Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review
Abstract
:1. Introduction
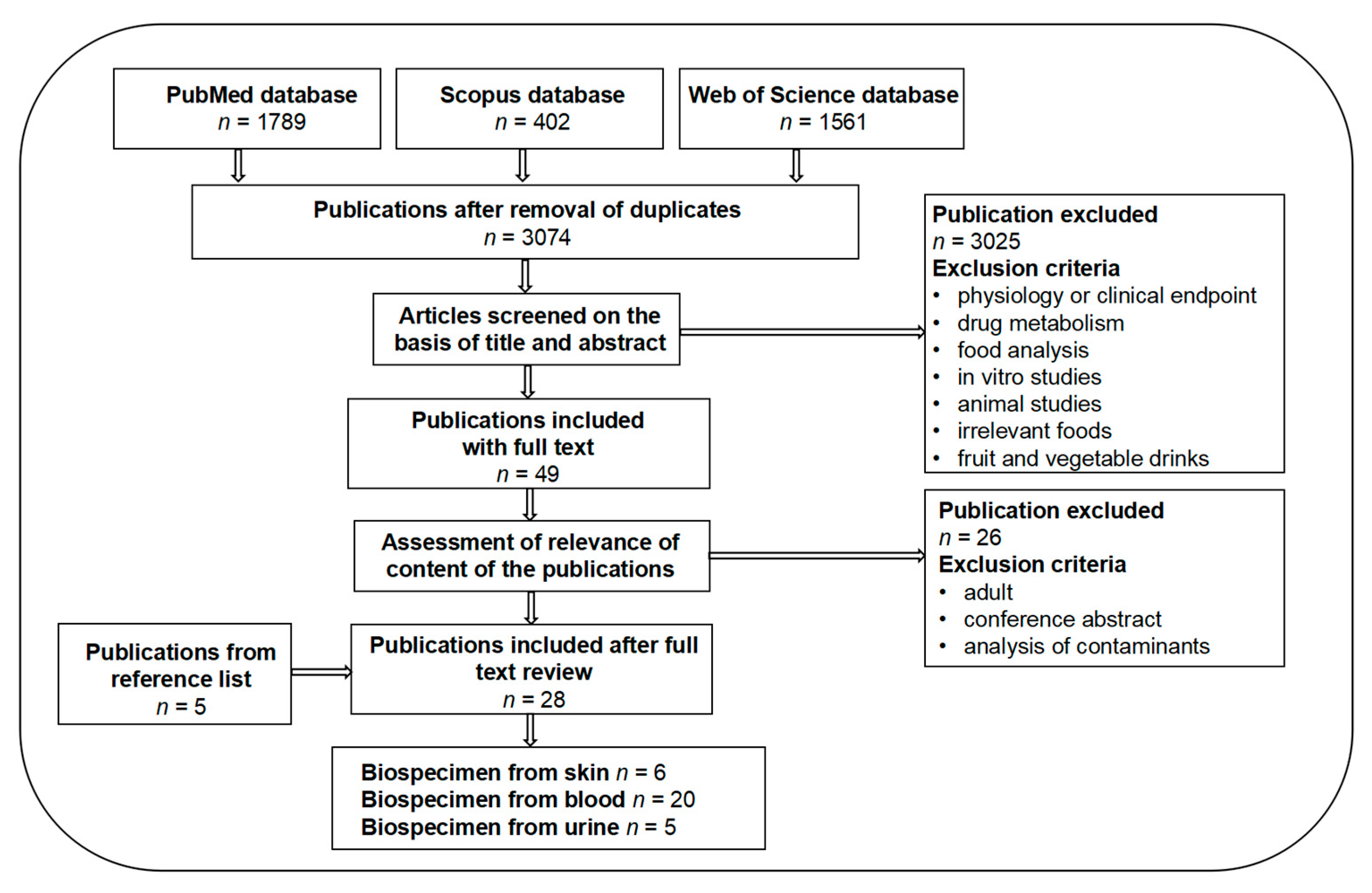
2. Methods
2.1. Selection of Food Groups
2.2. Literature Search for Biomarkers of Fruit and Vegetables Intake (BFVI) in Children and Adolescents
2.3. Identification and Characterization of Candidate BFVI
2.4. Evaluation of the Validation of Candidate BFVI
2.5. Risk of Bias and Quality of Study Assessment
3. Results
3.1. Skin Biomarkers
3.2. Blood Biomarkers
3.3. Urinary Biomarkers
3.4. Evaluation of Level of Validation of Candidate Biomarkers
3.5. Risk of Bias and Quality of Study Assessment
4. Discussion
4.1. Total FV Intake Biomarkers
4.2. Candidate Biomarkers for Intake of Specific Fruit and Vegetable
5. Strengths and Limitations
6. The Way Forward
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Lock, K.; Pomerleau, J.; Causer, L.; Altmann, D.R.; McKee, M. The global burden of disease attributable to low consumption of fruit and vegetables: Implications for the global strategy on diet. Bull. World Health Organ. 2005, 83, 100–108. [Google Scholar] [PubMed]
- Jääskeläinen, P.; Magnussen, C.; Pahkala, K.; Mikkilä, V.; Kähönen, M.; Sabin, M.; Fogelholm, M.; Hutri-Kähönen, N.; Taittonen, L.; Telama, R.; et al. Childhood Nutrition in Predicting Metabolic Syndrome in Adults: The Cardiovascular Risk in Young Finns Study. Diabetes Care 2012, 35, 1937–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasuk, V.S.; Brooker, A.-S. Interpreting epidemiologic studies of diet-disease relationships. J. Nutr. 1997, 127, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Robson, P.J.; Wallace, J.M. Issues in dietary intake assessment of children and adolescents. Br. J. Nutr. 2004, 92, S213–S222. [Google Scholar] [CrossRef]
- Smith, K.E. Who’s Minding the Kids?: Child Care Arrangements, Spring 1997; US Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2002.
- Iannotti, R.J.; Zuckerman, A.E.; Blyer, E.M.; O’Brien, R.W.; Finn, J.; Spillman, D.M. Comparison of Dietary Intake Methods with Young Children. Psychol. Rep. 1994, 74, 883–889. [Google Scholar] [CrossRef]
- Livingstone, M.B.E.; Robson, P.J. Measurement of dietary intake in children. Proc. Nutr. Soc. 2000, 59, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.K.; Newton, R.L., Jr.; Anton, S.D.; Allen, H.R.; Alfonso, A.; Han, H.; Stewart, T.; Sothern, M.; Willamson, D. Measurement of children’s food intake with digital photography and the effects of second servings upon food intake. Eat. Behav. 2007, 8, 148–156. [Google Scholar] [CrossRef]
- Baldrick, F.R.; Woodside, J.V.; Elborn, J.S.; Young, I.S.; McKinley, M.C. Biomarkers of Fruit and Vegetable Intake in Human Intervention Studies: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 795–815. [Google Scholar] [CrossRef]
- Couillard, C.; Lemieux, S.; Vohl, M.-C.; Couture, P.; Lamarche, B. Carotenoids as biomarkers of fruit and vegetable intake in men and women. Br. J. Nutr. 2016, 116, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Margalef, M.; Carres, L.I.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Age related differences in the plasma kinetics of flavanols in rats. J. Nutr. Biochem. 2016, 29, 90–96. [Google Scholar] [CrossRef]
