1. Introduction
Over the decades, numerous diets aimed at combatting obesity have risen and fallen in popularity. The concept of ‘eating like a pig’, however, has not been considered, to our knowledge. Pigs are typically portrayed as dirty, sloppy and obese; and if people call others ‘pigs’, it generally has a strongly negative connotation. Interestingly, pigs are inherently rather clean animals. Winston Churchill had an interesting quote: ‘I like pigs: cats look down on human beings, dogs look up to them, but pigs just treat us as their equals’. Physiologically, Churchill was exactly right.
Nutritionally, pigs and humans have a lot in common. In the wild, both would have consumed omnivore diets, scavenging a variety of plant products ranging from roots to fruit, as well as eggs, insects, honey and the like, and eating meat whenever it was accessible [
1]. Their digestive tracts, consequently, are remarkably similar [
2,
3], to the point that swine are high on the list of candidates as cross-species organ donors for man [
4]. Digestibility studies confirm this similarity: the digestibility coefficients match reasonably well [
5]. Last but not least, metabolically, again, there is great similarity. For example, prior to the introduction of biotechnology-produced insulin, pig-derived insulin was the primary treatment for type I diabetes, and digestive enzymes harvested from porcine pancreases are used to treat human pancreatic insufficiency.
A marked difference between modern man and pig is that modern man typically eats a diet driven by emotions and at times of the day that ‘fit’ with societal norms intertwined with convenience. Palatability trials with pigs indicate that they, e.g., dislike certain flavors; but when there is no choice, they eat a diet that is not their first choice. Moreover, pigs try hard to consume food at times that match their physiological needs.
Modern pigs are raised for meat production. In those production systems, food makes up roughly 70% of the total production costs. Consequently, a tremendous effort is put into understanding the fundamentals of nutrition and how this can be translated into diets that are most efficiently converted into muscle tissue. Adipose tissue also has value, as it harbors a lot of the meat’s taste, but given society’s drive for low-fat foods, swine production has adapted to producing pork with a low-fat content.
This knowledge has been converted into databases and computer models, so that diets can be optimized for maximum efficiency (e.g., INRAPorc from van Milgen and Noblet, [
6]; Watson from Ferguson [
7]). These models are continuously refined using data from large numbers of pigs, which followed exactly the prescribed diets. In strong contrast, controlled human studies are generally conducted with very small populations of subjects for very short durations, as emotions come into play. Humans are hard to persuade to follow a boring research diet, especially over a longer period of time, and humans are easily tempted to deviate from a protocol if peer pressure or desires are high. Even worse, in survey type experiments, people are asked what they ate for the past one or several days, and these data may well be subsequently extrapolated to patterns of behavior and then correlated with developments in health. Recalling what and especially how much a person ate yesterday is already a challenge for many, confounded even further by the desire not to include items that may be considered less acceptable. Extrapolating these data to lifetime impacts is, in our eyes, rather risky.
In man, obesity and related type II diabetes is the health challenge for the 21st century [
8]. A tremendous effort is put into understanding its causes and into finding solutions that ideally fit within the emotional constraints of a person. A number of theories have been proposed explaining obesity. What we will try to do next is to review several of those theories from the perspective of nutritionists whose work has focused on swine.
2. Calorie Counting
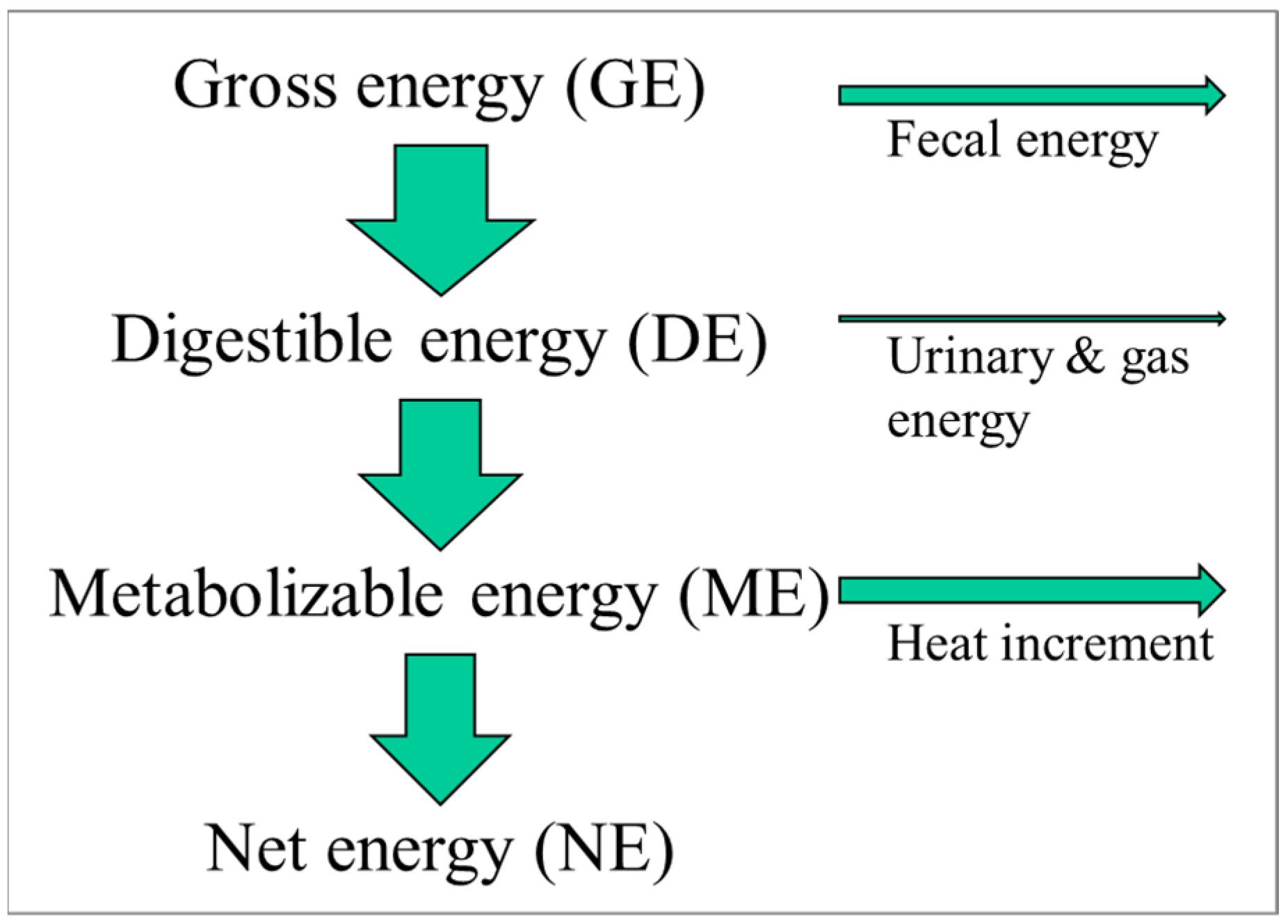
The energy content of foods is measured in calories (or joules) and can be determined accurately using a so-called bomb calorimeter. In such an instrument, a food item is simply burned, and the released energy (heat) is measured. This energy value represents the amount of gross energy a person would consume when eating such a food. Foods are typically not 100% digestible, while digestion itself also requires energy, which is ultimately dissipated as heat. Finally, there are urinary and gaseous losses of energy, albeit normally small.
In human nutrition, most calculations appear to be performed with the total energy contents of foods (e.g., EFSA and USDA dietary guidelines). Although efforts have been made to determine digestible energy content both in vivo (in actual people) and in vitro (in a test tube), these efforts have not been translated into comprehensive datasets, which allow a nutritionist to calculate the energy content of complex diets. Actually, most datasets on the nutritional value of foods aimed at humans are incomplete to the point that they provide little robust value for performing diet optimizations (e.g., USDA, NEVO). An important reason for this is that man has access to an enormous number of ever-changing foods, making it practically impossible to characterize them all.
