Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours
Abstract
:1. Introduction
2. Does Exercise Influence Neurocognitive Health?
3. Lipidomics
3.1. Why Lipids?
3.2. Measurement of the Lipidome
3.3. Challenges with Lipidomics
- (1)
- Characterisation of the lipid head group (e.g., phosphocholine (PC));
- (2)
- The length of the carbon chains (e.g., C12);
- (3)
- The enantiomeric configuration of linkages at the sn-1 and sn-2 positions on the glycerol backbone (e.g., acyl, alkyl or alkenyl);
- (4)
- The number, location and stereochemistry of any C=C bonds, or “unsaturated” bonds (e.g., 18:2 (9Z, 12Z) is a chain of 18 carbons with two C=C bonds at the ninth and twelfth carbon positions);
- (5)
- Any occurrences of modifications within these chains (e.g., 20:1(5Z)-OH(12S) is a chain of 20 carbons, with a C=C bond at the fifth carbon and a hydroxyl group on the twelfth carbon).
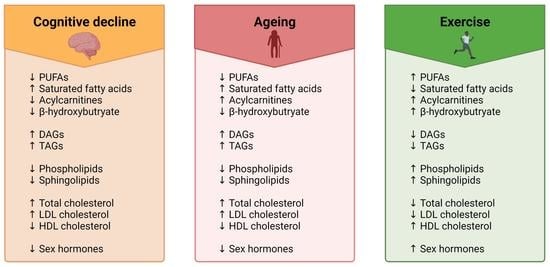
4. Impact of Ageing, Cognitive Dysfunction and Exercise on the Lipidome
4.1. Fatty Acyls
4.1.1. Fatty Acids
Fatty Acids in Ageing
Fatty Acids and Cognition
Fatty Acids and Exercise
4.1.2. Acylcarnitines
Acylcarnitines in Ageing
Acylcarnitines and Cognition
Acylcarnitines and Exercise
4.1.3. Ketone Bodies
Ketone Bodies in Ageing
Ketone Bodies and Cognition
Ketone Bodies and Exercise
4.2. Glycerolipids
4.2.1. Glycerolipids in Ageing
4.2.2. Glycerolipids and Cognition
4.2.3. Glycerolipids and Exercise
4.3. Glycerophospholipids
4.3.1. Glycerophospholipids in Ageing
4.3.2. Glycerophospholipids and Cognition
4.3.3. Glycerophospholipids and Exercise
4.4. Sphingolipids
4.4.1. Sphingolipids in Ageing
4.4.2. Sphingolipids and Cognition
4.4.3. Sphingolipids and Exercise
4.5. Sterols
4.5.1. Cholesterol
Cholesterol in Ageing
Cholesterol and Cognition
Cholesterol and Exercise
4.5.2. Steroid Hormones
Steroid Hormones and Ageing
Steroid Hormones and Cognition
Steroid Hormones and Exercise
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boss, G.R.; Seegmiller, J.E. Age-related physiological changes and their clinical significance. West. J. Med. 1981, 135, 434–440. [Google Scholar] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Aging 2017—Highlights. 2017. Available online: https://www.un.org/en/development/desa/population/theme/ageing/WPA2017.asp (accessed on 1 June 2022).
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Aging. 2019. Available online: https://www.un.org/development/desa/pd/content/world-population-ageing-2019 (accessed on 1 June 2022).
- Office, P.B. Australia’s Ageing Population: Understanding the Fiscal Impacts Over the Next Decade; Parliamentary Budget Office: Canberra, Australia, 2019. [Google Scholar]
- Sanders, L.M.J.; Hortobágyi, T.; la Bastide-van Gemert, S.; van der Zee, E.A.; van Heuvelen, M.J.G. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210036. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, W.M.; Scheltens, P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Amp. Psychiatry 2005, 76, v2–v7. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef]
- Shannon, K.; Montayre, J.; Neville, S. Nothing About us Without us: Research Methods Enabling Participation for Aged Care Residents Who Have Dementia. Int. J. Qual. Methods 2021, 20, 16094069211055938. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin Hear 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The influence of exercise on cognitive abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Young, J.; Angevaren, M.; Rusted, J.; Tabet, N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2014, 16, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Schwarck, S.; Schmicker, M.; Dordevic, M.; Rehfeld, K.; Müller, N.; Müller, P. Inter-Individual Differences in Cognitive Response to a Single Bout of Physical Exercise-A Randomized Controlled Cross-Over Study. J. Clin. Med. 2019, 8, 1101. [Google Scholar] [CrossRef]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Cadore, E.L.; Ramirez-Velez, R.; Izquierdo, M. Inter-individual variability in response to exercise intervention or usual care in hospitalized older adults. J. Cachexia Sarcopenia Muscle 2019, 10, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef]
- Carapeto, P.V.; Aguayo-Mazzucato, C. Effects of exercise on cellular and tissue aging. Aging 2021, 13, 14522–14543. [Google Scholar] [CrossRef]
- Gonzalez-Covarrubias, V. Lipidomics in longevity and healthy aging. Biogerontology 2013, 14, 663–672. [Google Scholar] [CrossRef]
- Yetukuri, L.; Ekroos, K.; Vidal-Puig, A.; Oresic, M. Informatics and computational strategies for the study of lipids. Mol. Biosyst. 2008, 4, 121–127. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.M.; Darzi, A.W.; Takats, Z.; Lindon, J.C. Metabolic phenotyping in clinical and surgical environments. Nature 2012, 491, 384–392. [Google Scholar] [CrossRef]
- Glisky, E.L. Changes in Cognitive Function in Human Aging. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2007. [Google Scholar]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Brown, B.; Shah, T.M. The Link Between Exercise and Mediation of Alzheimer’s Disease and Neurodegenerative Diseases. In Neurodegeneration and Alzheimer’s Disease; Wiley: Hoboken, NJ, USA, 2019; pp. 371–390. [Google Scholar]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public. Health Rep. 1985, 100, 126–131. [Google Scholar]
- Brown, B.M.; Peiffer, J.J.; Martins, R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874. [Google Scholar] [CrossRef]
- Weuve, J.; Kang, J.H.; Manson, J.E.; Breteler, M.M.B.; Ware, J.H.; Grodstein, F. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA 2004, 292, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef]
- Fabre, C.; Chamari, K.; Mucci, P.; Massé-Biron, J.; Préfaut, C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 2002, 23, 415–421. [Google Scholar] [CrossRef]
- Barnes, D.E.; Blackwell, T.; Stone, K.L.; Goldman, S.E.; Hillier, T.; Yaffe, K. Cognition in older women: The importance of daytime movement. J. Am. Geriatr. Soc. 2008, 56, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154. [Google Scholar] [CrossRef]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.; Steinman, L.; Mochan, K.; Grodstein, F.; Prohaska, T.R.; Thurman, D.J.; Brown, D.R.; Laditka, J.N.; Soares, J.; Zweiback, D.J.; et al. Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. J. Am. Geriatr. Soc. 2011, 59, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.; Fiatarone Singh, M.A.; Sachdev, P.S.; Valenzuela, M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry 2013, 21, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, 2018 Physical Activity Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018. Available online: https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf (accessed on 7 June 2022).
