Metabolomics and Self-Reported Depression, Anxiety, and Phobic Symptoms in the VA Normative Aging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. VA Normative Aging Study (NAS)
2.2. Brief Symptom Inventory
2.3. Metabolomic Profiling
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
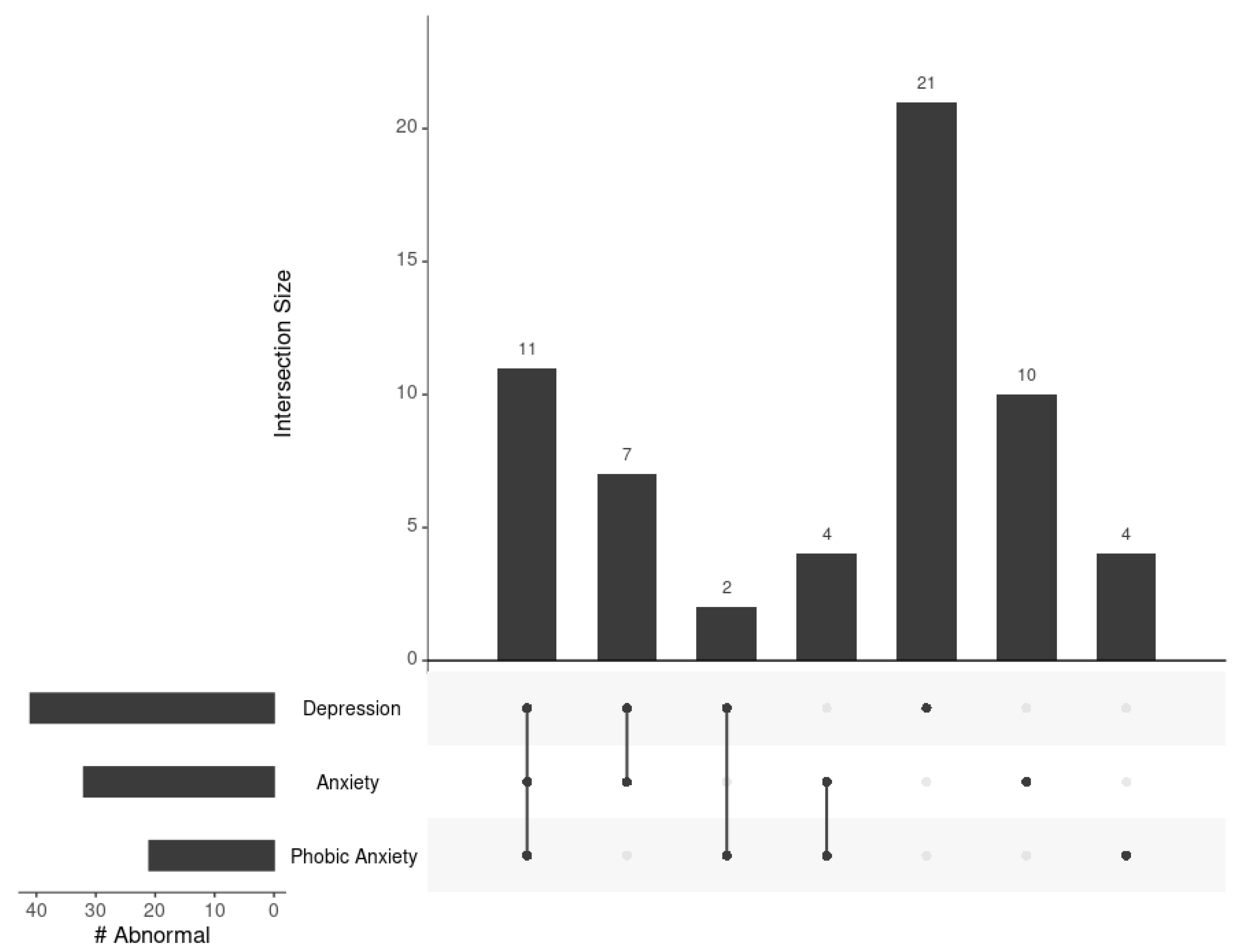
3.2. Relationships across BSI Depression, Anxiety, and Phobic Anxiety Subscales
