Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice
Abstract
:1. Introduction
2. Results
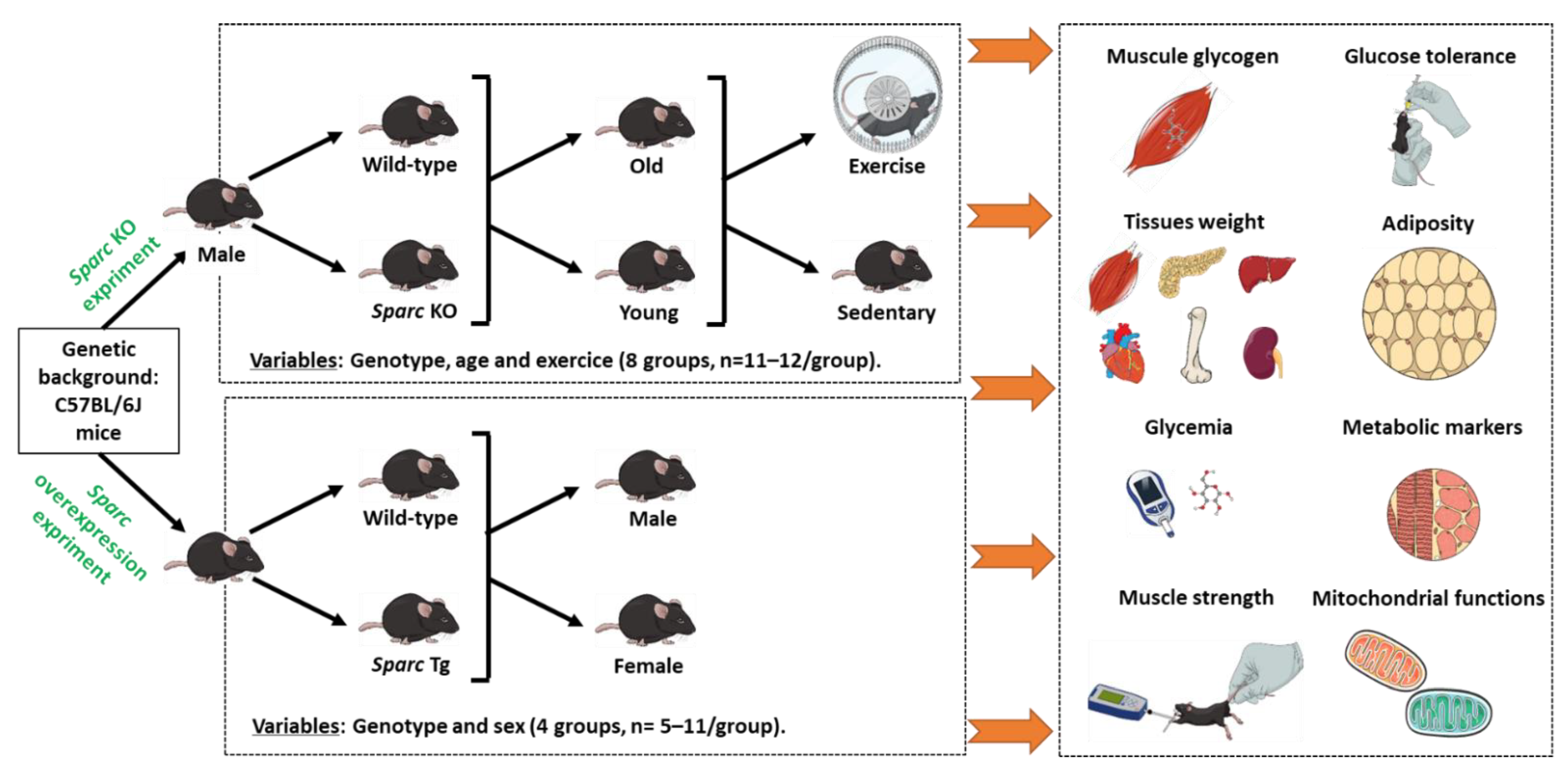
2.1. Sparc Knockout (KO) Mice, Exercise, and Ageing
2.1.1. Tissue Weights and Sizes
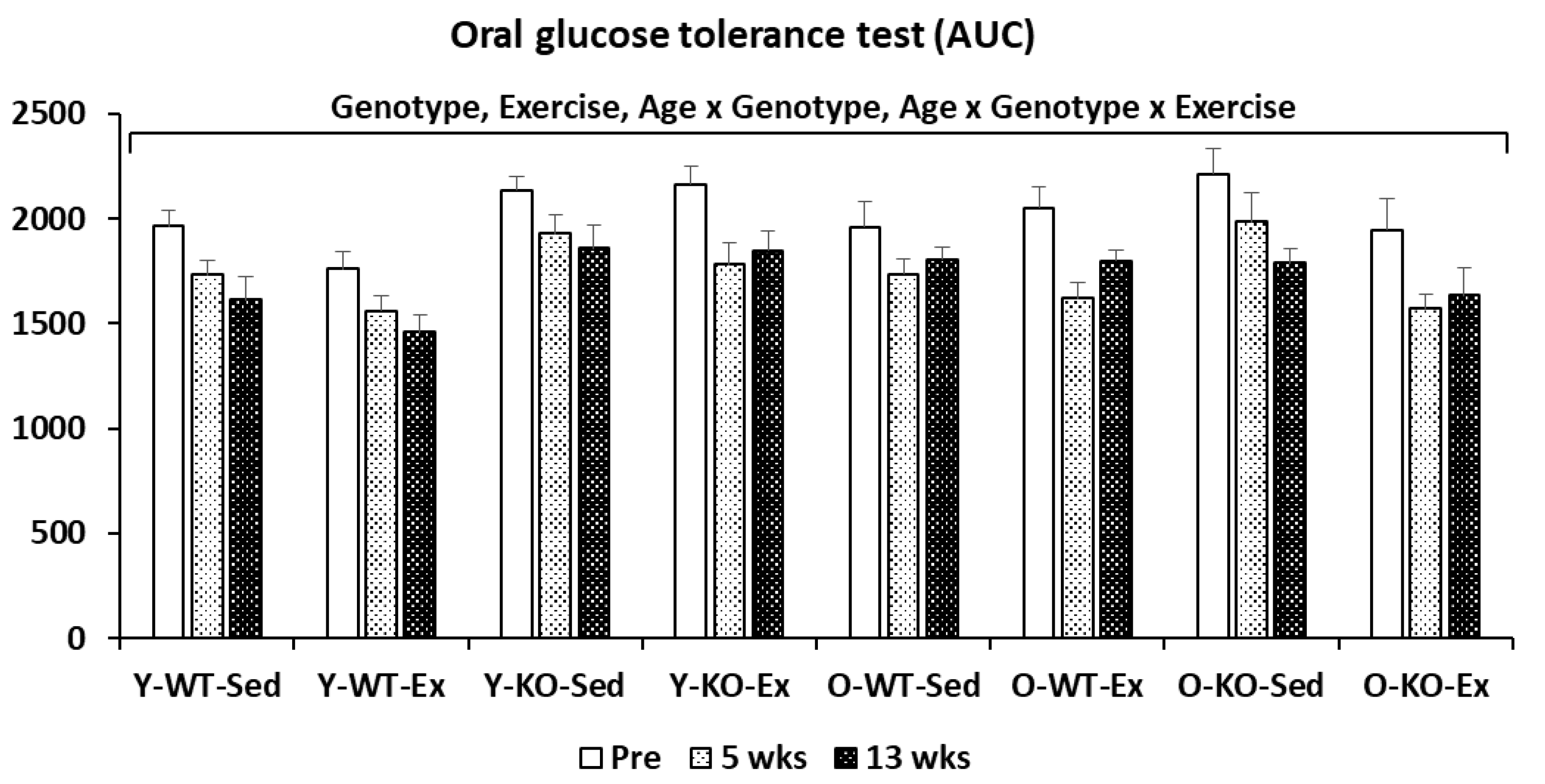
