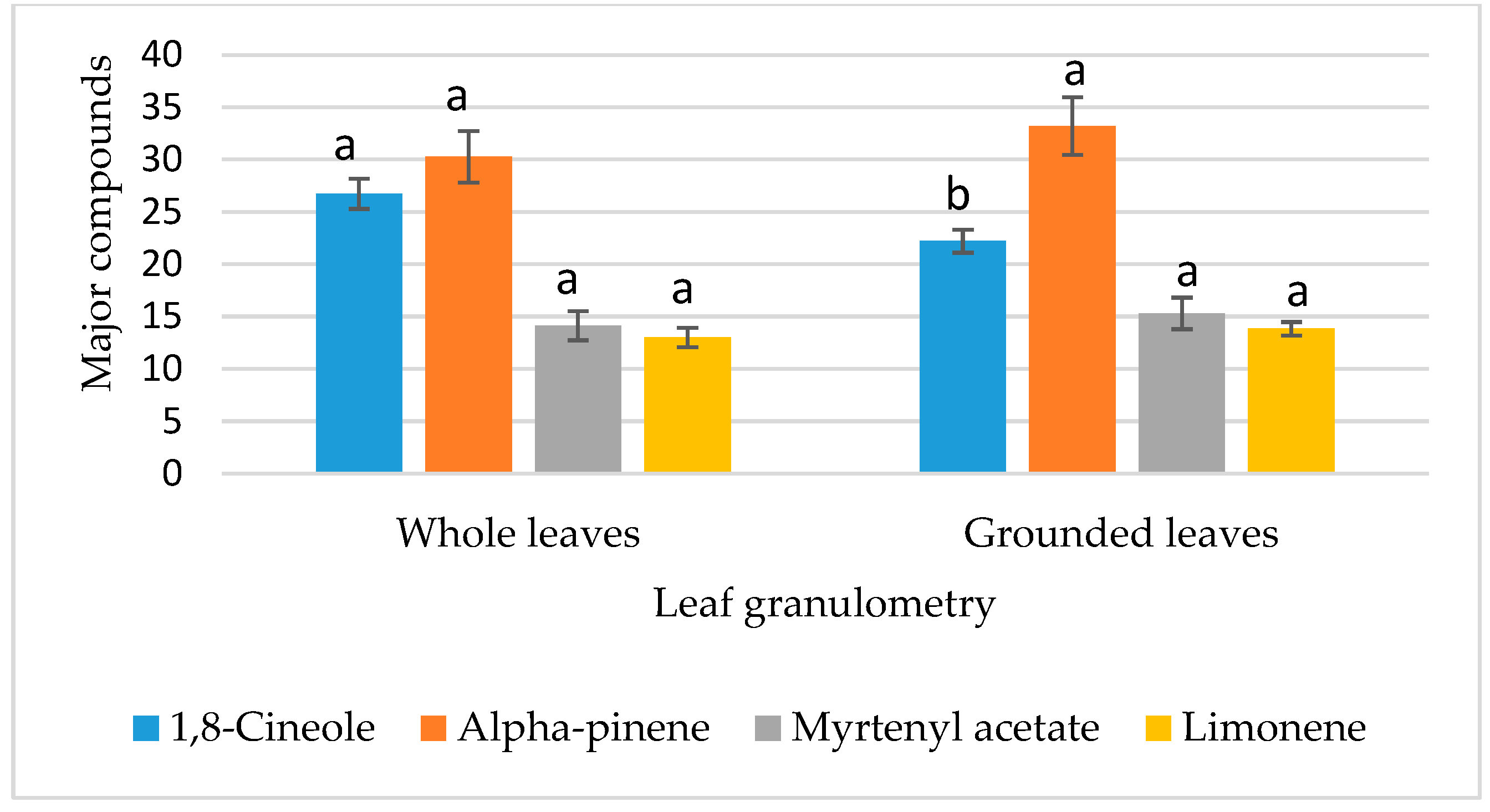
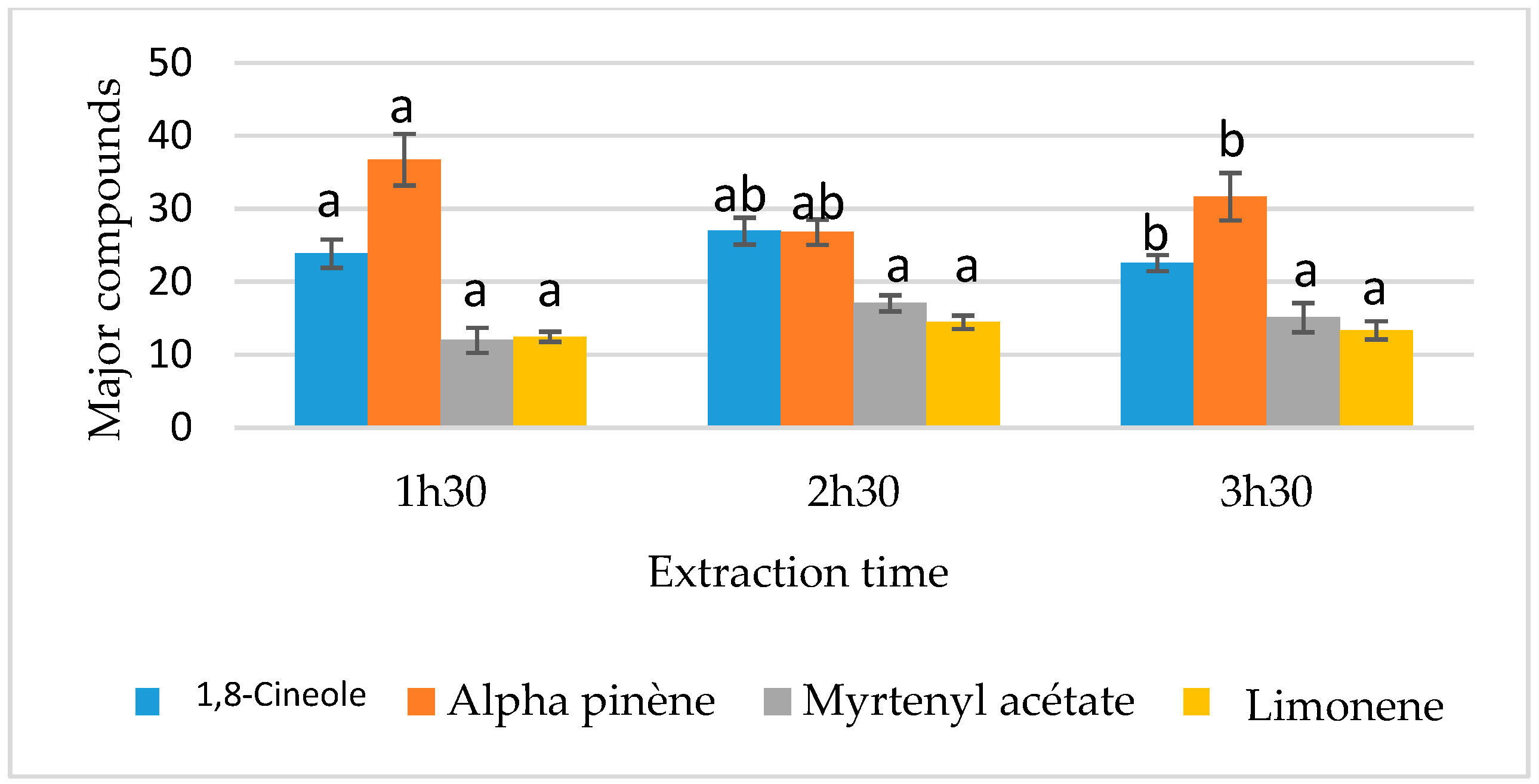
3.4.1. Analysis of the Effects of Factors on Chemical Composition
Analysis of the Effects of Factors on 1,8-Cineole Content
Analysis of the Effects of Factors on α-Pinene Content
Analysis of the Effects of Factors on Myrtenyl Acetate Content
Analysis of the Effects of Factors on the Content of Limonene
3.4.2. Effect of Interactions Between the Two Factors on 1,8-Cineole Content
3.5. Analysis of the Multi-Level Experimental Design
3.5.1. Block A: Fresh and Whole Leaves
Block A Multi-Response Optimization
3.5.2. Block B: Dry and Ground Leaves
Block B Multi-Response Optimization
3.5.3. Block C: Fresh and Ground Leaves
Block C Multi-Response Optimization
3.5.4. Block D: Dry and Whole Leaves
Block D Multi-Response Optimization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-diphényl 1-picrylhydrazyle |
| DW | dry weight |
| E.O | Essential oil |
| FW | Fresh weight |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| GDL | Ground dry leaves |
| GFL | Ground fresh Leaves |
| RWC | Relative water content |
| TE | Trolox equivalent |
| TW | Turgid weight |
| V/M | Water to plant material ratio |
| WDL | Whole dry Leaves |
| WFL | Whole fresh Leaves |
References
- Medda, S.; Fadda, A.; Dessena, L.; Mulas, M. Quantification of Total Phenols, Tannins, Anthocyanins Content in Myrtus communis L. and Antioxidant Activity Evaluation in Function of Plant Development Stages and Altitude of Origin Site. Agronomy 2021, 11, 1059. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef]
- Aidi Wannes, W.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar]
- Ammar, A.H.; Zagrouba, F.; Romdhane, M. Optimization of operating conditions of Tunisian myrtle (Myrtus communis L.) essential oil extraction by a hydrodistillation process using a 24 complete factorial design. Flavour Fragr. J. 2010, 25, 503–507. [Google Scholar] [CrossRef]
- Messaoud, C.; Afif, M.; Boulila, A.; Rejeb, M.N.; Boussaid, M. Genetic variation of Tunisian Myrtus communis L. (Myrtaceae) populations assessed by isozymes and RAPDs. Ann. For. Sci. 2007, 64, 845–853. [Google Scholar] [CrossRef][Green Version]
- Paap, T.; Santini, A.; Rodas, C.A.; Granados, G.M.; Pecori, F.; Wingfield, M.J. Myrtus communis in Europe threatened by the pandemic and South African strains of the myrtle rust pathogen Austropuccinia psidii (Sphaerophragmiaceae, Pucciniales). NeoBiota 2023, 84, 41–46. [Google Scholar] [CrossRef]
- Mohamadi, Y.; Lograda, T.; Ramdani, M.; Figueredo, G.; Chalard, P. Chemical composition and antimicrobial activity of Myrtus communis essential oils from Algeria. Biodiversitas 2021, 22. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Harassi, Y.; Tilaoui, M.; Idir, A.; Frédéric, J.; Baudino, S.; Ajouaoi, S.; Mouse, H.A.; Zyad, A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2019, 16, 20180100. [Google Scholar] [CrossRef]