For pigs, in contrast, only a limited number of dietary ingredients are used. For example, in the USA, pigs are typically fed a diet consisting of maize, soybean meal, and in recent decades, a maize-derived coproduct of ethanol production called dried distillers’ grains with solubles. The limited number of ingredients that change at a relatively slow pace with time has allowed the characterization of each of those ingredients in terms of its energy utilization by the animal (
Figure 1). We thus differentiate between the gross energy content of the food (as determined by a bomb calorimeter), the digestible energy content (subtracting fecal energy), the metabolizable energy content (subtracting the urinary and gaseous (e.g., methane) energy losses), and lastly, the net energy content. The net energy content is the amount of energy the pig has available to support metabolism or growth, e.g., to exercise or grow tissue, such as muscle or adipose tissue. As an example, maize contains the following in kcal/kg: gross energy, 3933; digestible energy, 3451; metabolizable energy, 3395; and net energy, 2672 [
9]. Not only have the energy inputs into the pigs been carefully characterized but also the energy outputs. The latter can be performed with a tool called an indirect calorimeter. Based on experiments with those indirect calorimeters, we know the energetic cost of exercise, of growing muscle or adipose tissue, of lactating, etc.
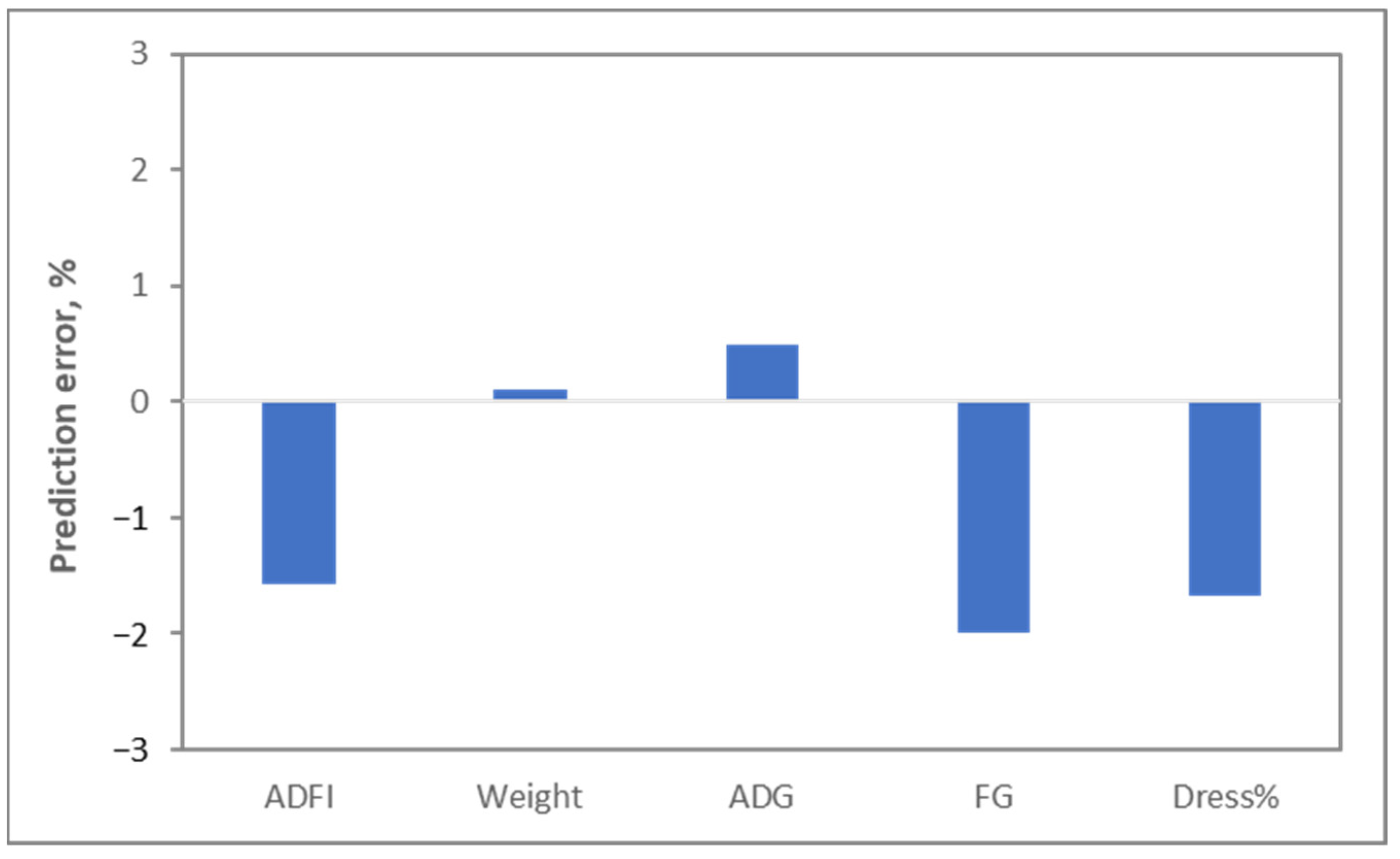
As indicated above, the data from all these sources have been included in computer models, which are used to determine what pigs all around the world should be fed, whether they are in Europe, where wheat is the primary cereal, or in the US, where maize is the primary cereal, or whether pigs are genetic lines selected for reproduction, meat production or not genetically improved at all, or whether pigs are infant, adult, male or female. Even more importantly, these models do a fantastic job in predicting what is happening with energy. A nice example is the Watson model developed by Ferguson [
7]. Validations of this model indicate that food intake can be predicted with an error of less than 2%, and weight gain can be predicted with an error of less than 0.5% (
Figure 2).
This returns us to the discussion on calories in versus calories out. Given the accuracy of these models, the answer to this discussion is quite easy. Energy metabolism is a reasonably simple calculation exercise in which the energy consumed, in the form of net energy, needs to find an outlet. These outlets can be tweaked, e.g., by exercising. The energy that is ‘left over’ will be used for growth. When the animal is a human being past puberty, most likely, this growth will be in the form of (typically undesirable) adipose mass. One must take into account the fact that a good basis of calorie counting exists, and arguably, a lot of obesity problems can be averted by either reducing calorie intake and/or by increasing calorie output, such that a net negative energy intake can be created.
These models, in practice, are not perfectly black and white. After all, there are some nuances in energy metabolism, which these models do not track. For example, a more recent finding is that the conversion of dietary net energy into ATP, the most important energy carrier within biology, is not as fixed as once believed [
10]. The change is associated with the health of mitochondria; when mitochondria function sub-optimally, this conversion can be as much as 25% less efficient than what many textbooks claim. Humans affected by this would generate more heat, and consequently, at the same energy intake, they have less energy available for becoming obese. The opposite, though, is difficult to find. It takes energy to deposit adipose tissue, and this energy can come from only one source: what we consume.
Another factor to consider is that for pigs, we are typically interested in a relatively short period of time, i.e., months. In humans, we hope for a healthy lifespan of close to a century. An error in the models of 1 g of adipose tissue per day would mean about 150 g of fat on a 100 kg pig; this is practically negligible. An error of 1 g for a human over a period of 60 years (post-puberty) equates instead to 22 kg of fat, enough to make one obese.
Nevertheless, we would argue that swine nutritionists in general believe that calorie counting would also apply to humans. The parameters in the equations would certainly be affected by sex, age and genotype. This may not be 100% accurate, and there may be a rare exception, but for the public at large, eating less and/or exercising more would achieve a negative energy balance and consequently a loss of adipose tissue weight.
3. Genetics
In humans, genetic variation exists, and this can certainly affect energy metabolism. In pigs, the same is the case. Two extreme examples are Piétrain and Meishan pigs. The former are typically very muscular (as if they worked out in the gym on a daily basis); the latter are typically chunky little pigs (as if they were couch potatoes). These distinct genetic lines differ in how the energy is utilized in the body. In the very lean Piétrain pigs, heat losses to the environment are higher under cold conditions, as they have limited insulation reserves that adipose tissue would provide. When energy is available for tissue growth prior to reaching sexual maturity, this energy is directed predominantly to the growth of muscles. In the Meishan pigs, the ability to grow muscle tissue is much more limited, and they mature faster. Consequently, in practice, a Meishan pig quite often appears to be obese.
The above rules of energy metabolism, nevertheless, still apply to both genetic lines of pigs. There are small differences in the model factors used. For example, van Milgen and Noblet (
Table 1), in developing the INRAPorc model, determined that in (castrated) Meishan pigs, the maintenance requirement was 799 kJ/kg
0.643, while this value was 762 for the Piétrain pigs (intact males), a difference of 5% (the maintenance requirement is the energy needed to not change weight, assuming limited physical activity and assuming that no food is being digested). Van Milgen and Noblet also have factors (c and d) describing how growth changes with age, and for that, there are big differences between the two lines. The efficiency of energy utilization for protein and fat deposition, however, was not sufficiently different between the two lines to assign separate factors. This would indeed mean that genetics does affect energy utilization and, in particular, muscle growth potential. The basic energy rules, though, vary very little.