- Agans, R.; Rigsbee, L.; Kenche, H.; Michail, S.; Khamis, H.J.; Paliy, O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011, 77, 404–412. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Cheng, J.; Ringel, Y.; Salojärvi, J.; Carroll, I.; Palva, A.; De Vos, W.M.; Satokari, R. Intestinal Microbiota in Healthy U.S. Young Children and Adults—A High Throughput Microarray Analysis. PLoS ONE 2013, 8, e64315. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; Van Der Wal, P.; Jong, J.C.K.-D.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.; Jaddoe, V.W.V.; et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beydoun, M.A.; Wang, Y. Parent–child dietary intake resemblance in the United States: Evidence from a large representative survey. Soc. Sci. Med. 2009, 68, 2137–2144. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Beydoun, M.A.; Li, J.; Liu, Y.; Moreno, L.A. Do children and their parents eat a similar diet? Resemblance in child and parental dietary intake: Systematic review and meta-analysis. J. Epidemiol. Community Health 2011, 65, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2008, 53, S194–S218. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Praticò, G.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Pedapati, S.H.; Afman, L.A.; Wishart, D.S.; et al. Guidelines for Biomarker of Food Intake Reviews (BFIRev): How to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Wirsching, J.; Graßmann, S.; Eichelmann, F.; Harms, L.M.; Schenk, M.; Barth, E.; Berndzen, A.; Olalekan, M.; Sarmini, L.; Zuberer, H.; et al. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS). BMC Med. Res. Methodol. 2018, 18, 122. [Google Scholar] [CrossRef]
- Lau, C.-H.E.; Siskos, A.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T.; et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, S.S.; Wengreen, H.J.; Lefevre, M.; Madden, G.J.; Gast, J. Skin Carotenoids: A Biomarker of Fruit and Vegetable Intake in Children. J. Acad. Nutr. Diet. 2014, 114, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Henebery, K.; Peracchio, H.; Cartmel, B.; Lin, H.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Duffy, V.B.; Mayne, S.T. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur. J. Clin. Nutr. 2012, 66, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguin-Fowler, R.A.; Hanson, K.L.; Marshall, G.A.; Belarmino, E.H.; Jilcott Pitts, S.B.; Kolodinsky, J.; Sitaker, M.; Ammerman, A. Fruit and Vegetable Intake Assessed by Repeat 24 h Recalls, but Not by A Dietary Screener, Is Associated with Skin Carotenoid Measurements in Children. Nutrients 2021, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch. Biochem. Biophys. 2015, 572, 73–80. [Google Scholar] [CrossRef]
- Whiteside-Mansell, L.; Swindle, T.; Davenport, K. Evaluation of “Together, We Inspire Smart Eating” (WISE) Nutrition Intervention for Young Children: Assessment of Fruit and Vegetable Consumption with Parent Reports and Measurements of Skin Carotenoids as Biomarkers. J. Hunger Environ. Nutr. 2019, 16, 235–245. [Google Scholar] [CrossRef]
- Martinelli, S.; Acciai, F.; Tasevska, N.; Ohri-Vachaspati, P. Using the Veggie Meter in Elementary Schools to Objectively Measure Fruit and Vegetable Intake: A Pilot Study. Methods Protoc. 2021, 4, 33. [Google Scholar] [CrossRef]
