The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells
Abstract
:1. Introduction
2. Results
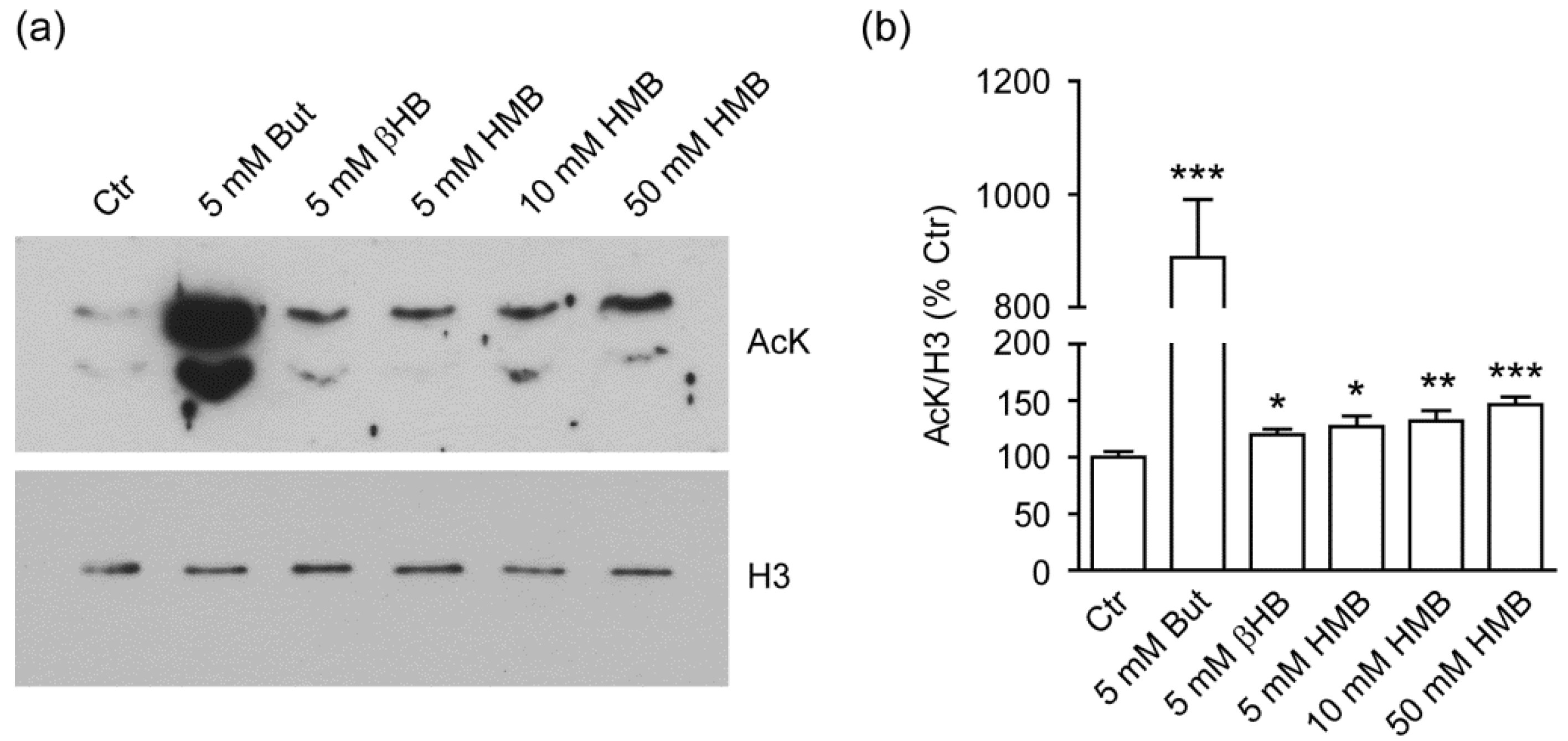
2.1. HMB Increases Global Histone Acetylation in C2C12 Murine Myoblasts
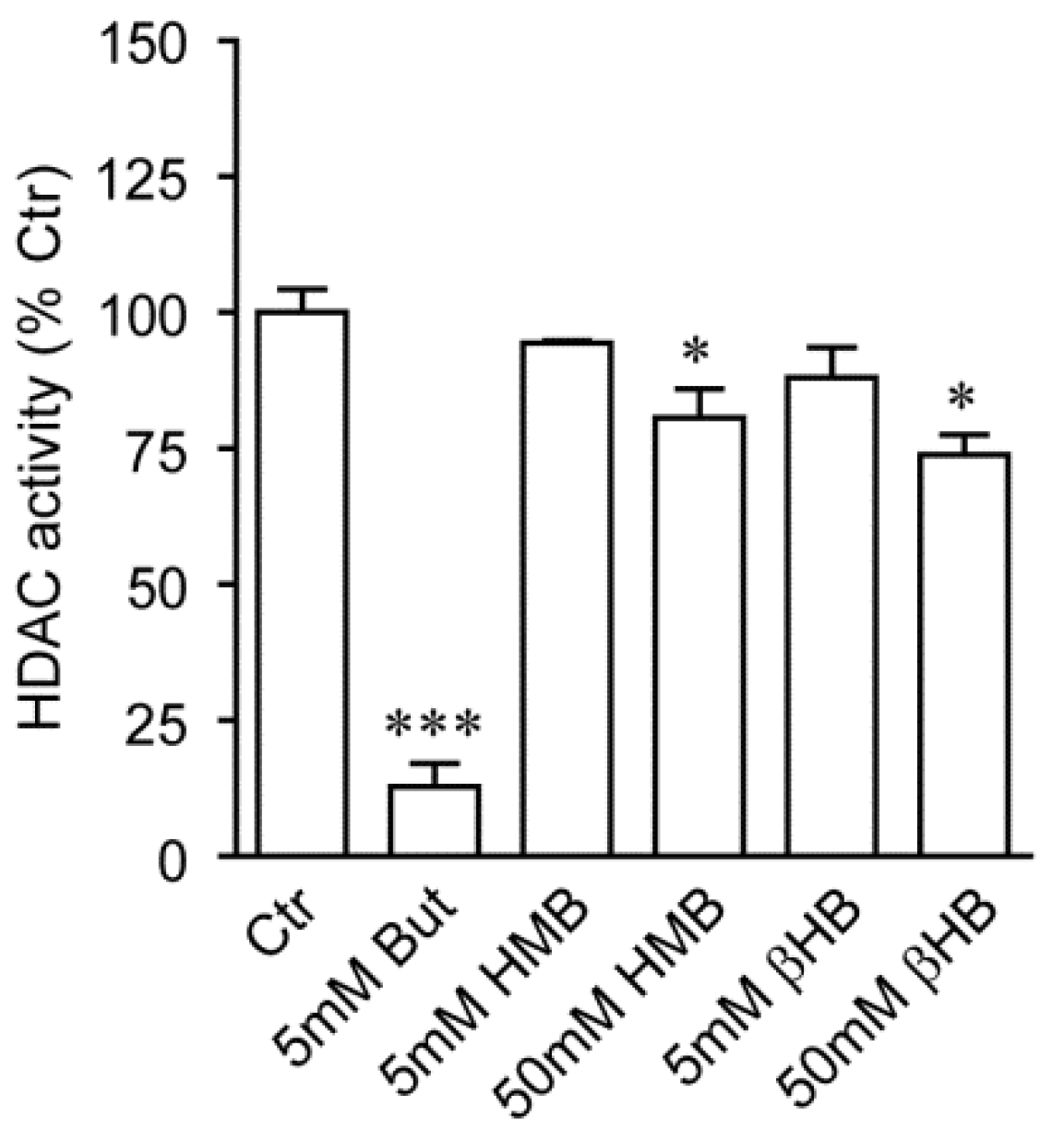
2.2. HMB and βHB Are Poor HDAC Inhibitors In Vitro
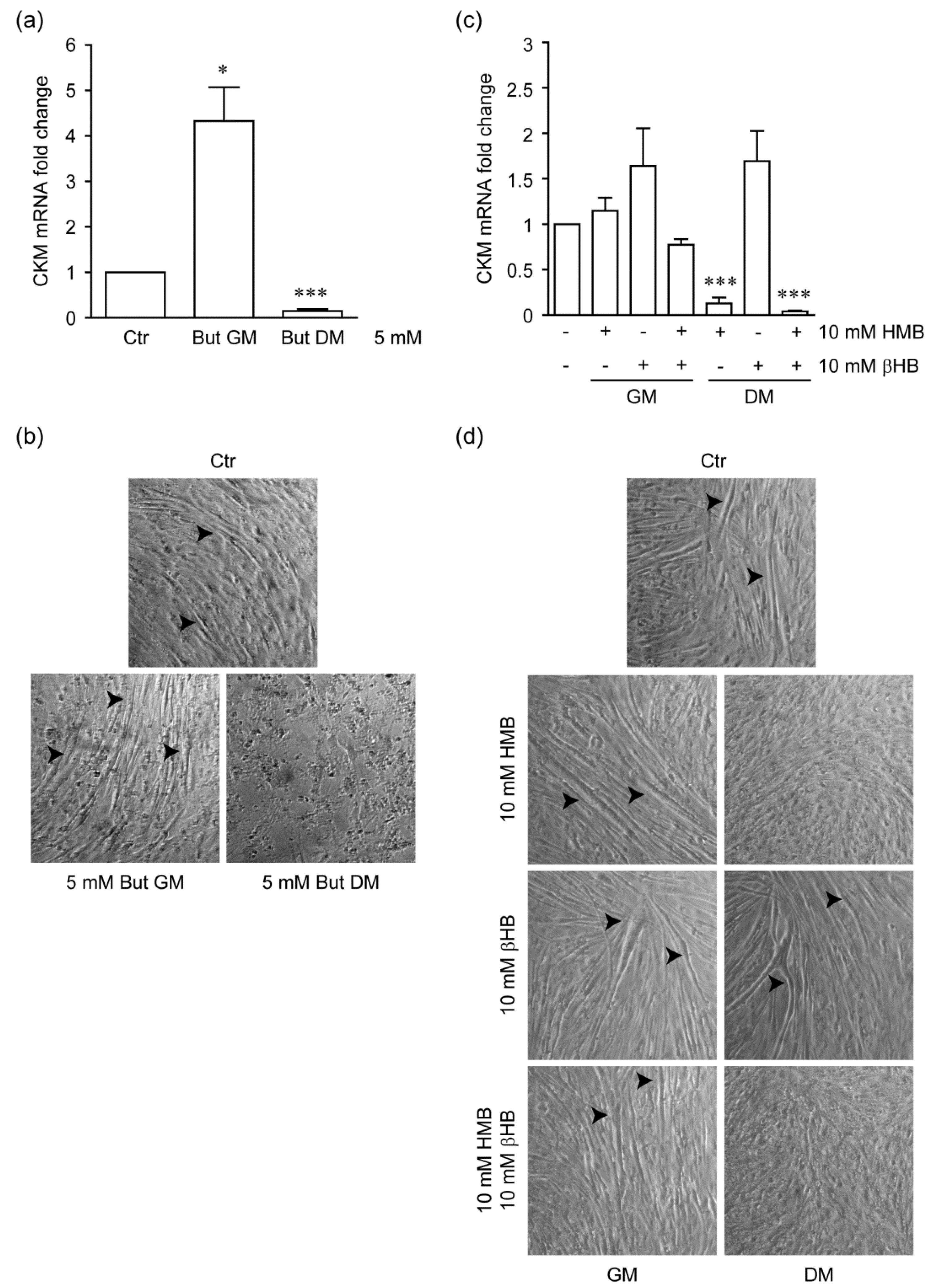
2.3. Stage-Specific Effect of HMB on Myogenic Differentiation of C2C12 Cells
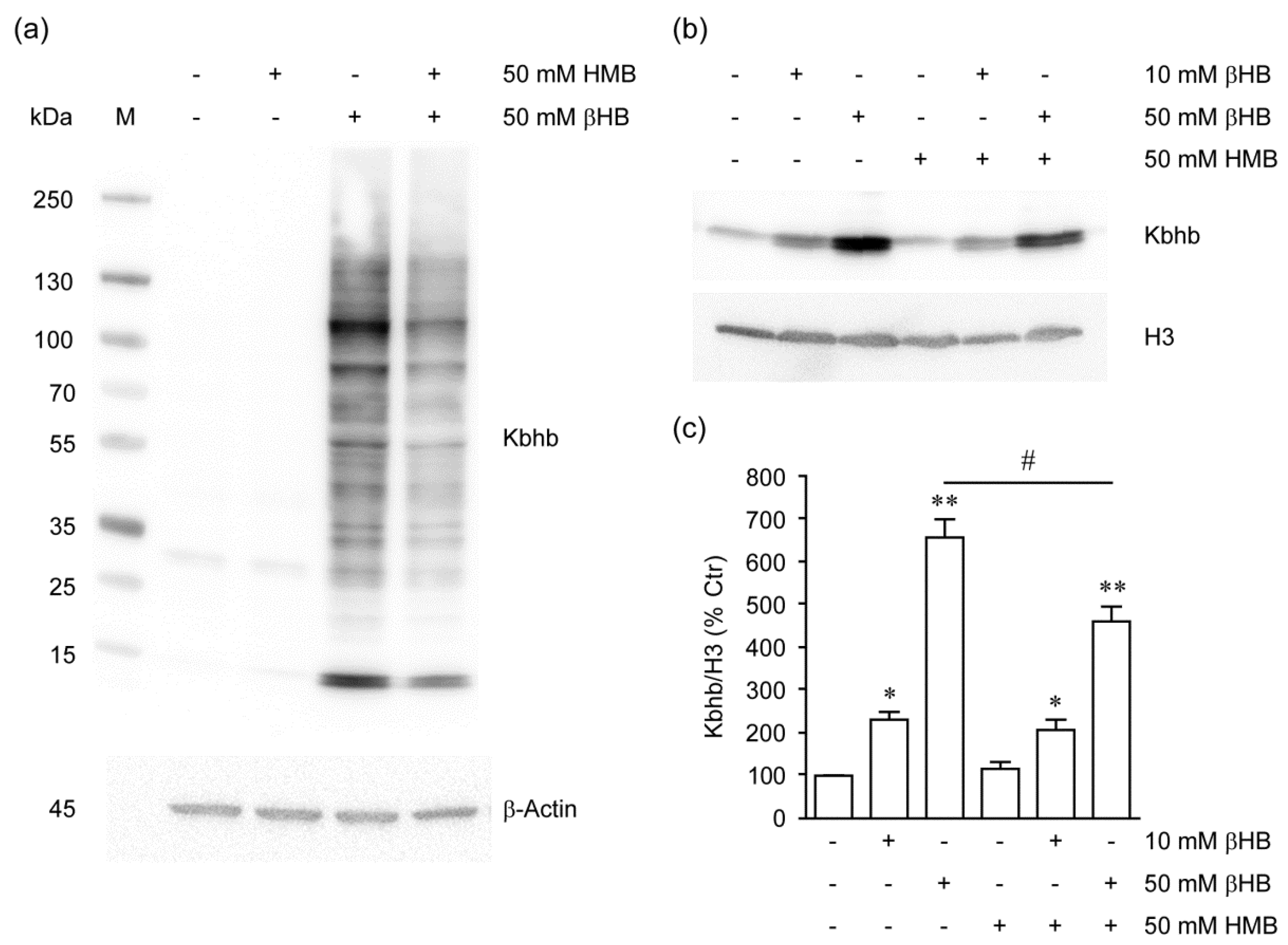
2.4. HMB Inhibits Histone β-Hydroxybutyrylation by βHB
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Histone Extraction
4.3. Total Lysates and Immunoblotting
4.4. Colorimetric Assay of Histone Deacetylase Activity
4.5. Quantitative Real-Time PCR
4.6. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Garcia, J.M. Sarcopenia, Cachexia and Aging: Diagnosis, Mechanisms and Therapeutic Options—A Mini-Review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [Green Version]
- McKendry, J.; Currier, B.S.; Lim, C.; Mcleod, J.C.; Thomas, A.C.Q.; Phillips, S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef]
- Little, J.P.; Phillips, S.M. Resistance exercise and nutrition to counteract muscle wasting. Appl. Physiol. Nutr. Metab. 2009, 34, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.W.P.D.; Dworatzek, E.; Ebner, N.; Von Haehling, S. Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin. Investig. Drugs. 2020, 29, 881–891. [Google Scholar] [CrossRef]