Notably, the kf is close to 1 (0.916), while the kp is only 0.511. What these values mean is that for every 1 kcal of net energy consumed, 0.916 kcal of fat can be formed; in contrast, if that 1 kcal was used for protein synthesis, then only 0.511 kcal of protein could be formed. The conversion of dietary energy into fat is thus extremely efficient. Fat has a high energy content, approximately 9 kcal/g; adipose tissue can actually replace water, so a person gaining 1 kg due to fat may have gained 10,000 kcal. Protein, in contrast, has an energy value upon catabolism of only 4 kcal (assuming nitrogen is converted into urea). Protein gain is invariably associated with water gain. Muscle typically consists of roughly 25% protein and 75% water. This means that a person gaining 1 kg of muscle has gained only 1000 kcal of usable energy. Protein tissue is also energetically expensive to maintain, as there is a continuous turnover of proteins, which requires energy. Adipose tissue, in contrast, has little turnover and consequently requires very little energy to maintain.
These strong differences in energy content per kg of body weight do mean that an obese person who starts exercising and dieting can actually gain body weight if adipose tissue is broken down while muscle tissue is accreted. The high energy content of adipose tissue also means that, in extreme obesity cases, a person can carry a year’s worth of energy in the form of adipose tissue, illustrating how hard it can be to correct serious obesity.
6. Glycemic Response
The glycemic response is the rise and fall in blood glucose observed after consuming a (standardized) meal containing a source of glucose (
Figure 5). Effectively, the glycemic response is a function of the amount of glucose in a diet, e.g., as starch or sucrose, and how easily/quickly these components are broken down into glucose in the intestinal tract and subsequently absorbed (the latter is very fast).
The glycemic response of a food is interesting, as our bodies try to prevent strong rises in glucose. High levels of glucose are not healthy, as glucose can react with other molecules in the body, resulting in unhealthy products, such as advanced glycation end (AGE) products [
18]. The lead mechanism for controlling blood glucose is through secretion of the hormone insulin. Insulin effectively tells the body to start metabolizing glucose. This can be for producing heat to stay warm, but it can also be for the production of fat. The latter, generalizing, occurs when the body is overloaded with glucose. Technically, if we were able to prevent blood glucose from rising above this threshold, we would prevent the production of excess fat.
The glycemic response of a diet is thus an important parameter in our fight against obesity. As explained, it is composed of two factors: how much glucose is consumed as, e.g., digestible starch or sugar, and how quickly this glucose is released into the blood stream. The glycemic response to a diet has received a lot of attention in both human and pig nutrition. For pigs, a database was constructed with over 700 samples of common dietary ingredients for which we had characterized this glycemic response (
Figure 6) [
15]. We performed this using a lab assay developed for use in human nutrition but which we also validated in actual pigs [
16,
17]. The pig data indeed indicated that some starches break down extremely fast after eating, giving a strong glycemic response, while others are broken down very slowly and also very poorly, and they thus hardly provide a glycemic response.
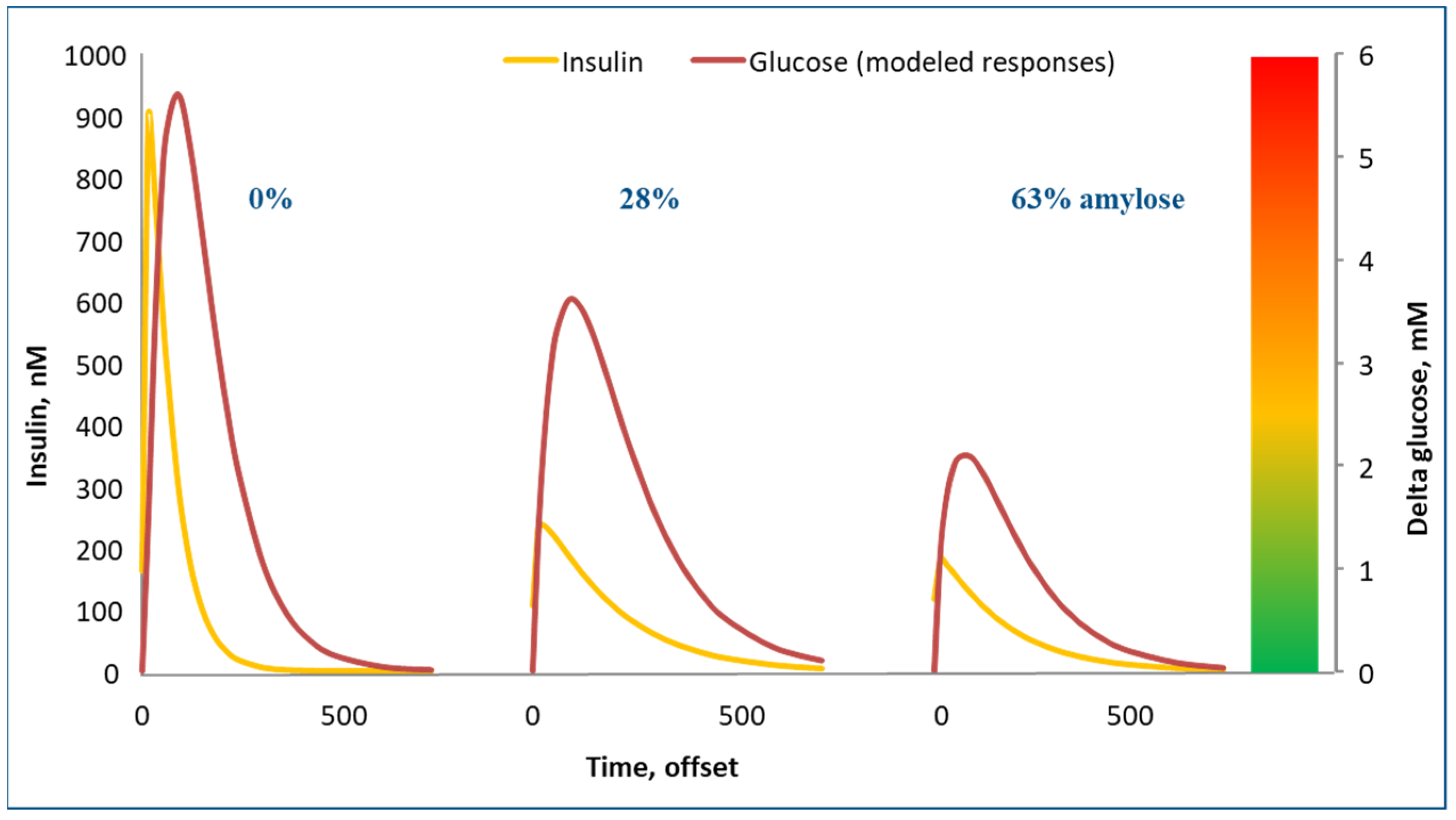
Figure 5 shows the blood glucose and insulin response to three starch sources. On the left in the graph, waxy maize starch (0% amylose) yielded very strong glucose and insulin peaks. On the right, high amylose (63%) maize starch resulted in very modest glucose and insulin peaks. Technically, it would be very interesting to switch our convenience food industry away from low amylose starches to high amylose starches, as they yield much lower glycemic responses and as they induce physical satiety for a much longer period of time. The crops to support this are already available, but they are not grown on a large scale. Swine trials with high amylose starches indeed confirmed these benefits [
15].
Notably, slowly digestible for starches also implies poorly digestible. For high amylose maize starch, a substantial portion of the starch is not digested but fermented by microbes in the large intestine [
17]. Fermented means that the starch is broken down by microbes, especially in the large intestine. Upon this breakdown, volatile fatty acids are formed but also heat. Thus, the net energy of the diet, as explained above, becomes lower. These slowly digestible starches are thus interesting in theory for preventing obesity. We indeed completed several piglet experiments, which confirmed this effect: exchanging rapidly digestible starches for slowly digestible starches resulted in leaner pigs!