- Hans, M.; Charpentier, M.; Huch, V.; Jauch, J.; Bruhn, T.; Bringmann, G.; Quandt, D. Stereoisomeric Composition of Natural Myrtucommulone A. J. Nat. Prod. 2015, 78, 2381–2389. [Google Scholar] [CrossRef]
- Sisay, M.; Gashaw, T. Ethnobotanical, Ethnopharmacological, and Phytochemical Studies of Myrtus communis Linn: A Popular Herb in Unani System of Medicine. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1035–1043. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Milani, F.; Todero, S.; Berera, P.; Maggi, F.; Santagostini, L.; Fico, G. Botanic Garden as a Factory of Molecules: Myrtus communis L. subsp. communis as a Case Study. Plants 2022, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Izgi, K.; Iskender, B.; Jauch, J.; Sezen, S.; Cakir, M.; Charpentier, M.; Canatan, H.; Sakalar, C. Myrtucommulone-A Induces both Extrinsic and Intrinsic Apoptotic Pathways in Cancer Cells: Myrtucommulone-A Effects Apoptotic Genes. J. Biochem. Mol. Toxicol. 2015, 29, 432–439. [Google Scholar] [CrossRef]
- Maxia, A.; Lancioni, M.C.; Balia, A.N.; Alborghetti, R.; Pieroni, A.; Loi, M.C. Medical ethnobotany of the Tabarkins, a Northern Italian (Ligurian) minority in south-western Sardinia. Genet. Resour. Crop Evol. 2008, 55, 911–924. [Google Scholar] [CrossRef]
- Uysal, S.; Sinan, K.I.; Zengin, G. Assessment of antioxidant and enzyme inhibition properties of Myrtus communis L. leaves. Int. J. Second. Metab. 2023, 10, 166–174. [Google Scholar] [CrossRef]
- Aykac, A.; Ozbeyli, D.; Uncu, M.; Ertaş, B.; Kılınc, O.; Şen, A.; Orun, O.; Sener, G. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene 2019, 689, 194–201. [Google Scholar] [CrossRef]
- Giuliani, C.; Moretti, R.M.; Bottoni, M.; Santagostini, L.; Fico, G.; Montagnani Marelli, M. The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells. Plants 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E. Selection of Optimal Operating Conditions for Extraction of Myrtus communis L. Essential Oil by the Steam Distillation Method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef]
- Zermane, A.; Larkeche, O.; Meniai, A.-H.; Crampon, C.; Badens, E. Optimization of essential oil supercritical extraction from Algerian Myrtus communis L. leaves using response surface methodology. J. Supercrit. Fluids 2014, 85, 89–94. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Madhumita, M.; Guha, P.; Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 2019, 138, 111578. [Google Scholar] [CrossRef]
- Tan, Q.; Kieu, X.; Hong, N. Application of response surface methodology (RSM) in conditionoptimization for essential oil production from Citrus latifolia. Emir. J. Food Agric. 2012, 24, 25. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Khouja, M.; Elaissi, A.; Ghazghazi, H.; Boussaid, M.; Khouja, M.L.; Khaldi, A.; Messaoud, C. Variation of Essential Oil Composition, Antioxidant and Anticholinesterase Activities between Pinus halepensis Mill. Plant Organs. J. Essent. Oil Bear. Plants 2020, 23, 1450–1462. [Google Scholar] [CrossRef]