- Herold, F.; Törpel, A.; Hamacher, D.; Budde, H.; Zou, L.; Strobach, T.; Müller, N.G.; Gronwald, T. Causes and Consequences of Interindividual Response Variability: A Call to Apply a More Rigorous Research Design in Acute Exercise-Cognition Studies. Front. Physiol. 2021, 12, 682891. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J. Do Non-Responders to Exercise Exist—And If So, What Should We Do About Them? Sports Med. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Hillard, C.J.; Spector, A.A.; Watkins, P.A. Brain Uptake and Utilization of Fatty Acids, Lipids and Lipoproteins: Application to Neurological Disorders. J. Mol. Neurosci. 2007, 33, 2–11. [Google Scholar] [CrossRef]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids1. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Shamim, A.; Mahmood, T.; Ahsan, F.; Kumar, A.; Bagga, P. Lipids: An insight into the neurodegenerative disorders. Clin. Nutr. Exp. 2018, 20, 1–19. [Google Scholar] [CrossRef]
- Alves, M.A.; Lamichhane, S.; Dickens, A.; McGlinchey, A.; Ribeiro, H.C.; Sen, P.; Wei, F.; Hyötyläinen, T.; Orešič, M. Systems biology approaches to study lipidomes in health and disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158857. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Ripatti, S. Integrating lipidomics and genomics: Emerging tools to understand cardiovascular diseases. Cell. Mol. Life Sci. 2021, 78, 2565–2584. [Google Scholar] [CrossRef] [PubMed]
- Zandl-Lang, M.; Züllig, T.; Trötzmüller, M.; Naegelin, Y.; Abela, L.; Wilken, B.; Scholl-Buergi, S.; Karall, D.; Kappos, L.; Köfeler, H.; et al. Changes in the Cerebrospinal Fluid and Plasma Lipidome in Patients with Rett Syndrome. Metabolites 2022, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Wong, M.W.; Thalamuthu, A.; Braidy, N.; Mather, K.A.; Liu, Y.; Ciobanu, L.; Baune, B.T.; Armstrong, N.J.; Kwok, J.; Schofield, P.; et al. Genetic and environmental determinants of variation in the plasma lipidome of older Australian twins. eLife 2020, 9, e58954. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Peng, X.-X. Functional metabolomics: From biomarker discovery to metabolome reprogramming. Protein. Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Merciai, F.; Carrizzo, A.; Sommella, E.; Di Pietro, P.; Caponigro, V.; Salviati, E.; Musella, S.; Sarno, V.D.; Rusciano, M.; et al. Untargeted lipidomics reveals specific lipid profiles in COVID-19 patients with different severity from Campania region (Italy). J. Pharm. Biomed. Anal. 2022, 217, 114827. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zan, J.; Wu, C.; Tan, W. Untargeted lipidomics reveals progression of early Alzheimer’s disease in APP/PS1 transgenic mice. Sci. Rep. 2020, 10, 14509. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; Bastos, M.L.; Guedes de Pinho, P. Metabolomics analysis for biomarker discovery: Advances and challenges. Curr. Med. Chem. 2013, 20, 257–271. [Google Scholar] [CrossRef]
- Oldiges, M.; Lütz, S.; Pflug, S.; Schroer, K.; Stein, N.; Wiendahl, C. Metabolomics: Current state and evolving methodologies and tools. Appl. Microbiol. Biotechnol. 2007, 76, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Holmes, E.; Nicholson, J.K. So what’s the deal with metabonomics? Anal. Chem. 2003, 75, 384a–391a. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass. Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Pfeifer, H. Principles of Nuclear Magnetic Resonance Microscopy. Z. Für Phys. Chem. 1992, 176, 132. [Google Scholar] [CrossRef]
- Neil, J.; Ackerman, J.J.H. Magnetic Resonance Spectroscopy. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 973–976. [Google Scholar]
- Davis, V.W.; Bathe, O.F.; Schiller, D.E.; Slupsky, C.M.; Sawyer, M.B. Metabolomics and surgical oncology: Potential role for small molecule biomarkers. J. Surg. Oncol. 2011, 103, 451–459. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Aru, V.; Lam, C.; Khakimov, B.; Hoefsloot, H.C.J.; Zwanenburg, G.; Lind, M.V.; Schäfer, H.; van Duynhoven, J.; Jacobs, D.M.; Smilde, A.K.; et al. Quantification of lipoprotein profiles by nuclear magnetic resonance spectroscopy and multivariate data analysis. TrAC Trends Anal. Chem. 2017, 94, 210–219. [Google Scholar] [CrossRef]
- Whiley, L.; Legido-Quigley, C. Current strategies in the discovery of small-molecule biomarkers for Alzheimer’s disease. Bioanalysis 2011, 3, 1121–1142. [Google Scholar] [CrossRef]
- Murphy, R.C. Challenges in Mass Spectrometry-based Lipidomics of Neutral Lipids. Trends Anal. Chem. 2018, 107, 91–98. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Accurate quantification of lipid species by electrospray ionization mass spectrometry-Meet a key challenge in lipidomics. Metabolites 2011, 1, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Rustam, Y.H.; Reid, G.E. Analytical Challenges and Recent Advances in Mass Spectrometry Based Lipidomics. Anal. Chem. 2018, 90, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Reid, G.E. Chemical Derivatization and Ultrahigh Resolution and Accurate Mass Spectrometry Strategies for "Shotgun" Lipidome Analysis. Acc. Chem. Res. 2016, 49, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; He, Z.; Zubkov, D.; Huang, S.; Kurochkin, I.; Yang, X.; Halene, T.; Willmitzer, L.; Giavalisco, P.; Akbarian, S.; et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol. Psychiatry 2020, 25, 2952–2969. [Google Scholar] [CrossRef] [PubMed]
- Emre, C.; Do, K.V.; Jun, B.; Hjorth, E.; Alcalde, S.G.; Kautzmann, M.-A.I.; Gordon, W.C.; Nilsson, P.; Bazan, N.G.; Schultzberg, M. Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Skowronska-Krawczyk, D.; Budin, I. Aging membranes: Unexplored functions for lipids in the lifespan of the central nervous system. Exp. Gerontol. 2020, 131, 110817. [Google Scholar] [CrossRef]
- Wilson, R.S.; Leurgans, S.E.; Boyle, P.A.; Bennett, D.A. Cognitive Decline in Prodromal Alzheimer Disease and Mild Cognitive Impairment. Arch. Neurol. 2011, 68, 351–356. [Google Scholar] [CrossRef]
- Karr, J.E.; Graham, R.B.; Hofer, S.M.; Muniz-Terrera, G. When does cognitive decline begin? A systematic review of change point studies on accelerated decline in cognitive and neurological outcomes preceding mild cognitive impairment, dementia, and death. Psychol. Aging 2018, 33, 195–218. [Google Scholar] [CrossRef]
- Moghadasian, M.H.; Shahidi, F. Fatty Acids. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 114–122. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Di Pasquale, M.G. The Essentials of Essential Fatty Acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, Y.; Shimada, K.; Tani, S.; Ogawa, T.; Ando, J.; Takahashi, M.; Yamamoto, M.; Shinozaki, T.; Miyazaki, T.; Miyauchi, K.; et al. Association between the docosahexaenoic acid to arachidonic acid ratio and acute coronary syndrome: A multicenter observational study. BMC Cardiovasc. Disord. 2016, 16, 143. [Google Scholar] [CrossRef]
- Muilwijk, M.; Vaz, F.M.; Celis-Morales, C.; Peters, R.J.G.; van Valkengoed, I.G.M. The Association of Acylcarnitines and Amino Acids With Age in Dutch and South-Asian Surinamese Living in Amsterdam. J. Clin. Endocrinol. Metab. 2018, 103, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Verri Hernandes, V.; Dordevic, N.; Hantikainen, E.M.; Sigurdsson, B.B.; Smárason, S.V.; Garcia-Larsen, V.; Gögele, M.; Caprioli, G.; Bozzolan, I.; Pramstaller, P.P.; et al. Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort. Metabolites 2022, 12, 205. [Google Scholar] [CrossRef]
