Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Patient and Public Involvement
2.3. Clinical and Biochemical Measurements
2.4. Definition of Increased ASCVD Risk and NAFLD
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Association between FLI and Metabolic Risk Factors
3.3. Associations of FLI with Increased 10-Year ASCVD Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ratziu, V.; Goodman, Z.; Sanyal, A. Current efforts and trends in the treatment of NASH. J. Hepatol. 2015, 62, S65–S75. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Valbusa, F.; Agnoletti, D.; Scala, L.; Grillo, C.; Arduini, P.; Bonapace, S.; Calabria, S.; Scaturro, G.; Mantovani, A.; Zoppini, G.; et al. Non-alcoholic fatty liver disease and increased risk of all-cause mortality in elderly patients admitted for acute heart failure. Int. J. Cardiol. 2018, 265, 162–168. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Shiina, K.; Matsumoto, C.; Nakano, H.; Fujii, M.; Yamashina, A.; Chikamori, T.; Tomiyama, H. Correlation of the Fatty Liver Index with the Pathophysiological Abnormalities Associated with Cardiovascular Risk Markers in Japanese Men without any History of Cardiovascular Disease: Comparison with the Fibrosis-4 Score. J. Atheroscler. Thromb. 2020, 28, 524–534. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: Findings from matched cohort study of 18 million European adults. BMJ 2019, 367, l5367. [Google Scholar] [CrossRef]
- Karanjia, R.N.; Crossey, M.M.; Cox, I.J.; Fye, H.K.; Njie, R.; Goldin, R.D.; Taylor-Robinson, S.D. Hepatic steatosis and fibrosis: Non-invasive assessment. World J. Gastroenterol. 2016, 22, 9880–9897. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Harrison, S.A.; Abdelmalek, M.F.; Anstee, Q.M.; Bedossa, P.; Castera, L.; Dimick-Santos, L.; Friedman, S.L.; Greene, K.; Kleiner, D.E.; et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology 2018, 67, 2001–2012. [Google Scholar] [CrossRef]
- Andrus, B.; Lacaille, D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J. Am. Coll. Cardiol. 2014, 63, 2886. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Nordestgaard, B.G.; Afzal, S.; Falk, E. ACC/AHA guidelines superior to ESC/EAS guidelines for primary prevention with statins in non-diabetic Europeans: The Copenhagen General Population Study. Eur. Heart J. 2017, 38, 586–594. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Hu, D.; Chen, J.; Li, Y.; Huang, J.; Liu, X.; Liu, F.; Cao, J.; Shen, C.; et al. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation 2016, 134, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Ning, G. Risk Evaluation of cAncers in Chinese diabeTic Individuals: A lONgitudinal (REACTION) study. J. Diabetes 2012, 4, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Lu, J.; Wang, W.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; Wan, Q.; et al. Cohort profile: Risk evaluation of cancers in Chinese diabetic individuals: A longitudinal (REACTION) study. J. Diabetes 2014, 6, 147–157. [Google Scholar] [CrossRef]
- Sun, K.; Li, F.; Qi, Y.; Lin, D.; Ren, M.; Xu, M.; Li, F.; Li, Y.; Yan, L. Sex difference in the association between habitual daytime napping and prevalence of diabetes: A population-based study. Endocrine 2016, 52, 263–270. [Google Scholar] [CrossRef]
- Sun, K.; Lin, D.; Li, F.; Qi, Y.; Feng, W.; Yan, L.; Chen, C.; Ren, M.; Liu, D. Fatty liver index, albuminuria and the association with chronic kidney disease: A population-based study in China. BMJ Open 2018, 8, e019097. [Google Scholar] [CrossRef]
- Tomioka, K.; Iwamoto, J.; Saeki, K.; Okamoto, N. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) in elderly adults: The Fujiwara-kyo Study. J. Epidemiol. 2011, 21, 459–465. [Google Scholar] [CrossRef]
- National Workshop on Fatty Liver; Alcoholic Liver Disease CSoH. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update. Chin. J. Hepatol. 2018, 26, 195–203.