- Aissi, O.; Boussaid, M.; Messaoud, C. Essential oil composition in natural populations of Pistacia lentiscus L. from Tunisia: Effect of ecological factors and incidence on antioxidant and antiacetylcholinesterase activities. Ind. Crops Prod. 2016, 91, 56–65. [Google Scholar] [CrossRef]
- Moura, D.; Vilela, J.; Saraiva, S.; Monteiro-Silva, F.; De Almeida, J.M.M.M.; Saraiva, C. Antimicrobial Effects and Antioxidant Activity of Myrtus communis L. Essential Oil in Beef Stored under Different Packaging Conditions. Foods 2023, 12, 3390. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Nenni, M.; Karahüseyin, S. Antioxidant Capacity of Essential Oils Obtained from Myrtus communis L. and Citrus sinensis (L.) Osbeck Plants Widely Consumed in Adana Region. Cumhur. Sci. J. 2023, 44, 470–473. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the smell of wood—Identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef] [PubMed]
- Zeller, A.; Rychlik, M. Character Impact Odorants of Fennel Fruits and Fennel Tea. J. Agric. Food Chem. 2006, 54, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Aroma-active compounds of Pinus densiflora (red pine) needles. Flavour Fragr. J. 2004, 19, 532–537. [Google Scholar] [CrossRef]
- Dhouibi, I.; Flamini, G.; Bouaziz, M. Comparative Study on the Essential Oils Extracted from Tunisian Rosemary and Myrtle: Chemical Profiles, Quality, and Antimicrobial Activities. ACS Omega 2023, 8, 6431–6438. [Google Scholar] [CrossRef]
- Mansour, R.B.; Beji, R.S.; Wasli, H.; Zekri, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Gastroprotective Effect of Microencapsulated Myrtus communis Essential Oil against Ethanol/HCl-Induced Acute Gastric Lesions. Molecules 2022, 27, 1566. [Google Scholar] [CrossRef]
- Ibrahim, F.; Fouad, R.; El-Hallouty, S.; Hendawy, S.; Omer, E.A.; Mohammed, R. Egyptian Myrtus communis L. Essential oil Potential role as invitro Antioxidant, Cytotoxic and α-amylase Inhibitor. Egypt. J. Chem. 2021, 64, 3005–3017. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.-A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative Study of Essential Oils Extracted from Algerian Myrtus communis L. Leaves Using Microwaves and Hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef]
- Messaoud, C.; Boussaid, M. Myrtus communis Berry Color Morphs: A Comparative Analysis of Essential Oils, Fatty Acids, Phenolic Compounds, and Antioxidant Activities. Chem. Biodivers. 2011, 8, 300–310. [Google Scholar] [CrossRef]