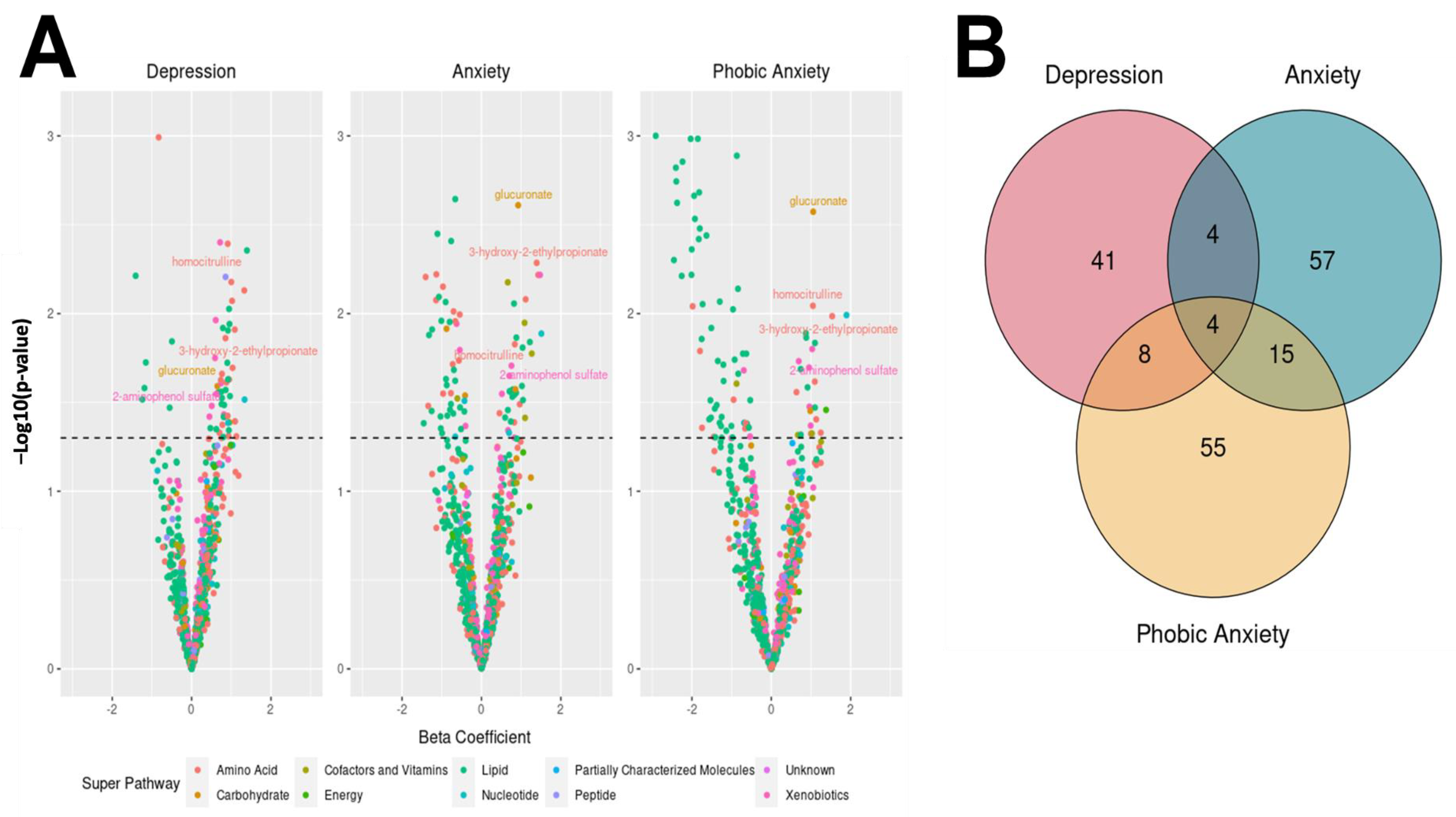
3.3. Associations between Metabolites and BSI Dimension Abnormality
3.4. Metabolite Levels between the Abnormality Groups
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Institute of Mental Health. Mental Illness. Available online: https://www.nimh.nih.gov/health/statistics/mental-illness (accessed on 10 April 2023).
