Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota
Abstract
1. Background
2. The History of Micro- and Nanoplastic Discovery
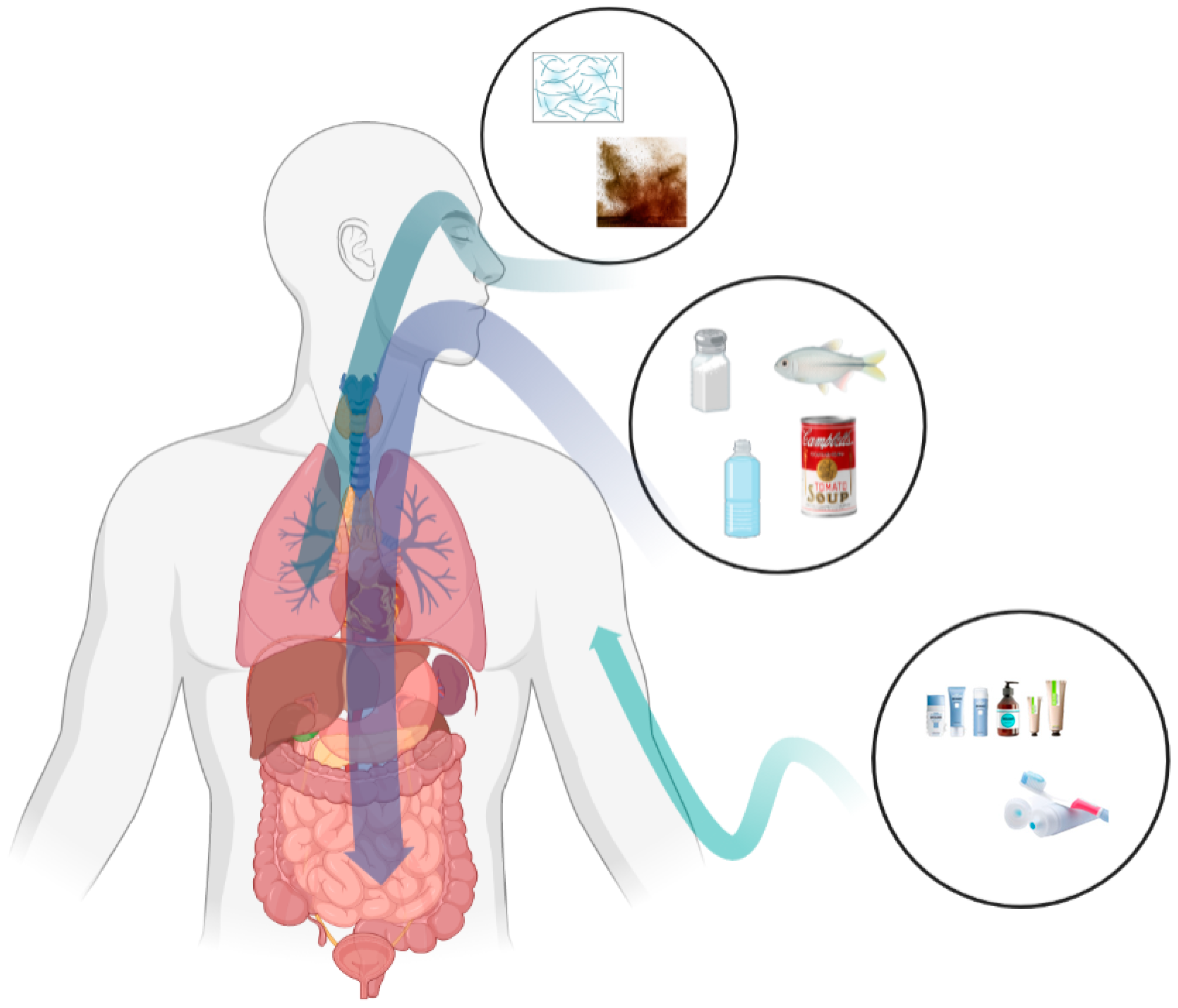
3. Micro- and Nanoplastic Exposure Routes
3.1. Oral Exposure
3.2. Respiratory Exposure
3.3. Dermal Exposure
4. Toxic Effects of Micro- and Nanoplastics
4.1. Eco-Toxicity
4.2. Marine Invertebrates and Vertebrates
4.3. Mouse Models
4.4. Impact of Micro- and Nanoplastic Exposure on the Gut Microbiota
4.4.1. Changes at the Compositional Level
4.4.2. Changes at the Metabolite Level
4.5. Micro- and Nanoplastics and Population
5. Preventive Strategies
| Species | Chemicals and Strategies | Prevention Approaches and Effects | Reference |
|---|---|---|---|
| Mouse and Caco2 cells | Polystyrene and C3G |
| [122] |
| Mouse | Polystyrene and C3G |
| [123] |
| Mouse | Polystyrene and C3G |
| [124] |
| C. elegans and Caco2 cells | Polystyrene and C3G |
| [125] |
| C. elegans | Polystyrene and C3G |
| [126] |
| C. elegans | Polystyrene and FMT |
| [127] |
6. Conclusions and Perspectives
- (i)
- Most of the current studies on the toxicity of micro- and nanoplastics have focused on the ecological environment and non-mammalian and laboratory mouse models. So far, what we know about micro- and nanoplastics and human health includes the fact that micro- and nanoplastics have accumulated in human tissues and organs, and relatively little research has been done on the harm they cause. However, the accumulation of micro- and nanoplastics in human tissues having not been discovered until recent years, the ethical limitations of collecting human specimens, and our current limited understanding of the toxicity of micro- and nanoplastics and the biomarkers that reflect their toxicity have limited scientists to conducting epidemiological studies. Determining whether micro- and nanoplastics have direct or indirect relationships with the occurrence and development of human diseases still requires scientists to continue efforts and exploration.
- (ii)
- There are limited data on the ecological, biological, and human toxicity of micro- and nanoplastics under environmentally relevant conditions. Exposure concentrations of the microplastics used in the laboratory study were significantly higher than those associated with the environment, so the scientists speculate that the laboratory results may overstate the harm caused by micro- and nanoplastics at the environmentally associated concentrations. In addition, extensive studies are still needed to elucidate the pathological mechanisms by which microplastics cause toxic hazards at the cellular and tissue levels and the health consequences of long-term exposure.
- (iii)
- In addition, factors affecting the toxicological role of microplastics, such as sex differences, the dose–response relationship, exposure frequency, and the type and size of microplastics have not yet been thoroughly investigated. Therefore, it is urgent to conduct more in-depth research on the factors influencing microplastics’ toxicity, microplastics-related knowledge, and potential risks, so as to provide a scientific basis for policy makers to cooperate with each other, solve this pressing environmental problem, and protect human health.