- Brada, M.; Tabti, N.; Boutoumi, H.; Wathelet, J.P.; Lognay, G. Composition of the essential oil of leaves and berries of Algerian myrtle (Myrtus communis L.). J. Essent. Oil Res. 2012, 24, 1–3. [Google Scholar] [CrossRef]
- Bazzali, O.; Tomi, F.; Casanova, J.; Bighelli, A. Occurrence of C8–C10 esters in Mediterranean Myrtus communis L. leaf essential oil. Flavour Fragr. J. 2012, 27, 335–340. [Google Scholar] [CrossRef]
- Yarahmadi, R.; Mumivand, H.; Ehtesham Nia, A.; Raji, M.R.; Argento, S. Natural Diversity in Total Phenol, Flavonoids, Antioxidant Properties, and Essential Oil Composition of Iranian Populations of Myrtus communis L. Plants 2024, 13, 3458. [Google Scholar] [CrossRef] [PubMed]
- Smeti, S.; Tibaoui, S.; Bertolín, J.R.; Yagoubi, Y.; Mekki, I.; Joy, M.; Atti, N. Effects of myrtle (Myrtus communis L.) essential oils as dietary antioxidant supplementation on carcass and meat quality of goat meat. J. Anim. Physiol. Anim. Nutr. 2021, 105, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Bahadırlı, N.P.; Kahramanoğlu, İ.; Wan, C. Exposure to Volatile Essential Oils of Myrtle (Myrtus communis L.) Leaves for Improving the Postharvest Storability of Fresh Loquat Fruits. J. Food Qual. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Dejam, M.; Farahmand, Y. Essential Oil Content and Composition of Myrtle (Myrtus communis L.) Leaves from South of Iran. J. Essent. Oil Bear. Plants 2017, 20, 869–872. [Google Scholar] [CrossRef]
- Karaoğul, E.; Alma, M.H. Solvent-free microwave and hydro-distillation extraction of essential oils from the sawdust of pines: Correlation with heat-map. BioResources 2019, 14, 8229–8240. [Google Scholar] [CrossRef]
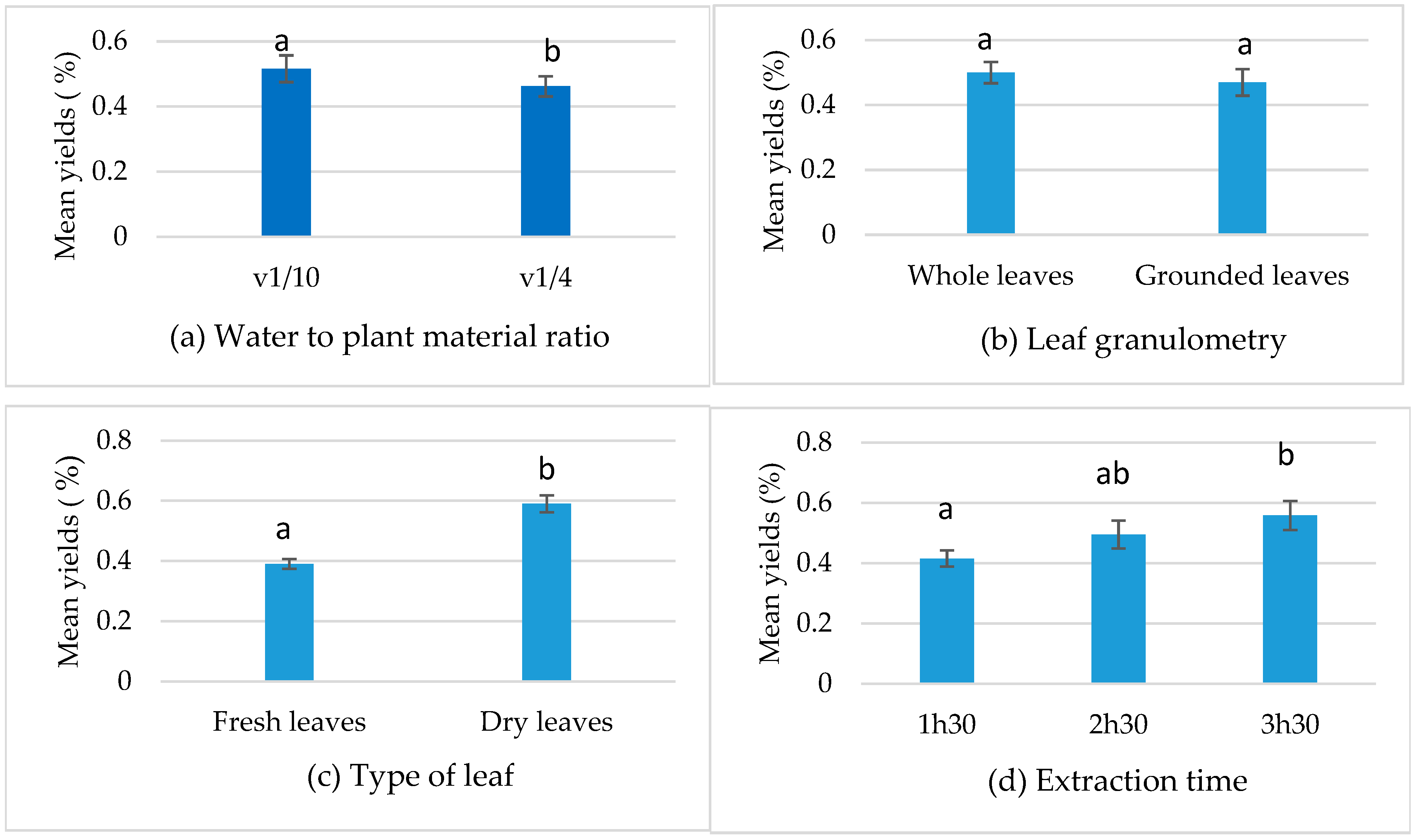

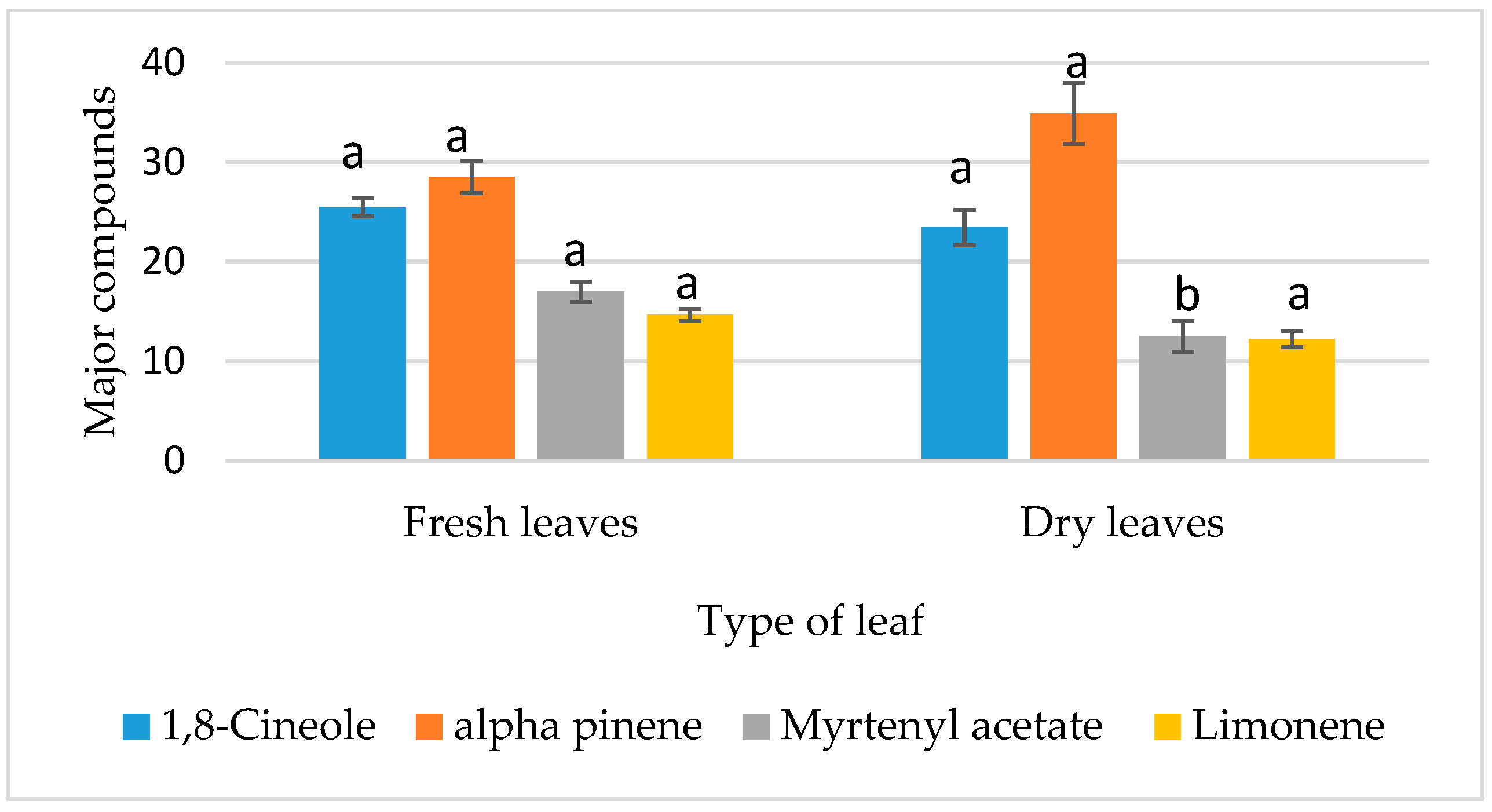

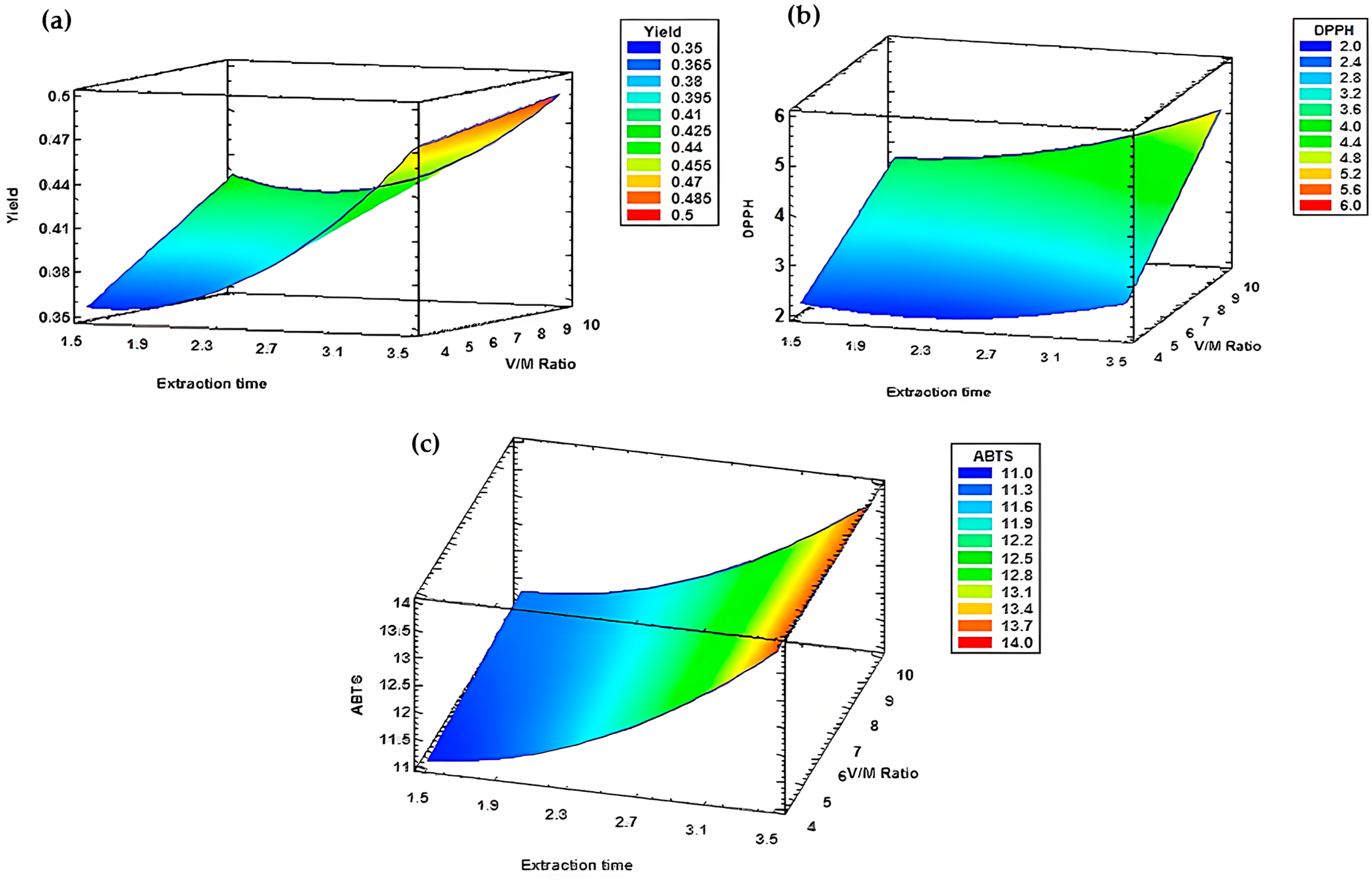


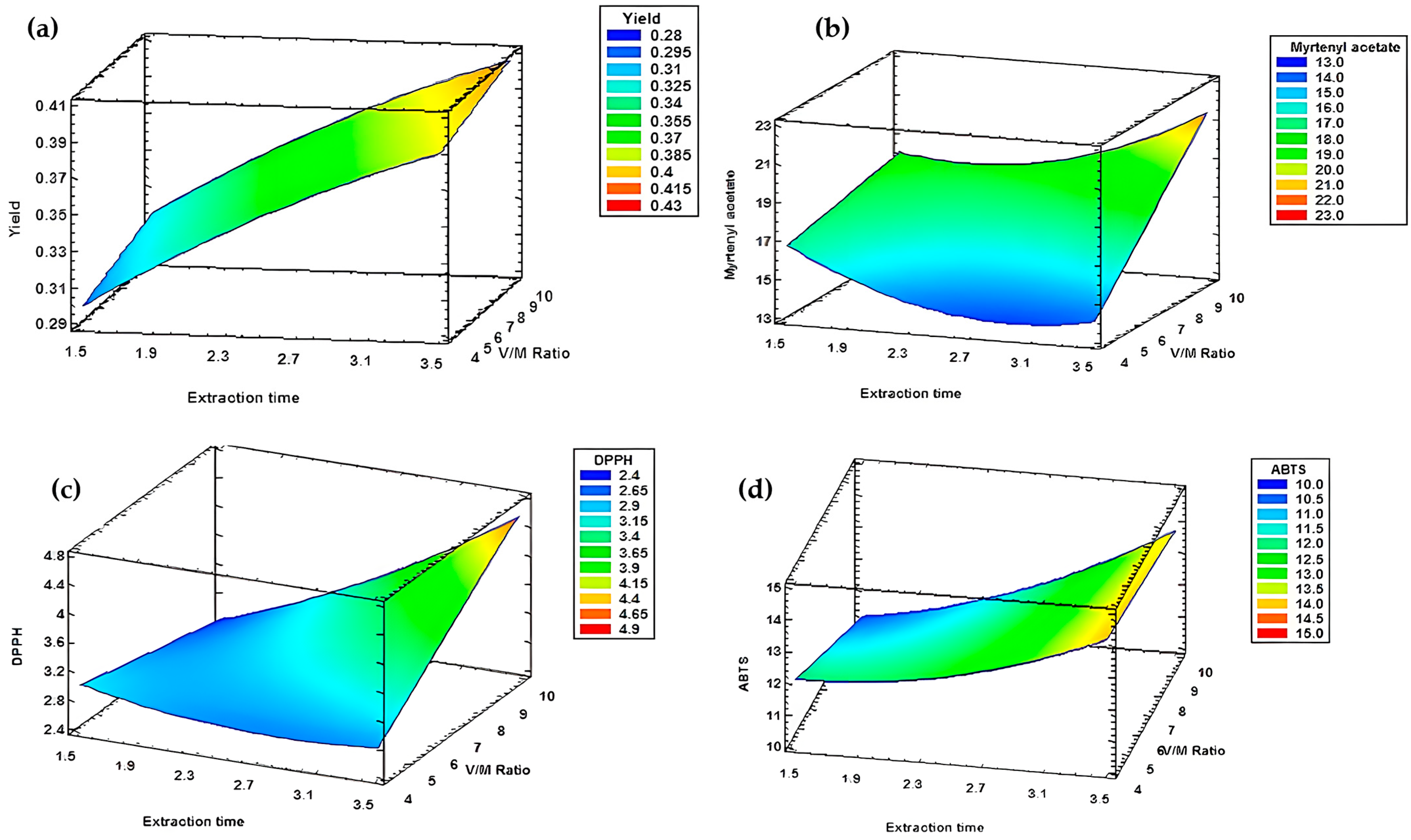
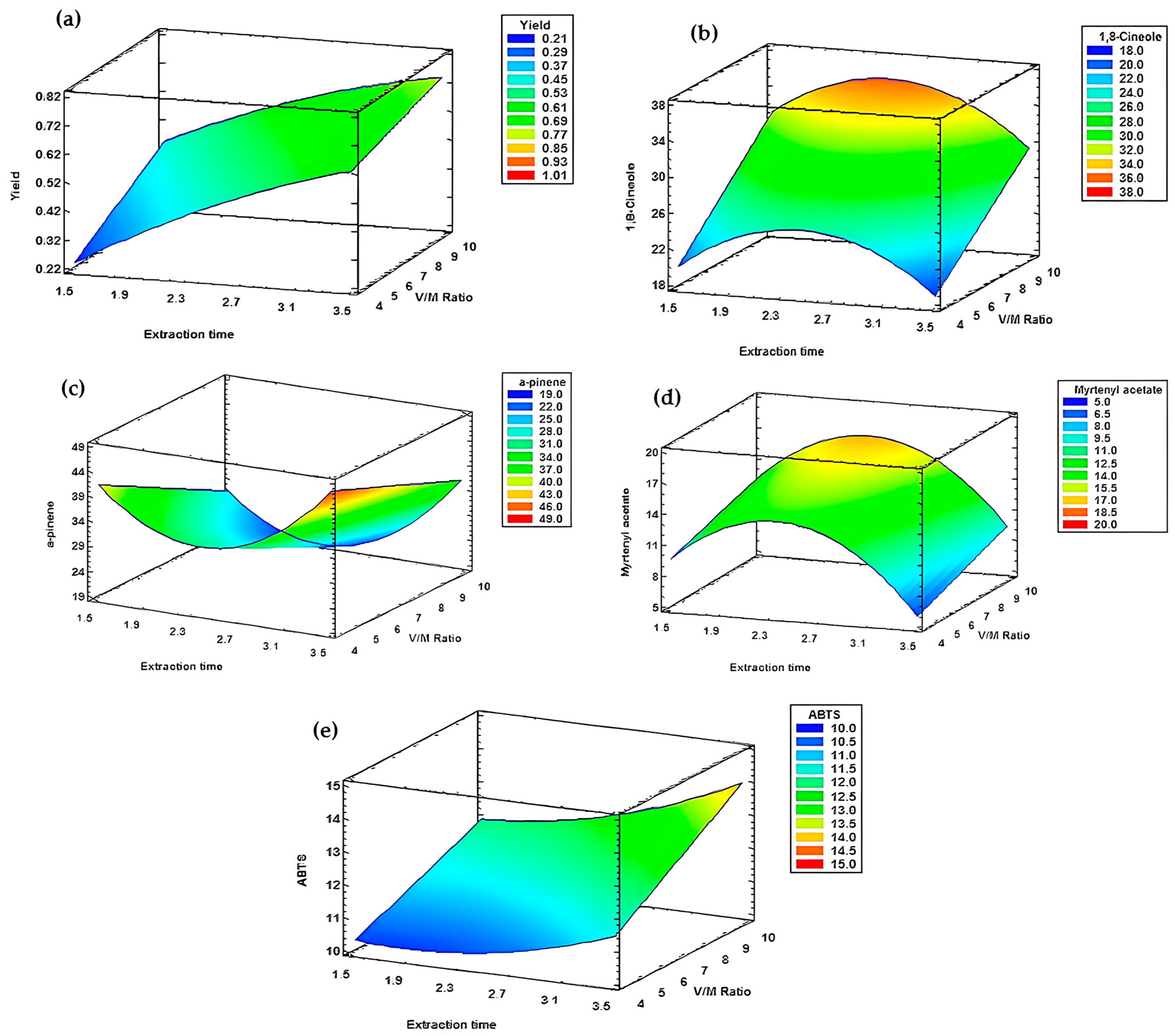
| Experiment | Hydrodistillation Modalities | E.O Yield (%) |
|---|---|---|
| H1 | WFL, Duration 1 h 30 min, V/M 1/10 | 0.42 ij ± 0.003 |
| H2 | WFL, Duration 2 h 30 min, V/M 1/10 | 0.43 i ± 0.003 |
| H3 | WFL, Duration 3 h 30 min, V/M 1/10 | 0.49 gh ± 0.003 |
| H4 | WFL, Duration 1 h 30 min, V/M 1/4 | 0.36 mn ± 0.005 |
| H5 | WFL, Duration 2 h 30 min, V/M 1/4 | 0.37 lm ± 0.003 |
| H6 | WFL, Duration 3 h 30 min, V/M 1/4 | 0.48 gh ± 0.006 |
| H7 | GFL, Duration 1 h 30 min, V/M 1/10 | 0.32 O ± 0.003 |
| H8 | GFL, Duration 2 h 30 min, V/M 1/10 | 0.37 m ± 0.005 |
| H9 | GFL, Duration 3 h 30 min, V/M 1/10 | 0.41 jk ± 0.003 |
| H10 | GFL, Duration 1 h 30 min, V/M 1/4 | 0.3 po ± 0.005 |
| H11 | GFL, Duration 2 h 30 min, V/M 1/4 | 0.35 n ± 0.000 |
| H12 | GFL, Duration 3 h 30 min, V/M 1/4 | 0.39 lk ± 0.005 |
| H13 | WDL, Duration 1 h 30 min, V/M 1/10 | 0.47 h ± 0.003 |
| H14 | WDL, Duration 2 h 30 min, V/M 1/10 | 0.61 d ± 0.008 |
| H15 | WDL, Duration 3 h 30 min, V/M 1/10 | 0.77 a ± 0.006 |
| H16 | WDL, Duration 1 h 30 min, V/M 1/4 | 0.47 q ± 0.014 |