- Biltoft-Jensen, A.P.; Bysted, A.; Trolle, E.; Christensen, T.; Knuthsen, P.; Damsgaard, C.T.; Andersen, L.F.; Brockhoff, P.B.; Tetens, I. Evaluation of Web-based Dietary Assessment Software for Children: Comparing reported fruit, juice and vegetable intakes with plasma carotenoid concentration and school lunch observations. Br. J. Nutr. 2012, 110, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Byers, T.; Treiber, F.; Gunter, E.; Coates, R.; Sowell, A.; Leonard, S.; Mokdad, A.; Jewell, S.; Miller, D.; Serdula, M.; et al. The accuracy of parental reports of their children’s intake of fruits and vegetables: Validation of a food frequency questionnaire with serum levels of carotenoids and vitamins C, A, and E. Epidemiology 1993, 350–355. [Google Scholar] [CrossRef]
- Notario-Barandiaran, L.; Navarrete-Muñoz, E.-M.; Valera-Gran, D.; Hernández-Álvarez, E.; Donoso-Navarro, E.; González-Palacios, S.; García-De-La-Hera, M.; Fernández, M.; Freire, C.; Vioque, J. Biochemical Validation of a Self-Administered Food Frequency Questionnaire to Assess Diet Using Carotenoids and Vitamins E and D in Male Adolescents in Spain. Antioxidants 2021, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Rock, C.L.; Eldridge, A.L.; Kristal, A.R.; Patterson, R.E.; Cooper, D.A.; Neumark-Sztainer, D.; Cheskin, L.J.; Thornquist, M.D. Serum concentrations of retinol, α-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J. Nutr. 2001, 131, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrows, T.L.; Warren, J.M.; Colyvas, K.; Garg, M.L.; Collins, C.E. Validation of Overweight Children’s Fruit and Vegetable Intake Using Plasma Carotenoids. Obesity 2008, 17, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hillesheim, E.; Toffano, R.B.D.; de Barros, T.T.; Salomão, R.G.; Mathias, M.G.; Coelho-Landell, C.D.A.; Almada, M.O.R.D.V.; Camarneiro, J.M.; Camelo-Junior, J.S.; Ued, F.D.V.; et al. Biomarker-based validity of a food frequency questionnaire estimating intake in Brazilian children and adolescents. Int. J. Food Sci. Nutr. 2020, 72, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Sasaki, S.; Bando, N.; Hashimoto, M.; Kunitsugu, I.; Sugiyama, S.; Terao, J.; Hobara, T. Carotenoid, Tocopherol, and Fatty Acid Biomarkers and Dietary Intake Estimated by Using a Brief Self-Administered Diet History Questionnaire for Older Japanese Children and Adolescents. J. Nutr. Sci. Vitaminol. 2009, 55, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Slater, B.; Enes, C.C.; López, R.V.M.; Damasceno, N.R.T.; Voci, S.M. Validation of a food frequency questionnaire to assess the consumption of carotenoids, fruits and vegetables among adolescents: The method of triads. Cad. Saúde Pública 2010, 26, 2090–2100. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Takkinen, H.-M.; Uusitalo, L.; Tapanainen, H.; Ovaskainen, M.-L.; Alfthan, G.; Erlund, I.; Ahonen, S.; Åkerlund, M.; Toppari, J.; et al. Carotenoid Intake and Serum Concentration in Young Finnish Children and Their Relation with Fruit and Vegetable Consumption. Nutrients 2018, 10, 1533. [Google Scholar] [CrossRef] [Green Version]
- Irwig, M.; El-Sohemy, A.; Baylin, A.; Rifai, N.; Campos, H. Frequent Intake of Tropical Fruits That Are Rich in β-Cryptoxanthin Is Associated with Higher Plasma β-Cryptoxanthin Concentrations in Costa Rican Adolescents. J. Nutr. 2002, 132, 3161–3167. [Google Scholar] [CrossRef] [Green Version]
- Vuong, L.T.; Dueker, S.R.; Murphy, S.P. Plasma β-carotene and retinol concentrations of children increase after a 30-d supplementation with the fruit Momordica cochinchinensis (gac). Am. J. Clin. Nutr. 2002, 75, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Lala, V.R.; Reddy, V. Absorption of β-Carotene from Green Leafy Vegetables in Undernourished Children. Am. J. Clin. Nutr. 1970, 23, 110–113. [Google Scholar] [CrossRef]