- Ceriotti, F.; Henny, J.; Queraltó, J.; Ziyu, S.; Özarda, Y.; Chen, B.; Boyd, J.C.; Panteghini, M. Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT) in serum: Results from an IFCC multicenter study. Clin. Chem. Lab. Med. 2010, 48, 1593–1601. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Gitto, S.; Fogacci, F.; Rosticci, M.; Giovannini, M.; D’Addato, S.; Andreone, P.; Borghi, C. Fatty liver index is associated to pulse wave velocity in healthy subjects: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2018, 53, 29–33. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Huo, Y.; Xy, L. Chinese Guidelines for cardiovascular disease prevention (2017). Chin. J. Cardiol. 2018, 1, 10–25. [Google Scholar]
- Huang, X.; Xu, M.; Chen, Y.; Peng, K.; Huang, Y.; Wang, P.; Ding, L.; Lin, L.; Xu, Y.; Chen, Y.; et al. Validation of the Fatty Liver Index for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Chinese. Medicine 2015, 94, e1682. [Google Scholar] [CrossRef] [PubMed]
- Olubamwo, O.O.; Virtanen, J.K.; Voutilainen, A.; Kauhanen, J.; Pihlajamäki, J.; Tuomainen, T.P. Association of fatty liver index with the risk of incident cardiovascular disease and acute myocardial infarction. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.L.; Wu, W.C.; Fang, K.C.; Wang, Y.C.; Huo, T.I.; Huang, Y.H.; Yang, H.I.; Su, C.W.; Lin, H.C.; Lee, F.Y.; et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE 2015, 10, e0120443. [Google Scholar]
- Han, E.; Lee, Y.H.; Kim, Y.D.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H.; et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am. J. Gastroenterol. 2020, 115, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Giral, P.; Khan, J.F.; Rosenbaum, D.; Housset, C.; Poynard, T.; Ratziu, V. Fatty liver is an independent predictor of early carotid atherosclerosis. J. Hepatol. 2016, 65, 95–102. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Xu, C.Y.; Chang, X.X.; Li, W.W.; Sun, L.Y.; Yang, X.B.; Yu, L.F. Fatty liver index correlates with non-alcoholic fatty liver disease, but not with newly diagnosed coronary artery atherosclerotic disease in Chinese patients. BMC Gastroenterol. 2013, 13, 110. [Google Scholar] [CrossRef]