2.1.2. Oral Glucose Tolerance Test (OGTT)
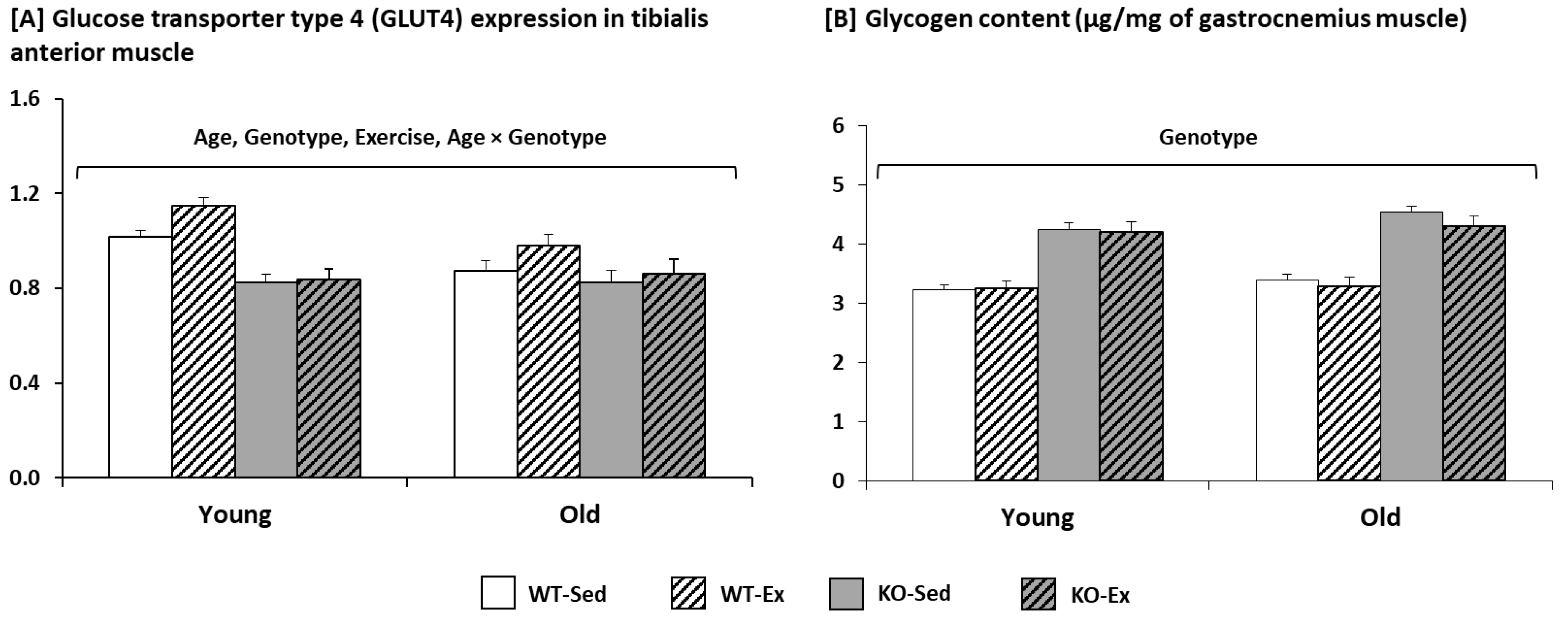
2.1.3. Glycogen Content and Glucose Transporter Type 4 (GLUT4) Expression in the Muscle
2.2. Sparc Transgeneic (Tg) Mice
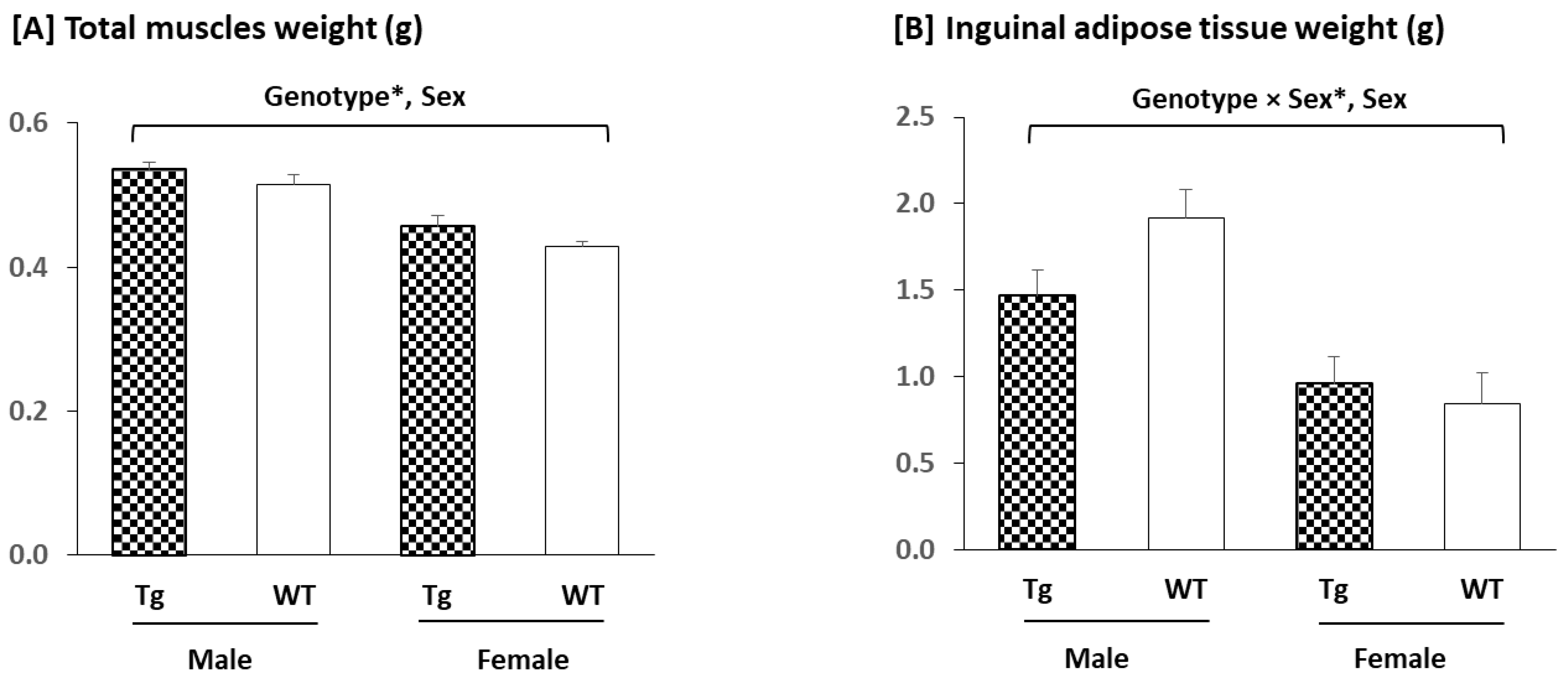
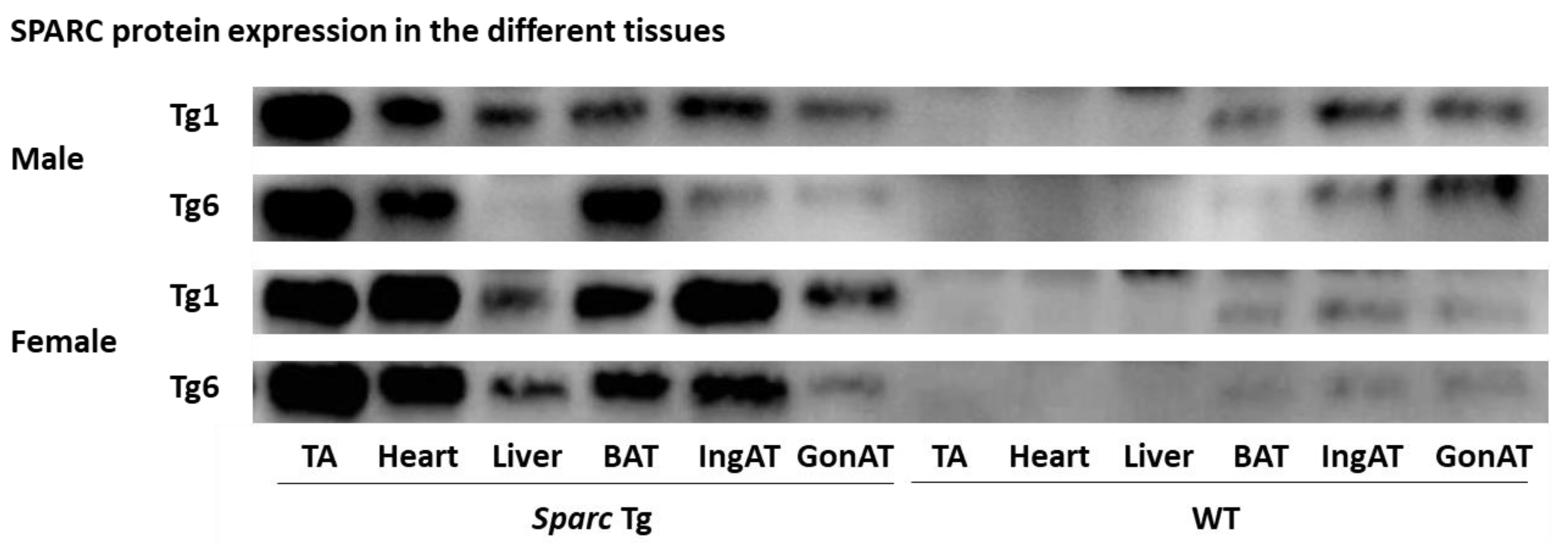