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Wu, M.; Tu, C.; Liu, G.; Zhong, H. Time to Safeguard the Future Generations from the Omnipresent Microplastics. Bull. Environ. Contam. Toxicol. 2021, 107, 793–799. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.-A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Brun, N.R.; van Hage, P.; Hunting, E.R.; Haramis, A.-P.G.; Vink, S.C.; Vijver, M.G.; Schaaf, M.J.M.; Tudorache, C. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol. 2019, 2, 382. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro-and nano-plastics and human health. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 343–366. [Google Scholar]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Castle, L. Chemical migration into food: An overview. In Chemical Migration and Food Contact Materials; Woodhead Publishing Ltd.: Cambridge, UK, 2007; pp. 1–13. [Google Scholar]
- Bumbudsanpharoke, N.; Choi, J.; Ko, S. Applications of Nanomaterials in Food Packaging. J. Nanosci. Nanotechnol. 2015, 15, 6357–6372. [Google Scholar] [CrossRef] [PubMed]
- Addo Ntim, S.; Norris, S.; Goodwin, D.G.; Breffke, J.; Scott, K.; Sung, L.; Thomas, T.A.; Noonan, G.O. Effects of consumer use practices on nanosilver release from commercially available food contact materials. Food Addit. Contam. Part A 2018, 35, 2279–2290. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Verschoor, A.; De Poorter, L.; Roex, E.; Bellert, B. Quick scan and prioritization of microplastic sources and emissions. RIVM Lett. Rep. 2014, 156, 1–41. [Google Scholar]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Guideline, A. Interactions Affecting the Achievement of Acceptable Indoor Environments; ASHRAE: Atlanta, GA, USA, 2011. [Google Scholar]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Marine, P.I.T.C.T.; Litter, M. Are We Polluting the Environment through Our Personal Care? UNEP: Nairobi, Kenya, 2015. [Google Scholar]
- Sun, Q.; Ren, S.-Y.; Ni, H.-G. Incidence of microplastics in personal care products: An appreciable part of plastic pollution. Sci. Total Environ. 2020, 742, 140218. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Tsoi, K.M.; Hwang, D.M.; Chan, W.C.W. Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nat. Commun. 2014, 5, 3796. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- López-Rojo, N.; Pérez, J.; Alonso, A.; Correa-Araneda, F.; Boyero, L. Microplastics have lethal and sublethal effects on stream invertebrates and affect stream ecosystem functioning. Environ. Pollut. 2020, 259, 113898. [Google Scholar] [CrossRef]
- Green, D.S.; Jefferson, M.; Boots, B.; Stone, L. All that glitters is litter? Ecological impacts of conventional versus biodegradable glitter in a freshwater habitat. J. Hazard. Mater. 2021, 402, 124070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germin Response of soil dissolved organic matter to microplastic addition in Chinese loess soil ation and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Nolte, T.M.; Hartmann, N.B.; Kleijn, J.M.; Garnæs, J.; van de Meent, D.; Jan Hendriks, A.; Baun, A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 2017, 183, 11–20. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef]