- Nawiri, M.P.; Nyambaka, H.; Murungi, J.I. Sun-dried cowpeas and amaranth leaves recipe improves beta-carotene and retinol levels in serum and hemoglobin concentration among preschool children. Eur. J. Nutr. 2012, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Vandevijvere, S.; Geelen, A.; Gonzalez-Gross, M.; Van’t Veer, P.; Dallongeville, J.; Mouratidou, T.; Dekkers, A.; Börnhorst, C.; Breidenassel, C.; Crispim, S.P.; et al. Evaluation of food and nutrient intake assessment using concentration biomarkers in European adolescents from the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br. J. Nutr. 2012, 109, 736–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, A.P.; Vally, H.; Morris, P.; Daniel, M.; Esterman, A.; Karschimkus, C.S.; O’Dea, K. Nutritional impacts of a fruit and vegetable subsidy programme for disadvantaged Australian Aboriginal children. Br. J. Nutr. 2013, 110, 2309–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collese, T.S.; De Moraes, A.C.F.; Rendo-Urteaga, T.; Luzia, L.A.; Rondó, P.H.D.C.; Marchioni, D.M.L.; Carvalho, H.B. The Validity of Children’s Fruit and Vegetable Intake Using Plasma Vitamins A, C, and E: The SAYCARE Study. Nutrients 2019, 11, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pee, S.; West, C.E.; Permaesih, D.; Martuti, S.; Muhilal; Hautvast, J.G. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am. J. Clin. Nutr. 1998, 68, 1058–1067. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Riley, M.D.; Whiting, S. Association between urinary potassium, urinary sodium, current diet, and bone density in prepubertal children. Am. J. Clin. Nutr. 2001, 73, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Barfoot, K.L.; Istas, G.; Feliciano, R.P.; Lamport, D.J.; Riddell, P.; Rodriguez-Mateos, A.; Williams, C.M. Effects of daily consumption of wild blueberry on cognition and urinary metabolites in school-aged children: A pilot study. Eur. J. Nutr. 2021, 60, 4263–4278. [Google Scholar] [CrossRef]
- Krupp, D.; Doberstein, N.; Shi, L.; Remer, T. Hippuric Acid in 24-Hour Urine Collections Is a Potential Biomarker for Fruit and Vegetable Consumption in Healthy Children and Adolescents. J. Nutr. 2012, 142, 1314–1320. [Google Scholar] [CrossRef] [Green Version]
- Penczynski, K.J.; Krupp, D.; Bring, A.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relative validation of 24-hour urinary hippuric acid excretion as a biomarker for dietary flavonoid intake from fruit and vegetables in healthy adolescents. Eur. J. Nutr. 2015, 56, 757–766. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. Int. Rev. J. 2014, 5, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Iglesia, I.; Mouratidou, T.; Gonzalez-Gross, M.; Huybrechts, I.; Breidenassel, C.; Santabárbara, J.; Díaz, L.-E.; Hällström, L.; De Henauw, S.; Gottrand, F.; et al. Foods contributing to vitamin B(6), folate, and vitamin B(12) intakes and biomarkers status in European adolescents: The HELENA study. Eur. J. Nutr. 2015, 56, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Van Horn, L.; Tinker, L.F.; Neuhouser, M.L.; Carbone, L.; Mossavar-Rahmani, Y.; Thomas, F.; Prentice, R.L. Measurement error corrected sodium and potassium intake estimation using 24-h urinary excretion. Hypertension 2014, 63, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled Results From 5 Validation Studies of Dietary Self-Report Instruments Using Recovery Biomarkers for Potassium and Sodium Intake. Am. J. Epidemiol. 2015, 181, 473–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Lees, H.J.; Swann, J.R.; Wilson, I.D.; Nicholson, J.K.; Holmes, E. Hippurate: The Natural History of a Mammalian–Microbial Cometabolite. J. Proteome Res. 2013, 12, 1527–1546. [Google Scholar] [CrossRef]