Rapidly digestible starches can trigger adiposity through their high glycemic index, but they also result in very rapid passage through the small intestine (
Figure 7). This means that they induce physical satiety only for a short period of time; our stomachs will start growling for food much quicker after a meal based on rapidly digestible starches.
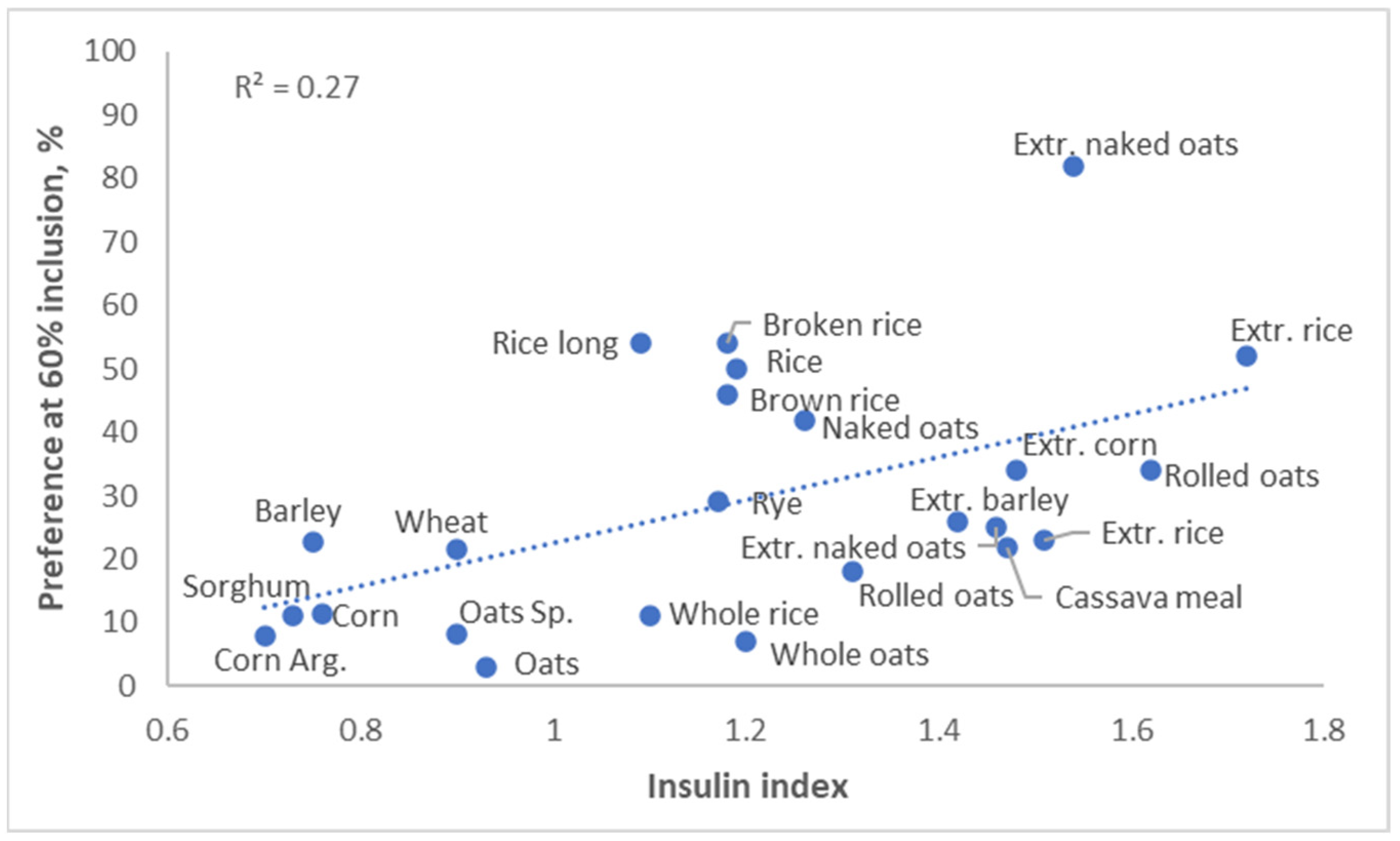
The glycemic response to a diet affects not only obesity, but it also affects palatability. Pigs preferred diets with a high insulin index, and humans appear to have the same preference (
Figure 8, Solà-Oriol and van Kempen, unpublished). This is quite logical; a high glycemic index generally results in a food tasting sweet, and both pigs and humans have a serious sweet tooth.
High glycemic foodstuffs thus have three downsides against them:
High palatability, which stimulates intake;
Rapid intestinal passage rate, which induces limited physical satiety;
Strong stimulation of insulin and blood glucose, which triggers conversion of glucose into fat.
Sweeteners are a way of tricking us into thinking that a product has a high glycemic index but without providing a strong glycemic response. Technically, this sounds like an interesting solution. Physiologically, however, a big question is how our bodies respond to those sweeteners. Our work implied that the insulin response in pigs is already steered by the diet’s glycemic response, as measured by taste receptors in the mouth (
Figure 5) [
16]. This remains a point of debate, but if the sweet taste does indeed induce insulin, but subsequently, our bodies do not receive the glucose to be processed under the influence of this insulin, is that a sign to the body to ignore the insulin signal?
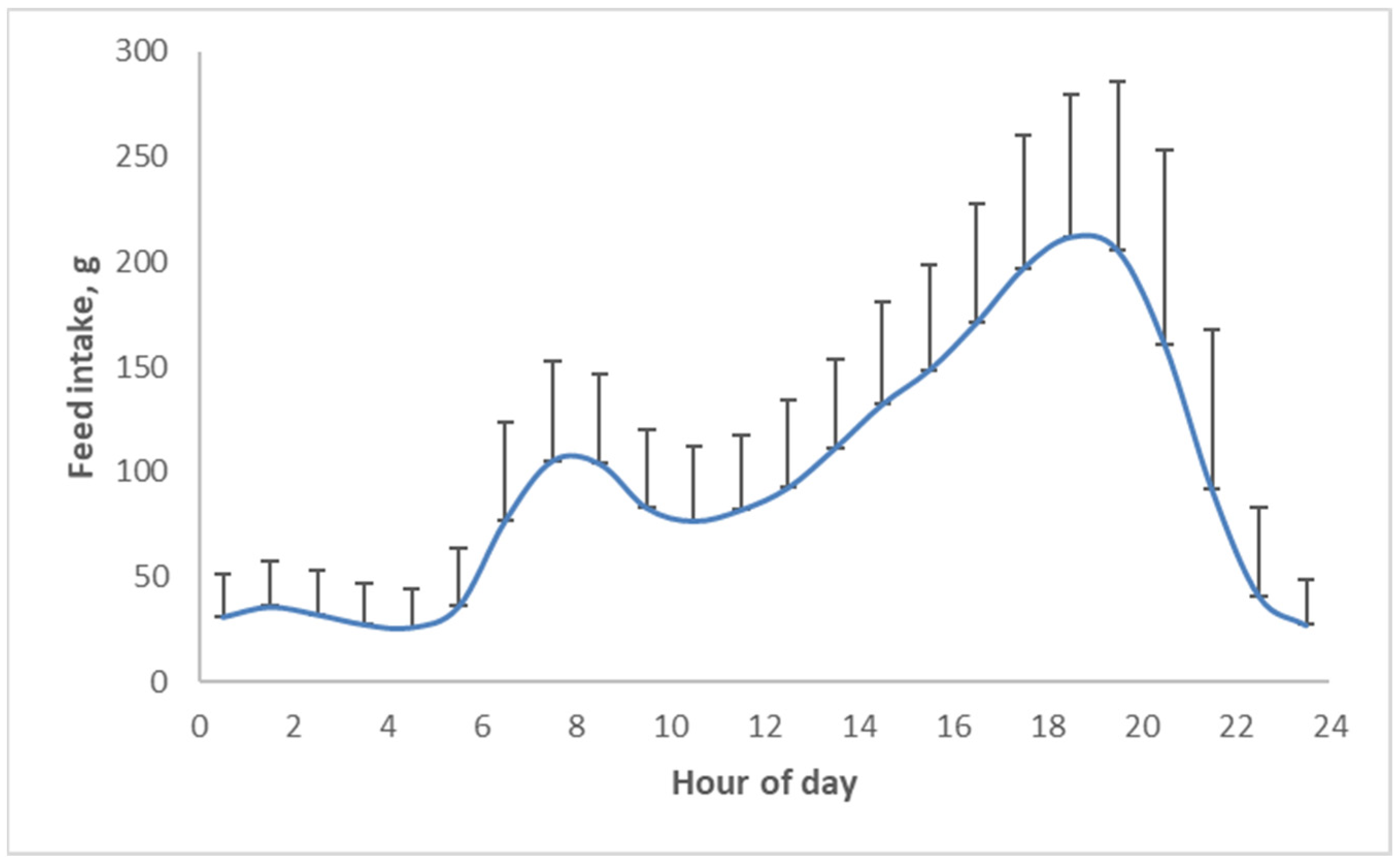
The glycemic response is not only a function of the type and amount of carbohydrates in the diet but also of meal size. The larger the meal, the stronger the glycemic response it can induce. In one group of pigs, we tracked how they consumed food over a period of roughly three months. This food intake behavior remained reasonably consistent throughout this period for individual pigs. Hence, we tried to correlate the number of meals, meal size and food intake to adiposity of the pigs.