- Carlsson, H.; Rollborn, N.; Herman, S.; Freyhult, E.; Svenningsson, A.; Burman, J.; Kultima, K. Metabolomics of Cerebrospinal Fluid from Healthy Subjects Reveal Metabolites Associated with Ageing. Metabolites 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G.; et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer’s Dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yang, J.; Zhu, M.; Pang, Y.; Wang, Q.; Wei, Q.; Li, Y.; Li, T.; Li, F.; Wang, Q.; et al. A metabolite panel that differentiates Alzheimer’s disease from other dementia types. Alzheimer’s Dement. 2021, 18, 1345–1356. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Murphy, N.E.; Carrigan, C.T.; Castellani, J.W.; Madslien, E.H.; Teien, H.-K.; Martini, S.; Montain, S.J.; Pasiakos, S.M. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol. Rep. 2017, 5, e13407. [Google Scholar] [CrossRef]
- Nemkov, T.; Skinner, S.C.; Nader, E.; Stefanoni, D.; Robert, M.; Cendali, F.; Stauffer, E.; Cibiel, A.; Boisson, C.; Connes, P.; et al. Acute Cycling Exercise Induces Changes in Red Blood Cell Deformability and Membrane Lipid Remodeling. Int. J. Mol. Sci. 2021, 22, 896. [Google Scholar] [CrossRef]
- Howe, C.C.F.; Alshehri, A.; Muggeridge, D.; Mullen, A.B.; Boyd, M.; Spendiff, O.; Moir, H.J.; Watson, D.G. Untargeted Metabolomics Profiling of an 80.5 km Simulated Treadmill Ultramarathon. Metabolites 2018, 8, 14. [Google Scholar] [CrossRef]
- Manaf, F.A.; Lawler, N.G.; Peiffer, J.J.; Maker, G.L.; Boyce, M.C.; Fairchild, T.J.; Broadhurst, D. Characterizing the plasma metabolome during and following a maximal exercise cycling test. J. Appl. Physiol. 2018, 125, 1193–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrell, Z.R.; Smith, M.R.; Hu, X.; Orr, M.; Liu, K.H.; Quyyumi, A.A.; Jones, D.P.; Go, Y.M. Plasma acylcarnitine levels increase with healthy aging. Aging 2020, 12, 13555–13570. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Zhong, X.; Cheema, A.K.; Orquiza, M.H.; Chidambaram, S.; Tan, M.T.; Gresenz, C.R.; FitzGerald, K.T.; Nalls, M.A.; Singleton, A.B.; et al. Plasma 24-metabolite Panel Predicts Preclinical Transition to Clinical Stages of Alzheimer’s Disease. Front. Neurol. 2015, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Gillitt, N.D.; Pappan, K.L.; Lila, M.A. Serum Metabolic Signatures Induced By a Three-Day Intensified Exercise Period Persist After 14 h of Recovery in Runners. J. Proteome Res. 2013, 12, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Yu, L.; Yang, J.; Zhu, Y.; Bennett, D.A.; Zhao, J. Brain and blood metabolome for Alzheimer’s dementia: Findings from a targeted metabolomics analysis. Neurobiol. Aging 2020, 86, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Kastenmüller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis–mass spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef]
- Nieman, D.C.; Sha, W.; Pappan, K.L. IL-6 Linkage to Exercise-Induced Shifts in Lipid-Related Metabolites: A Metabolomics-Based Analysis. J. Proteome Res. 2017, 16, 970–977. [Google Scholar] [CrossRef]
- Olazarán, J.; Gil-de-Gómez, L.; Rodríguez-Martín, A.; Valentí-Soler, M.; Frades-Payo, B.; Marín-Muñoz, J.; Antúnez, C.; Frank-García, A.; Acedo-Jiménez, C.; Morlán-Gracia, L.; et al. A blood-based, 7-metabolite signature for the early diagnosis of Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 1157–1173. [Google Scholar] [CrossRef]
- Yu, Z.; Zhai, G.; Singmann, P.; He, Y.; Xu, T.; Prehn, C.; Römisch-Margl, W.; Lattka, E.; Gieger, C.; Soranzo, N.; et al. Human serum metabolic profiles are age dependent. Aging Cell 2012, 11, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Cheong, Y.J.; Bhatnagar, A.; Goozee, K.; Wu, Y.; McKay, M.; Martins, I.J.; Lim, W.L.F.; Pedrini, S.; Tegg, M.; et al. Plasma metabolites associated with biomarker evidence of neurodegeneration in cognitively normal older adults. J. Neurochem. 2020, 159, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.F.; Huynh, K.; Chatterjee, P.; Martins, I.; Jayawardana, K.S.; Giles, C.; Mellett, N.A.; Laws, S.M.; Bush, A.I.; Rowe, C.C.; et al. Relationships Between Plasma Lipids Species, Gender, Risk Factors, and Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2020, 76, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Chen, Y.-J.; Kondoh, H.; Fukuji, Y.; Yanagida, M. Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc. Natl. Acad. Sci. USA 2021, 118, e2022857118. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Yang, Y.; Ou, W.; Chen, X.; Huang, B.; Wang, H.; Liu, M. Metabolic Biomarkers of Aging and Aging-related Diseases in Chinese Middle-Aged and Elderly Men. J. Nutr. Health Aging 2018, 22, 1189–1197. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Rupérez, F.J.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic-Driven Elucidation of Serum Disturbances Associated with Alzheimer’s Disease and Mild Cognitive Impairment. Curr. Alzheimer Res. 2016, 13, 641–653. [Google Scholar] [CrossRef]
- Chak, C.M.; Lacruz, M.E.; Adam, J.; Brandmaier, S.; Covic, M.; Huang, J.; Meisinger, C.; Tiller, D.; Prehn, C.; Adamski, J.; et al. Ageing Investigation Using Two-Time-Point Metabolomics Data from KORA and CARLA Studies. Metabolites 2019, 9, 44. [Google Scholar] [CrossRef]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; St-Pierre, V.; Myette-Côté, É.; Langlois, F.; Roy, M.; Morin, M.C.; Bocti, C.; Fulop, T.; Godin, J.P.; et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Cipolla, M.; Chiang, J.; Arakaki, X.; Harrington, M.G. Human Cerebrospinal Fluid Fatty Acid Levels Differ between Supernatant Fluid and Brain-Derived Nanoparticle Fractions, and Are Altered in Alzheimer’s Disease. PLoS ONE 2014, 9, e100519. [Google Scholar] [CrossRef]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baierle, M.; Vencato, P.H.; Oldenburg, L.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; Duarte, M.M.; Veit, J.C.; Somacal, S.; Emanuelli, T.; et al. Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly. Nutrients 2014, 6, 3624–3640. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.; Chatterjee, P.; James, I.; Shen, K.; Sohrabi, H.R.; Asih, P.R.; Dave, P.; Ball, B.; ManYan, C.; Taddei, K.; et al. Alterations in erythrocyte fatty acid composition in preclinical Alzheimer’s disease. Sci. Rep. 2017, 7, 676. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Kaufman, J.S.; Satia, J.A.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Qin, Q.; Long, Z.; Yang, Y.; Peng, C.; Xi, C.; Li, L.; Wu, Z.; Daria, V.; Zhao, Y.; et al. High-Throughput Metabolomics for Discovering Potential Biomarkers and Identifying Metabolic Mechanisms in Aging and Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 602887. [Google Scholar] [CrossRef]
- Heude, B.; Ducimetière, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [CrossRef]
- Fang, J.; Cui, L.; Sun, Y.; Feng, L.; Ong, C.N. Targeted metabolomics reveals altered oxylipin profiles in plasma of mild cognitive impairment patients. Metabolomics 2017, 13, 112. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Scott, T.M.; Harris, W.S.; Tucker, K.L. Prospective Associations of Erythrocyte Composition and Dietary Intake of n-3 and n-6 PUFA with Measures of Cognitive Function. Nutrients 2018, 10, 1253. [Google Scholar] [CrossRef]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; De la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef]
