Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract from Cynara Scolymus L. (Artichoke)
2.2. Animals
2.3. Brain Tissue Homogenates
2.4. Biochemical Analyses
2.4.1. Assay of Brain GSH
2.4.2. Assay of GPX1
2.4.3. Assay of GABA
2.4.4. Assay of IL-6
2.4.5. Assay of IL-10
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swensson, C.; Smedman, A.; Henriksson, M.; Lindmark-Månsson, H.; Edman, A.-K.M. Protein efficiency in intensive dairy production: A Swedish example. J. Sci. Food Agric. 2017, 97, 4890–4897. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in autism spectrum disorders: Exercise as a “pharmacological” tool. Neurosci. Biobehav. Rev. 2021, 129, 63. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.A.; Chung, H.J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.S.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef] [PubMed]
- Vismara, L.A.; Rogers, S.J. Behavioral treatments in autism spectrum disorder: What do we know? Annu. Rev. Clin. Psychol. 2010, 6, 447–468. [Google Scholar] [CrossRef]
- Penzol, M.J.; Salazar de Pablo, G.; Llorente, C.; Moreno, C.; Hernández, P.; Dorado, M.L.; Parellada, M. Functional Gastrointestinal Disease in Autism Spectrum Disorder: A Retrospective Descriptive Study in a Clinical Sample. Front. Psychiatry 2019, 10, 179. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Psychiatr. Clin. N. Am. 2021, 44, 69–81. [Google Scholar] [CrossRef]
- Alamoudi, M.U.; Hosie, S.; Shindler, A.E.; Wood, J.L.; Franks, A.E.; Hill-Yardin, E.L. Comparing the Gut Microbiome in Autism and Preclinical Models: A Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 905841. [Google Scholar] [CrossRef]
- Mehra, A.; Arora, G.; Sahni, G.; Kaur, M.; Singh, H.; Singh, B.; Kaur, S. Gut microbiota and Autism Spectrum Disorder: From pathogenesis to potential therapeutic perspectives. J. Tradit. Complement. Med. 2022, 13, 135–149. [Google Scholar] [CrossRef]