Figure 9A shows that an inverse relationship exists between the number of meals per day and the meal size. Multiplying these two values equals the total food intake per day. The correlation analysis (
Figure 9B) indicates that food intake was positively correlated with adiposity; eating more food resulted in slightly fatter and less muscular pigs (this is very much calorie counting). The number of meals/day (i.e., the inverse of the meal size) also had an effect; pigs that ate more frequently and thus had smaller meals were less fat and more muscular. In fact, this is quite logical, given the glycemia story above. Smaller meals mean less stimulation of blood glucose and insulin; glucose ends up less in the ‘red’ (‘danger’) zone (
Figure 5).
8. Hyper-Processed Food
The diet that the typical person consumes has changed drastically over time, especially in recent decades. The fraction of carbohydrates has increased, and many of these carbohydrates are categorized as hyper-processed. For example, maize contains a lot of carbohydrates in the form of starch, which is contained inside a matrix, making this starch less accessible by digestive enzymes (intact maize kernels can actually pass through a pig’s digestive tract completely intact). Maize, however, is often processed to extract this starch. The resulting starch yields a much higher glycemic index than the same amount of starch in the maize itself. Processing this starch into, e.g., glucose syrup, sometimes used in soft drinks, results in an even higher glycemic index. Generalizing, hyper-processed foods are foods that result in much stronger glycemic responses than non-processed foods. As such, they can contribute to the development of obesity, as outlined above, simply by augmenting the glycemic response.
Although we do support the opinion that hyper-processed foods can contribute to obesity, experiments with pigs do not always corroborate this. One project we executed involved processing maize into three fractions: the endosperm containing the starch, the germ containing most of the protein and the hull containing most of the fiber. We subsequently tested diets based on these three different fractions. The aim of this experiment was to design a diet that yielded less feces, as the disposal of feces can be a challenge in livestock production. Textbooks claim that diets based on only endosperm, thus hardly any fiber, cause health problems and obesity, but we did not observe this in our pigs [
24,
25]. In addition, this contradicts the story above on glycemic response!
Another confounding observation to the glycemia hypothesis is based on an animal feed mill in the Netherlands. This feed mill has taken circular economy to the next level. Already in Western Europe, pigs are fed diets consisting of byproducts from the food industry as their primary ingredients (e.g., coproducts obtained from wheat after extracting starch). This feed mill, though, collects all kinds of foods rejected for human consumption, and they create their swine diets using these materials. In this plant, there are sheds full of cookies, caramel, whipped cream, gummy bears, bread, etc. To turn these into a pig diet, this food material is ground and heat treated. Thus, if the cookies were hyper-processed to start out with, they are hyper-hyper-processed when fed to the pigs. Interestingly, pigs fed diets based on this food material are just as lean as pigs fed more conventional diets based on regular feedstuffs (personal communication). This would imply that hyper-processing is NOT the cause of obesity, despite what we know about the glycemic index. A high glycemic food thus seems to pose an extra challenge to humans, but by itself, it may not induce obesity.
To make matters worse, a large portion of pigs that receive these hyper-processed diets are fed with so-called liquid feeding systems. This means that the food is mixed with water into a porridge, which is distributed several times per day. In contrast to the natural feeding pattern of the pigs outlined above, when pigs are fed these liquid diets, they typically receive three or four meals per day. The timing of these meals is very much dictated by the farmer and the available food mixing and distribution equipment; little attention is given to align this with natural behavior. Worse, survival of the fittest kicks in when the pigs are fed; consequently, they generally consume this porridge in a matter of minutes. From a meal-pattern perspective, this eating pattern is not ideal because the meals are not aligned with natural endocrinology, and moreover, very large meals with a high glycemic index are consumed. Multiplying this implies that the pigs are exposed to a giant glycemic response. Nevertheless, obesity is no worse than when pigs are fed more naturally. The reason for this may be that pigs only received diets that were balanced for all nutrients.
9. Imbalanced Diets
The hyper-processing of food influences human food in a way that it does not affect the feed for pigs. The above experiments with the fractionation of maize can be used as an example. The germ fraction does not only contain most of the protein but also other essential nutrients, such as minerals and vitamins (referred to as micronutrients). The endosperm, in contrast, contains mostly energy in the form of starch. In
Figure 11, relative nutrient and micronutrient levels are shown for wheat flour versus wheat meal (whole wheat, ground) and corn starch versus corn meal (whole corn, ground). The wheat flour or corn starch, as expected, contains more carbohydrates, mainly starch, as compared to the whole grains. The micronutrients, however, are drastically lower or even absent in the flour. It should be pointed out that, already for decades, seed companies have selected grains high in starch, which will compromise micronutrients as well [
26].
When we manufactured our experimental swine diets, we took these differences in micronutrient content into consideration. Thus, we supplemented the diets based on endosperm with extra micronutrients, such that in the end, the diets were deemed identical from a micronutrient perspective! For the feed mill processing food that has been rejected for human consumption, animal diets are also formulated to be sufficient in all micronutrients!
In human nutrition, formulating individual foods for all nutrients is generally not carried out. For example, sodas, potato chips and the like are formulated for taste and product appearance. Nutritional value is not a concern. For some (primary) food products, micronutrients such as vitamins may well be added, but there is effectively no effort to balance the entire daily diet for all nutrients (meaning that the diet meets or exceeds the requirements for all nutrients known to be required for optimal growth and health). In fact, the information is not even available in the public human food composition databases, despite decades of research! Additionally, neither the USDA nor EFSA specify the requirements for all vitamins or for any of the amino acids. In line with this, diets used in human-focused obesity research may well be nutritionally inadequate. For example, Lee et al. [
28] used a high-calorie atherogenic diet for Ossabaw pigs containing only 8% protein; this level of protein is unlikely to meet the amino acid requirements of the animals, which would predispose them to obesity. Detailed information on essential nutrients, however, was missing from the paper and appears missing from the website of the company that manufactured the diets (
Testdiet.com; accessed on 8 January 2023). Suriano et al. [
29] evaluated high-fat diets for their impact on obesity. Their high-fat diets contained 36% more calories than their control diet, but neither the micronutrients nor the protein were adjusted for this increase. In both cases, the conclusions are thus severely confounded by micronutrient/energy ratios.
In animal nutrition, we even go a step further than simply formulating a diet for nutrient content; we focus on what we call the bio-available nutrient content. This means that we painstakingly study what fraction of nutrients is absorbed from a diet, and we determine the requirement based on the absorbable amount of that nutrient. As an example, an important but often ignored micronutrient is phosphate. Phosphate is a mineral, which plays a role in effectively every metabolic pathway in our body. Above, we mentioned ATP as the energy currency. ATP stands for Adenosine Tri Phosphate, and it obtains its energy from the number of phosphate groups, which are attached to it (tri is 3). Phosphate has a strong interplay with insulin. Insulin signals to the body to increase metabolic activity as the energy is arriving from the diet, which needs to be processed somehow. This increase in metabolic activity creates a demand for phosphate. This also means that the phosphate status of a person is dependent on what the person is doing at that moment. If the person has just consumed a big bag of processed starch (e.g., potato chips), then metabolism is triggered to process this starch into heat, activity or, e.g., adipose tissue, for which phosphate is pulled from blood into cells in order to support metabolism. Processing the starch into fat apparently requires the least amount of phosphate within the body. Thus, when phosphate is in short supply, we predispose ourselves to adiposity when consuming high glycemic foods without concomitantly providing phosphate. Metabolically, this is apparently ‘the body’s easy way out.