- Saito, K.; Hattori, K.; Hidese, S.; Sasayama, D.; Miyakawa, T.; Matsumura, R.; Tatsumi, M.; Yokota, Y.; Ota, M.; Hori, H.; et al. Profiling of Cerebrospinal Fluid Lipids and Their Relationship with Plasma Lipids in Healthy Humans. Metabolites 2021, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Lee, J.S.; Scott, T.; Kelly, R.S.; Bhupathiraju, S.N.; Bigornia, S.J.; Tucker, K.L. Circulating Plasma Metabolites and Cognitive Function in a Puerto Rican Cohort. J. Alzheimer’s Dis. 2020, 76, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Poljak, A.; Braidy, N.; Crawford, J.; Sachdev, P. Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2020, 60, 101043. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Hung, C.F.; Ponnusamy, V.K.; Chen, K.C.; Chen, N.C. Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimer’s Disease: Two-Year Follow-Up. Nutrients 2022, 14, 1159. [Google Scholar] [CrossRef]
- van der Lee, S.J.; Teunissen, C.E.; Pool, R.; Shipley, M.J.; Teumer, A.; Chouraki, V.; Melo van Lent, D.; Tynkkynen, J.; Fischer, K.; Hernesniemi, J.; et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimer’s Dement. 2018, 14, 707–722. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef]
- Hussain, G.; Schmitt, F.; Loeffler, J.-P.; Gonzalez De Aguilar, J. Fatting the brain: A brief of recent research. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Carver, J.D.; Benford, V.J.; Han, B.; Cantor, A.B. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull 2001, 56, 79–85. [Google Scholar] [CrossRef]
- Rohrbach, S. Effects of dietary polyunsaturated fatty acids on mitochondria. Curr. Pharm. Des. 2009, 15, 4103–4116. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Wang, L.; Xu, R.J.; Chen, Z.Y. DHA depletion in rat brain is associated with impairment on spatial learning and memory. Biomed. Environ. Sci. 2006, 19, 474–480. [Google Scholar]
- Bach, S.A.; de Siqueira, L.V.; Müller, A.P.; Oses, J.P.; Quatrim, A.; Emanuelli, T.; Vinadé, L.; Souza, D.O.; Moreira, J.D. Dietary omega-3 deficiency reduces BDNF content and activation NMDA receptor and Fyn in dorsal hippocampus: Implications on persistence of long-term memory in rats. Nutr. Neurosci. 2014, 17, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, F.; Walle, R.; Contini, A.; Oummadi, A.; Caraballo, B.; van der Veldt, S.; Boyer, M.-L.; Aby, F.; Tolentino-Cortez, T.; Helbling, J.-C.; et al. Causal Link between n-3 Polyunsaturated Fatty Acid Deficiency and Motivation Deficits. Cell Metab. 2020, 31, 755–772.e757. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.S.; Harris, W.S.; Beiser, A.S.; Au, R.; Himali, J.J.; Debette, S.; Pikula, A.; Decarli, C.; Wolf, P.A.; Vasan, R.S.; et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 2012, 78, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Valdés Hernández, M.C.; Muñoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef]
- Kou, J.; Kovacs, G.G.; Höftberger, R.; Kulik, W.; Brodde, A.; Forss-Petter, S.; Hönigschnabl, S.; Gleiss, A.; Brügger, B.; Wanders, R.; et al. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011, 122, 271–283. [Google Scholar] [CrossRef]
- Ahlborg, G.; Felig, P.; Hagenfeldt, L.; Hendler, R.; Wahren, J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J. Clin. Investig. 1974, 53, 1080–1090. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Watt, M.J.; Heigenhauser, G.J.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969–978. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Zhang, X.J.; Wolfe, R.R. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J. Appl. Physiol. 1995, 79, 1939–1945. [Google Scholar] [CrossRef]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Chytrova, G.; Ying, Z.; Gomez-Pinilla, F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2010, 1341, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Scriver, C.R. The Metabolic & Molecular Bases of Inherited Disease; McGraw-Hill: New York, NY, USA, 2001; Volume 4. [Google Scholar]
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin Med. Genet. 2006, 142c, 77–85. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef]
- Pertiwi, K.; Küpers, L.K.; Wanders, A.J.; de Goede, J.; Zock, P.L.; Geleijnse, J.M. Associations of dairy and fiber intake with circulating odd-chain fatty acids in post-myocardial infarction patients. Nutr. Metab. 2019, 16, 78. [Google Scholar] [CrossRef]
- Fu, L.; Huang, M.; Chen, S. Primary carnitine deficiency and cardiomyopathy. Korean Circ. J. 2013, 43, 785–792. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; Arnold, M.; MahmoudianDehkordi, S.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Serum metabolites associated with brain amyloid beta deposition, cognition and dementia progression. Brain Commun. 2021, 3, fcab139. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; de Glisezinski, I.; Larrouy, D.; Harant, I.; Moro, C. Influence of Acute and Chronic Exercise on Abdominal Fat Lipolysis: An Update. Front. Physiol. 2020, 11, 575363. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.; Churchward-Venne, T.A.; Bailey, D.; van Loon, L.J. Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Med. 2017, 47, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Blázquez, E.; Hurtado-Carneiro, V.; LeBaut-Ayuso, Y.; Velázquez, E.; García-García, L.; Gómez-Oliver, F.; Ruiz-Albusac, J.M.; Ávila, J.; Pozo, M.Á. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front. Endocrinol. 2022, 13, 873301. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef]
- Edwards, C.; Copes, N.; Bradshaw, P.C. D-ß-hydroxybutyrate: An anti-aging ketone body. Oncotarget 2015, 6, 3477–3478. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N. Chapter 1-Chemical Basis of Lipid Biochemistry. In Brain Lipids in Synaptic Function and Neurological Disease; Fantini, J., Yahi, N., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–28. [Google Scholar]
- Reue, K.; Brindley, D.N. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 2008, 49, 2493–2503. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Brion, J.P.; Couck, A.M.; Passareiro, E.; Flament-Durand, J. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J. Submicrosc. Cytol. 1985, 17, 89–96. [Google Scholar]
- Banks, W.A.; Farr, S.A.; Salameh, T.S.; Niehoff, M.L.; Rhea, E.M.; Morley, J.E.; Hanson, A.J.; Hansen, K.M.; Craft, S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. 2018, 42, 391–397. [Google Scholar] [CrossRef]
- Fanelli, F.; Mezzullo, M.; Belluomo, I.; Di Lallo, V.D.; Baccini, M.; Ibarra Gasparini, D.; Casadio, E.; Mastroroberto, M.; Vicennati, V.; Gambineri, A.; et al. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol. Metab. 2017, 6, 406–415. [Google Scholar] [CrossRef]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular Choreography of Acute Exercise. Cell 2020, 181, 1112–1130.e1116. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, Y.; Yokozeki, K.; Sawada, S.; Sakurai, R.; Fujiwara, Y.; Shinkai, S.; Goda, N.; Suzuki, K. Effects of aging on serum levels of lipid molecular species as determined by lipidomics analysis in Japanese men and women. Lipids Health Dis. 2018, 17, 135. [Google Scholar] [CrossRef]