- Tlili, S.; Jemai, D.; Brinis, S.; Regaya, I. Microplastics mixture exposure at environmentally relevant conditions induce oxidative stress and neurotoxicity in the wedge clam Donax trunculus. Chemosphere 2020, 258, 127344. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Bourdages, M.P.T.; Provencher, J.F.; Baak, J.E.; Mallory, M.L.; Vermaire, J.C. Breeding seabirds as vectors of microplastics from sea to land: Evidence from colonies in Arctic Canada. Sci. Total Environ. 2021, 764, 142808. [Google Scholar] [CrossRef]
- Provencher, J.F.; Gaston, A.J.; Mallory, M.L.; O’Hara, P.D.; Gilchrist, H.G. Ingested plastic in a diving seabird, the thick-billed murre (Uria lomvia), in the eastern Canadian Arctic. Mar. Pollut. Bull. 2010, 60, 1406–1411. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Lok-Shun Lai, N.; Zhang, W.; Hua, J.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Acute exposure to PBDEs at an environmentally realistic concentration causes abrupt changes in the gut microbiota and host health of zebrafish. Environ. Pollut. 2018, 240, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Lee, W.T.; Chan, A.K.Y.; Lo, H.S.; Shin, P.K.S.; Cheung, S.G. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull. 2017, 124, 798–802. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, N.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.; Liu, H.-P.; Xu, Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018, 195, 67–76. [Google Scholar] [CrossRef]
- Greven, A.-C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, W.; Hu, F.; Song, X.; Cheng, Z.; Zhou, J. Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells. Ecotoxicol. Environ. Saf. 2021, 227, 112882. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, M.; Wang, Y.; Xiao, Y.; Cai, D.; Xiao, F. Keap1-Nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J. Hazard. Mater. 2021, 402, 123933. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Freitas, Í.N.; Mubarak, N.M.; Rahman, M.M.; Rodrigues, F.P.; Rodrigues, A.S.d.L.; Barceló, D.; Islam, A.R.M.T.; Malafaia, G. Exposure to polystyrene nanoplastics induces an anxiolytic-like effect, changes in antipredator defensive response, and DNA damage in Swiss mice. J. Hazard. Mater. 2023, 442, 130004. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Zhang, M.; Ding, G.; Sun, J.; Du, M.; Liu, Q.; Cong, Y.; Jin, F.; Zhang, W.; et al. The uptake and elimination of polystyrene microplastics by the brine shrimp, Artemia parthenogenetica, and its impact on its feeding behavior and intestinal histology. Chemosphere 2019, 234, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal Barrier Function-Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Round, J.L.; O’Connell, R.M.; Mazmanian, S.K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 2010, 34, J220–J225. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Byeon, E.; Jeong, H.; Kim, M.-S.; Chen, Q.; Lee, J.-S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of polyethylene microplastics on the microbiome and metabolism in larval zebrafish. Environ. Pollut. 2021, 282, 117039. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.-L.; An, X.-L.; Yang, X.-R.; Christie, P.; Ke, X.; Wu, L.-H.; Zhu, Y.-G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Huang, W.; Yin, H.; Yang, Y.; Jin, L.; Lu, G.; Dang, Z. Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: Simulation in vitro with human cell Caco-2 and gut microbiota. Sci. Total Environ. 2021, 778, 146264. [Google Scholar] [CrossRef]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.-P.; Pei, D.-S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Sun, Y.; Xiao, S.; Chen, J.; Zhou, X.; Wu, W.-M.; Zhang, Y. Influence of Polymer Size on Polystyrene Biodegradation in Mealworms (): Responses of Depolymerization Pattern, Gut Microbiome, and Metabolome to Polymers with Low to Ultrahigh Molecular Weight. Environ. Sci. Technol. 2022, 56, 17310–17320. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xu, C.; Zou, X.; Xia, Z.; Su, L.; Qiu, N.; Cai, L.; Yu, F.; Wang, Q.; Zhao, X.; et al. Long-term exposure to microplastics induces intestinal function dysbiosis in rare minnow (Gobiocypris rarus). Ecotoxicol. Environ. Saf. 2022, 246, 114157. [Google Scholar] [CrossRef]
- Jiang, P.; Yuan, G.-H.; Jiang, B.-R.; Zhang, J.-Y.; Wang, Y.-Q.; Lv, H.-J.; Zhang, Z.; Wu, J.-L.; Wu, Q.; Li, L. Effects of microplastics (MPs) and tributyltin (TBT) alone and in combination on bile acids and gut microbiota crosstalk in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112345. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, Y.; Kulyar, M.F.-E.A.; Iqbal, M.; Lai, R.; Zhu, H.; Li, K. Environmental microplastics exposure decreases antioxidant ability, perturbs gut microbial homeostasis and metabolism in chicken. Sci. Total Environ. 2023, 856, 159089. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Huang, J.-N.; Wen, B.; Xu, L.; Ma, H.-C.; Li, X.-X.; Gao, J.-Z.; Chen, Z.-Z. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): Insight from brain-gut-microbiota axis. J. Hazard. Mater. 2022, 421, 126830. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; An, L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci. Total Environ. 2023, 856, 159060. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Duru, C.E.; Ovuoraye, P.E.; Wang, Q. Evaluation of nanoplastics toxicity to the human placenta in systems. J. Hazard. Mater. 2023, 446, 130600. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, G.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total Environ. 2022, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association Between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct accumulation of nanoplastics in human intestinal organoids. Sci. Total Environ. 2022, 838, 155811. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; López-Mesas, M.; Fuentes-Cebrian, V.; Marcos, R.; Hernández, A. Hazard Assessment of Polystyrene Nanoplastics in Primary Human Nasal Epithelial Cells, Focusing on the Autophagic Effects. Biomolecules 2023, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, M.; Niu, S.; Shang, M.; Chang, X.; Sun, Z.; Zhang, R.; Shen, X.; Xue, Y. ROS and DRP1 interactions accelerate the mitochondrial injury induced by polystyrene nanoplastics in human liver HepG2 cells. Chem. Biol. Interact. 2023, 379, 110502. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Wang, M.; Yang, L.; Pan, K.; Miao, A.-J. Bioaccumulation of differently-sized polystyrene nanoplastics by human lung and intestine cells. J. Hazard. Mater. 2022, 439, 129585. [Google Scholar] [CrossRef]