- Seal, K.H.; Metzler, T.J.; Gima, K.S.; Bertenthal, D.; Maguen, S.; Marmar, C.R. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Am. J. Public Health 2009, 99, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.B. Metabolomics Biomarkers for Precision Psychiatry. Adv. Exp. Med. Biol. 2019, 1161, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Vogt, D.S.; Tyrell, F.A.; Bramande, E.A.; Nillni, Y.I.; Taverna, E.C.; Finley, E.P.; Perkins, D.F.; Copeland, L.A. U.S. Military Veterans’ Health and Well-Being in the First Year After Service. Am. J. Prev. Med. 2020, 58, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.W.; Oh, G.H.; Jung, S.; Moon, J.Y.; Son, K.L.; Kim, W.H.; Jung, D.; Baik, M.; Shim, E.J.; Moon, H.; et al. Prevalence and comorbidities of adult adhd in male military conscripts in korea: Results of an epidemiological survey of mental health in korean military service. Psychiatry Res. 2020, 293, 113401. [Google Scholar] [CrossRef]
- Wang, J.; Ursano, R.J.; Gifford, R.K.; Dinh, H.; Farooq, S.; Broshek, C.E.; Cohen, G.H.; Sampson, L.; Galea, S.; Fullerton, C.S. Mental Health and Suicidality in Separating U.S. Reserve and National Guard Personnel. Psychiatry 2020, 83, 166–175. [Google Scholar] [CrossRef]
- Theriault, F.L.; Gardner, W.; Momoli, F.; Garber, B.G.; Kingsbury, M.; Clayborne, Z.; Cousineau-Short, D.Y.; Sampasa-Kanyinga, H.; Landry, H.; Colman, I. Mental Health Service Use in Depressed Military Personnel: A Systematic Review. Mil. Med. 2020, 185, e1255–e1262. [Google Scholar] [CrossRef]
- Moradi, Y.; Dowran, B.; Sepandi, M. The global prevalence of depression, suicide ideation, and attempts in the military forces: A systematic review and Meta-analysis of cross sectional studies. BMC Psychiatry 2021, 21, 510. [Google Scholar] [CrossRef]
- Hakak-Zargar, B.; Tamrakar, A.; Voth, T.; Sheikhi, A.; Multani, J.; Schutz, C.G. The Utility of Research Domain Criteria in Diagnosis and Management of Dual Disorders: A Mini-Review. Front. Psychiatry 2022, 13, 805163. [Google Scholar] [CrossRef]
- Maruish, M.E. 4.18—Therapeutic Assessment: Linking Assessment and Treatment. In Comprehensive Clinical Psychology; Bellack, A.S., Hersen, M., Eds.; Pergamon: Oxford, UK, 1998; pp. 525–561. [Google Scholar]
- Gerszten, R.E.; Wang, T.J. The search for new cardiovascular biomarkers. Nature 2008, 451, 949–952. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Yang, J.; Chen, T.; Sun, L.; Zhao, Z.; Qi, X.; Zhou, K.; Cao, Y.; Wang, X.; Qiu, Y.; Su, M.; et al. Potential metabolite markers of schizophrenia. Mol. Psychiatry 2013, 18, 67–78. [Google Scholar] [CrossRef]
- Hagihara, H.; Horikawa, T.; Irino, Y.; Nakamura, H.K.; Umemori, J.; Shoji, H.; Yoshida, M.; Kamitani, Y.; Miyakawa, T. Peripheral blood metabolome predicts mood change-related activity in mouse model of bipolar disorder. Mol. Brain 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Pieh, C.; Probst, T. Metabolomic Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 4784. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Turecki, G.; Lam, R.W.; Milev, R.V.; Frey, B.N.; MacQueen, G.M.; Muller, D.J.; Rotzinger, S.; Kennedy, S.H.; Foster, J.A.; et al. Metabolomic signatures associated with depression and predictors of antidepressant response in humans: A CAN-BIND-1 report. Commun. Biol. 2021, 4, 903. [Google Scholar] [CrossRef] [PubMed]