- Soltysova, M.; Tomova, A.; Ostatnikova, D. Gut Microbiota Profiles in Children and Adolescents with Psychiatric Disorders. Microorganisms 2022, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Brandão, T.L.S.; Silva, J.C.L.; Campos, S.; Évelin, D.; Francelino, J.O. Supplementation of Prebiotics and Probiotics in Autistic Children: Integrative Review. RSD 2022, 11, e12811124061. [Google Scholar] [CrossRef]
- Pranckutė, R.; Kaunietis, A.; Kuisienė, N.; Čitavičius, D.J. Combining prebiotics with probiotic bacteria can enhance bacterial growth and secretion of bacteriocins. Int. J. Biol. Macromol. 2016, 89, 669–676. [Google Scholar] [CrossRef]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bäuerlein, M.; Frohberg, C.; Landschütze, V.; Gibson, G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76 (Suppl. S1), 4–15. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; Macfabe, D.F. Propionic Acid Animal Model of Autism Propionic Acid Animal Model of Autism. Compr. Guide Autism 2014, 1755–1778. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.; Kim, J.; Lee, S.; Hong, Y. Pathophysiological and Neurobehavioral Characteristics of a Propionic Acid-Mediated Autism-like Rat Model. PLoS ONE 2018, 13, e0192925. [Google Scholar]
- Abuaish, S.; Al-Otaibi, N.M.; Aabed, K.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; Bhat, R.S.; Arzoo, S.; Algahtani, N.; Moubayed, N.M.; et al. The Efficacy of Fecal Transplantation and Bifidobacterium Supplementation in Ameliorating Propionic Acid-Induced Behavioral and Biochemical Autistic Features in Juvenile Male Rats. J. Mol. Neurosci. 2022, 72, 372–381. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.K.; Bacha, A.B.; Kotb, M. Etiology of Autistic Features: The Persisting Neurotoxic Effects of Propionic Acid. J. Neuroinflamm. 2012, 9, 74. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef]
- Issazadeh, K.; Ali Abadi, M.A.; Kazemi Darsanaki, R.; Alikhani, F.; Dadras, H.; Tajehmiri, A. Isolation, identification and analysis of probiotic properties of Lactobacillus spp from traditional yoghurts in North of Iran. J. Pure Appl. Microbiol. 2013, 7, 2965–2971. [Google Scholar]