- Pero, R.W. Health Consequences of Catabolic Synthesis of Hippuric Acid in Humans. Curr. Clin. Pharmacol. 2010, 5, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Kay, C.D.; Clifford, M.N.; Crozier, A. Flavanones. Dietary Polyphenols: Their Metabolism and Health Effects; Wiley: Hoboken, NJ, USA, 2020; pp. 439–495. [Google Scholar]
- Heinzmann, S.S.; Brown, I.J.; Chan, Q.; Bictash, M.; Dumas, M.-E.; Kochhar, S.; Stamler, J.; Holmes, E.; Elliott, P.; Nicholson, J.K. Metabolic profiling strategy for discovery of nutritional biomarkers: Proline betaine as a marker of citrus consumption. Am. J. Clin. Nutr. 2010, 92, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Posma, J.M.; Garcia-Perez, I.; Heaton, J.C.; Burdisso, P.; Mathers, J.C.; Draper, J.; Lewis, M.; Lindon, J.C.; Frost, G.; Holmes, E.; et al. Integrated Analytical and Statistical Two-Dimensional Spectroscopy Strategy for Metabolite Identification: Application to Dietary Biomarkers. Anal. Chem. 2017, 89, 3300–3309. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.D. Nδ-acetylornithine and S-methylcysteine in blood plasma. Biochim. Biophys. Acta (BBA) Gen. Subj. 1979, 587, 638–642. [Google Scholar] [CrossRef]
- Groenen, P.M.; Merkus, H.M.; Sweep, F.C.; Wevers, R.A.; Janssen, F.S.; Steegers-Theunissen, R.P. Kinetics of myo-inositol loading in women of reproductive age. Ann. Clin. Biochem. Int. J. Lab. Med. 2003, 40, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.D.; Pitts, S.J.; Jahns, L.; Firnhaber, G.C.; Loofbourrow, B.M.; Zeng, A.; Scherr, R.E. Criterion-Related Validity of Spectroscopy-Based Skin Carotenoid Measurements as a Proxy for Fruit and Vegetable Intake: A Systematic Review. Adv. Nutr. 2020, 11, 1282–1299. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef]
- Parker, R.S.; Swanson, J.E.; You, C.-S.; Edwards, A.J.; Huang, T. Bioavailability of carotenoids in human subjects. Proc. Nutr. Soc. 1999, 58, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Umbreen, H.; Zia-Ul-Haq, M. Carotenoids and Skin Diseases. In Carotenoids: Structure and Function in the Human Body; Springer: Cham, Switzerland, 2021; p. 721. [Google Scholar]
- Darvin, M.E.; Fluhr, J.W.; Caspers, P.; Van Der Pool, A.; Richter, H.; Patzelt, A.; Sterry, W.; Lademann, J. In vivo distribution of carotenoids in different anatomical locations of human skin: Comparative assessment with two different Raman spectroscopy methods. Exp. Dermatol. 2009, 18, 1060–1063. [Google Scholar] [CrossRef]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in human skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in human skin. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158588. [Google Scholar] [CrossRef]