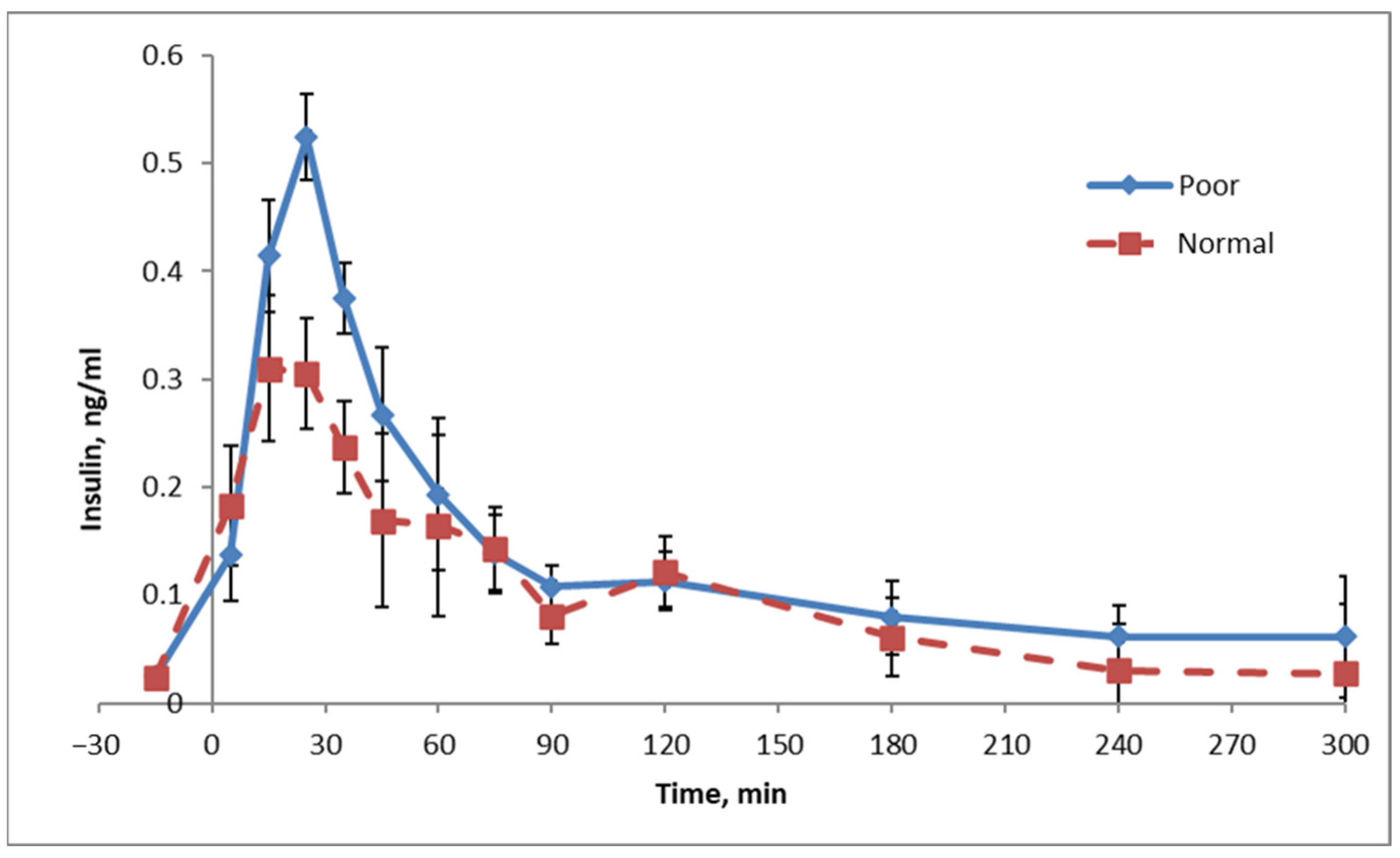
For pigs, we perform experiments to determine the optimum level of each nutrient in a diet. These types of dose–response data provide interesting insights with respect to obesity. The nutrient phosphate can again be used as an example. Bertram [
30] performed dose–response studies in which the bioavailable phosphate content ranged from 0.08 to 0.475% of the diet. For this experiment, two genetic lines of pigs were used: a highly muscular line and a moderately muscular line. The key results from this experiment are summarized in
Figure 12. Pigs fed diets that were deficient in phosphate ate somewhat less (−4%), although not spectacularly so. Their growth, however, was strongly compromised (−14%). The conversion of food into growth, consequently, was also strongly compromised (−11%). If we go back to calorie counting, the energy intake of the pigs was thus reduced by 4%, but growth was compromised much more drastically. The explanation for this is that the energy was used to grow a tissue, which is energetically very expensive to grow: adipose tissue. Indeed, backfat increased by 12–13%, kidney fat by 23–29% (which is especially unhealthy) and total body fat by 17–21%. This fat gain occurred at the expense of muscle growth; the longissimus dorsi (or loin eye) muscle was 8–12% smaller, and the total muscle content was reduced by 6–8%. Although there were small differences between the two genetic lines of pigs, none of those were statistically significant.
Similar datasets exist for other micronutrients, both minerals and B vitamins (and for amino acids). Many of these micronutrients, when deficient, compromise metabolic health, which often hinders the body’s ability to grow muscle. The growth of adipose tissue, however, is apparently a metabolically ‘simpler’ process, and consequently, dietary energy is often funneled into adipose tissue as fat when deficiencies occur.
Consequently, based on this mechanism, the requirement for phosphate and several other micronutrients is actually a function of the glycemic load of the diet. Diets with a low glycemic load will not result in strong insulin peaks; therefore, low amounts of phosphate and related micronutrients are required to process the glucose. Diets with a strong glycemic response, however, will require a lot of phosphate and related micronutrients during this glycemic load. Khattab et al. [
31] elegantly showed that blood phosphate levels were inversely related to blood glucose levels during a glycemia challenge; this drop in blood phosphate could be prevented by supplementation of phosphate at the start of the glucose challenge (
Figure 13). This level of sophistication has not yet been implemented in swine nutrition, and it may well explain why diets with an extremely high glycemic index can still lead to problems with adiposity as outlined above despite formulating for all micronutrients.