- Chung, T.H.; Kim, J.K.; Kim, J.H.; Lee, Y.J. Fatty Liver Index as a Simple and Useful Predictor for 10-year Cardiovascular Disease Risks Determined by Framingham Risk Score in the General Korean Population. J. Gastrointest. Liver Dis. 2021, 30, 221–226. [Google Scholar] [CrossRef]
- Onat, A.; Can, G.; Kaya, A.; Akbaş, T.; Özpamuk-Karadeniz, F.; Şimşek, B.; Çakır, H.; Yüksel, H. Fatty liver disease: Disparate predictive ability for cardiometabolic risk and all-cause mortality. World J. Gastroenterol. 2015, 21, 13555–13565. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C.J. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, J.L.; Pencina, K.M.; Massaro, J.M.; Hoffmann, U.; Seshadri, S.; Fox, C.S.; O’Donnell, C.J.; Speliotes, E.K. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J. Hepatol. 2015, 63, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, P.; Zhang, D.; Sun, Y.; Chen, Y.; Liang, J.; Wu, J.; Zhang, J.; Lu, P.; Lin, H.; et al. Evaluation of Atherosclerotic Cardiovascular Risk Prediction Models in China: Results From the CHERRY Study. JACC Asia 2022, 2, 33–43. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.S.; Curzen, N.P.; Calder, P.C.; Byrne, C.D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur. Heart J. 2012, 33, 1190–1200. [Google Scholar] [CrossRef]
- Oni, E.T.; Agatston, A.S.; Blaha, M.J.; Fialkow, J.; Cury, R.; Sposito, A.; Erbel, R.; Blankstein, R.; Feldman, T.; Al-Mallah, M.H.; et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013, 230, 258–267. [Google Scholar] [CrossRef]

| Characteristics | Total | FLI < 30 | 30 ≤ FLI < 60 | FLI ≥ 60 | p |
|---|---|---|---|---|---|
| n (%) | 9044 (100.0) | 6052 (66.9) | 2090 (23.1) | 902 (10.0) | |
| Fatty liver index | 18.86 (8.53–37.21) | 11.54 (6.03–19.03) | 41.37 (34.96–49.48) | 71.74 (65.26–80.44) | <0.0001 |
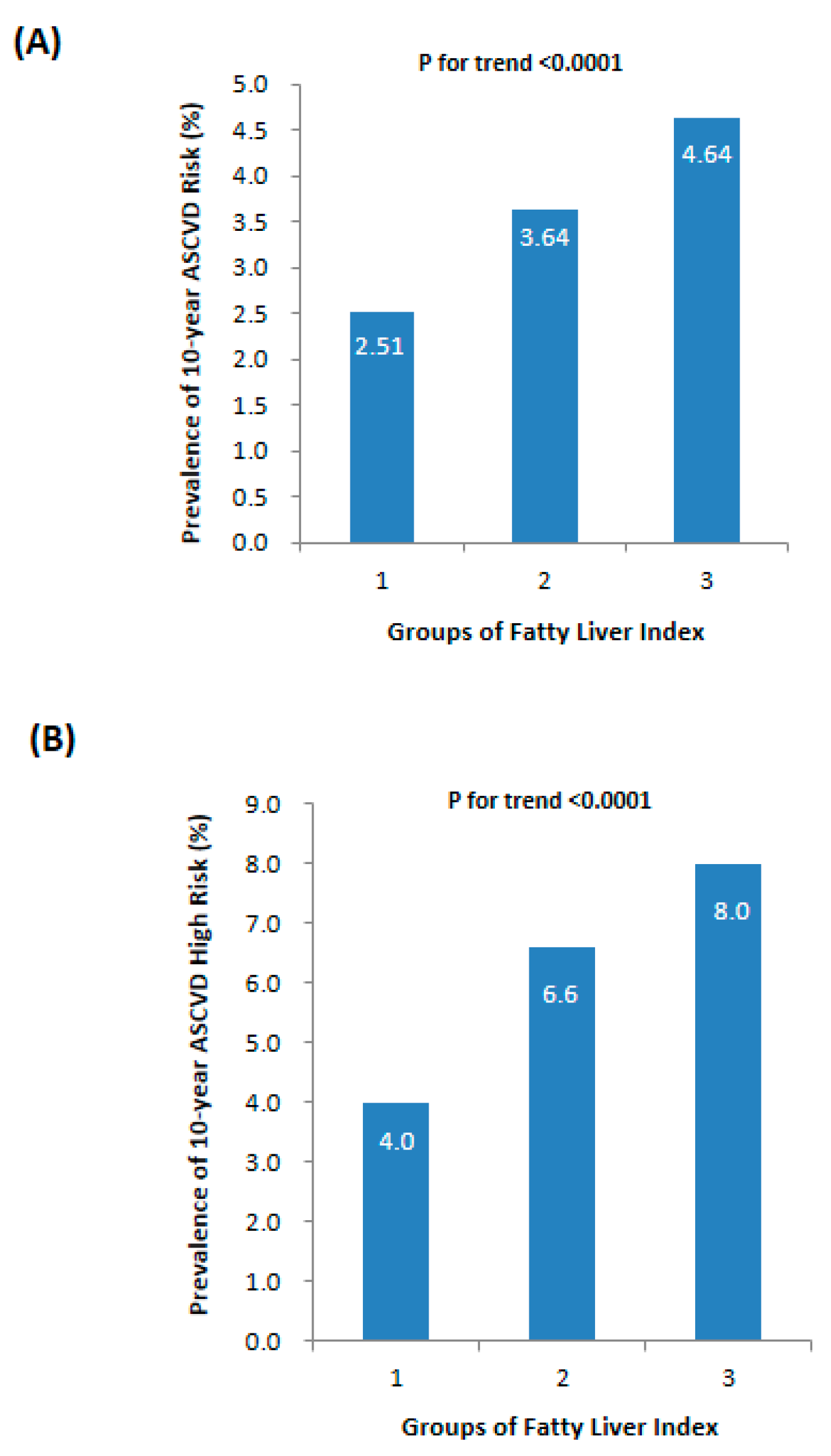
| ASCVD risk (%) | 2.99 ± 4.52 | 2.51 ± 4.11 | 3.64 ± 4.65 * | 4.64 ± 6.04 *† | <0.0001 |
| ASCVD HIGH risk, n (%) | 458 (5.1) | 242 (4.0) | 137 (6.6) * | 79 (8.8) *† | <0.0001 |
| Age (yr) | 55.0 ± 7.9 | 54.6 ± 7.8 | 56.0 ± 8.0 * | 55.8 ± 8.2 * | <0.0001 |
| Male, n (%) | 2572 (28.4) | 1396 (23.1) | 751 (35.9) * | 425 (47.1) *† | <0.0001 |
| BMI (kg/m2) | 23.60 ± 3.15 | 22.30 ± 2.45 | 25.50 ± 2.27 * | 27.88 ± 3.07 *† | <0.0001 |