- Pennant, M.; Steur, M.; Moore, C.; Butterworth, A.; Johnson, L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Tasevska, N.; Runswick, S.A.; Bingham, S.A. Urinary Potassium Is as Reliable as Urinary Nitrogen for Use as a Recovery Biomarker in Dietary Studies of Free Living Individuals. J. Nutr. 2006, 136, 1334–1340. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Estévez-Santiago, R. Dietary beta-Cryptoxanthin and alpha-Carotene Have Greater Apparent Bioavailability Than beta-Carotene in Subjects from Countries with Different Dietary Patterns. Nutrients 2020, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; Manach, C. Biomarkers of intake for tropical fruits. Genes Nutr. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Directive 2008/128/EC of 22 December 2008 laying down specific purity criteria concerning colours for use in foodstuffs (codified version). Off. J. Eur. Union 2009, 6, 20–62. [Google Scholar]
- Authority, E.F.S. Use of Lycopene as a food colour-Scientific Opinion of the Panel on Food additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 6, 674. [Google Scholar]
- Authority, E.F.S. Revised exposure assessment for lycopene as a food colour. EFSA J. 2010, 8, 1444. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef]
- Ogata, M.; Taguchi, T. Simultaneous determination of urinary creatinine and metabolites of toluene, xylene, styrene, ethylbenzene and phenol by automated high performance liquid chromatography. Int. Arch. Occup. Environ. Health 1988, 61, 131–140. [Google Scholar] [CrossRef]
- Tomokuni, K.; Ogata, M. Direct Colorimetric Determination of Hippuric Acid in Urine. Clin. Chem. 1972, 18, 349–351. [Google Scholar] [CrossRef]
- Pujos-Guillot, E.; Hubert, J.; Martin, J.-F.; Lyan, B.; Quintana, M.; Claude, S.; Chabanas, B.; Rothwell, J.; Bennetau-Pelissero, C.; Scalbert, A.; et al. Mass Spectrometry-based Metabolomics for the Discovery of Biomarkers of Fruit and Vegetable Intake: Citrus Fruit as a Case Study. J. Proteome Res. 2013, 12, 1645–1659. [Google Scholar] [CrossRef]
- Lang, R.; Lang, T.; Bader, M.; Beusch, A.; Schlagbauer, V.; Hofmann, T. High-Throughput Quantitation of Proline Betaine in Foods and Suitability as a Valid Biomarker for Citrus Consumption. J. Agric. Food Chem. 2017, 65, 1613–1619. [Google Scholar] [CrossRef]
- Gibbons, H.; Michielsen, C.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; Brennan, L. Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol. Nutr. Food Res. 2017, 61, 1700037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2014, 14, 765–798. [Google Scholar] [CrossRef]
- Evans, L.S.; Tramontano, W.A. Trigonelline and promotion of cell arrest in G2 of various legumes. Phytochemistry 1984, 23, 1837–1840. [Google Scholar] [CrossRef]
- Perera, T.; Young, M.R.; Zhang, Z.; Murphy, G.; Colburn, N.H.; Lanza, E.; Hartman, T.J.; Cross, A.J.; Bobe, G. Identification and monitoring of metabolite markers of dry bean consumption in parallel human and mouse studies. Mol. Nutr. Food Res. 2015, 59, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnard, R.; Narasimhan, B.; Pliskamatyshak, G.; Murthy, P. Characterization of Scyllo-Inositol-Containing Phosphatidylinositol in Plant Cells. Biochem. Biophys. Res. Commun. 1995, 210, 549–555. [Google Scholar] [CrossRef]
- Ichimura, K.; Kohata, K.; Yamaguchi, Y.; Douzono, M.; Ikeda, H.; Koketsu, M. Identification ofL-Inositol and Scyllitol and Their Distribution in Various Organs in Chrysanthemum. Biosci. Biotechnol. Biochem. 2000, 64, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Badmaev, V. Coconut Water and Its the Method of Preparation. 2010. Available online: https://www.researchgate.net/publication/260064797_COCONUT_WATER_AND_ITS_THE_METHOD_OF_PREPARATION (accessed on 10 June 2021).