- Suvitaival, T.; Mantere, O.; Kieseppa, T.; Mattila, I.; Poho, P.; Hyotylainen, T.; Suvisaari, J.; Oresic, M. Serum metabolite profile associates with the development of metabolic co-morbidities in first-episode psychosis. Transl. Psychiatry 2016, 6, e951. [Google Scholar] [CrossRef]
- LN, F.G.C.; Carneiro, B.A.; Alves, G.S.; Lins Silva, D.H.; Faria Guimaraes, D.; Souza, L.S.; Bandeira, I.D.; Beanes, G.; Miranda Scippa, A.; Quarantini, L.C. Metabolomics of Major Depressive Disorder: A Systematic Review of Clinical Studies. Cureus 2022, 14, e23009. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Hertel, J.; Johar, H.; Pietzner, M.; Lukaschek, K.; Atasoy, S.; Kunze, S.; Volzke, H.; Nauck, M.; Friedrich, N.; et al. A metabolome-wide association study in the general population reveals decreased levels of serum laurylcarnitine in people with depression. Mol. Psychiatry 2021, 26, 7372–7383. [Google Scholar] [CrossRef]
- Pedrini, M.; Cao, B.; Nani, J.V.S.; Cerqueira, R.O.; Mansur, R.B.; Tasic, L.; Hayashi, M.A.F.; McIntyre, R.S.; Brietzke, E. Advances and challenges in development of precision psychiatry through clinical metabolomics on mood and psychotic disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 182–188. [Google Scholar] [CrossRef]
- Bell, B.; Rose, C.L.; Damon, A. The Normative Aging Study: An Interdisciplinary and Longitudinal Study of Health and Aging. Aging Hum. Dev. 1972, 3, 5–17. [Google Scholar] [CrossRef]
- Rhodes, D.; Spiro, A., 3rd; Aro, A.; Hu, H. Relationship of bone and blood lead levels to psychiatric symptoms: The normative aging study. J. Occup. Environ. Med. 2003, 45, 1144–1151. [Google Scholar] [CrossRef]
- Payton, M.; Riggs, K.M.; Spiro, A., 3rd; Weiss, S.T.; Hu, H. Relations of bone and blood lead to cognitive function: The VA Normative Aging Study. Neurotoxicol. Teratol. 1998, 20, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Bayne, H.; Spiro, A., 2nd; Vokonas, P.; Sparrow, D.; Weiss, S.T.; Schwartz, J.; Nassan, F.L.; Lee-Sarwar, K.; Huang, M.; et al. Metabolomic signatures of lead exposure in the VA Normative Aging Study. Environ. Res. 2020, 190, 110022. [Google Scholar] [CrossRef] [PubMed]
- Derogatis, L.R.; Melisaratos, N. The Brief Symptom Inventory: An introductory report. Psychol. Med. 1983, 13, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Stewart, I.D.; Bayne, H.; Kachroo, P.; Spiro, A., 3rd; Vokonas, P.; Sparrow, D.; Weiss, S.T.; Knihtila, H.M.; Litonjua, A.A.; et al. Metabolomic differences in lung function metrics: Evidence from two cohorts. Thorax 2022, 77, 919–928. [Google Scholar] [CrossRef]
- Rajan, P.; Kelsey, K.T.; Schwartz, J.D.; Bellinger, D.C.; Weuve, J.; Sparrow, D.; Spiro, A., 3rd; Smith, T.J.; Nie, H.; Hu, H.; et al. Lead burden and psychiatric symptoms and the modifying influence of the delta-aminolevulinic acid dehydratase (ALAD) polymorphism: The VA Normative Aging Study. Am. J. Epidemiol. 2007, 166, 1400–1408. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- de Kluiver, H.; Jansen, R.; Milaneschi, Y.; Bot, M.; Giltay, E.J.; Schoevers, R.; Penninx, B. Metabolomic profiles discriminating anxiety from depression. Acta Psychiatr. Scand. 2021, 144, 178–193. [Google Scholar] [CrossRef]