- Alghamdi, M.A.; Al-Ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-Ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562. [Google Scholar] [CrossRef]
- Abu-Elsaad, N.; El-Karef, A. Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacol. Rep. 2019, 71, 688–694. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Gibson, G.R.; Saavedra, J.M.; MsFarlane, S.; McFarlane, G.T. Probiotics and intestinal infections. In Probiotics 2: Applications and Practical Aspects; Fuller, R., Ed.; Chapman & Hall: New York, NY, USA, 1997; pp. 10–38. [Google Scholar]
- Saavedra, J.M.; Bauman, N.A.; Oung, I.; Perman, J.A.; Yolken, R.H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994, 344, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Jeewanthi, R.K.; Park, E.H.; Paik, H.D. Short communication: Physicochemical and antioxidant properties of Cheddar-type cheese fortified with Inula britannica extract. J. Dairy Sci. 2016, 99, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lee, P.; Denney, D.R.; Spaeth, K.; Nast, O.; Ptomey, L.; Roth, A.K.; Lierman, J.A.; Sullivan, D.K. Dairy intake is associated with brain glutathione concentration in older adults. Am. J. Clin. Nutr. 2015, 101, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Salekzamani, S.; Ebrahimi-Mameghani, M.; Rezazadeh, K. The antioxidant activity of artichoke (Cynara scolymus): A systematic review and meta-analysis of animal studies. Phytother. Res. 2019, 33, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Di Matteo, G.; Spano, M.; Acciaro, E.; Campiglia, E.; Mannina, L.; Sobolev, A.P. Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study. Molecules 2023, 28, 1363. [Google Scholar] [CrossRef]
- Raines, T.; Jones, P.; Moe, N.; Duncan, R.; McCall, S.; Ceremuga, T.E. Investigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male Sprague-Dawley rat. AANA J. 2009, 77, 33–36. [Google Scholar]