| H17 | WDL, Duration 2 h 30 min, V/M 1/4 | 0.49 gh ± 0.005 |
| H18 | WDL, Duration 3 h 30 min, V/M 1/4 | 0.61 d ± 0.003 |
| H19 | GDL, Duration 1 h 30 min, V/M 1/10 | 0.51 f ± 0.012 |
| H20 | GDL, Duration 2 h 30 min, V/M 1/10 | 0.69 c ± 0.005 |
| H21 | GDL, Duration 3 h 30 min, V/M 1/10 | 0.72 b ± 0.011 |
| H22 | GDL, Duration 1 h 30 min, V/M 1/4 | 0.47 h ± 0.006 |
| H23 | GDL, Duration 2 h 30 min, V/M 1/4 | 0.57 e ± 0 |
| H24 | GDL, Duration 3 h 30 min, V/M 1/4 | 0.61 d ± 0.003 |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 0.175 | 1 | 0.175 | 115.00 | 0 ** |
| B: Leaf granulometry | 0.000 | 1 | 0.000 | 0.00 | 0.959 ns |
| C: Water to plant material ratio | 0.039 | 1 | 0.039 | 25.75 | 0.001 ** |
| D: Extraction time | 0.122 | 2 | 0.061 | 39.95 | 0 ** |
| Experiment | DPPH (mg TE/g E.O) | ABTS (mg TE/g E.O) |
|---|---|---|
| H1 | 3.788 fg ± 0.011 | 11.330 h ± 0.058 |
| H2 | 3.988 f ± 0.012 | 12.033 f ± 0.118 |
| H3 | 5.133 d ± 0.006 | 13.706 c ± 0.097 |
| H4 | 2.166 n ± 0.012 | 11.080 i ± 0.096 |
| H5 | 2.230 mn ± 0.010 | 11.796 g ± 0.029 |
| H6 | 2.573 lm ± 0.012 | 13.830 bc ± 0.022 |
| H7 | 2.623 kl ± 0.040 | 10.480 j ± 0.097 |
| H8 | 3.076 hij ± 0.020 | 11.217 hi ± 0.040 |
| H9 | 4.710 e ± 0.014 | 13.950 ab ± 0.049 |
| H10 | 2.853 jkl ± 0.054 | 11.896 fg ± 0.029 |
| H11 | 3.000 ijk ± 0.011 | 12.836 d ± 0.022 |
| H12 | 2.640 kl ± 0.592 | 13.930 ab ± 0.011 |
| H13 | 2.614 kl ± 0.027 | 11.986 fg ± 0.022 |
| H14 | 2.743 jkl ± 0.031 | 12.506 e ± 0.029 |
| H15 | 5.637 c ± 0.057 | 14.053 a ± 0.011 |
| H16 | 2.997 ijk ± 0.054 | 10.283 k ± 0.022 |
| H17 | 3.227 hi ± 0.005 | 10.466 j ± 0.017 |
| H18 | 3.427 gh ± 0.025 | 11.384 h ± 0.047 |
| H19 | 1.340 o ± 0 | 8.780 n ± 0.019 |
| H20 | 3.610 g ± 0.027 | 9.350 m ± 0.009 |
| H21 | 5.453 cd ± 0.024 | 9.930 l ± 0.009 |
| H22 | 2.108 n ± 0.036 | 8.101 o ± 0.043 |
| H23 | 5.100 d ± 0 | 9.334 m ± 0 |
| H24 | 7.477 a ± 0.016 | 11.986 fg ± 0.205 |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 2.007 | 1 | 2.007 | 3.50 | 0.094 ns |
| B: Leaf granulometry | 0.493 | 1 | 0.493 | 0.86 | 0.378 ns |
| C: V/M ratio | 1.000 | 1 | 1.000 | 1.75 | 0.219 ns |
| D: Extraction time | 17.429 | 2 | 8.714 | 15.20 | 0.001 ** |
| Source | Sum of Squares | DF | Mean square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 16.517 | 1 | 16.517 | 36.73 | 0 ** |
| B: Leaf granulometry | 6.668 | 1 | 6.668 | 14.83 | 0.004 ** |
| C: V/M ratio | 0.246 | 1 | 0.246 | 0.55 | 0.478 ns |
| D: Extraction time | 23.407 | 2 | 11.703 | 26.03 | 0 ** |
| Hydrodistillation | α-Pinene | Limonene | 1,8-Cineole | Myrtenyl-Acetate |
|---|---|---|---|---|
| RT:5.479 | RT:7.911 | RT: 7.973 | RT: 17.375 | |
| RI: 936 | RI: 1033 | RI: 1035 | RI: 1333 | |
| H1 | 30.66 m ± 0.008 | 12.06 n ± 0.020 | 27.84 f ± 0.023 | 14.11 l ± 0.020 |