2.2.1. Tissues Weights
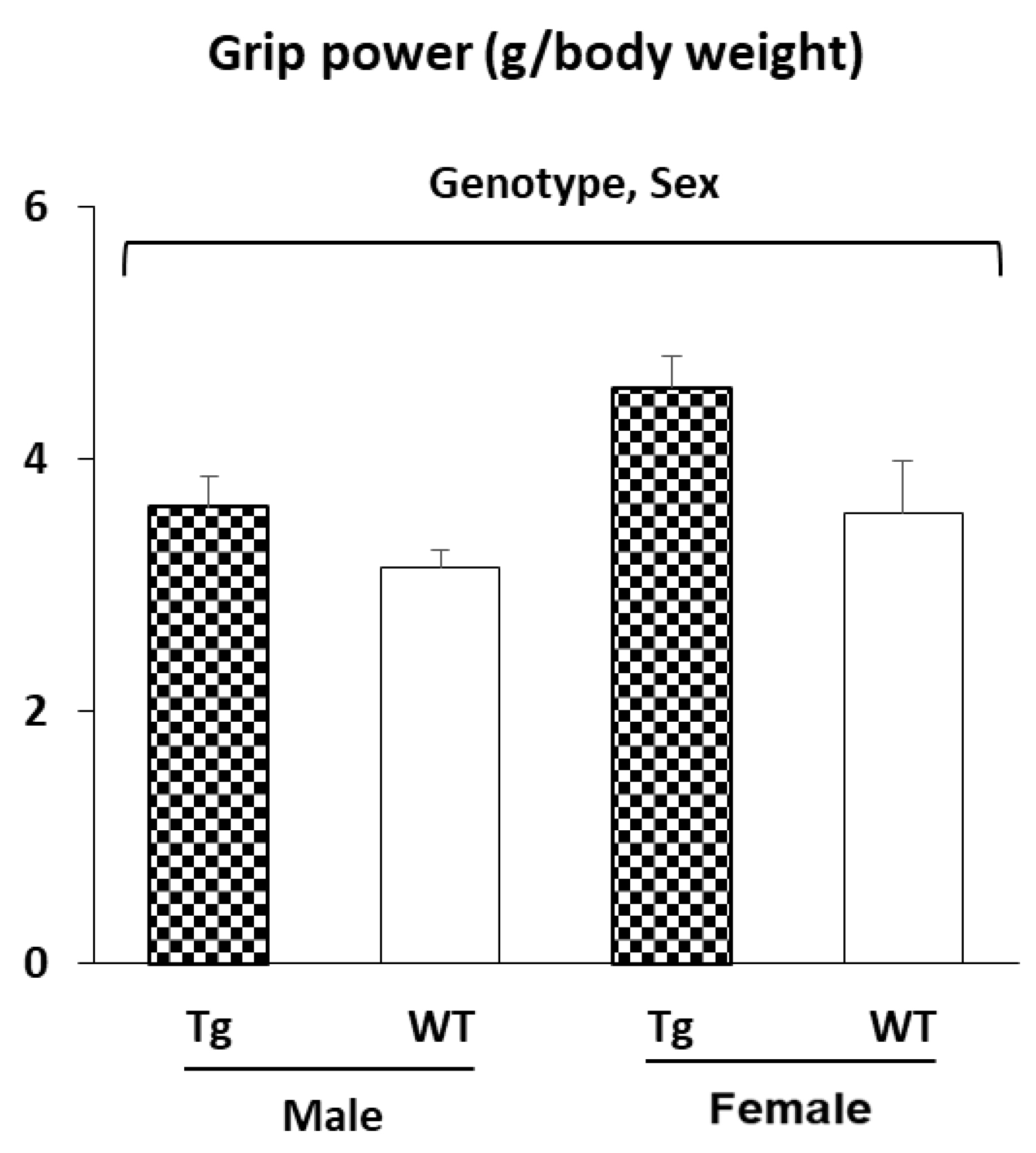
2.2.2. Muscle Strength (Grip Power Test)
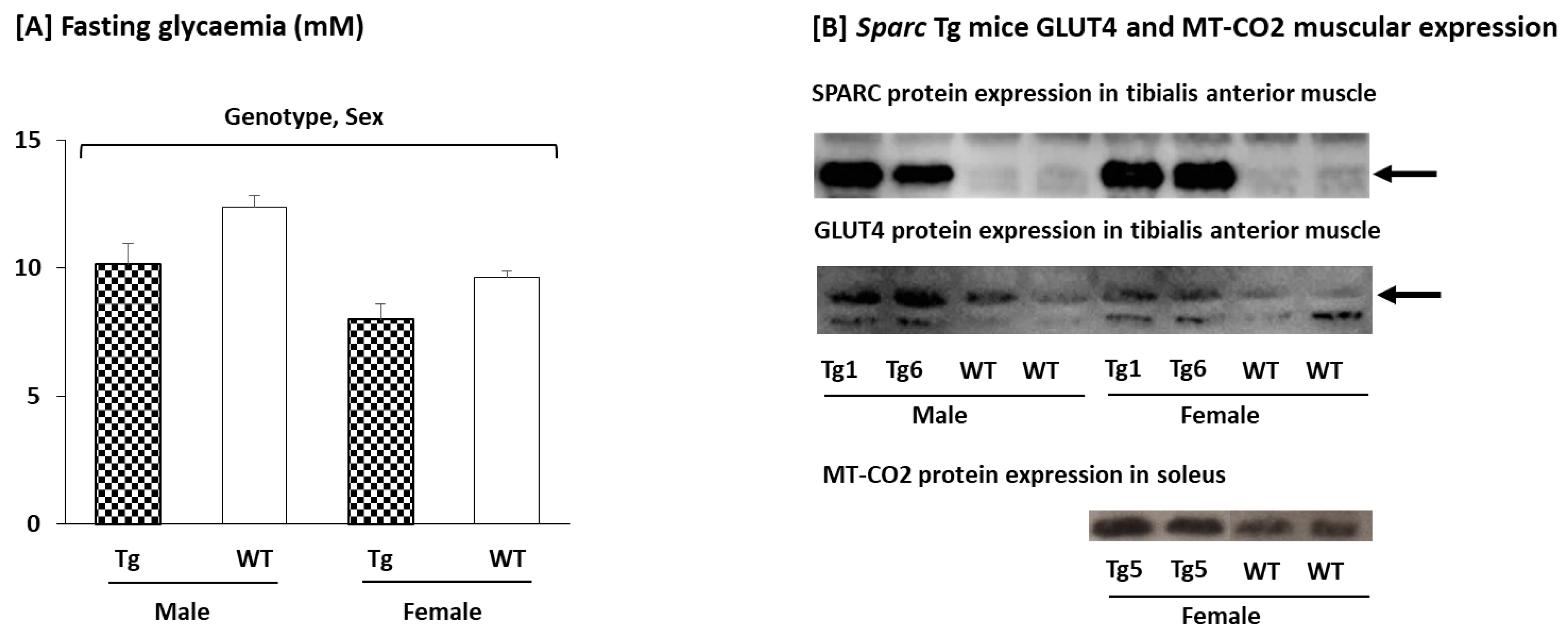
2.2.3. Glycemia, Glucose Transporter Type 4 (GLUT4), and Mitochondrially Encoded Cytochrome c Oxidase II (MT-CO2) Expression in the Muscle