- Šmidák, R.; Köfeler, H.C.; Hoeger, H.; Lubec, G. Comprehensive identification of age-related lipidome changes in rat amygdala during normal aging. PLoS ONE 2017, 12, e0180675. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.K.; Braidy, N.; Pickford, R.; Vafaee, F.; Crawford, J.; Muenchhoff, J.; Schofield, P.; Attia, J.; Brodaty, H.; Sachdev, P.; et al. Plasma lipidome variation during the second half of the human lifespan is associated with age and sex but minimally with BMI. PLoS ONE 2019, 14, e0214141. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, I.; Scherer, M.; Beguelin, F.; DaSilva, L.; Mari, D.; Salvioli, S.; Martin, F.-P.J.; Capri, M.; Bucci, L.; Ostan, R.; et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging 2014, 6, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, N.; Zhang, X.; Raftery, D.; Gu, H.; Hu, S.C.; Montine, T.J.; Quinn, J.F.; Chung, K.A.; Hiller, A.L.; Wang, D.; et al. A Metabolomic Aging Clock Using Human Cerebrospinal Fluid. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 744–754. [Google Scholar] [CrossRef]
- Harchaoui, K.E.; Visser, M.E.; Kastelein, J.J.; Stroes, E.S.; Dallinga-Thie, G.M. Triglycerides and cardiovascular risk. Curr. Cardiol. Rev. 2009, 5, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Huang, C.Q.; Dong, B.R.; Wu, H.M.; Zhang, Y.L.; Wu, J.H.; Lu, Z.C.; Flaherty, J.H. Association of cognitive impairment with serum lipid/lipoprotein among Chinese nonagenarians and centenarians. Dement Geriatr. Cogn. Disord. 2009, 27, 111–116. [Google Scholar] [CrossRef]
- Yin, Z.-X.; Shi, X.-M.; Kraus, V.B.; Fitzgerald, S.M.; Qian, H.-z.; Xu, J.-w.; Zhai, Y.; Sereny, M.D.; Zeng, Y. High normal plasma triglycerides are associated with preserved cognitive function in Chinese oldest-old. Age Ageing 2012, 41, 600–606. [Google Scholar] [CrossRef]
- Mougios, V.; Kotzamanidis, C.; Koutsari, C.; Atsopardis, S. Exercise-induced changes in the concentration of individual fatty acids and triacylglycerols of human plasma. Metabolism 1995, 44, 681–688. [Google Scholar] [CrossRef]
- Bourre, J.M. 12-Brain lipids and ageing. In Food for the Ageing Population; Raats, M., de Groot, L., van Staveren, W., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 219–251. [Google Scholar]
- Xie, M. Phospholipids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 214–217. [Google Scholar]
- Huang, Q.; Lei, H.; Dong, M.; Tang, H.; Wang, Y. Quantitative analysis of 10 classes of phospholipids by ultrahigh-performance liquid chromatography tandem triple-quadrupole mass spectrometry. Analyst 2019, 144, 3980–3987. [Google Scholar] [CrossRef]
- Hancock, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. Changes in Phospholipid Composition of the Human Cerebellum and Motor Cortex during Normal Ageing. Nutrients 2022, 14, 2495. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Dwivedi, S.; Singh, M.P.; Mishra, R.; Chandy, A. Basic and modern concepts on cholinergic receptor: A review. Asian Pac. J. Trop. Dis. 2013, 3, 413–420. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Sultzer, D.L.; Lim, A.C.; Gordon, H.L.; Yarns, B.C.; Melrose, R.J. Cholinergic receptor binding in unimpaired older adults, mild cognitive impairment, and Alzheimer’s disease dementia. Alzheimer’s Res. Ther. 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease:: Getting on and staying on. Curr. Res. Clin. Exp 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Svennerholm, L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schick, P.K.; Schick, B.P.; Brandeis, G.; Mills, D.C. Distribution of phosphatidylethanolamine arachidonic acid in platelet membranes. Biochim. Biophys. Acta 1981, 643, 659–662. [Google Scholar] [CrossRef]
- Villacara, A.; Spatz, M.; Dodson, R.F.; Corn, C.; Bembry, J. Effect of arachidonic acid on cultured cerebromicrovascular endothelium: Permeability, lipid peroxidation and membrane “fluidity”. Acta Neuropathol. 1989, 78, 310–316. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.M.; Van Antwerpen, P.; Govaerts, C. Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. J. Biol. Chem. 2016, 291, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Heacock, A.M.; Fisher, S.K. Chapter 23-Phosphoinositides. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 442–454. [Google Scholar]
- Raghu, P.; Joseph, A.; Krishnan, H.; Singh, P.; Saha, S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front. Mol. Neurosci. 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Collino, S.; Montoliu, I.; Martin, F.-P.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic Signatures of Extreme Longevity in Northern Italian Centenarians Reveal a Complex Remodeling of Lipids, Amino Acids, and Gut Microbiota Metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Metabolomic study of lipids in serum for biomarker discovery in Alzheimer’s disease using direct infusion mass spectrometry. J. Pharm. Biomed. Anal. 2014, 98, 321–326. [Google Scholar] [CrossRef]
- Shao, Y.; Ouyang, Y.; Li, T.; Liu, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Alteration of Metabolic Profile and Potential Biomarkers in the Plasma of Alzheimer’s Disease. Aging Dis. 2020, 11, 1459–1470. [Google Scholar] [CrossRef]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Chiang, J.; Cipolla, M.; Hale, J.; Diallo, F.; Chirino, A.; Arakaki, X.; Harrington, M.G. Alterations in cerebrospinal fluid glycerophospholipids and phospholipase A2 activity in Alzheimer’s disease. J. Lipid. Res. 2013, 54, 2884–2897. [Google Scholar] [CrossRef]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; García-Gómez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1864–1884. [Google Scholar] [CrossRef]
- Namba, T.; Nakamuta, S.; Funahashi, Y.; Kaibuchi, K. The role of selective transport in neuronal polarization. Dev. Neurobiol. 2011, 71, 445–457. [Google Scholar] [CrossRef]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, C.; Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef]
- Schneider, N.; Hauser, J.; Oliveira, M.; Cazaubon, E.; Mottaz, S.C.; O’Neill, B.V.; Steiner, P.; Deoni, S.C.L. Sphingomyelin in Brain and Cognitive Development: Preliminary Data. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Fote, G.; Wu, J.; Mapstone, M.; Macciardi, F.; Fiandaca, M.S.; Federoff, H.J. Plasma Sphingomyelins in Late-Onset Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 1161–1171. [Google Scholar] [CrossRef]
- Varga, T.V.; Ali, A.; Herrera, J.A.R.; Ahonen, L.L.; Mattila, I.M.; Al-Sari, N.H.; Legido-Quigley, C.; Skouby, S.; Brunak, S.; Tornberg, Å.B. Lipidomic profiles, lipid trajectories and clinical biomarkers in female elite endurance athletes. Sci. Rep. 2020, 10, 2349. [Google Scholar] [CrossRef]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1β. PLoS ONE 2017, 12, e0176793. [Google Scholar] [CrossRef]
- McBurney, M.I.; Tintle, N.L.; Vasan, R.S.; Sala-Vila, A.; Harris, W.S. Using an erythrocyte fatty acid fingerprint to predict risk of all-cause mortality: The Framingham Offspring Cohort. Am. J. Clin. Nutr. 2021, 114, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Marzolini, S.; Mielke, M.M.; Andreazza, A.; Oh, P.I.; Vattem Venkata, S.L.; Haughey, N.J.; Lanctôt, K.L. Association Between Sphingolipids and Cardiopulmonary Fitness in Coronary Artery Disease Patients Undertaking Cardiac Rehabilitation. J. Gerontol. Ser. A 2018, 75, 671–679. [Google Scholar] [CrossRef]
- Justin, B.N.; Turek, M.; Hakim, A.M. Heart disease as a risk factor for dementia. Clin. Epidemiol. 2013, 5, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids1. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554–567. [Google Scholar] [CrossRef]