Our bodies seem to have recognized the importance of phosphate; it is one of the few nutrients for which we have taste receptors [
32]. Consequently, animals, including strict herbivores, which suffer from hypophosphatemia, will actively search for phosphate sources. These sources include bones of deceased animals (osteophagia), resulting in interesting pictures of, e.g., giraffes chewing bones. Research with lab animals confirms that induction of hypophosphatemia leads to an appetite for phosphate [
33]. Bassil and Obeid [
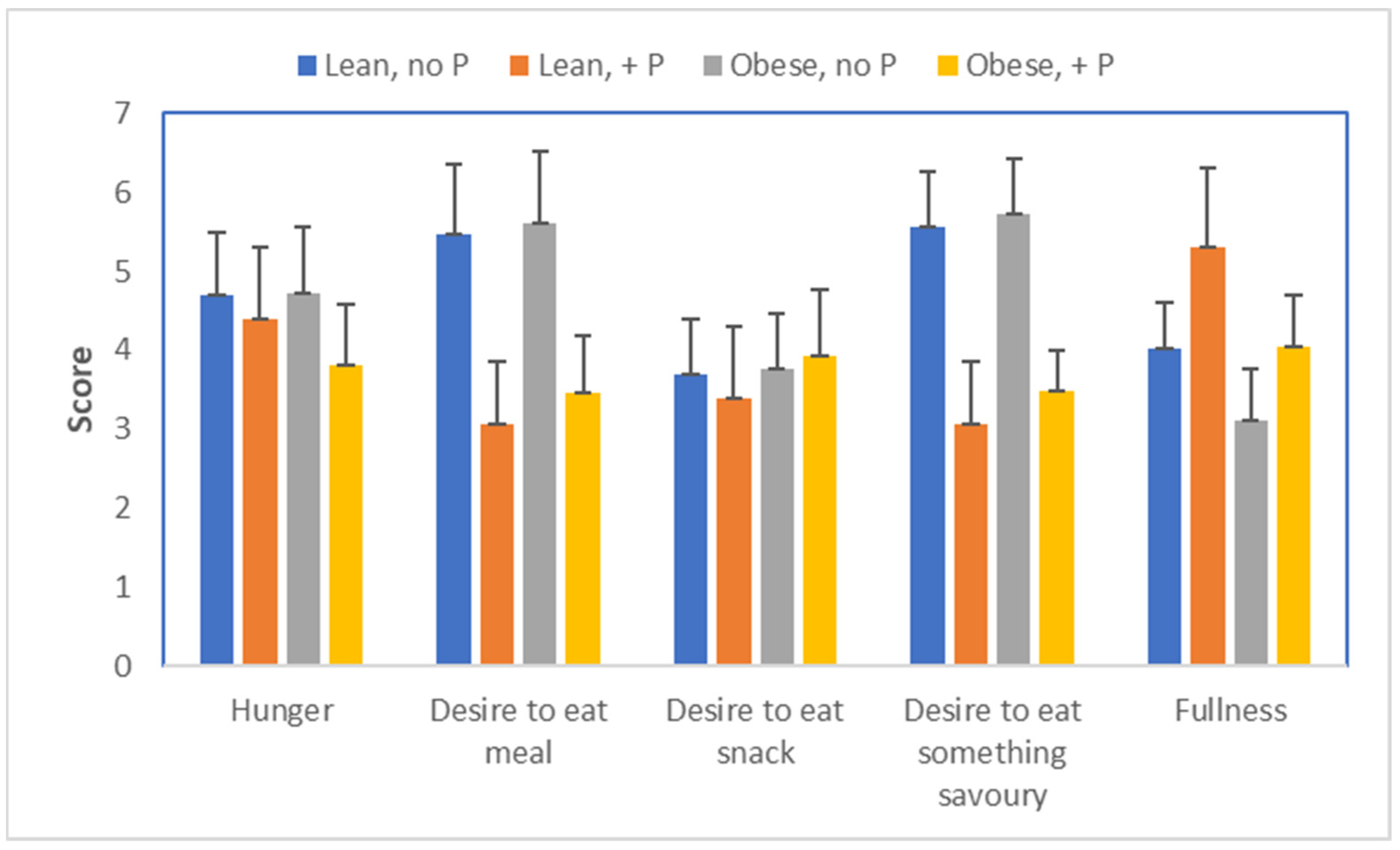
34] actually showed that appetite is also stimulated in both lean and obese humans who are hypophosphatemic (
Figure 14). Their work also indicated that extra phosphate stimulated glucose oxidation in obese individuals, implying that glucose oxidation was impeded by the low phosphate status. As explained, carbohydrates that are not oxidized will likely end up being converted into fat.
In maize and many other plants, phosphate is mainly present in the germ and stored in an ester with inositol (a sugar) called inositol phosphate or phytic acid. This nifty molecule poses a challenge for the digestive system of both humans and pigs, as neither can break it down. This means that the inositol phosphate we eat passes through the small intestine without releasing any of its phosphate, and consequently, the phosphate is not absorbed. Germ phosphate, in fact, has a digestibility in swine of only 8% [
25]. Animal-derived food, in contrast, contains phosphate in a bioavailable form. Vegans are thus more prone to shortages of phosphate than omnivores. In pig nutrition, we characterized the bioavailability of phosphate for all ingredients used; therefore, we incorporated this information when formulating a diet (
Figure 15). This ensures that there is no risk of our diets being deficient in phosphate. For humans, bioavailability is generally not considered, as the data are lacking. This may well mean, though, that for a diet containing ample amounts of phosphate, most of it is not absorbed, so the diet still leads to a deficiency of phosphate.
Phosphate, from a diabetes and obesity perspective, is especially interesting, as numerous reports link phosphate to insulin action. Moreover, people with type II diabetes and/or obesity are prone to be deficient in phosphate [
35,
36]. Although we could debate whether this is a cause or effect, the above data on hyper-processed diets fed to pigs would indicate that it is causative. After all, pigs were fed a diet, which included ‘human-targeted junk food’ but was adequate in phosphate, and they experienced no issues.
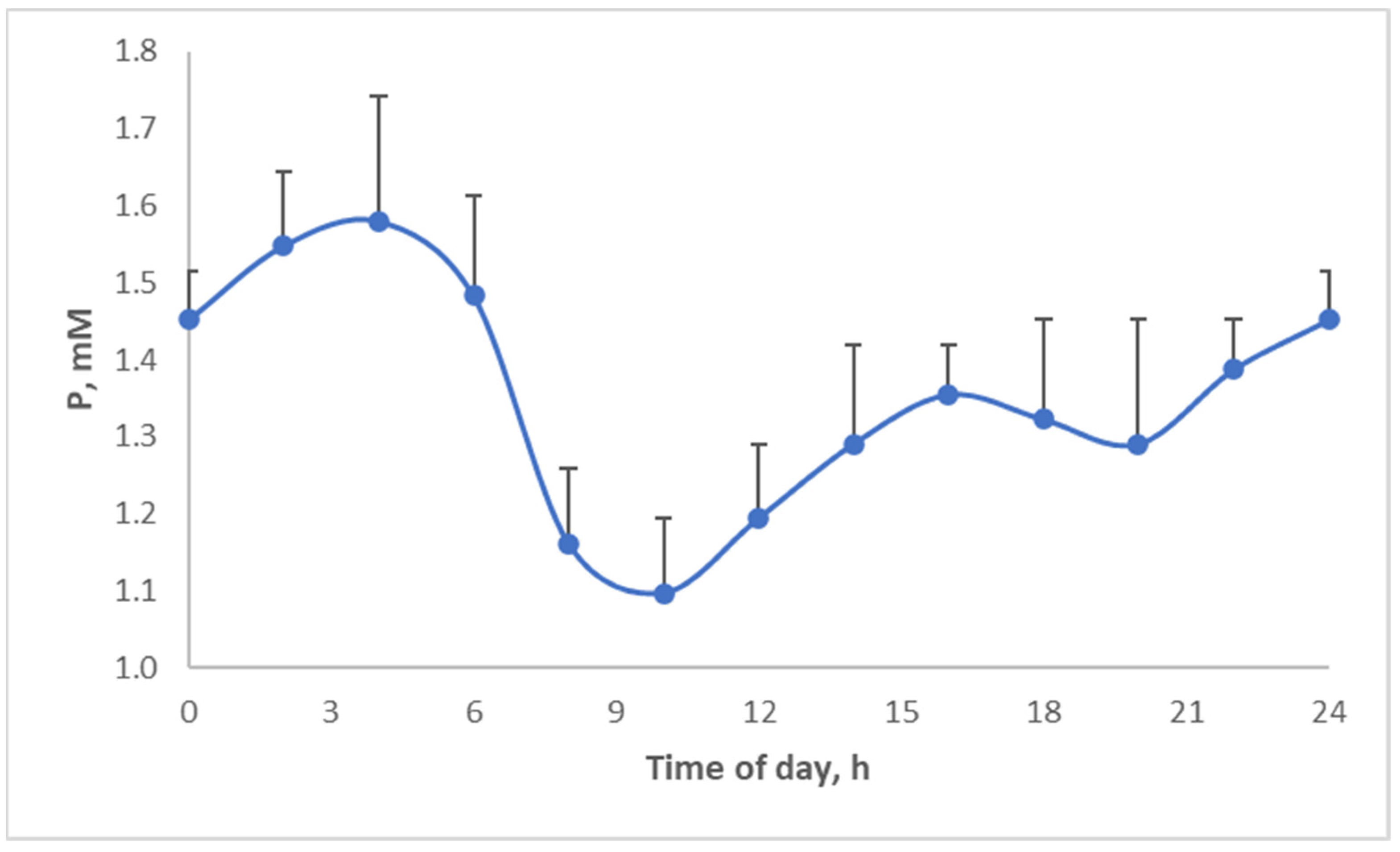
Above, the diurnal food intake patterns were discussed. Several micronutrients and hormones actually display diurnal patterns. For example, phosphate shows a strong circadian pattern (
Figure 16), which is actually quite similar to the food intake pattern of pigs (
Figure 10) but shifted in time. Thus, by changing when we eat, we may aggravate the discrepancies between micronutrient availability and requirement.