3. Discussion
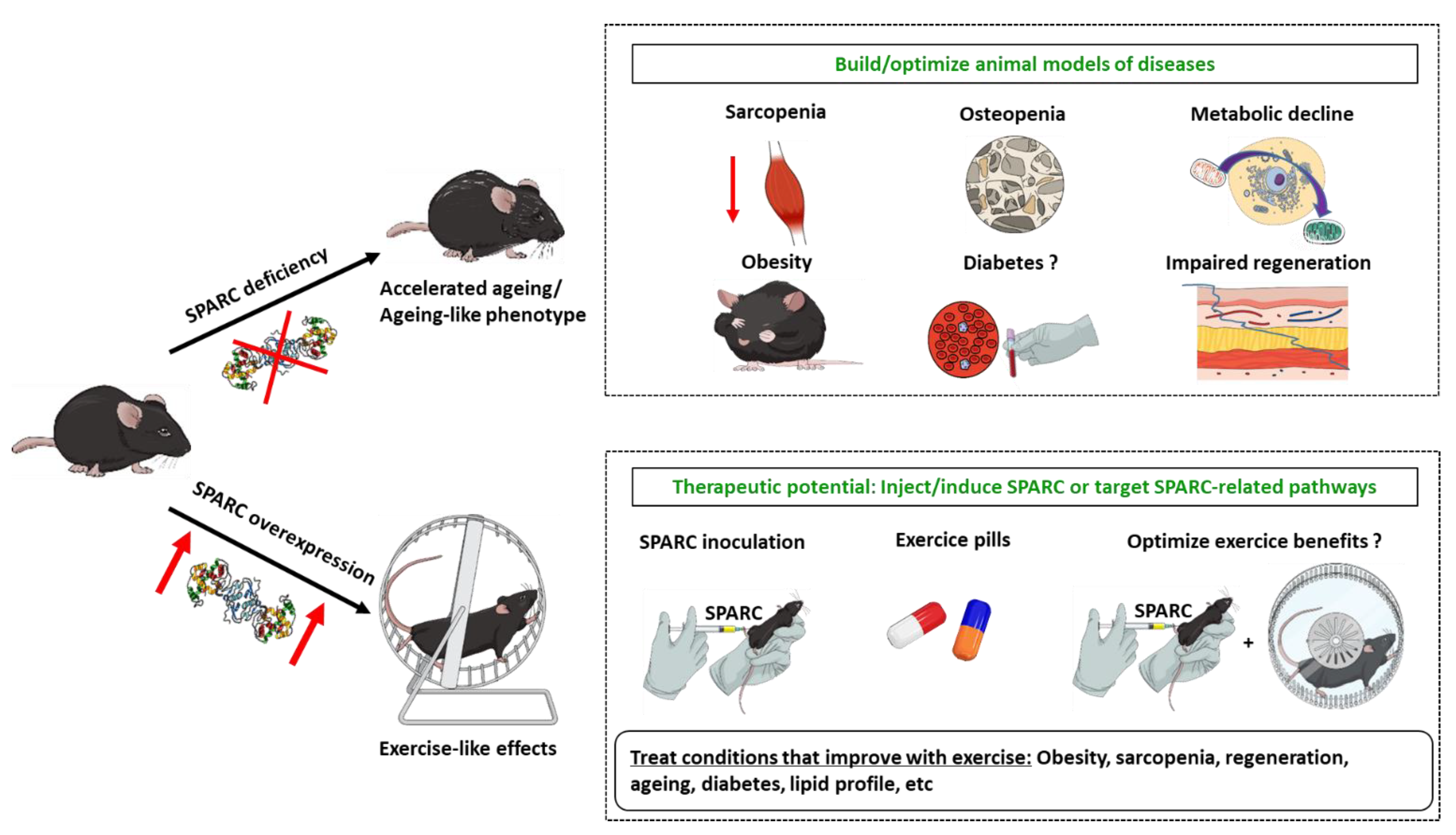
3.1. Sparc KO to Optimize (Accelerated) Ageing Animal Models
3.2. Sparc KO as Type 1 Diabetes Model?
3.3. Sparc KO to Optimize Sarcopenic Obesity
3.4. SPARC Overexpression Mimics Exercise Benefits
4. Materials and Methods
4.1. Sparc KO Experiment
4.1.1. Mice Sacrifice Tissue Weights
4.1.2. Oral Glucose Tolerance Test (OGTT)
4.1.3. Muscle Glucose Transporter Type 4 (GLUT4) Expression (Western Blot)
4.1.4. Skeletal Muscle Glycogen Content
4.1.5. Histological Analysis of the White Adipose Tissue
4.1.6. Statistical Analyses
4.2. Sparc Overexpression (Sparc Tg) Experiment
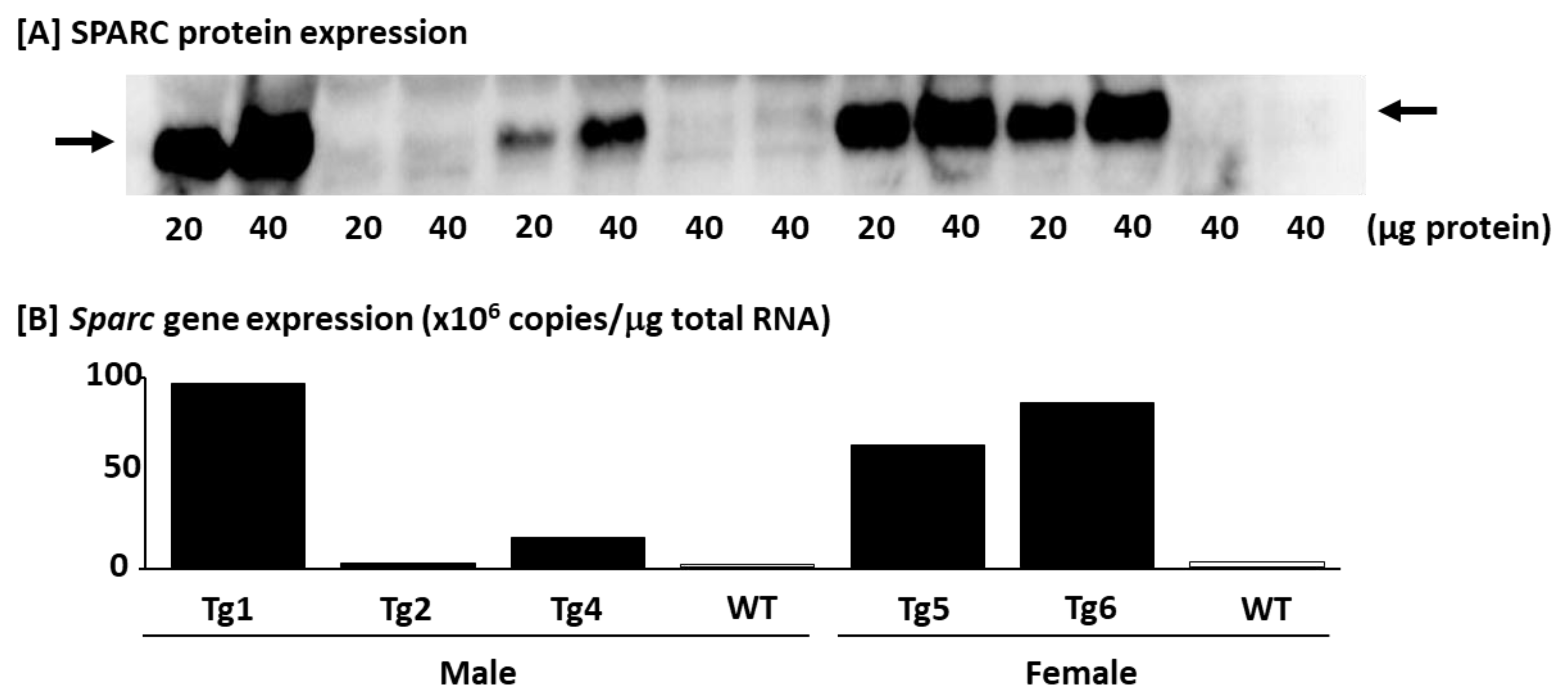
4.2.1. Confirming the SPARC/Sparc Gene Overexpression and Selecting Mice (Western blot and Q_RT-PCR)
4.2.2. Body Weight and Tissue Weights
4.2.3. Grip Power Test
4.2.4. Blood Glucose (Glycemia)
4.2.5. Muscular GLUT4 and Mitochondrially Encoded Cytochrome c Oxidase II (MT-CO2) Expression (Western Blot)
4.2.6. Statistical Analyses
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D. Influence of age on skeletal muscle glucose transport and glycogen metabolism. Med. Sci. Sports Exerc. 1994, 26, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L. Aerobic exercise in the elderly: A key to successful aging. Discov. Med. 2012, 13, 223–228. [Google Scholar] [PubMed]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef]
- Lee, P.G.; Jackson, E.A.; Richardson, C.R. Exercise Prescriptions in Older Adults. Am. Fam. Physician 2017, 95, 425–432. [Google Scholar]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef] [Green Version]
- Riedl, I.; Yoshioka, M.; Nishida, Y.; Tobina, T.; Paradis, R.; Shono, N.; Tanaka, H.; St-Amand, J. Regulation of skeletal muscle transcriptome in elderly men after 6 weeks of endurance training at lactate threshold intensity. Exp. Gerontol. 2010, 45, 896–903. [Google Scholar] [CrossRef]
- Melouane, A.; Yoshioka, M.; Kanzaki, M.; St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019, 229, 277–287. [Google Scholar] [CrossRef]
- Aoi, W.; Sakuma, K. Skeletal muscle: Novel and intriguing characteristics as a secretory organ. BioDiscovery 2013, 7, e8942. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020, 10, 9108. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Measuring Exercise-Induced Secreted Protein Acidic and Rich in Cysteine Expression as a Molecular Tool to Optimize Personalized Medicine. Genes 2021, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Melouane, A.; Carbonell, A.; Yoshioka, M.; Puymirat, J.; St-Amand, J. Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE 2018, 13, e0192714. [Google Scholar] [CrossRef] [Green Version]
- Garneau, L.; Parsons, S.A.; Smith, S.R.; Mulvihill, E.E.; Sparks, L.M.; Aguer, C. Plasma Myokine Concentrations After Acute Exercise in Non-obese and Obese Sedentary Women. Front. Physiol. 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yamanouchi, K.; Nishihara, M. Secreted protein acidic and rich in cysteine internalization and its age-related alterations in skeletal muscle progenitor cells. Aging Cell 2014, 13, 175–184. [Google Scholar] [CrossRef]
- Delany, A.M.; Kalajzic, I.; Bradshaw, A.D.; Sage, E.H.; Canalis, E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology 2003, 144, 2588–2596. [Google Scholar] [CrossRef] [Green Version]
- Motamed, K. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol. 1999, 31, 1363–1366. [Google Scholar] [CrossRef]
- Scavelli, K.; Chatterjee, A.; Rhee, D.J. Secreted Protein Acidic and Rich in Cysteine in Ocular Tissue. J. Ocul. Pharmacol. Ther. 2015, 31, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Sage, H.; Johnson, C.; Bornstein, P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 1984, 259, 3993–4007. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biol. 2000, 19, 569–580. [Google Scholar] [CrossRef]
- Norose, K.; Clark, J.I.; Syed, N.A.; Basu, A.; Heber-Katz, E.; Sage, E.H.; Howe, C.C. SPARC deficiency leads to early-onset cataractogenesis. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2674–2680. [Google Scholar]
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999, 47, 1495–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Wang, L.Y.; Li, C.Y.; Wu, J.Y.; Zhang, Y.T.; Pang, K.P.; Wei, Y.; Du, L.Q.; Liu, M.; Wu, X.Y. SPARC promotes self-renewal of limbal epithelial stem cells and ocular surface restoration through JNK and p38-MAPK signaling pathways. Stem Cells 2020, 38, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alachkar, H.; Santhanam, R.; Maharry, K.; Metzeler, K.H.; Huang, X.; Kohlschmidt, J.; Mendler, J.H.; Benito, J.M.; Hickey, C.; Neviani, P.; et al. SPARC promotes leukemic cell growth and predicts acute myeloid leukemia outcome. J. Clin. Investig. 2014, 124, 1512–1524. [Google Scholar] [CrossRef] [Green Version]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000, 105, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Barker, T.H.; Baneyx, G.; Cardó-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005, 280, 36483–36493. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 2009, 3, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. Biochem. Cell Biol. 2019, 117, 105627. [Google Scholar] [CrossRef]