| H2 | 21.02 w ± 0.015 | 17.05 b ± 0.026 | 31.15 b ± 0.046 | 21.33 c ± 0.017 |
| H3 | 27.92 r ± 0.012 | 16.5 e ± 0.014 | 24.14 l ± 0.066 | 15.22 j ± 0.014 |
| H4 | 31.74 j ± 0.008 | 13.11 l ± 0.017 | 27.19 h ± 0.020 | 12.63 o ± 0.018 |
| H5 | 30.04 n ± 0.020 | 11.68 q ± 0.008 | 26.10 i ± 0.031 | 14.62 k ± 0.015 |
| H6 | 18.55 y ± 0.017 | 17.77 a ± 0.014 | 23.66 m ± 0.031 | 23.04 a ± 0.029 |
| H7 | 26.42 t ± 0.014 | 16.06 f ± 0.027 | 27.43 g ± 0.017 | 18.04 e ± 0.029 |
| H8 | 31.23 k ± 0.014 | 15.05 g ± 0.028 | 24.84 j ± 0.011 | 18.52 d ± 0.015 |
| H9 | 22.27 u ± 0.012 | 16.92 c ± 0.018 | 20.18 q ± 0.014 | 21.35 c ± 0.023 |
| H10 | 36.49 f ± 0.012 | 13.79 i ± 0.020 | 24.30 k ± 0.012 | 16.82 f ± 0.014 |
| H11 | 29.89 o ± 0.003 | 13.49 k ± 0.012 | 28.27 e ± 0.012 | 13.65 m ± 0.020 |
| H12 | 36.23 g ± 0.014 | 12.14 m ± 0.024 | 20.64 p ± 0.008 | 14.08 l ± 0.017 |
| H13 | 26.74 s ± 0.020 | 11.83 g ± 0.017 | 30.72 c ± 0.015 | 13.02 n ± 0.017 |
| H14 | 20.51 x ± 0.018 | 9.92 s ± 0.017 | 37.23 a ± 0.006 | 16.32 g ± 0.014 |
| H15 | 35.96 i ± 0.014 | 9.75 t ± 0.017 | 28.76 d ± 0.008 | 9.91 p ± 0.011 |
| H16 | 41.55 e ± 0.017 | 11.27 r ± 0.012 | 20.72 o ± 0.003 | 9.25 r ± 0.024 |
| H17 | 31.09 l ± 0.037 | 16.76 d ± 0.018 | 23.67 m ± 0.005 | 14.65 k ± 0.017 |
| H18 | 47.48 b ± 0.020 | 8.32 v ± 0.017 | 19.48 r ± 0.008 | 5.47 s ± 0.017 |
| H19 | 45.53 d ± 0.016 | 12.12 m ± 0.014 | 15.67 t ± 0.006 | 9.36 q ± 0.023 |
| H20 | 21.25 v ± 0.017 | 16.91 c ± 0.017 | 20.69 po ± 0.003 | 21.75 b ± 0.017 |
| H21 | 36.06 h ± 0.023 | 11.77 p ± 0.014 | 20.15 p ± 0.012 | 15.50 i ± 0.008 |
| H22 | 54.79 a ± 0.015 | 9.46 u ± 0.023 | 17.02 s ± 0.015 | 2.67 t ± 0.020 |
| H23 | 29.51 p ± 0.015 | 14.69 h ± 0.020 | 23.70 m ± 0.008 | 15.63 h ± 0.018 |
| H24 | 28.84 q ± 0.023 | 13.63 j ± 0.014 | 23.54 n ± 0.01 | 16.27 g ± 0.014 |
| Experience | Hydrodistillation Modalities | Desired Compound | Optimum Value | ||
|---|---|---|---|---|---|
| Type and granulometry of leaves | Extraction time | V/M | |||
| H14 | Whole and dry leaves | 2 h 30 min | 1/10 | 1,8-cineole | 37.23 ± 0.006 |
| H22 | Ground and dry leaves | 1 h 30 min | 1/4 | α-pinene | 54.79 ± 0.015 |
| H6 | Whole and fresh leaves | 3 h 30 min | 1/4 | Myrtenyl acetate | 23.43 ± 0.029 |
| H6 | Whole and fresh leaves | 3 h 30 min | 1/4 | Limonene | 17.77 ± 0.014 |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 24.827 | 1 | 24.827 | 2.47 | 0.150 ns |
| B: Leaf granulometry | 122.447 | 1 | 122.447 | 12.19 | 0.007 ** |
| C: V/M ratio | 38.786 | 1 | 38.786 | 3.86 | 0.081 ns |
| D: Extraction time | 81.395 | 2 | 50.697 | 5.05 | 0.05 * |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 246.016 | 1 | 246.016 | 4.07 | 0.074 ns |
| B: Leaf granulometry | 51.744 | 1 | 51.744 | 0.86 | 0.379 ns |
| C: V/M ratio | 207.917 | 1 | 207.917 | 3.44 | 0.096 ns |