- Kessler, R.C.; Heeringa, S.G.; Stein, M.B.; Colpe, L.J.; Fullerton, C.S.; Hwang, I.; Naifeh, J.A.; Nock, M.K.; Petukhova, M.; Sampson, N.A.; et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry 2014, 71, 504–513. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional therapies for mental disorders. Nutr. J. 2008, 7, 2. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Zhong, Q.; Bai, S.J.; Zhou, C.J.; Tian, T.; Chen, J.J. Associations Between Disordered Microbial Metabolites and Changes of Neurotransmitters in Depressed Mice. Front. Cell Infect. Microbiol. 2022, 12, 906303. [Google Scholar] [CrossRef] [PubMed]
- Gropman, A.L.; Summar, M.; Leonard, J.V. Neurological implications of urea cycle disorders. J. Inherit. Metab. Dis. 2007, 30, 865–879. [Google Scholar] [CrossRef] [PubMed]
- 2 Final Report on the Safety Assessment of p-Aminophenol, m-Aminophenol, and o-Aminophenol. J. Am. Coll. Toxicol. 1988, 7, 279–333. [CrossRef]
- Elhaj, R.; Reynolds, J.M. Chemical exposures and suspected impact on Gulf War Veterans. Mil. Med. Res. 2023, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ge, S.; Singh, R.; Basu, S.; Shatzer, K.; Zen, M.; Liu, J.; Tu, Y.; Zhang, C.; Wei, J.; et al. Glucuronidation: Driving factors and their impact on glucuronide disposition. Drug Metab. Rev. 2017, 49, 105–138. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Dratter, J.; Wang, C.; Tunge, J.A.; Desaire, H. Identification of sulfation sites of metabolites and prediction of the compounds’ biological effects. Anal. Bioanal. Chem. 2006, 386, 666–674. [Google Scholar] [CrossRef]
- Schneider, M.; Levant, B.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Muller, C.P. Lipids in psychiatric disorders and preventive medicine. Neurosci. Biobehav. Rev. 2017, 76, 336–362. [Google Scholar] [CrossRef]
- Pinto, B.; Conde, T.; Domingues, I.; Domingues, M.R. Adaptation of Lipid Profiling in Depression Disease and Treatment: A Critical Review. Int. J. Mol. Sci. 2022, 23, 2032. [Google Scholar] [CrossRef]
- Sethi, S.; Hayashi, M.A.; Barbosa, B.S.; Pontes, J.G.; Tasic, L.; Brietzke, E. Lipidomics, Biomarkers, and Schizophrenia: A Current Perspective. Adv. Exp. Med. Biol. 2017, 965, 265–290. [Google Scholar] [CrossRef]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Muhle, C.; Bilbao Canalejas, R.D.; Kornhuber, J. Sphingomyelin Synthases in Neuropsychiatric Health and Disease. Neurosignals 2019, 27, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Thomas, E.A. Sphingolipid abnormalities in psychiatric disorders: A missing link in pathology? Front. Biosci. (Landmark Ed) 2011, 16, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- van Kruining, D.; Luo, Q.; van Echten-Deckert, G.; Mielke, M.M.; Bowman, A.; Ellis, S.; Oliveira, T.G.; Martinez-Martinez, P. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv. Drug Deliv. Rev. 2020, 159, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Vega, S.; Garcia-Juarez, M.; Camacho-Morales, A. Contribution of ceramides metabolism in psychiatric disorders. J. Neurochem. 2023, 164, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Deng, K.; Xue, Y.; Yang, R.; Yang, R.; Gong, Z.; Tang, M. Carnitine and Depression. Front. Nutr. 2022, 9, 853058. [Google Scholar] [CrossRef]