- Zha, H.; Cai, Y.; Yin, Y.; Wang, Z.; Li, K.; Zhu, Z.-J. SWATHtoMRM: Development of High-Coverage Targeted Metabolomics Method Using SWATH Technology for Biomarker Discovery. Anal. Chem. 2018, 90, 4062–4070. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Dietary Factor | Sample Size | Country | Age (Years) | Study Design | Potential Biomarkers 1 | Primary References |
|---|---|---|---|---|---|---|
| Skin biomarkers | ||||||
| F/V | 45 (20 boys) | USA | 5–17 | Cross-sectional | Total carotenoids | [24] |
| F/V | 381 (193 boys) | USA | 3–5 | Cross-sectional | Total carotenoids | [25] |
| F/V | 177 (83 boys) | USA | 2–12 | Cross-sectional | Total carotenoids | [26] |
| F/V | 166 (62 boys) | USA | 9–12 | Cross-sectional | Total carotenoids | [27] |
| F/V | 374 (N) | NM | NM | Noncontrolled dietary intervention | Total carotenoids | [28] |
| F/V | 143 (68 boys) | USA | 9–11 | Cross-sectional | Total carotenoids | [29] |
| Blood biomarkers | ||||||
| F/V | 1192 (651 boys) | France, Greece, Lithuania, Norway, Spain, UK | 6–11 | Cross-sectional | Acetylornithine | [23] |
| F/V | 45 (20 boys) | USA | 5–17 | Cross-sectional | Total carotenoids | [24] |
| F/V | 166 (62 boys) | USA | 9–12 | Cross-sectional | Total carotenoids | [27] |
| F/V | 81 (34 boys) | Danish | 8–11 | Cross-sectional | α- and β-Carotene, β-cryptoxanthin | [30] |
| F/V | 97 (43 boys) | USA | 6–10 | Cross-sectional | Total carotenoids and vitamin C | [31] |
| F/V | 122 boys | Spain | 15–17 | Cross-sectional | Total carotenoids | [32] |
| F/V | 285 (boys 153) | USA | 12–17 | Cross-sectional | α-Carotene | [33] |
| F/V | 93 (N) | Australia | 5–12 | Cross-sectional | β-Carotene, lycopene α-Carotene, cryptoxanthin | [34] |
| DGOV/green vegetable | 210 (99 boys) | Brazil | 9–13 | Cross-sectional | β-carotene, 5-MTHF | [35] |
| Fruit/green-yellow vegetable | 398 (214 boys) | Japan | 10–11 and 13–14 | Cross-sectional | β-Carotene, cryptoxanthin, | [36] |
| F/V | 80 (23 boys) | Brazil | 13.0 ± 1.1 | Cross-sectional | β-Carotene | [37] |
| Fruit/root vegetable | 207 (129 boys) | Finnish | 1–3 | Cohort study | α- and β-Carotene | [38] |
| Papaya | 159 (81 boys) | Costa Rican | 12–20 | Cross-sectional | β-Cryptoxanthin | [39] |
| Momordica cochinchinensis (gac) | 185 (N) | Vietnam | 2–6 | Controlled dietary intervention | α- and β-Carotene, retinol, lycopene, zeaxanthin | [40] |
| Amaranth | 35 (N) | India | 2–6 | Controlled dietary intervention | Vitamin A | [41] |
| Sun-dried cowpea and amaranth leaves | 152 (N) | Kenya | 2.5–6 | Controlled dietary intervention | β-Carotene, retinol | [42] |
| F/V | 390 (163 boys) | Austria, Belgium, France, Germany, Greece, Hungary, Italy, Spain, and Sweden | 12.5–17.5 | Cross-sectional | Vitamin C, β-carotene | [43] |
| F/V | 174 (82 boys) | Australia | 0–17 | Noncontrolled dietary intervention | β-Cryptoxanthin, lutein–zeaxanthin, vitamin C | [44] |
| F/V | 45 (21 boys) | Brazil | 6–10 | Cross-sectional | Combination of β-carotene, retinol, vitamin C and α-tocopherol | [45] |
| Orange fruit/ dark-green leafy vegetable | 238 (104 boys) | Indonesia | 7–11 | Controlled dietary intervention | Retinol, β-carotene, β-cryptoxanthin, lutein, lycopene | [46] |