15. Healthy Foods
‘An apple a day keeps the doctor away’ is a well-known phrase in the English language. Indeed, apples are deemed as healthy by many, including many health professionals. To look at the health benefits of apples, we analyzed them in the same manner as the ingredients for use in swine diets. In order to achieve this, we took the nutritional composition, as listed in the NEVO [
27] database, and the recommended dietary intake for a 40-year-old person. Based on this, we calculated for each nutrient how much apple one would have to eat each day to meet the nutritional requirement. Indeed, this is an odd calculation, as we do not live on apples alone, but it hopefully makes the numbers more tangible (
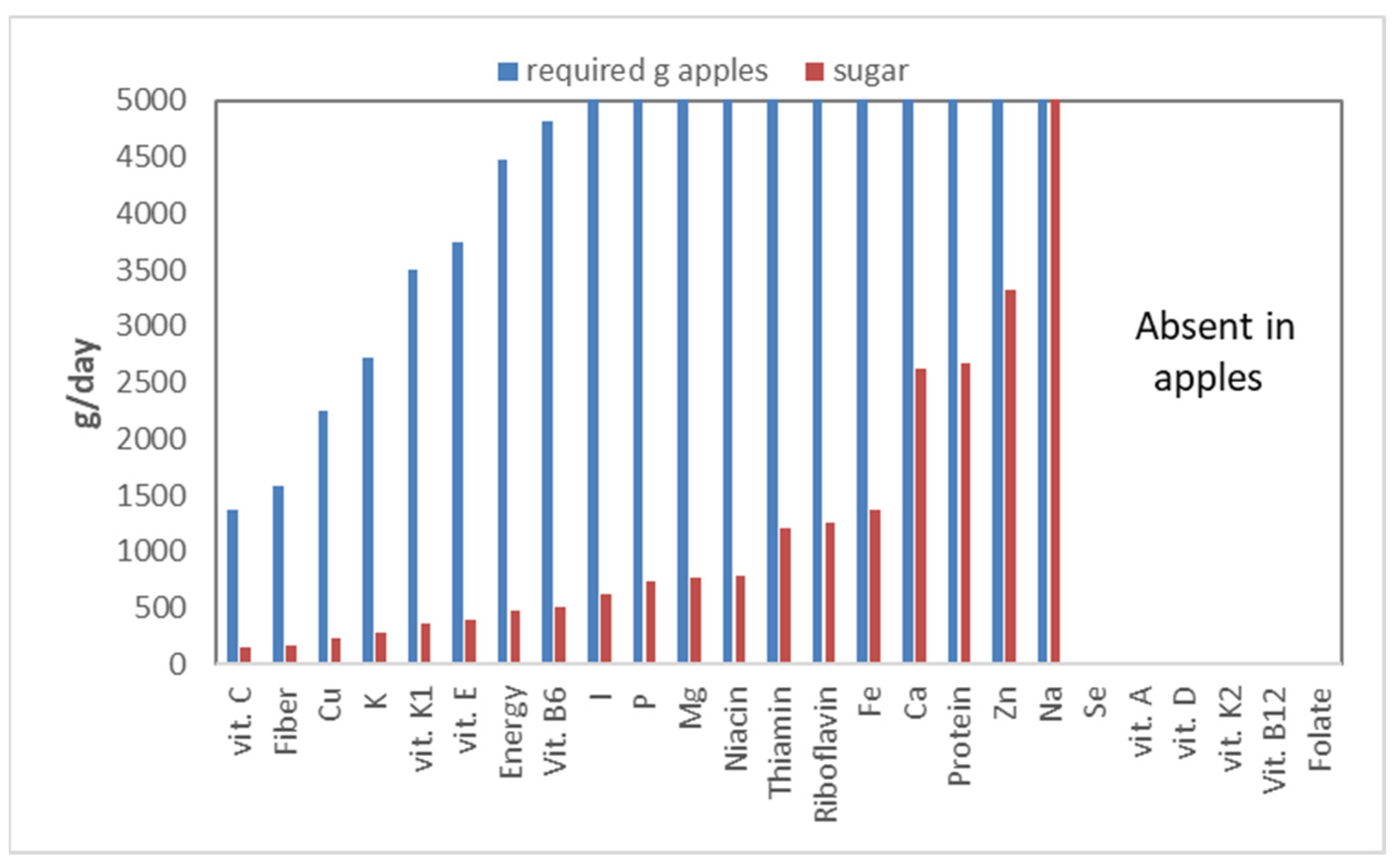
Figure 18).
Apples turn out to be a reasonable source of vitamin C: one would have to eat 1375 g of apples a day to meet the vitamin C requirement. Apples are also a good source of fiber, requiring only 1575 g of apples a day. The dominant fiber in apples, pectin, is a ‘sticky’ fiber, which will not work for everybody, as it strongly stimulates fermentation. Apples, however, are also an important source of sugars: 10.5%. This number becomes even more staggering when expressed on a dry matter basis: 72%! Even worse, apples are high in fructose, a sugar that is hard on the body, as explained above. Calculating the sugar intake, which would accompany the various nutrients that apples contain, shows that to meet one’s vitamin C requirement, the total sugar intake would be 144 g, which is far above what is deemed healthy! This means that unhealthy nutrients (sugar) are relatively more important in apples than vitamin C, the nutrient for which apples are a relatively good source.
Several nutrients (selenium, folate, and vitamin A, D, K
2, B
12) are not provided by apples—hence no bars in
Figure 18—while for iodine (I) to sodium (Na), the required intake is above 5000 g/d, or well above 500 g of sugars per day. This graph also ignores bio-availability; not all nutrients contained in apples are indeed accessible for absorption by the intestines. Thus, in reality, the required intakes should be even higher.
Apples do contain phytonutrients, such as quercetin. Quercetin is a well-researched polyphenol, which can act as a potent antioxidant, and indeed, many health claims are described for quercetin. Quercetin, though, is contained exclusively in the peel, so it would have to be eaten for any health benefits. Unfortunately, quercetin is difficult to absorb by our intestines, so an important question is whether it can provide any benefit. To our knowledge, the European regulatory agencies have not formally approved any of these health claims, and trials with quercetin in pigs failed to show any benefit. Based on this assessment, it is thus more logical to conclude that apples are very much like candy, providing some vitamin C and fiber.






 ), and oral phosphate (500 mg) dose (
), and oral phosphate (500 mg) dose ( ), or the combination of an oral glucose tolerance test plus oral phosphate (
), or the combination of an oral glucose tolerance test plus oral phosphate ( ). * p-value < 0.05: paired t-test in the same treatment in comparison with baseline (time 0) value. a p-value < 0.05: paired t-test, phosphorus vs. glucose treatments at each time point. b p-value < 0.05: paired t-test, phosphorus vs. glucose + phosphorus treatments at each time point. c p-value < 0.05: paired t-test, glucose vs. glucose + phosphorus treatments at each time point [31].
). * p-value < 0.05: paired t-test in the same treatment in comparison with baseline (time 0) value. a p-value < 0.05: paired t-test, phosphorus vs. glucose treatments at each time point. b p-value < 0.05: paired t-test, phosphorus vs. glucose + phosphorus treatments at each time point. c p-value < 0.05: paired t-test, glucose vs. glucose + phosphorus treatments at each time point [31].
 ), and oral phosphate (500 mg) dose (
), and oral phosphate (500 mg) dose ( ), or the combination of an oral glucose tolerance test plus oral phosphate (
), or the combination of an oral glucose tolerance test plus oral phosphate ( ). * p-value < 0.05: paired t-test in the same treatment in comparison with baseline (time 0) value. a p-value < 0.05: paired t-test, phosphorus vs. glucose treatments at each time point. b p-value < 0.05: paired t-test, phosphorus vs. glucose + phosphorus treatments at each time point. c p-value < 0.05: paired t-test, glucose vs. glucose + phosphorus treatments at each time point [31].
). * p-value < 0.05: paired t-test in the same treatment in comparison with baseline (time 0) value. a p-value < 0.05: paired t-test, phosphorus vs. glucose treatments at each time point. b p-value < 0.05: paired t-test, phosphorus vs. glucose + phosphorus treatments at each time point. c p-value < 0.05: paired t-test, glucose vs. glucose + phosphorus treatments at each time point [31].
 ) for some foodstuffs for pigs [9].
) for some foodstuffs for pigs [9].
 ) for some foodstuffs for pigs [9].
) for some foodstuffs for pigs [9].