- Onorato, A.M.; Fiore, E.; Bayo, J.; Casali, C.; Fernandez-Tomé, M.; Rodríguez, M.; Domínguez, L.; Argemi, J.; Hidalgo, F.; Favre, C.; et al. SPARC inhibition accelerates NAFLD-associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int. 2021, 41, 1677–1693. [Google Scholar] [CrossRef]
- Song, H.; Ding, L.; Zhang, S.; Wang, W. MiR-29 family members interact with SPARC to regulate glucose metabolism. Biochem. Biophys. Res. Commun. 2018, 497, 667–674. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020, 10, 2388. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, S.; Baicu, C.F.; Heymans, S.; Bradshaw, A.D. Cardiac extracellular matrix remodeling: Fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J. Mol. Cell. Cardiol. 2010, 48, 544–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersson, S.J.; Jørgensen, L.H.; Andersen, D.C.; Nørgaard, R.C.; Jensen, C.H.; Schrøder, H.D. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol. Histopathol. 2013, 28, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Galvão, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-induced myokines and their effect on prostate cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as a Molecular Physiological and Pathological Biomarker. Biomolecules 2021, 11, 1689. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegeman, R.; Weake, V.M. Transcriptional Signatures of Aging. J. Mol. Biol. 2017, 429, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Vasan, R.S. Age as a risk factor. Med. Clin. N. Am. 2012, 96, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheitlin, M.D. Cardiovascular physiology-changes with aging. Am. J. Geriatr. Cardiol. 2003, 12, 9–13. [Google Scholar] [CrossRef]
- Kõks, S.; Dogan, S.; Tuna, B.G.; González-Navarro, H.; Potter, P.; Vandenbroucke, R.E. Mouse models of ageing and their relevance to disease. Mech. Ageing Dev. 2016, 160, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Libert, C. The mouse as a model organism in aging research: Usefulness, pitfalls and possibilities. Ageing Res. Rev. 2013, 12, 8–21. [Google Scholar] [CrossRef]
- Azzu, V.; Valencak, T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology 2017, 63, 327–336. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Jørgensen, L.H.; Jepsen, P.L.; Boysen, A.; Dalgaard, L.B.; Hvid, L.G.; Ørtenblad, N.; Ravn, D.; Sellathurai, J.; Møller-Jensen, J.; Lochmüller, H.; et al. SPARC Interacts with Actin in Skeletal Muscle in Vitro and in Vivo. Am. J. Pathol. 2017, 187, 457–474. [Google Scholar] [CrossRef] [Green Version]
- Kjær, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wagner, A.; Gehwolf, R.; Yan, W.; Passini, F.S.; Thien, C.; Weissenbacher, N.; Lin, Z.; Lehner, C.; Teng, H.; et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 2021, 13, eabe5738. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Schaffer, S.; Halliwell, B. The mitochondrial free radical theory of ageing--where do we stand? Front. Biosci. 2008, 13, 6554–6579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A. Glucose Intolerance and Aging. Diabetes Care 1981, 4, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Kos, K.; Wong, S.; Tan, B.; Gummesson, A.; Jernas, M.; Franck, N.; Kerrigan, D.; Nystrom, F.H.; Carlsson, L.M.; Randeva, H.S.; et al. Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes 2009, 58, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Lee, J.A.; Park, H.S.; Song, Y.S.; Jang, Y.J.; Kim, J.H.; Lee, Y.J.; Heo, Y. Associations among SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolic parameters in Korean women. Obesity 2013, 21, 2296–2302. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choi, M.J.; Kim, S.W.; Yun, J.W. Secreted protein acidic and rich in cysteine (SPARC) regulates thermogenesis in white and brown adipocytes. Mol. Cell. Endocrinol. 2020, 506, 110757. [Google Scholar] [CrossRef]