| WC (cm) | 81.6 ± 9.5 | 77.5 ± 7.2 | 87.6 ± 6.0 * | 95.2 ± 9.4 *† | <0.0001 |
| SBP (mm Hg) | 125.9 ± 16.5 | 123.0 ± 15.8 | 130.9 ± 15.7 * | 134.4 ± 17.1 *† | <0.0001 |
| DBP (mm Hg) | 75.3 ± 9.9 | 73.5 ± 9.4 | 78.3 ± 9.7 * | 80.7 ± 10.0 *† | <0.0001 |
| Treated hypertension, n (%) | 808 (8.9) | 405 (6.7) | 252 (12.1) * | 151 (16.7) *† | <0.0001 |
| ALT (U/L) | 13.0 (9.0–18.0) | 12.0 (9.0–16.0) | 15.0 (11.0–20.0) * | 19.0 (13.0–28.0) *† | <0.0001 |
| AST (U/L) | 18.0(15.0–22.0) | 18.0 (14.0–21.0) | 19.0 (16.0–23.0) * | 22.0 (18.0–27.0) *† | <0.0001 |
| γ-GGT (U/L) | 20.0 (14.0–28.0) | 17.0 (13.0–22.0) | 26.0 (20.0–36.0) * | 40.0 (27.0–63.0) *† | <0.0001 |
| TGs (mmol/L) | 1.59 ± 1.25 | 1.18 ± 0.53 | 2.05 ± 1.09 * | 3.34 ± 2.53 *† | <0.0001 |
| TC (mmol/L) | 5.22 ± 1.22 | 5.07 ± 1.23 | 5.44 ± 1.15 * | 5.67 ± 1.23 *† | <0.0001 |
| HDL-C (mmol/L) | 1.32 ± 0.36 | 1.39 ± 0.37 | 1.23 ± 0.29 * | 1.16 ± 0.35 *† | <0.0001 |
| LDL-C (mmol/L) | 3.16 ± 0.95 | 3.09 ± 0.94 | 3.31 ± 0.95 * | 3.28 ± 0.99 * | <0.0001 |
| Treated dyslipidemia, n (%) | 96 (1.1) | 57 (0.9) | 26 (1.2) | 13 (1.4) | 0.256 |
| FPG (mmol/L) | 5.68 ± 1.34 | 5.49 ± 1.14 | 5.91 ± 1.40 * | 6.38 ± 2.00 *† | <0.0001 |
| Fasting Insulin (μIU/mL) | 7.10 (5.20–9.90) | 6.20 (4.60–8.20) | 9.10 (7.00–12.10) * | 11.70 (8.90–15.40) *† | <0.0001 |
| HbA1c (%) | 6.04 ± 0.90 | 5.92 ± 0.78 | 6.22 ± 0.99 * | 6.48 ± 1.22 *† | <0.0001 |
| Diabetes, n (%) | 623 (6.9) | 352 (5.8) | 174 (8.3) * | 97 (10.8) *† | <0.0001 |
| SCr (μmol/L) | 69.8 ± 16.7 | 67.9 ± 16.3 | 72.4 ± 15.1 * | 76.9 ± 20.0 *† | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 112.9 ± 23.3 | 115.0 ± 24.3 | 109.6 ± 19.9 * | 106.6 ± 22.0 *† | <0.0001 |
| Physical activity (MET-hour/week) | 21.0 (10.5–45.0) | 24.0 (10.5–46.0) | 21.0 (10.5–42.0) * | 21.0 (9.0–45.1) * | 0.013 |
| Current smoker, n (%) | 924 (10.2) | 518 (8.6) | 245 (11.7) * | 161 (17.8) *† | 0.032 |
| Drinker, n (%) | 65 (0.7) | 26 (0.4) | 21 (1.0) * | 18 (2.0) *† | <0.001 |
| r | p | Standardized β | p | |
|---|---|---|---|---|
| ASCVD risk (%) | 0.183 | <0.0001 | 0.076 | 0.004 |
| Age (yr) | 0.092 | <0.0001 | −0.039 | 0.004 |
| BMI (kg/m2) | 0.716 | <0.0001 | ||
| WC (cm) | 0.758 | <0.0001 | ||