- Banerjee, A.; Billey, L.O.; McGarvey, A.M.; Shelver, W.L. Effects of polystyrene micro/nanoplastics on liver cells based on particle size, surface functionalization, concentration and exposure period. Sci. Total Environ. 2022, 836, 155621. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, D.; Yang, S.; Shi, Z.; Pan, H.; Jin, Q.; Ding, Z. Neurotoxicity of polystyrene nanoplastics with different particle sizes at environment-related concentrations on early zebrafish embryos. Sci. Total Environ. 2023, 872, 162096. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Li, T.; Yu, L.; Qi, Y.; Tian, G.; He, F.; Li, X.; Sun, N.; Liu, R. Size-dependent effects of nanoplastics on structure and function of superoxide dismutase. Chemosphere 2022, 309, 136768. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, Y.; Huang, F.; Su, Y.; Zhou, X.; Wang, A.-J.; Gao, S.-H. Distinct responses of Pseudomonas aeruginosa PAO1 exposed to different levels of polystyrene nanoplastics. Sci. Total Environ. 2022, 852, 158214. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, M.; Jiang, Q.; Wu, D.; Huang, Y.; Jiao, Y.; Zhang, M.; Zhao, Y. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard. Mater. 2020, 398, 122990. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, X.A.; Zheng, X. Protective effect of mulberry fruit anthocyanin on human hepatocyte cells (LO2) and Caenorhabditis elegans under hyperglycemic conditions. Food Res. Int. 2017, 102, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chu, Q.; Ye, X.; Sun, Y.; Liu, Y.; Jia, R.; Li, Y.; Tu, P.; Tang, Q.; Yu, T.; et al. Canidin-3-glucoside prevents nano-plastics induced toxicity via activating autophagy and promoting discharge. Environ. Pollut. 2021, 274, 116524. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tu, P.; Ye, X.; Tang, Q.; Yu, T.; Zheng, X. Cyanidin-3-O-glucoside impacts fecal discharge of polystyrene microplastics in mice: Potential role of microbiota-derived metabolites. Toxicol. Appl. Pharmacol. 2022, 453, 116212. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, R.; Ye, X.; Sun, Y.; Tang, Q.; Liu, Y.; Yan, F.; Yu, T.; Zheng, X.; Tu, P. Food-derived cyanidin-3-O-glucoside reverses microplastic toxicity promoting discharge and modulating the gut microbiota in mice. Food Funct. 2022, 13, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ye, X.; Tang, Q.; Yu, T.; Tu, P.; Zheng, X. Cyanidin-3-O-glucoside reduces nanopolystyrene-induced toxicity and accumulation: Roles of mitochondrial energy metabolism and cellular efflux. Environ. Sci. Nano 2022, 9, 2572–2586. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Shan, S.; Wu, A.; Zhao, C.; Ye, X.; Zheng, X.; Zhu, R. Cyanidin-3-O-glucoside promotes stress tolerance and lifespan extension of Caenorhabditis elegans exposed to polystyrene via DAF-16 pathway. Mech. Ageing Dev. 2022, 207, 111723. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Zhang, S.; Yu, X.; Wang, Y.; Zhang, M.; Zheng, X. Fecal microbiota transplantation attenuates nano-plastics induced toxicity in Caenorhabditis elegans. Sci. Total Environ. 2021, 779, 146454. [Google Scholar] [CrossRef] [PubMed]


| Properties of Microplastics Used | Toxicity | Reference |