- Hughes, H.K.; Onore, C.E.; Careaga, M.; Rogers, S.J.; Ashwood, P. Increased Monocyte Production of IL-6 after Toll-like Receptor Activation in Children with Autism Spectrum Disorder (ASD) Is Associated with Repetitive and Restricted Behaviors. Brain Sci. 2022, 12, 220. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Gotou, A.; Sashihara, T.; Hachimura, S.; Shioya, N.; Suzuki, S.; Asami, Y. Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients 2020, 12, 374. [Google Scholar] [CrossRef]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The potential effects of probiotics and ω-3 fatty acids on chronic low-grade inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.J.; Lim, S.M.; Lee, N.K.; Paik, H.D. Probiotic Properties and Neuroprotective Effects of Lactobacillus buchneri KU200793 Isolated from Korean Fermented Foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- EI-Alfy, M.S.E.; Youssef, A.; Sabrey, R. A Study on Effect of Probiotic Supplementation on Gastrointestinal Symptoms, Cognition and Behavior in Egyptian Children with Autism Spectrum Disorder. Egypt. J. Pediatr. 2019, 36, 327–337. [Google Scholar] [CrossRef]
- Li, Y.Q.; Sun, Y.H.; Liang, Y.P.; Zhou, F.; Yang, J.; Jin, S.L. Effect of probiotics combined with applied behavior analysis in the treatment of children with autism spectrum disorder: A prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 1103–1110. [Google Scholar]
- He, X.; Liu, W.; Tang, F.; Chen, X.; Song, G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2023, 15, 1415. [Google Scholar] [CrossRef]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2020, 137, 257–264. [Google Scholar] [CrossRef]
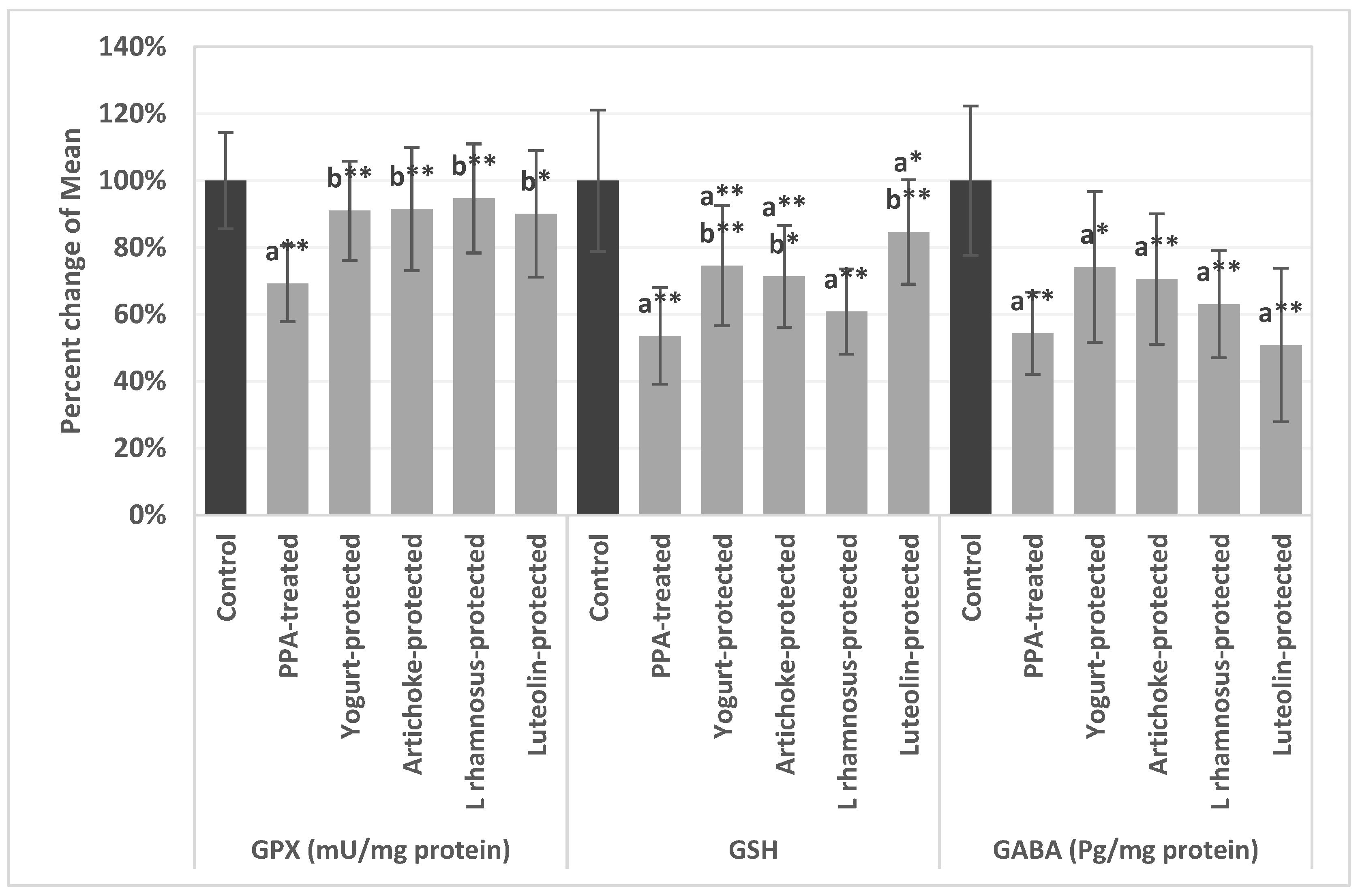
| Parameters | Groups | Mean ± S.D. | p Value |
|---|---|---|---|
| GPX (mU/mg protein) | Control | 108.60 ± 11.05 | 0.001 |
| PPA | 75.19 ± 9.72 a** | ||
| Yogurt-protected | 98.82 ± 12.58 b** | ||
| Artichoke-protected | 99.40 ± 17.30 b** | ||
| L rhamnosus GG-protected | 102.82 ± 14.28 b** | ||
| Luteolin-protected | 97.79 ± 17.95 b* | ||
| GSH | Control | 15.18 ± 2.27 | 0.001 |
| PPA | 8.13 ± 1.82 a** | ||
| Yogurt-protected | 11.32 ± 2.14 a**b** | ||
| Artichoke-protected | 10.83 ± 1.64 a**b* | ||
| L rhamnosus GG-protected | 9.24 ± 1.35 a** | ||
| Luteolin-protected | 12.85 ± 1.39 a*b** | ||