- Sgrò, P.; Sansone, M.; Sansone, A.; Sabatini, S.; Borrione, P.; Romanelli, F.; Di Luigi, L. Physical exercise, nutrition and hormones: Three pillars to fight sarcopenia. Aging Male 2019, 22, 75–88. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and Sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Jiao, J.; Demontis, F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr. Opin. Pharmacol. 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.W.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKβ/NF-ΚB Activation Causes Severe Muscle Wasting in Mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.J.; Liu, S.L.; Lei, S.F.; Papasian, C.J.; Deng, H.W. Molecular genetic studies of gene identification for sarcopenia. Hum. Genet. 2012, 131, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J. Beta-Hydroxy-Beta-Methyl Butyrate (HMB): From Experimental Data to Clinical Evidence in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 668–672. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.-M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial Pathways in Sarcopenia of Aging and Disuse Muscle Atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [Green Version]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-Methylbutyrate and Its Impact on Skeletal Muscle Mass and Physical Function in Clinical Practice: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Rosa, J.C.; Lira, F.S.; Zanchi, N.E.; Ropelle, E.R.; Oyama, L.M.; Oller do Nascimento, C.M.; de Mello, M.T.; Tufik, S.; Santos, R.V. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr. Metab. 2011, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [PubMed]
- Girón, M.D.; Vílchez, J.D.; Shreeram, S.; Salto, R.; Manzano, M.; Cabrera, E.; Campos, N.; Edens, N.K.; Rueda, R.; López-Pedrosa, J.M. β-Hydroxy-β-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PLoS ONE 2015, 10, e0117520. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Alamdari, N.; Castillero, E.; Muscaritoli, M.; Rossi Fanelli, F.; Hasselgren, P.O. β-Hydroxy-β-methylbutyrate (HMB) prevents dexamethasone-induced myotube atrophy. Biochem. Biophys. Res. Commun. 2012, 423, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal Muscle Atrophy: Potential Therapeutic Agents and Their Mechanisms of Action. Pharmacol. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The Evolving Metabolic Landscape of Chromatin Biology and Epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Hiltunen, M.; Kaarniranta, K. Krebs cycle intermediates regulate DNA and histone methylation: Epigenetic impact on the aging process. Ageing