- Daniels, T.F.; Killinger, K.M.; Michal, J.J.; Wright, R.W., Jr.; Jiang, Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int. J. Biol. Sci. 2009, 5, 474–488. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [Google Scholar] [CrossRef]
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy aging and the blood-brain barrier. Nat. Aging 2021, 1, 243–254. [Google Scholar] [CrossRef]
- Brandon, J.A.; Farmer, B.C.; Williams, H.C.; Johnson, L.A. APOE and Alzheimer’s Disease: Neuroimaging of Metabolic and Cerebrovascular Dysfunction. Front. Aging Neurosci. 2018, 10, 180. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- de Menezes, K.J.; Peixoto, C.; Nardi, A.E.; Carta, M.G.; Machado, S.; Veras, A.B. Dehydroepiandrosterone, Its Sulfate and Cognitive Functions. Clin. Pract. Epidemiol. Ment. Health 2016, 12, 24–37. [Google Scholar] [CrossRef]
- Thelen, K.M.; Falkai, P.; Bayer, T.A.; Lütjohann, D. Cholesterol synthesis rate in human hippocampus declines with aging. Neurosci. Lett. 2006, 403, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Feringa, F.M.; van der Kant, R. Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front Aging Neurosci. 2021, 13, 690372. [Google Scholar] [CrossRef] [PubMed]
- Koudinov, A.R.; Koudinova, N.V. Cholesterol, synaptic function and Alzheimer’s disease. Pharmacopsychiatry 2003, 36 (Suppl. S2), S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A.; Genové, G.; Li, T.; Lütjohann, D.; Olin, M.; Mast, N.; Pikuleva, I.A.; Crick, P.; Wang, Y.; Griffiths, W.; et al. Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J. Biol. Chem. 2014, 289, 23712–23722. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef]
- van Zutphen, E.M.; Rijnhart, J.J.M.; Rhebergen, D.; Muller, M.; Huisman, M.; Beekman, A.; Kok, A.; Appelman, Y. Do Cardiovascular Risk Factors and Cardiovascular Disease Explain Sex Differences in Cognitive Functioning in Old Age? J. Alzheimers Dis. 2021, 80, 1643–1655. [Google Scholar] [CrossRef]
- Leon, A.S.; Sanchez, O.A. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc. 2001, 33, S502–S515. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Tenover, J.L. Aging and declining testosterone: Past, present, and hopes for the future. J. Androl. 2012, 33, 1111–1118. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Mohr, B.A.; Guay, A.T.; O’Donnell, A.B.; McKinlay, J.B. Normal, bound and nonbound testosterone levels in normally ageing men: Results from the Massachusetts Male Ageing Study. Clin. Endocrinol. 2005, 62, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pardo, E.; Holland, C.A.; Cano, A. Sex Hormones and Healthy Psychological Aging in Women. Front. Aging Neurosci. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Bélanger, A.; Cusan, L.; Gomez, J.L.; Candas, B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef]
- Sorwell, K.G.; Urbanski, H.F. Causes and consequences of age-related steroid hormone changes: Insights gained from nonhuman primates. J. Neuroendocr. 2013, 25, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- Muller, M.; Aleman, A.; Grobbee, D.E.; de Haan, E.H.; van der Schouw, Y.T. Endogenous sex hormone levels and cognitive function in aging men: Is there an optimal level? Neurology 2005, 64, 866–871. [Google Scholar] [CrossRef]
- Yaffe, K.; Lui, L.Y.; Zmuda, J.; Cauley, J. Sex hormones and cognitive function in older men. J. Am. Geriatr. Soc. 2002, 50, 707–712. [Google Scholar] [CrossRef]
- Gurvich, C.; Hoy, K.; Thomas, N.; Kulkarni, J. Sex Differences and the Influence of Sex Hormones on Cognition through Adulthood and the Aging Process. Brain Sci. 2018, 8, 163. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Milne, M.R.; Haug, C.A.; Ábrahám, I.M.; Kwakowsky, A. Estradiol modulation of neurotrophin receptor expression in female mouse basal forebrain cholinergic neurons in vivo. Endocrinology 2015, 156, 613–626. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. The Molecular and Neuroanatomical Basis for Estrogen Effects in the Central Nervous System. J. Clin. Endocrinol. Metab. 1999, 84, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocr. 2008, 29, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Katayama, K.; Ishida, K.; Kanao, Y.; Saito, M. Responses of sex steroid hormones to different intensities of exercise in endurance athletes. Exp. Physiol. 2016, 101, 168–175. [Google Scholar] [CrossRef] [Green Version]

| Subclass | Species | Change with Ageing | Change with Cognitive Decline | Change with Exercise |
|---|---|---|---|---|
| Acylcarnitine | Acetylcarnitine (C2) | ↑ plasma [81] ↑ serum [82] ↑ CSF [83] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] | ↑ plasma [86,87,88,89] |
| Propionylcarnitine (C3:0) | ↑ plasma [81,90] | ↓ baseline plasma levels in MCI/AD converters than CN [91] ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ↓ plasma [86] ↑ plasma [88] ↑ serum [93] | |
| Malonylcarnitine (C3-DC) | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with slower decline in SM [94] | ||
| Butyrylcarnitine (C4) | ↑ plasma [81] ↑ serum [82] | ↑ plasma [86,88] ↑ serum[93] | ||
| Hexanoylcarnitine (C6) | ↑ plasma [81] ↑ serum [82] | ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] | ↑ plasma [86,88,89] ↑ serum [93] | |
| Hexenoylcarnitine (C6:1) | ↑ serum [82] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] ↑ baseline serum levels in CN and MCI associated with reduced AD risk and slower decline in GC, EM and SM [94] | ↑ plasma [88] | |
| Pimelylcarnitine (C7DC) | ↓ plasma [90] | ↑ baseline serum levels in CN and MCI associated with reduced AD risk and slower decline in GC, EM and SM [94] | ↑ plasma [86] | |
| Octanoylcarnitine (C8:0) | ↑ plasma [81,90,95] ↑ serum [82] | ↑ serum levels in MCI and then decreased slightly in AD (CN < AD < MCI) [96] ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] | ↑ plasma [86,88,97] ↑ serum [93] | |
| Nonanoylcarnitine (C9:0) | ↓ plasma [90] ↑ serum [82] | ↓ baseline plasma levels in MCI/AD converters than CN [91] | ↑ plasma [88] | |
| Decanoylcarnitine (C10) | ↑ plasma [81] ↑ serum [82] | ↑ serum levels progressively increased from CN < MCI < AD [96] ↑ baseline serum levels in CN and MCI associated with reduced AD risk and slower decline in GC and SM [94] ↓ serum (CN > SMC > MCI > AD) [84] ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] ↑ plasma levels progressively increased from CN < MCI < AD [98] | ↑ plasma [86,88,89,97]↑ serum [93] | |
| Decenoylcarnitine (C10:1) | ↑ plasma [81] ↑ serum [82,99] | ↑ baseline plasma levels in MCI/AD converters than CN [91] ↑ serum levels increased in AD but not MCI when compared to CN [96] ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] ↑ plasma levels progressively increased from CN < MCI < AD [98] | ↑ plasma [86,88,97] | |
| Decadienoylcarnitine (C10:2) | ↑ plasma [90,100] ↑ serum [82] | ↓ baseline plasma levels in MCI/AD converters than CN [91] | ↑ plasma [88] | |
| Dodecanoylcarnitine (C12) | ↑ plasma [101] ↑ serum [82] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] | ↑ plasma [86,97] | |
| Myristoylcarnitine (C14:0) | ↑ plasma [81,90,102] ↑ serum [82] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] | ↑ plasma [86,97] | |
| Tetradecenoyl- carnitine (C14:1) | ↑ plasma [81,101,103,104] ↑ serum [82] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] | ↑ plasma [86] | |