| GABA (Pg/mg protein) | Control | 65.91 ± 10.41 | 0.004 |
| PPA | 35.80 ± 5.82 a** | ||
| Yogurt-protected | 48.90 ± 12.70 a* | ||
| Artichoke-protected | 46.50 ± 10.55 a** | ||
| L rhamnosus GG-protected | 41.52 ± 8.26 a** | ||
| Luteolin-protected | 33.51 ± 14.19 a** |
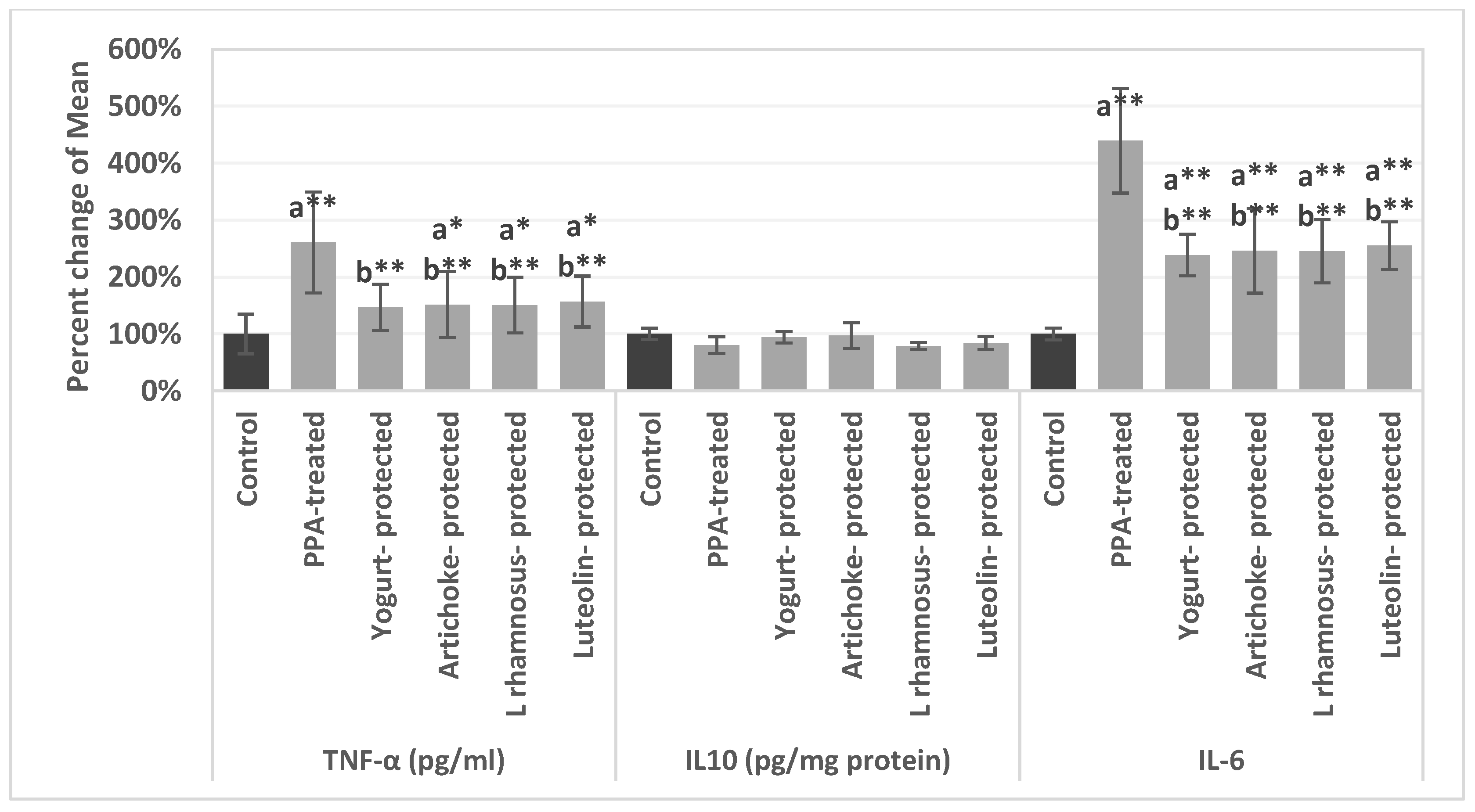
| Parameters | Groups | Mean ± S.D. | p value |
|---|---|---|---|
| TNF-α (pg/mL) | Control | 14.40 ± 3.53 | 0.001 |
| PPA | 37.53 ± 8.89 a** | ||
| Yogurt-protected | 21.13 ± 2.82 b** | ||
| Artichoke-protected | 21.82 ± 6.41 a*b** | ||
| L rhamnosus GG-protected | 21.70 ± 4.64 a*b** | ||
| Luteolin-protected | 22.60 ± 3.28 a*b** | ||
| IL10 (pg/mg protein) | Control | 49.74 ± 3.50 | 0.331 |
| PPA-pretreated | 40.11 ± 6.76 | ||
| Yogurt-protected | 46.82 ± 3.76 | ||
| Artichoke-protected | 48.42 ± 10.57 | ||
| L rhamnosus GG-protected | 39.18 ± 1.18 | ||
| Luteolin-protected | 41.82 ± 5.00 | ||
| IL-6 | Control | 10.86 ± 0.80 | 0.001 |
| PPA-pretreated | 47.69 ± 9.33 a** | ||
| Yogurt-protected | 25.91 ± 3.46 a**b** | ||
| Artichoke-protected | 26.75 ± 7.86 a**b** | ||
| L rhamnosus GG-protected | 26.61 ± 5.69 a**b** | ||
| Luteolin-protected | 27.72 ± 4.02 a**b** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsubaiei, S.R.M.; Alfawaz, H.A.; Bhat, R.S.; El-Ansary, A. Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups. Metabolites 2023, 13, 738. https://doi.org/10.3390/metabo13060738
Alsubaiei SRM, Alfawaz HA, Bhat RS, El-Ansary A. Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups. Metabolites. 2023; 13(6):738. https://doi.org/10.3390/metabo13060738
Chicago/Turabian StyleAlsubaiei, Sana Razhan M., Hanan A. Alfawaz, Ramesa Shafi Bhat, and Afaf El-Ansary. 2023. "Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups" Metabolites 13, no. 6: 738. https://doi.org/10.3390/metabo13060738
APA StyleAlsubaiei, S. R. M., Alfawaz, H. A., Bhat, R. S., & El-Ansary, A. (2023). Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups. Metabolites, 13(6), 738. https://doi.org/10.3390/metabo13060738