- Guo, S.S.; Zeller, C.; Chumlea, W.C.; Siervogel, R.M. Aging, body composition, and lifestyle: The Fels Longitudinal Study. Am. J. Clin. Nutr. 1999, 70, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Sackmann-Sala, L.; Berryman, D.E.; Munn, R.D.; Lubbers, E.R.; Kopchick, J.J. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity 2012, 20, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Bradshaw, A.D.; Delany, A.M.; Sage, E.H. Inactivation of SPARC enhances high-fat diet-induced obesity in mice. Connect. Tissue Res. 2011, 52, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musarò, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.B.; Brack, A.S. Muscle Stem Cells and Aging. Curr. Top. Dev. Biol. 2018, 126, 299–322. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as A Regeneration Factor: Beyond the Tissue Repair. Life 2021, 11, 38. [Google Scholar] [CrossRef]
- Mansergh, F.C.; Wells, T.; Elford, C.; Evans, S.L.; Perry, M.J.; Evans, M.J.; Evans, B.A. Osteopenia in Sparc (osteonectin)-deficient mice: Characterization of phenotypic determinants of femoral strength and changes in gene expression. Physiol. Genom. 2007, 32, 64–73. [Google Scholar] [CrossRef]
- Mansergh, F.C.; Wride, M.A.; Walker, V.E.; Adams, S.; Hunter, S.M.; Evans, M.J. Gene expression changes during cataract progression in Sparc null mice: Differential regulation of mouse globins in the lens. Mol. Vis. 2004, 10, 490–511. [Google Scholar]
- Rempel, S.A.; Hawley, R.C.; Gutiérrez, J.A.; Mouzon, E.; Bobbitt, K.R.; Lemke, N.; Schultz, C.R.; Schultz, L.R.; Golembieski, W.; Koblinski, J.; et al. Splenic and immune alterations of the Sparc-null mouse accompany a lack of immune response. Genes Immun. 2007, 8, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Whittal, M.C.; Molladavoodi, S.; Zwambag, D.P.; Millecamps, M.; Stone, L.S.; Gregory, D.E. Mechanical Consequence of Induced Intervertebral Disc Degeneration in the SPARC-Null Mouse. J. Biomech. Eng. 2021, 143, 024501. [Google Scholar] [CrossRef]
- Gruber, H.E.; Sage, E.H.; Norton, H.J.; Funk, S.; Ingram, J.; Hanley, E.N., Jr. Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J. Histochem. Cytochem. 2005, 53, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Atorrasagasti, C.; Onorato, A.; Gimeno, M.L.; Andreone, L.; Garcia, M.; Malvicini, M.; Fiore, E.; Bayo, J.; Perone, M.J.; Mazzolini, G.D. SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin. Sci. 2019, 133, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Harries, L.W.; McCulloch, L.J.; Holley, J.E.; Rawling, T.J.; Welters, H.J.; Kos, K. A role for SPARC in the moderation of human insulin secretion. PLoS ONE 2013, 8, e68253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; He, F.; Huang, M.; Zhao, Q.; Cheng, L.; Said, N.; Zhou, Z.; Liu, F.; Dai, Y.S. SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Sci. Rep. 2020, 10, 17581. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [Green Version]
- Songsorn, P.; Ruffino, J.; Vollaard, N.B. No effect of acute and chronic supramaximal exercise on circulating levels of the myokine SPARC. Eur. J. Sport Sci. 2017, 17, 447–452. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obese Animals as Models for Numerous Diseases: Advantages and Applications. Medicina 2021, 57, 399. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and cancer: A homeostatic hormone? Cytokine 2020, 127, 154996. [Google Scholar] [CrossRef]
- Aoi, W.; Hirano, N.; Lassiter, D.G.; Björnholm, M.; Chibalin, A.V.; Sakuma, K.; Tanimura, Y.; Mizushima, K.; Takagi, T.; Naito, Y.; et al. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J. 2019, 33, 10551–10562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.M. Muscle growth and exercise. Crit. Rev. Food Sci. Nutr. 1990, 29, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Francaux, M.; Deldicque, L. Exercise and the control of muscle mass in human. Pflug. Arch.-Eur. J. Physiol. 2019, 471, 397–411. [Google Scholar] [CrossRef]
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology 2019, 34, 56–70. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and inflammation: Another homeostatic property? Cytokine 2020, 133, 155179. [Google Scholar] [CrossRef] [PubMed]
- Rowlatt, C.; Chesterman, F.C.; Sheriff, M.U. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab. Anim. 1976, 10, 419–442. [Google Scholar] [CrossRef] [PubMed]
- Kunstyr, I.; Leuenberger, H.G. Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. J. Gerontol. 1975, 30, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Mouisel, E.; Roblot, N.; Melki, J. Inter- and intrastrain variation in mouse critical running speed. J. Appl. Physiol. 2005, 98, 1258–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]
- Teklad Global 18% Protein Rodent Diet (Sterilizable). Available online: https://insights.envigo.com/hubfs/resources/data-sheets/2018s-datasheet-0915.pdf (accessed on 13 December 2020).