| D: Extraction time | 393.893 | 2 | 196.947 | 3.26 | 0.05 * |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 119.751 | 1 | 119.751 | 6.45 | 0.032 * |
| B: Leaf granulometry | 8.225 | 1 | 8.225 | 0.44 | 0.522 ns |
| C: V/M ratio | 52.955 | 1 | 52.955 | 2.85 | 0.126 ns |
| D: Extraction time | 104.621 | 2 | 52.311 | 2.82 | 0.112 ns |
| Source | Sum of Squares | DF | Mean Square | F | Probability |
|---|---|---|---|---|---|
| Main effects | |||||
| A: Type of leaf | 35.527 | 1 | 35.527 | 4.85 | 0.055 ns |
| B: Leaf Granulometry | 4.150 | 1 | 4.150 | 0.57 | 0.471 ns |
| C: V/M ratio | 4.002 | 1 | 4.002 | 0.55 | 0.479 ns |
| D: Extraction time | 15.719 | 2 | 7.860 | 1.07 | 0.382 ns |
| Response | Model Adequacy (R2) | Model Equation | Optimum | Optimal Value |
|---|---|---|---|---|
| Yield | 98.57% | Yield = 0.392153 − 0.110833 X + 0.0176389 Y + 0.0375 X2 − 0.00416667 XY | X = 3 h 30 Y = 1/10 | 0.5 |
| % 1,8-cineole | 82.15% | -------- | -------- | ------- |
| % α-pinene | 62.08% | -------- | -------- | ------- |
| %Myrtenyl acetate | 64.32% | -------- | -------- | ------- |
| % Limonene | 61.35% | -------- | -------- | ------- |
| DPPH | 99.48% | DPPH = 3.00833 − 1.65833 X + 0.134167 Y + 0.31 X2 + 0.0783333 XY | X = 3 h 30 Y = 1/10 | 5.08 |
| ABTS | 99.86% | ABTS = 11.6297 − 1.38917 X + 0.0976389 Y + 0.5775 X2 − 0.0308333 XY | X = 3 h 30 Y = 1/4 | 13.80 |
| Response | Optimal Value | Optimal Modalities |
|---|---|---|
| Yield | 0.49 | X = 3 h 30 min Y = 1/10 |
| DPPH | 5.09 | |
| ABTS | 13.74 |
| Response | Model Adequacy (R2) | Model Equation | Optimum | Optimal Value |
|---|---|---|---|---|
| Yield | 98.60% | Yield = 0.0860417 + 0.390833 X − 0.000416667 Y − 0.0525 X2 − 0.00583333 XY | X = 3 h 30 Y = 1/4 | 0.73 |
| % 1,8-cineole | 99.75% | Cin = −4.11757 + 17.0808 X + 0.00652778 Y − 3.1025 X2 + 0.169167 XY | X = 3 h Y = 1/10 | 24.34 |
| % α-pinene | 97.69% | Pin = 119.01 − 78.8667 X + 4.00556 Y + 15.925 X2 − 1.37333 XY | X = 1 h 30 Y = 1/10 | 55.99 |
| % Myrtenyl acetate | 98.49% | Myrt = −26.4912 + 39.3083 X − 2.2225 Y − 7.745 X2 + 0.621667 XY | X = 2 h 42 Y = 1/4 | 21.00 |
| % Limonene | 96.72% | Lim = −4.12611 + 18.5683 X − 1.11056 Y − 4.05 X2 + 0.376667 XY | X = 2 h 29 Y = 1/4 | 16.31 |
| DPPH | 99.99% | DPPH = −3.02583 + 2.935 X − 0.0241667 Y − 0.26 X2 + 0.105 XY | X = 3 h 30 Y = 1/10 | 7.50 |
| ABTS | 98.16% | ABTS = 11.8768 − 2.11333 X − 0.494722 Y + 0.355 X2 + 0.228333 XY | X = 3 h 30 Y = 1/10 | 11.87 |
| Response | Optimum | Optimal Modalities |
|---|---|---|
| Yield | 0.60 | X = 3 h 30 min Y = 1/9.9 |
| Limonene | 13.32 | |
| 1,8-cineole | 23.62 | |
| Myrtenyl acetate | 15.75 | |
| α-pinene | 30.08 | |
| DPPH | 7.48 | |
| ABTS | 11.86 |
| Response | Model Adequacy (R2) | Model Equation | Optimum | Optimal Value |
|---|---|---|---|---|
| Yield | 99.62% | Yield = 0.220833 + 0.045 X + 0.00333333 Y | X = 3 h 30 Y = 1/10 | 0.41 |