Res. Rev. 2014, 16, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tang, K.; Ma, J.; Zhou, L.; Liu, J.; Zeng, L.; Zhu, L.; Xu, P.; Chen, J.; Wei, K.; et al. Ketogenesis-Generated β-Hydroxybutyrate Is an Epigenetic Regulator of CD8+ T-Cell Memory Development. Nat. Cell Biol. 2020, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Van Remmen, H. Emerging Roles for Histone Deacetylases in Age-Related Muscle Atrophy. Nutr. Healthy Aging 2016, 4, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The Histone Deacetylase Inhibitor Butyrate Improves Metabolism and Reduces Muscle Atrophy during Aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent Action of Butyrate over β-Hydroxybutyrate as Histone Deacetylase Inhibitor, Transcriptional Modulator and Anti-Inflammatory Molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Iezzi, S.; Cossu, G.; Nervi, C.; Sartorelli, V.; Puri, P.L. Stage-Specific Modulation of Skeletal Myogenesis by Inhibitors of Nuclear Deacetylases. Proc. Natl. Acad. Sci. USA 2002, 99, 7757–7762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, S.L.; Abumrad, N.N. Nutritional Role of the Leucine Metabolite β-Hydroxy β-Methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pani, G.; Cavallucci, V.; Bartoccioni, E. Age-Related Sarcopenia: Diabetes of the Muscle? J. Clin. Mol. Endocrinol. 2016, 1, 29. [Google Scholar] [CrossRef]