- Smeland, O.B.; Meisingset, T.W.; Borges, K.; Sonnewald, U. Chronic acetyl-L-carnitine alters brain energy metabolism and increases noradrenaline and serotonin content in healthy mice. Neurochem. Int. 2012, 61, 100–107. [Google Scholar] [CrossRef]
- Eaton, W.W.; Neufeld, K.; Chen, L.S.; Cai, G. A comparison of self-report and clinical diagnostic interviews for depression: Diagnostic interview schedule and schedules for clinical assessment in neuropsychiatry in the Baltimore epidemiologic catchment area follow-up. Arch. Gen. Psychiatry 2000, 57, 217–222. [Google Scholar] [CrossRef]
- Panyard, D.J.; Yu, B.; Snyder, M.P. The metabolomics of human aging: Advances, challenges, and opportunities. Sci. Adv. 2022, 8, eadd6155. [Google Scholar] [CrossRef]
- Hu, J.; Yao, J.; Deng, S.; Balasubramanian, R.; Jimenez, M.C.; Li, J.; Guo, X.; Cruz, D.E.; Gao, Y.; Huang, T.; et al. Differences in Metabolomic Profiles Between Black and White Women and Risk of Coronary Heart Disease: An Observational Study of Women from Four US Cohorts. Circ. Res. 2022, 131, 601–615. [Google Scholar] [CrossRef]
- Huang, M.; Kelly, R.S.; Chu, S.H.; Kachroo, P.; Gürdeniz, G.; Chawes, B.L.; Bisgaard, H.; Weiss, S.T.; Lasky-Su, J. Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial. Metabolites 2021, 11, 65. [Google Scholar] [CrossRef]
| Characteristics | |
|---|---|
| Age (years), Mean [SD] | 74.98 [6.61] |
| BMI (kg/m2), Mean [SD] | 27.65 [4.14] |
| Race, N (%) | |
| White | 400 (98.8%) |
| Black | 3 (0.7%) |
| Hispanic White | 2 (0.5%) |
| Health, N (%) | |
| Excellent | 52 (12.8%) |
| Good | 252 (62.2%) |
| Fair | 71 (17.5%) |
| Poor/Very Poor | 12 (3%) |
| Missing | 18 (4.4%) |
| Income, N (%) | |
| Less than USD 25,000 | 54 (13.3%) |
| USD 25,000-USD 50,000 | 130 (32.1%) |
| USD 50,000-USD 75,000 | 83 (20.5%) |
| More than USD 75,000 | 80 (19.8%) |
| Missing | 58 (14.3%) |
| Ever Married | |
| No | 14 (3.5%) |
| Yes | 379 (93.6%) |
| Missing | 12 (3.0%) |
| Smoking Status, N (%) | |
| Never Smoked | 61 (15.1%) |
| Ex-Smoker | 315 (77.8%) |
| Current Smoker | 18 (4.4%) |
| Missing | 11 (2.7%) |
| Drinking Status, N (%) | |
| Non-Drinker | 61 (15.1%) |
| Light Drinker | 189 (46.7%) |
| Heavy Drinker | 108 (26.7%) |
| Other | 11 (2.7%) |
| Missing | 36 (8.9%) |
| Employment Status, N (%) | |
| Employed | 22 (5.4%) |
| Unemployed/Retired | 355 (87.7%) |
| Missing | 28 (6.9%) |
| Abnormal Depression Score, 1 N (%) | 41 (10.1%) |
| Depression Score, Mean [SD] | 0.26 [0.47] |
| Abnormal Anxiety Score, 1 N (%) | 32 (7.9%) |
| Anxiety Score, Mean [SD] | 0.24 [0.44] |
| Abnormal Phobic Anxiety Score, 1 N (%) | 21 (5.2%) |
| Phobic Anxiety Score, Mean [SD] | 0.12 [0.37] |
| Metabolite | Super Pathway | Sub Pathway | Depression (N = 41 Men) | Anxiety (N = 32 Men) | Phobic Anxiety (N = 21 Men) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | Lower 95% | Upper 95% | p | OR | Lower 95% | Upper 95% | p | OR | Lower 95% | Upper 95% | p | |||
| Homocitrulline | Amino Acid | Urea cycle; arginine and proline metabolism | 2.49 | 1.33 | 4.66 | 0.004 | 2.32 | 1.17 | 4.58 | 0.01 | 2.86 | 1.29 | 6.31 | 0.009 |