| % 1,8-cineole | 86.57% | -------- | ------- | --------- |
| % α-pinene | 61.38% | -------- | ------- | -------- |
| % Myrtenyl acetate | 99.69% | Myrt = 28.653 − 10.8242 X − 0.518194 Y + 1.4875 X2 + 0.504167 XY | X = 3 h 30 Y = 1/10 | 21.45 |
| % Limonene | 80.04% | -------- | --------- | --------- |
| DPPH | 92.47% | DPPH = 5.5441 − 1.7175 X − 0.375139 Y + 0.1675 X2 + 0.1925 XY | X = 3 h 30 Y = 1/10 | 4.57 |
| ABTS | 97.31% | ABTS = 15.2331 − 2.165 X − 0.467222 Y + 0.54 X2 + 0.12 XY | X = 3 h 30 Y = 1/4 | 14.08 |
| Response | Optimum | Optimal Modalities |
|---|---|---|
| Yield | 0.41 | X = 3 h 30 min Y = 1/10 |
| Myrtenyl acetate | 21.45 | |
| DPPH | 4.57 | |
| ABTS | 13.80 |
| Response | Model Adequacy (R2) | Model Equation | Optimum | Optimal Value |
|---|---|---|---|---|
| Yield | 98.83% | Yield = −0.401458 + 0.3825 X + 0.0470833 Y − 0.0325 X2 − 0.0075 XY | X = 3 h 30 Y = 1/10 | 0.75 |
| % 1,8-cineole | 97.76% | Cin = −15.9336 + 27.27 X + 1.97444 Y − 5.53 X2 − 0.06 XY | X = 2 h 25 Y = 1/10 | 35.97 |
| % α-pinene | 99.54% | Pin = 111.311 − 58.7942 X − 2.73597 Y + 12.1325 X2 + 0.274167 XY | X = 3 h 30 Y = 1/4 | 47.05 |
| % Myrtenyl acetate | 97.50% | Myrt = −21.0236 + 28.24 X + 0.411944 Y − 6.07 X2 + 0.055 XY | X = 2 h 23 Y = 1/10 | 17.23 |
| % Limonene | 52.96% | ---------- | --------- | --------- |
| DPPH | 89.68% | ---------- | --------- | --------- |
| ABTS | 99.93% | ABTS = 11.1795 − 1.98583 X + 0.155694 Y + 0.4425 X2 + 0.0808333 XY | X = 3 h 30 Y = 1/10 | 14.04 |
| Response | Optimum | Optimal Modalities |
|---|---|---|
| Yield | 0.73 | X = 3 h 20 min Y = 1/10 |
| 1,8-cineole | 31.16 | |
| α-pinene | 32.18 | |
| Myrtenyl acetate | 11.50 | |
| ABTS | 13.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayani, R.; BenSalem, E.; Khouja, M.; Bouhjar, A.; Boussaid, M.; Messaoud, C. Optimization of Tunisian Myrtus communis L. Essential Oil Extraction by Complete Factorial Experimental Design. Metabolites 2025, 15, 369. https://doi.org/10.3390/metabo15060369
Zayani R, BenSalem E, Khouja M, Bouhjar A, Boussaid M, Messaoud C. Optimization of Tunisian Myrtus communis L. Essential Oil Extraction by Complete Factorial Experimental Design. Metabolites. 2025; 15(6):369. https://doi.org/10.3390/metabo15060369
Chicago/Turabian StyleZayani, Rania, Eya BenSalem, Mariem Khouja, Amani Bouhjar, Mohamed Boussaid, and Chokri Messaoud. 2025. "Optimization of Tunisian Myrtus communis L. Essential Oil Extraction by Complete Factorial Experimental Design" Metabolites 15, no. 6: 369. https://doi.org/10.3390/metabo15060369
APA StyleZayani, R., BenSalem, E., Khouja, M., Bouhjar, A., Boussaid, M., & Messaoud, C. (2025). Optimization of Tunisian Myrtus communis L. Essential Oil Extraction by Complete Factorial Experimental Design. Metabolites, 15(6), 369. https://doi.org/10.3390/metabo15060369