| Urinary biomarkers | ||||||
| F/V | 330 (215 boys) | Australia | 8 | Cross-sectional | Potassium | [47] |
| Wild blueberry | 15 (7 boys) | UK | 7–10 | Controlled dietary intervention | Hippuric acid, dihydro caffeic acid 3-O-sulfate | [48] |
| F/V | 240 (120 boys) | Germany | 9–10 and 12–15 | Cross-sectional | Hippuric acid | [49] |
| FlavFV | 287 (48% boys) | Germany | 9–16 | Cross-sectional | Hippuric acid | [50] |
| F/V | 1192 (651 boys) | France, Greece, Lithuania, Norway, Spain, UK | 6–11 | Cross-sectional | Hippurate, proline betaine, NMNA, scyllo-inositol, acetate | [23] |
| Metabolites | HMDB ID | PubChem CID | Biofluid | Specificity | Reason | References |
|---|---|---|---|---|---|---|
| α-Tocopherol | 1893 | 57393415 | Blood | no | Common for many sources | [51] |
| 5-MTHF | 1396 | 135398561 | Blood | no | Common for many sources | [52] |
| Potassium | 586 | 5462222 | Urine | no | Common for many sources | [53,54,55] |
| Acetate | - | 175 | Urine | no | Common for many sources | [56] |
| Hippuric acid | 714 | 464 | Urine | yes | Specific to polyphenolic compounds | [57,58] |
| Dihydro caffeic acid 3-O-sulfate | 41721 | 49844181 | Urine | yes | Combined biomarker | [59] |
| Proline betaine | 4827 | 115244 | Urine | yes | Specific to citrus | [60] |
| NMNA | - | 5571 | Urine | yes | Specific to beans | [61] |
| Acetylornithine | 3357 | 6992102 | Blood | yes | Combined biomarker | [62] |
| Scyllo-Inositol | 6088 | 892 | Urine | yes | Specific to coconut | [63] |
| Substrate | Biomarker | Plausibility | Dose–Response | Time–Response | Robustness | Reliability | Stability | Analytical Performance | Reproducibility | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenolic compounds | Urine | Dihydro caffeic acid 3-O-sulfate | Y | U | U | Y | U | U | U | U |
| Polyphenolic compounds | Urine | Hippuric acid | Y | U | U | U | Y | Y | Y | Y |
| Citrus | Urine | Proline betaine | Y | U | U | U | U | U | Y | U |
| Beans | Urine | NMNA | Y | U | U | U | U | U | Y | U |
| Coconut | Urine | Scyllo-inositol | Y | U | U | U | U | U | Y | U |
| Beans | Serum | Acetylornithine | Y | U | U | U | U | U | Y | U |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Muli, S.; Huybrechts, I.; Nöthlings, U.; Ahrens, W.; Scalbert, A.; Floegel, A. Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review. Metabolites 2022, 12, 126. https://doi.org/10.3390/metabo12020126
Yuan L, Muli S, Huybrechts I, Nöthlings U, Ahrens W, Scalbert A, Floegel A. Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review. Metabolites. 2022; 12(2):126. https://doi.org/10.3390/metabo12020126
Chicago/Turabian StyleYuan, Li, Samuel Muli, Inge Huybrechts, Ute Nöthlings, Wolfgang Ahrens, Augustin Scalbert, and Anna Floegel. 2022. "Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review" Metabolites 12, no. 2: 126. https://doi.org/10.3390/metabo12020126
APA StyleYuan, L., Muli, S., Huybrechts, I., Nöthlings, U., Ahrens, W., Scalbert, A., & Floegel, A. (2022). Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review. Metabolites, 12(2), 126. https://doi.org/10.3390/metabo12020126