| 3-hydroxy-2-ethylpropionate | Amino Acid | Leucine, isoleucine, and valine metabolism | 2.81 | 1.18 | 6.78 | 0.02 | 4.02 | 1.53 | 10.87 | 0.005 | 4.64 | 1.45 | 15.40 | 0.01 |
| Glucuronate | Carbohydrate | Aminosugar metabolism | 1.91 | 1.06 | 3.36 | 0.02 | 2.52 | 1.36 | 4.59 | 0.002 | 2.87 | 1.41 | 5.70 | 0.003 |
| 2-aminophenol sulfate | Xenobiotics | Chemical | 1.87 | 1.08 | 3.31 | 0.03 | 2.13 | 1.14 | 4.09 | 0.02 | 2.59 | 1.19 | 5.92 | 0.02 |
| Super Pathway | Sub Pathway | Depression | Anxiety | Phobic Anxiety |
|---|---|---|---|---|
| Amino Acid | Alanine and Aspartate Metabolism | 2 | 1 | 1 |
| Amino Acid | Glutamate Metabolism | 2 | 1 | 1 |
| Amino Acid | Leucine Isoleucine and Valine Metabolism | 5 | 1 | 1 |
| Amino Acid | Methionine, Cysteine, SAM, and Taurine Metabolism | 2 | 4 | 3 |
| Amino Acid | Tyrosine Metabolism | 1 | 2 | 3 |
| Amino Acid | Urea cycle; Arginine and Proline Metabolism | 3 | 2 | 2 |
| Xenobiotics | Chemical | 2 | 2 | 1 |
| Xenobiotics | Food Component/Plant | 4 | 3 | 4 |
| Carbohydrate | Aminosugar Metabolism | 1 | 1 | 1 |
| Lipid | Fatty Acid Metabolism (Acyl Carnitine) | 2 | 9 | 3 |
| Lipid | Fatty Acid Dicarboxylate | 1 | 3 | 2 |
| Lipid | Phosphatidylethanolamine (PE) | 7 | 1 | 1 |
| Lipid | Sphingomyelins | 3 | 2 | 22 |
| Metabolite | No Abnormality (N = 346) | Depression Only (N = 21) | Anxiety Only (N = 10) | Phobic Anxiety Only (N = 4) | All (N = 11) | η2 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Glucuronate | −0.04 | 0.47 | −0.11 | 0.26 | −0.02 | 0.55 | 0.31 | 1.03 | 0.68 | 0.81 | 0.065 | <0.001 |
| Homocitrulline | −0.08 | 0.50 | −0.02 | 0.46 | 0.02 | 0.40 | 0.00 | 0.93 | 0.58 | 0.71 | 0.046 | 0.001 |
| 2-aminophenol sulfate | −0.06 | 0.63 | −0.01 | 0.62 | 0.08 | 0.62 | −0.21 | 0.74 | 0.57 | 0.43 | 0.030 | 0.020 |
| 3-hydroxy-2-ethylpropionate | −0.05 | 0.38 | −0.01 | 0.31 | 0.06 | 0.29 | 0.29 | 0.31 | 0.22 | 0.44 | 0.023 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prince, N.; Stav, M.; Cote, M.; Chu, S.H.; Vyas, C.M.; Okereke, O.I.; Palacios, N.; Litonjua, A.A.; Vokonas, P.; Sparrow, D.; et al. Metabolomics and Self-Reported Depression, Anxiety, and Phobic Symptoms in the VA Normative Aging Study. Metabolites 2023, 13, 851. https://doi.org/10.3390/metabo13070851
Prince N, Stav M, Cote M, Chu SH, Vyas CM, Okereke OI, Palacios N, Litonjua AA, Vokonas P, Sparrow D, et al. Metabolomics and Self-Reported Depression, Anxiety, and Phobic Symptoms in the VA Normative Aging Study. Metabolites. 2023; 13(7):851. https://doi.org/10.3390/metabo13070851
Chicago/Turabian StylePrince, Nicole, Meryl Stav, Margaret Cote, Su H. Chu, Chirag M. Vyas, Olivia I. Okereke, Natalia Palacios, Augusto A Litonjua, Pantel Vokonas, David Sparrow, and et al. 2023. "Metabolomics and Self-Reported Depression, Anxiety, and Phobic Symptoms in the VA Normative Aging Study" Metabolites 13, no. 7: 851. https://doi.org/10.3390/metabo13070851
APA StylePrince, N., Stav, M., Cote, M., Chu, S. H., Vyas, C. M., Okereke, O. I., Palacios, N., Litonjua, A. A., Vokonas, P., Sparrow, D., Spiro, A., III, Lasky-Su, J. A., & Kelly, R. S. (2023). Metabolomics and Self-Reported Depression, Anxiety, and Phobic Symptoms in the VA Normative Aging Study. Metabolites, 13(7), 851. https://doi.org/10.3390/metabo13070851