| Tetradecadienoyl- carnitine (C14:2) | ↑ plasma [81,90] ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with reduced AD risk and slower decline in GC and SM [94] | ||
| Tetradecadien- carnitine (C14:2) | ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] | |||
| Palmitoylcarnitine (C16:0) | ↑ plasma [81,102] ↑ serum [82] | ↑ serum levels in AD, but no change in MCI and CN [104] | ↑ plasma [86,88,89] | |
| Hexadecenoyl- Hydroxy-carnitine (C16:1-OH) | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | |||
| Hexadecenoyl- carnitine (C16:1) | ↑ plasma [81,90,102]↑ serum [82] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] | ↑ plasma [86,88] ↑ serum [93] | |
| Hexadecadienoyl- carnitine (C16:2) | ↑ plasma [90] | ↑ baseline plasma levels in MCI/AD converters than CN [91] | ↑ plasma [88] | |
| Octadecanoyl- carnitine (C18:0) | ↑ plasma [81,102] ↑ serum [82,105] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] ↑ serum levels in MCI and then decreased slightly in AD (CN < AD < MCI) [104] | ↑ plasma [86] | |
| Octadecenoyl- carnitine (C18:1) | ↑ plasma [81,90] ↑ serum [82,99] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ↑ plasma [86,89] | |
| Octadecadienyl- carnitine (C18:2) | ↑ plasma [81] | ↓ serum levels progressively decreased from CN > SMC > MCI > AD [84] ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ↑ plasma [86] | |
| Ketone bodies | Beta-hydroxy butyrate | ↑ plasma [106] | ↓ levels in AD plasma [103] ↑ plasma levels in MCI improvements in cognitive function and positively correlated with plasma ketone levels [107] | ↑ plasma [86,97] ↑ serum [93] |
| Saturated Fatty acids | Capric acid (C10:0) | ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | ↑ plasma [86,88,97] ↑ serum [109] | |
| Undecylic acid (C11:0) | ↑ CSF levels progressively increased from CN < MCI < AD[108] | |||
| Myristic acid (C14:0) | ↑ serum levels in MCI > CN [110] ↑ erythrocyte levels in SMC [111] ↑ CSF levels progressively increased from CN < MCI < AD [108] | ↑ plasma [86,88,97] ↑ serum [109] | ||
| Pentadecylic acid (C15:0) | ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | |||
| Palmitic acid (C16:0) | ↑ levels associated with increased risk of cognitive decline [112] ↑ serum levels in MCI > CN [110] ↓ plasma levels progressively decreased from CN > MCI > AD [113] ↑ CSF levels progressively increased from CN < MCI < AD [108] ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ↑ plasma [86,88,97] | ||
| Margaric acid (C17:0) | ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | ↑ plasma [86,88,97] | ||
| Stearic acid (C18:0) | ↑ plasma [106] plasma [114] | ↓ plasma levels decreased from CN > MCI, then increased slightly in AD (CN > AD > MCI) [113] ↑ levels associated with greater risk of cognitive decline [115] ↑ CSF levels decreased from CN to MCI and then increased in AD (MCI < CN < AD) [108] | ↑ plasma [86,97] | |
| Behenic acid (C22:0) | ↓ serum levels in MCI > CN [110] | ↑ plasma [86,97] | ||
| MUFA | Pentadecenoic acid (C15:1) | ↑ CSF levels progressively increased from CN < MCI < AD [108] | ||
| Palmitoleic acid (C16:1) | ↑ serum levels in MCI > CN [110] ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | ↑ plasma [86,88,97] ↑ serum [109] | ||
| Heptadecenoic acid (C17:1) | ↑ plasma[95] | ↑ plasma [86,88,97] | ||
| Oleic acid (C18:1) | ↑ plasma [106] | ↓ plasma levels progressively decreased from CN > MCI > AD [113] ↑ CSF levels increased from CN to MCI and then declined below CN levels in AD (AD < CN < MCI) [108] ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ↑ plasma [86,89,97] ↑ serum [109] | |
| Nonadecenoic acid (C19:1) | ↑ CSF levels progressively increased from CN < MCI < AD [108] | ↑ plasma [86,88,97] | ||
| Nervonic acid (C24:1) | ↓ serum levels in MCI > CN [110] | ↑ plasma [86,88,97] | ||
| PUFA | Linoleic acid (C18:2) | ↑ plasma [106] ↓ erythrocyte [111] | ↓ plasma levels progressively decreased from CN > MCI > AD [113] ↑ plasma levels in MCI > CN [116] ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | ↑ plasma [86,89,97] ↑ serum [93] |
| Linolenic acid (C18:3) | ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] | ↑ plasma [86,89,97] | ||
| Eicosadienoic acid (C20:2n-6) | ↓ plasma [95] | ↑ erythrocyte levels associated with lower MMSE and executive function scores [117] ↑ CSF levels progressively increased from CN < MCI < AD [108] ↓ plasma levels decreased from CN < MCI then increased slightly in AD (CN > AD > MCI) [98] | ↑ plasma [86,89,97] | |
| Eicosatrienoic acid (C20:3) | ↑ CSF levels increased from CN to MCI and then slightly decreased in AD (CN < AD < MCI) [108] ↓ plasma levels decreased from CN < MCI then increased slightly in AD (CN > AD > MCI) [98] | ↑ serum [86,89,97] | ||
| Arachidonic acid [AA,(C20:4n-6)] | ↑ plasma [106] | ↑ levels associated with increased risk of cognitive decline [112] ↑ erythrocyte levels predicted cognitive impairment [117] ↑ CSF levels decreased from CN > MCI, then increased in AD (MCI < CN < AD) [108] | ↑ plasma[86] | |
| Eicosapentanoic acid [EPA, (C20:5n-3)] | ↓ plasma [118] ↑ plasma [95] ↑ CSF [119] | ↓ levels associated with increased risk of cognitive decline [112] ↑ CSF levels increased from CN < MCI then declined below CN levels in AD (AD < CN < MCI) [108] ↑ serum levels positively associated with cognition [120] ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ↑ plasma [86] ↑ serum [93] | |
| Docosapentaenoic acid [DPA, (C22:5n-3)] | ↑ CSF [119] | ↑ CSF levels progressively increased from CN < MCI < AD [108] ↓ plasma levels decreased from CN < MCI then increased slightly in AD (CN > AD > MCI) [98] | ↑ plasma [86,88,97] ↑ serum [93] | |
| Docosahexaenoic acid [DHA, (C22:6n-3)] | ↑ CSF [119] ↓ brain [121] ↓ erythrocyte [111] | ↓ levels associated with increased risk of cognitive decline [112] ↓ plasma levels decreased from CN > MCI then returned to normal levels in AD (CN = AD > MCI)[113] ↓ serum levels in MCI > CN [110] ↓ serum levels in MCI > CN [122] ↓ baseline levels in AD were associated with a higher risk of cognitive decline [123] ↑ blood levels associated with lower risk of AD and dementia [124] ↓ levels associated with declines in memory and executive function [125] ↑ CSF levels progressively increased from CN < MCI < AD [108] ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ↑ plasma [86,88] ↑ serum [93] |
| Subclass | Species | Change with Ageing | Change with Cognitive Decline | Change with Exercise |
|---|---|---|---|---|
| MAG | 2AG | ↑ plasma [168] | ||
| DAG | DAG 16:0/18:1 | ↓ plasma [86,169] | ||
| DAG 16:0/18:2 | ↓ plasma [86,169] | |||
| DAG 16:1/18:2 | ↓ plasma [86,169] | |||
| DAG 18:1/18:1 | ↓ plasma [86,169] | |||
| DAG 18:1/18:2 | ↓ plasma [86,169] | |||
| TAG | TAG 48:1 | ↑ serum [170] | ||
| TAG 50:0 | ↑ serum [170] | |||
| TAG 52:1 | ↑ serum [170] | |||
| TAG 56:7 | ↓ plasma [100] ↑ CSF [119] | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | ||
| TAG 56:8 | ↓ plasma [101] ↑ CSF [119] | ↓ plasma levels progressively decreased from CN > MCI > AD [98] |
| Subclass | Species | Change with Ageing | Change with Cognitive Decline | Change with Exercise |
|---|---|---|---|---|
| PC | Glycero- phosphocholine | ↓ serum [173] ↑ plasma [106] | ↓ serum levels associated with worse cognition and lower MMSE scores in AD [85] | |
| LysoPC | LPC a C18:2 | ↓ serum [196] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | |
| LPC 20:5 | ↓ serum levels progressively declined in CN > MCI > AD [104] | |||
| LPC 22:6 | ↓ serum levels progressively declined in CN > MCI > AD [104] | |||
| PC | PC 16:0/16:0 | ↑ CSF [174] | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | |
| PPC 16:0/18:2 | ↓ serum levels progressively declined in CN > MCI > AD [104] | |||
| PC 16:0/18:2 | ↑ serum [173] | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ||
| PC 16:1/22:6 | ↓ serum levels in MCI and then increased in AD (CN > AD > MCI) [104] | |||
| PC 18:1/20:4 | ↑ CSF [174] | ↓ serum (AD) [197] | ↑ plasma [169] | |
| PC ae C26:1 | ↑ baseline serum levels in CN and MCI associated with faster decline in SM [94] | |||
| PC aa 30:0 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in GC [94] | ||
| PC ae 30:0 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in GC and SM [94] | ||
| PC ae C34:0 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in GC, EM, PS and SM [94] | ||
| PC ae C34:1 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in GC [94] | ||
| PC ae C36:2 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in GC and SM [94] | ||
| PC O-36:4 | ↓ plasma levels decreased from CN > MCI then stayed the same for AD (CN > MCI = AD) [98] | |||
| PC 36:5 | ↑ CSF [174] ↓ plasma [100,198] | ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ||
| PC aa C36:5 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with slower decline in PS [94] | ||
| PC aa 36:6 | ↑ serum [82] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC 37:6 | ↓ plasma levels decreased from CN < MCI then slightly increased in AD (CN > AD > MCI) [98] | |||
| PC aa 38:0 | ↑ serum [82] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC aa C38:3 | ↑ serum [82] | ↑ baseline serum levels in CN and MCI associated with faster decline in SM [94] | ||
| PC aa 38:5 | ↓ plasma levels decreased from CN < MCI then slightly increased in AD (CN > AD > MCI) [98] | |||
| PC aa C38:5 | ↑ CSF [174] ↓ plasma [100] ↓ CSF [108] | ↑ baseline serum levels in CN and MCI associated with slower decline in PS [94] | ||
| PC 38:6 | ↓ plasma [100] ↓ CSF [108] | ↓ plasma levels decreased from CN < MCI, then slightly increased in AD (CN > AD > MCI) [98] | ||
| PC aa C40:1 | ↑ serum [82] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC aa C40:2 | ↑ serum [82] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC aa 40:5 | ↑ serum [82] ↓ serum [105] | ↓ plasma levels decreased from CN < MCI, then slightly increased in AD (CN > AD > MCI) [98] | ||
| PC aa 40:6 | ↓ plasma [100] | ↓ plasma levels progressively decreased from CN < MCI < AD [98] | ||
| PC aa C40:6 | ↑ serum [82] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC ae C40:6 | ↓ plasma [95] | ↓ plasma levels progressively decreased from CN > Converterpre > MCI/AD [92] | ||
| PC ae C44:4 | ↑ baseline serum levels in CN and MCI associated with faster decline in GC and EM [94] | |||
| PE | PE 16:0/18:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ||
| PE 36:4 | ↑ plasma levels increased from CN < MCI, then stayed the same in AD (CN < MCI = AD) [98] | |||
| PE 38:5 | ↑ plasma levels increased from CN < MCI, then decreased slightly in AD (CN < AD < MCI) [98] | |||
| PE 38:7 | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | |||
| PE 40:6 | ↓ CSF[119] | ↓ plasma levels decreased from CN > MCI, then the stayed same in AD (CN > MCI = AD) [98] | ||
| LysoPE | LPE 18:0/0:0 | ↑ plasma levels progressively increased from CN < MCI < AD [98] | ||
| LPE 18:1/0:0 | ↑ plasma levels increased from CN < MCI, then decreased slightly in AD (CN < AD < MCI) [98] | |||
| PI | PI 40:6 | ↑ plasma levels decreased from CN > MCI, then increased slightly in AD (CN > AD > MCI) [98] | ||
| LysoPI | LPI 18:0/0:0 | ↑ plasma levels positively associated with cognition [120] |
| Subclass | Species | Change with Ageing | Change with Cognitive Decline | Change with Exercise |
|---|---|---|---|---|
| Ceramides | Hex-CER 18:1/16:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ||
| Hex-CER 18:1/18:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | |||
| Hex-CER 18:1/24:1(2OH) | ↑ plasma levels negatively associated with cognition [120] | |||
| Lac-CER 18:1/14:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | |||
| Lac-CER 18:1/16:1 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | |||
| Lac-CER 18:1/16:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | |||
| CER 18:1/16:0 | ↑ serum levels progressively increased from CN < MCI < AD [104] | |||
| CER 39:1 | ↑ plasma levels decreased from CN > MCI, then increased slightly in AD (CN > AD > MCI) [98] | |||
| CER 40:1 | ↑ plasma levels decreased from CN > MCI, then increased slightly in AD (CN > AD > MCI) [98] | |||
| CER 41:1 | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | |||
| CER 42:1 | ↑ plasma levels decreased from CN > MCI, then increased slightly in AD (CN > AD > MCI) [98] | |||
| CER 43:1 | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | |||
| Sphingo- myelins | SM 18:1/14:0 | ↑ plasma [95] | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ↓ plasma [86] |
| SM 18:1/16:0 | ↑ plasma [95] | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] ↓ CSF (MCI) [207] | ↑ plasma [86] ↓ plasma [86] | |
| SM 18:1/18:1 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ↑ plasma [86] | ||
| SM 18:1/18:0 | ↑ serum levels in MCI and then decreased in AD (CN < AD < MCI) [104] | ↑ plasma [86] ↓ plasma [86] | ||
| SM 18:1/22:0 | ↓ plasma [101] | ↓ CSF (MCI) [207] | ↑ plasma [86] | |
| SM 39:1 | ↓ plasma [100] ↓ CSF [119] | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | ||
| SM 41:1 | ↓ plasma [100] | ↓ plasma levels progressively decreased from CN > MCI > AD [98] | ↑ serum [208] | |
| SM 42:1 | ↓ plasma [100] | ↓ plasma levels progressively decreased from CN > MCI > AD [98] |
| Subclass | Species | Change with Ageing | Change with Cognitive Decline | Change with Exercise |
|---|---|---|---|---|
| Sterols | Cholesterol | ↑ plasma [106,200] ↑ plasma (F) [199] | ↓ brain (AD) [222] | ↓ plasma [86] |
| Cholesteryl esters | CE 20:3 | ↑ levels associated with increased risk of global cognitive decline [112] | ||
| CE 18:2 | ↓ plasma [100] | ↓ levels associated with increased risk of global cognitive decline [112] | ||
| Steroids | Dehydro- epiandrosterone-sulfate (DHEA-S) | ↓ plasma [106] | Positive correlation between DHEA-S and global cognition in M and F. Positive correlations between DHEA-S on working memory, attention and verbal fluency in F only [223] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrov, M.; Whiley, L.; Brown, B.; Erickson, K.I.; Holmes, E. Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours. Metabolites 2022, 12, 822. https://doi.org/10.3390/metabo12090822
Kadyrov M, Whiley L, Brown B, Erickson KI, Holmes E. Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours. Metabolites. 2022; 12(9):822. https://doi.org/10.3390/metabo12090822
Chicago/Turabian StyleKadyrov, Maria, Luke Whiley, Belinda Brown, Kirk I. Erickson, and Elaine Holmes. 2022. "Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours" Metabolites 12, no. 9: 822. https://doi.org/10.3390/metabo12090822
APA StyleKadyrov, M., Whiley, L., Brown, B., Erickson, K. I., & Holmes, E. (2022). Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours. Metabolites, 12(9), 822. https://doi.org/10.3390/metabo12090822