| SBP (mm Hg) | 0.299 | <0.0001 | 0.036 | 0.014 |
| DBP (mm Hg) | 0.306 | <0.0001 | 0.146 | <0.0001 |
| ALT (U/L) | 0.348 | <0.0001 | ||
| AST (U/L) | 0.246 | <0.0001 | ||
| GGT (U/L) | 0.405 | <0.0001 | ||
| TC (mmol/L) | 0.216 | <0.0001 | ||
| TGs (mmol/L) | 0.602 | <0.0001 | ||
| HDL-C (mmol/L) | −0.264 | <0.0001 | ||
| LDL-C (mmol/L) | 0.139 | <0.0001 | ||
| FPG (mmol/L) | 0.241 | <0.0001 | 0.030 | 0.032 |
| Fasting insulin (μIU/mL) | 0.570 | <0.0001 | 0.445 | <0.0001 |
| SCr (μmol/L) | 0.208 | <0.0001 | 0.186 | <0.0001 |
| eGFR (ml/min per 1.73 m2) | −0.160 | <0.0001 | 0.040 | 0.003 |
| HbA1c (%) | 0.238 | <0.0001 | 0.098 | <0.0001 |
| Physical activity (MET-hour/week) | −0.027 | 0.012 | −0.008 | 0.404 |
| FLI < 30 | 30 ≤ FLI < 60 | FLI ≥ 60 | p | ||
|---|---|---|---|---|---|
| 10-year ASCVD HIGH risk | MODEL 1 | 1 | 1.68 (1.36–2.09) | 2.31 (1.77–3.00) | <0.0001 |
| MODEL 2 | 1 | 1.87 (1.32–2.67) | 3.91 (2.52–6.08) | <0.0001 | |
| MODEL 3 | 1 | 1.89 (1.28–2.77) | 3.88 (2.40–6.28) | <0.0001 | |
| MODEL 4 | 1 | 1.41 (0.91–2.18) | 2.61 (1.52–4.49) | <0.0001 |
| FLI < 30 | 30 ≤ FLI < 60 | FLI ≥ 60 | p | |
|---|---|---|---|---|
| The PCE model | 1 | 1.68 (1.36–2.09) | 2.31 (1.77–3.00) | <0.001 |
| The China-PAR model | 1 | 2.77 (2.29–3.35) | 5.16 (4.16–6.38) | <0.001 |
| The Framingham Risk Score | 1 | 2.31 (2.02–2.65) | 3.66 (3.10–4.32) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Fan, J.; Zhang, X.; You, L.; Lin, D.; Huang, C.; Li, F.; Sun, K. Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China. Metabolites 2023, 13, 850. https://doi.org/10.3390/metabo13070850
Zhou J, Fan J, Zhang X, You L, Lin D, Huang C, Li F, Sun K. Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China. Metabolites. 2023; 13(7):850. https://doi.org/10.3390/metabo13070850
Chicago/Turabian StyleZhou, Jing, Jing Fan, Xiaoyun Zhang, Lili You, Diaozhu Lin, Chulin Huang, Feng Li, and Kan Sun. 2023. "Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China" Metabolites 13, no. 7: 850. https://doi.org/10.3390/metabo13070850
APA StyleZhou, J., Fan, J., Zhang, X., You, L., Lin, D., Huang, C., Li, F., & Sun, K. (2023). Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China. Metabolites, 13(7), 850. https://doi.org/10.3390/metabo13070850