- Norose, K.; Lo, W.K.; Clark, J.I.; Sage, E.H.; Howe, C.C. Lenses of SPARC-null mice exhibit an abnormal cell surface-basement membrane interface. Exp. Eye Res. 2000, 71, 295–307. [Google Scholar] [CrossRef]
- Nishida, Y.; Tokuyama, K.; Nagasaka, S.; Higaki, Y.; Shirai, Y.; Kiyonaga, A.; Shindo, M.; Kusaka, I.; Nakamura, T.; Ishibashi, S.; et al. Effect of moderate exercise training on peripheral glucose effectiveness, insulin sensitivity, and endogenous glucose production in healthy humans estimated by a two-compartment-labeled minimal model. Diabetes 2004, 53, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Shindo, M. The benefits of the low intensity training. Ann. Physiol. Anthr. 1992, 11, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, M.; Sunami, Y.; Kinoshita, F.; Kiyonaga, A.; Tanaka, H.; Shindo, M.; Irie, T.; Urata, H.; Sasaki, J.; Arakawa, K. Blood pressure lowering effect of low intensity aerobic training in elderly hypertensive patients. Med. Sci. Sports Exerc. 1998, 30, 818–823. [Google Scholar] [CrossRef]
- Sunami, Y.; Motoyama, M.; Kinoshita, F.; Mizooka, Y.; Sueta, K.; Matsunaga, A.; Sasaki, J.; Tanaka, H.; Shindo, M. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism 1999, 48, 984–988. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Moriguch, Y.; Mitani, T.; Aoyama, T.; Arai, H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Shimizu, Y.; Matsuo, Y.; Otaru, T.; Kanzawa, Y.; Miyamae, N.; Yamada, E.; Katsuno, T. Effects of exercise intensity and duration on a myokine, secreted protein acidic and rich in cysteine. Eur. J. Sport Sci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Potteiger, J.A.; Jacobsen, D.J.; Donnelly, J.E. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.C.; Taylor, A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [CrossRef] [Green Version]
- De Giorgio, M.R.; Yoshioka, M.; Riedl, I.; Moreault, O.; Cherizol, R.G.; Shah, A.A.; Blin, N.; Richard, D.; St-Amand, J. Trefoil factor family member 2 (Tff2) KO mice are protected from high-fat diet-induced obesity. Obesity 2013, 21, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cyagen.com/us/en/service/piggybac-transgenic-mouse.html (accessed on 15 December 2020).
- Luu-The, V.; Paquet, N.; Calvo, E.; Cumps, J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. BioTechniques 2005, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Hicks, P.S.; Ortiz, L.R.; Martinez, M.J.; Mandler, R.N. Quantitative measurement of muscle strength in the mouse. J. Neurosci. Methods 1995, 62, 15–19. [Google Scholar] [CrossRef]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef]
- Capaldi, R.A.; Malatesta, F.; Darley-Usmar, V.M. Structure of cytochrome c oxidase. Biochim. Biophys. Acta 1983, 726, 135–148. [Google Scholar] [CrossRef]
- García-Horsman, J.A.; Barquera, B.; Rumbley, J.; Ma, J.; Gennis, R.B. The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 1994, 176, 5587–5600. [Google Scholar] [CrossRef] [Green Version]
- Saraste, M. Structure and evolution of cytochrome oxidase. Antonie Leeuwenhoek 1994, 65, 285–287. [Google Scholar] [CrossRef]
- Azzi, A. Cytochrome c oxidase. Towards a clarification of its structure, interactions and mechanism. Biochim. Biophys. Acta 1980, 594, 231–252. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/gene/17709 (accessed on 16 January 2022).
- Dittmer, A.; Dittmer, J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis 2006, 27, 2844–2845. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.M.; Dang, Q.; Lin, C.J.; Lo, U.G.; Feldkoren, B.; Dang, A.; Hernandez, E.; Li, F.; Panwar, V.; Lee, C.F.; et al. SPARC is a key mediator of TGF-β-induced renal cancer metastasis. J. Cell. Physiol. 2021, 236, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Wei, K.; Jacobs, S.S.; Upadhyay, D.; Weill, D.; Rosen, G.D. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J. Biol. Chem. 2010, 285, 8196–8206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Zhao, Y.; Yuan, L.; Yi, W.; Zhao, R.; Yi, Q.; Yong, T. SPARC is over-expressed in adipose tissues of diet-induced obese rats and causes insulin resistance in 3T3-L1 adipocytes. Acta Histochem. 2014, 116, 158–166. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Yang, M.; Liu, H.; Yang, G. Elevated plasma levels of SPARC in patients with newly diagnosed type 2 diabetes mellitus. Eur. J. Endocrinol. 2011, 165, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Yiu, G.K.; Chan, W.Y.; Ng, S.W.; Chan, P.S.; Cheung, K.K.; Berkowitz, R.S.; Mok, S.C. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am. J. Pathol. 2001, 159, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Nakano, S.; Miyoshi, T.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging 2012, 4, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Chang, B.; Traktuev, D.O.; Sun, J.; March, K.; Chan, L.; Sage, E.H.; Pasqualini, R.; Arap, W.; Kolonin, M.G. IFATS collection: Combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells 2008, 26, 2735–2745. [Google Scholar] [CrossRef] [Green Version]