|---|---|---|
| Polystyrene microplastics (5–20 μm) |
| [74] |
| Polystyrene microplastics (5 μm) |
| [26] |
| Polystyrene nanoplastics (0.5 μm) |
| [75] |
| Polystyrene microplastics (5 μm) |
| [76] |
| Polystyrene micro- and nanoplastics (0.5 μm and 50 μm) |
| [77] |
| Polystyrene microplastics (10–150 μm) |
| [78] |
| Polystyrene microplastics (5 μm and 20 μm) |
| [24] |
| Polyethylene micro- and nanoplastics (3–16 μm, 100 nm, and 600 nm) Polystyrene micro- and nanoplastics (10 μm, 40 nm, and 250 nm) |
| [79] |
| Polypropylene microplastics (<200 μm) |
| [80] |
| Polystyrene microplastics (1 μm, 4 μm, and 10 μm) |
| [81] |
| Polystyrene nanoplastics (23–26 nm) |
| [82] |
| Polystyrene microplastics (5 μm) |
| [83] |
| Species | Properties of Microplastics Used | Changes in Intestinal Microbiota | Reference |
|---|---|---|---|
| Chinese mitten crab (Eriocheir sinesis) | Polystyrene microplastics (5 μm) |
| [93] |
| Marine medaka (Oryzias melastigma) | Polystyrene micro- and nanoplastics (45 μm and 50 nm) |
| [94] |
| Larval zebrafish | Polystyrene microplastics (1–4 μm) |
| [95] |
| Collembolans | Polyvinyl chloride microplastics |
| [96] |
| Mouse | Polyethylene microplastics (10–150 μm) |
| [78] |
| Mouse | Polystyrene micro- and nanoplastics (50 μm and 0.5 μm) |
| [77] |
| Mouse | Polystyrene microplastics (5 μm) |
| [26] |
| Species | Properties of Microplastics Used | Changes in Gut Microbiota Metabolome | Reference |
|---|---|---|---|
| Simulation in vitro with human cell Caco-2 and gut microbiota | Polyethylene microplastics (30–140 μm) |
| [97] |
| Marine medaka (Oryzias melastigma) | Polystyrene microplastics (2.5 μm) |
| [98] |
| Mealworms (Tenebrio molitor) | Polystyrene microplastics |
| [99] |
| Mouse | Polystyrene microplastics (5 μm) |
| [26] |
| Rare minnow (Gobiocypris rarus) | Polystyrene microplastics (1 μm) |
| [100] |
| Mouse | Polystyrene microplastics (5 μm) |
| [101] |
| Chicken | Polyethylene microplastics |
| [102] |
| Mouse | Polystyrene micro- and nanoplastics (5 μm and 70 nm) |
| [103] |
| Discus fish (Symphysodon aequifasciatus) | Polystyrene nanoplastics (~80 nm) |
| [104] |
| Marine medaka (Oryzias melastigma) | Polystyrene micro- and nanoplastics (45 μm and 50 nm) |
| [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, H.; Liu, S.; Jiang, Y.; Hu, Y.; Li, Y.; He, L.; Xing, M.; Li, X.; Wu, L.; Chen, Z.; et al. Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota. Metabolites 2023, 13, 739. https://doi.org/10.3390/metabo13060739
Niu H, Liu S, Jiang Y, Hu Y, Li Y, He L, Xing M, Li X, Wu L, Chen Z, et al. Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota. Metabolites. 2023; 13(6):739. https://doi.org/10.3390/metabo13060739
Chicago/Turabian StyleNiu, Huixia, Shaojie Liu, Yujie Jiang, Yang Hu, Yahui Li, Luyang He, Mingluan Xing, Xueqing Li, Lizhi Wu, Zhijian Chen, and et al. 2023. "Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota" Metabolites 13, no. 6: 739. https://doi.org/10.3390/metabo13060739
APA StyleNiu, H., Liu, S., Jiang, Y., Hu, Y., Li, Y., He, L., Xing, M., Li, X., Wu, L., Chen, Z., Wang, X., & Lou, X. (2023). Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota. Metabolites, 13(6), 739. https://doi.org/10.3390/metabo13060739