- Girón, M.D.; Vílchez, J.D.; Salto, R.; Manzano, M.; Sevillano, N.; Campos, N.; Argilés, J.M.; Rueda, R.; López-Pedrosa, J.M. Conversion of Leucine to β-Hydroxy-β-Methylbutyrate by α-Keto Isocaproate Dioxygenase Is Required for a Potent Stimulation of Protein Synthesis in L6 Rat Myotubes. J. Cachexia Sarcopenia Muscle 2016, 7, 68–78. [Google Scholar] [CrossRef]
- Fuller, J.C.; Sharp, R.L.; Angus, H.F.; Khoo, P.Y.; Rathmacher, J.A. Comparison of Availability and Plasma Clearance Rates of β-Hydroxy-β-Methylbutyrate Delivery in the Free Acid and Calcium Salt Forms. Br. J. Nutr. 2013, 114, 1403–1409. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallucci, V.; Pani, G. The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. Metabolites 2021, 11, 512. https://doi.org/10.3390/metabo11080512
Cavallucci V, Pani G. The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. Metabolites. 2021; 11(8):512. https://doi.org/10.3390/metabo11080512
Chicago/Turabian StyleCavallucci, Virve, and Giovambattista Pani. 2021. "The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells" Metabolites 11, no. 8: 512. https://doi.org/10.3390/metabo11080512
APA StyleCavallucci, V., & Pani, G. (2021). The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. Metabolites, 11(8), 512. https://doi.org/10.3390/metabo11080512