- Jacunski, M.; Melville, P.; Currie, G.P. Exercise: The panacea in management of many ills. Now is the time to engage. J. R. Coll. Physicians Edinb. 2021, 51, 120–122. [Google Scholar] [CrossRef]
| Young | Old | 3-Way ANOVA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild-Type | Sparc Knockout | Wild-Type | Sparc Knockout | |||||||||||
| Sedentary | Exercise | Sedentary | Exercise | Sedentary | Exercise | Sedentary | Exercise | Age | Genotype | Exercise | Age × Genotype | Genotype × Exercise | ||
| Achilles tendon | mg | 4.3 ± 0.6 | 4.0 ± 0.2 | 3.1 ± 0.2 | 2.8 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.4 | 4.8 ± 0.2 | 5.1 ± 0.5 | O ˃ Y | WT ˃ KO | - | WT: O > Y *, KO: O >> Y | - |
| % | 0.015 ± 0.002 | 0.014 ± 0.001 | 0.011 ± 0.001 | 0.011 ± 0.001 | 0.013 ± 0.001 | 0.014 ± 0.001 | 0.016 ± 0.001 | 0.017 ± 0.002 | - | - | - | KO: O >> Y | - | |
| Brown adipose tissue | mg | 93 ± 13 | 103 ± 16 | 103 ± 8 | 130 ± 33 | 159 ± 27 | 142 ± 13 | 135 ± 12 | 192 ± 32 | O ˃ Y | - | - | - | - |
| % | 0.31 ± 0.04 | 0.35 ± 0.05 | 0.37 ± 0.03 | 0.50 ± 0.10 | 0.41 ± 0.04 | 0.40 ± 0.03 | 0.42 ± 0.02 | 0.63 ± 0.10 | O ˃ Y * | KO ˃ WT | Ex > Sed | - | Ex: KO > WT, | |
| Inguinal adipose tissue | mg | 19 ± 4 | 16 ± 4 | 18 ± 6 | 17 ± 3 | 41 ± 11 | 23 ± 1 | 22 ± 3 | 21 ± 2 | O ˃ Y | - | - | - | - |
| % | 0.065 ± 0.014 | 0.054 ± 0.014 | 0.063 ± 0.023 | 0.068 ± 0.015 | 0.100 ± 0.020 | 0.065 ± 0.004 | 0.069 ± 0.008 | 0.069 ± 0.007 | - | - | - | - | - | |
| µm2 | 1076 ± 109 | 1020 ± 126 | 1157 ± 87 | 1201 ± 102 | 3335 ± 646 | 3262 ± 542 | 2949 ± 613 | 1834 ± 215 | O ˃ Y | - | - | WT: O > Y | - | |
| N | 844 ± 140 | 471 ± 134 | 793 ± 206 | 411 ± 173 | 616 ± 144 | 332 ± 67 | 619 ± 161 | 578 ± 130 | Y > O | - | - | - | - | |
| Abdominal adipose tissue | mg | 982 ± 204 | 781 ± 126 | 919 ± 133 | 651 ± 125 | 2831 ± 347 | 2603 ± 175 | 1760 ± 221 | 1463 ± 162 | O ˃ Y | WT ˃ KO | Sed > Ex * | WT: O >> Y, KO: O > Y | - |
| % | 3.23 ± 0.58 | 2.61 ± 0.36 | 3.24 ± 0.43 | 2.49 ± 0.38 | 7.32 ± 0.63 | 7.31 ± 0.33 | 5.38 ± 0.49 | 4.71 ± 0.43 | O ˃ Y | WT ˃ KO | - | WT: O >> Y, KO: O > Y | - | |
| Retroperitoneal adipose tissue | mg | 177 ± 38 | 125 ± 19 | 182 ± 34 | 119 ± 23 | 435 ± 40 | 410 ± 27 | 247 ± 30 | 230 ± 25 | O ˃ Y | WT ˃ KO | Sed > Ex * | WT: O >> Y, KO: O > Y | - |
| % | 0.58 ± 0.11 | 0.42 ± 0.05 | 0.64 ± 0.11 | 0.46 ± 0.07 | 1.15 ± 0.08 | 1.15 ± 0.05 | 0.76 ± 0.07 | 0.74 ± 0.07 | O ˃ Y | WT ˃ KO | - | WT: O >> Y, KO: O > Y | - | |
| Epididymal adipose tissue | mg | 305 ± 67 | 246 ± 42 | 293 ± 41 | 205 ± 39 | 870 ± 101 | 838 ± 54 | 556 ± 70 | 502 ± 58 | O ˃ Y | WT ˃ KO | - | WT: O >> Y, KO: O > Y | - |
| % | 1.00 ± 0.19 | 0.82 ± 0.12 | 1.03 ± 0.13 | 0.78 ± 0.12 | 2.27 ± 0.21 | 2.36 ± 0.10 | 1.71 ± 0.17 | 1.61 ± 0.16 | O ˃ Y | WT ˃ KO | - | WT: O >> Y, KO: O > Y | - | |
| µm2 | 2501 ± 196 | 2417 ± 226 | 3007 ± 230 | 2456 ± 255 | 4739 ± 635 | 4722 ± 512 | 4686 ± 562 | 4115 ± 287 | O ˃ Y | - | - | - | - | |
| N | 3397 ± 437 | 7037 ± 2648 | 2924 ± 291 | 6037 ± 2164 | 2553 ± 250 | 2856 ± 463 | 2392 ± 219 | 2805 ± 314 | O > Y | WT > KO | - | WT: O > Y | - | |
| Mesenteric adipose tissue | mg | 195 ± 35 | 164 ± 25 | 152 ± 21 | 122 ± 25 | 656 ± 126 | 518 ± 46 | 400 ± 63 | 230 ± 31 | O ˃ Y | WT ˃ KO | Sed > Ex | WT: O >> Y, KO: O > Y | - |
| % | 0.64 ± 0.10 | 0.55 ± 0.07 | 0.54 ± 0.07 | 0.46 ± 0.08 | 1.63 ± 0.22 | 1.44 ± 0.10 | 1.19 ± 0.14 | 0.74 ± 0.09 | O ˃ Y | WT ˃ KO | Sed > Ex | WT: O >> Y, KO: O > Y | - | |
| Gastrocnemius | mg | 325 ± 9 | 316 ± 10 | 258 ± 8 | 244 ± 7 | 308 ± 7 | 307 ± 4 | 242 ± 5 | 237 ± 6 | Y ˃ O | WT ˃ KO | - | - | - |
| % | 1.11 ± 0.04 | 1.10 ± 0.03 | 0.93 ± 0.04 | 0.99 ± 0.04 | 0.84 ± 0.04 | 0.88 ± 0.03 | 0.80 ± 0.04 | 0.78 ± 0.02 | Y ˃ O | WT ˃ KO | - | - | - | |
| Soleus | mg | 11.5 ± 1.8 | 9.9 ± 0.2 | 9.1 ± 0.2 | 9.0 ± 0.4 | 10.7 ± 0.3 | 10.7 ± 0.3 | 9.0 ± 0.6 | 10.0 ± 0.6 | - | WT ˃ KO | - | - | - |
| % | 0.039 ± 0.005 | 0.034 ± 0.001 | 0.033 ± 0.001 | 0.036 ± 0.001 | 0.029 ± 0.001 | 0.031 ± 0.001 | 0.029 ± 0.001 | 0.033 ± 0.002 | Y ˃ O | - | - | - | Sed: WT > KO, Ex: KO > WT | |
| Extensor digitorum longus | mg | 25 ± 2 | 25 ± 2 | 20 ± 2 | 19 ± 1 | 23 ± 2 | 27 ± 1 | 23 ± 1 | 21 ± 1 | - | WT ˃ KO | - | - | - |
| % | 0.086 ± 0.005 | 0.089 ± 0.007 | 0.070 ± 0.007 | 0.075 ± 0.006 | 0.065 ± 0.004 | 0.078 ± 0.003 | 0.078 ± 0.007 | 0.069 ± 0.003 | Y > O * | - | - | WT: Y > O | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites 2022, 12, 125. https://doi.org/10.3390/metabo12020125
Ghanemi A, Melouane A, Yoshioka M, St-Amand J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites. 2022; 12(2):125. https://doi.org/10.3390/metabo12020125
Chicago/Turabian StyleGhanemi, Abdelaziz, Aicha Melouane, Mayumi Yoshioka, and Jonny St-Amand. 2022. "Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice" Metabolites 12, no. 2: 125. https://doi.org/10.3390/metabo12020125
APA StyleGhanemi, A., Melouane, A., Yoshioka, M., & St-Amand, J. (2022). Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites, 12(2), 125. https://doi.org/10.3390/metabo12020125








