Abstract
Skeletal muscle contraction relies on both high-fidelity calcium (Ca2+) signals and robust capacity for adenosine triphosphate (ATP) generation. Ca2+ release units (CRUs) are highly organized junctions between the terminal cisternae of the sarcoplasmic reticulum (SR) and the transverse tubule (T-tubule). CRUs provide the structural framework for rapid elevations in myoplasmic Ca2+ during excitation–contraction (EC) coupling, the process whereby depolarization of the T-tubule membrane triggers SR Ca2+ release through ryanodine receptor-1 (RyR1) channels. Under conditions of local or global depletion of SR Ca2+ stores, store-operated Ca2+ entry (SOCE) provides an additional source of Ca2+ that originates from the extracellular space. In addition to Ca2+, skeletal muscle also requires ATP to both produce force and to replenish SR Ca2+ stores. Mitochondria are the principal intracellular organelles responsible for ATP production via aerobic respiration. This review provides a broad overview of the literature supporting a role for impaired Ca2+ handling, dysfunctional Ca2+-dependent production of reactive oxygen/nitrogen species (ROS/RNS), and structural/functional alterations in CRUs and mitochondria in the loss of muscle mass, reduction in muscle contractility, and increase in muscle damage in sarcopenia and a wide range of muscle disorders including muscular dystrophy, rhabdomyolysis, central core disease, and disuse atrophy. Understanding the impact of these processes on normal muscle function will provide important insights into potential therapeutic targets designed to prevent or reverse muscle dysfunction during aging and disease.
1. Ca2+ Signaling in Skeletal Muscle
Calcium (Ca2+) is a universal second messenger used by virtually all mammalian cells to control a wide range of physiological/biological processes including differentiation, apoptosis, gene transcription, migration, excitability, neurotransmitter secretion, and muscle contraction. Ca2+ signaling occurs when the free cytosolic Ca2+ concentration, maintained around 100 nM under resting conditions, rises rapidly upon Ca2+ release from intracellular stores and/or Ca2+ entry from the extracellular space, due to the combination of the opening of Ca2+-permeable channels and a steep concentration gradient (~4 orders of magnitude).
1.1. Excitation–Contraction (EC) Coupling in Skeletal Muscle
Muscle contraction and relaxation are regulated by rapid changes of myoplasmic Ca2+. To accomplish this task, skeletal muscle fibers utilize a highly organized sarcotubular membrane system consisting of a dense network of specialized invaginations of the sarcolemma, termed transverse tubules (T-tubules), and a continuous system of internal membranes of sarcoplasmic reticulum (SR). The SR is composed of two distinct functional and morphological compartments in direct continuity with each other: (1) the SR terminal cisternae and (2) longitudinal or free SR [1,2]. The Ca2+ release unit (CRU), or “triad”, is composed of a central T-tubule flanked by adjacent junctions with two SR terminal cisternae [3] (Figure 1). The CRU is the fundamental structure that mediates excitation-contraction (EC) coupling, a mechanism whereby an action potential in the T-tubule membrane is converted into a rapid and massive increase of myoplasmic Ca2+ concentration. In skeletal muscle, EC coupling involves a unique physical or mechanical interaction between two different types of Ca2+ channels: voltage-gated L-type Ca2+ channels (CaV1.1) or dihydropiridine receptors (DHPRs), which function as voltage sensors in the T-tubule membrane, and type-1 ryanodine receptor (RyR1) Ca2+ release channels in the SR [4,5,6,7] (Figure 1). Following depolarization of the T-tubule membrane, DHPR voltage sensor proteins undergo a conformational change that is mechanically coupled to the opening of nearby RyR1 Ca2+ release channels [8,9,10,11]. The subsequent rapid and massive release of Ca2+ from the SR into the myoplasm provides the chemical signal used to drive muscle contraction. The high amount of free (~300–500 µM) and bound Ca2+ within the SR [12,13,14], coupled with steep gradient across the SR membrane, is achieved by two fundamental proteins that work in concert: calsequestrin-1 (CASQ1) and sarco/endoplasmic Ca2+ ATPases (SERCA). CASQ1, a highly acidic protein resident in the SR terminal cisternae, has two major roles during EC coupling: (1) to bind/buffer a large amount of Ca2+ needed for release and activation of muscle contraction [15,16,17,18] and (2) to regulate RyR1 activity by reducing channel open probability during SR Ca2+ depletion [19,20,21,22]. SERCA is a high-affinity, ATP-dependent Ca2+ pump densely packed in the free SR [23,24]. SERCA-mediated SR Ca2+ reuptake is the primary mechanism for Ca2+ clearance in skeletal muscle following release during EC coupling, allowing muscle relaxation [23,25]. In fact, it is estimated that ~90% of the Ca2+ increase during a single twitch contraction (e.g., the SR release induced by a single action potential) is cleared from the myoplasm through SERCA pumps. As a result, SERCA-mediated Ca2+ reuptake is responsible for muscle relaxation and recovery of SR Ca2+ to levels needed for subsequent cycles of EC coupling.
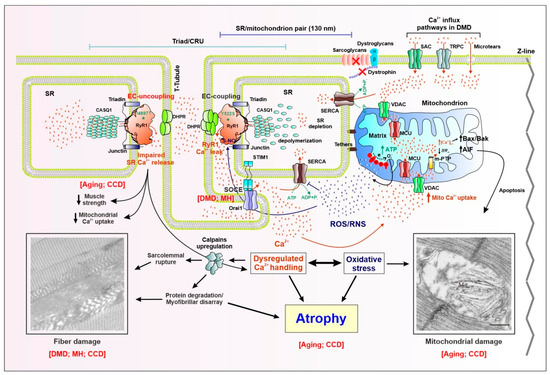
Figure 1.
Schematic model showing proposed molecular mechanisms for altered Ca2+ signaling, mitochondrial function, and muscle fiber damage/atrophy in skeletal muscle disease and aging. Skeletal muscle contraction relies on a rapid and massive release of Ca2+ from the sarcoplasmic reticulum (SR) terminal cisternae upon depolarization of the sarcolemma. Excitation–contraction (EC) coupling, the process whereby an action potential in the surface membrane is converted into Ca2+ release from SR, is mediated by a functional coupling between the dihydropyridine receptor (DHPR) voltage sensor in the transverse tubule (T-tubule) membrane and the type-1 ryanodine receptor (RyR1) Ca2+ release channel in the SR. The fundamental structure that mediates EC coupling is the Ca2+ release unit (CRU), or “triad”, which is composed of a central T-tubule flanked by adjacent junctions with two SR terminal cisternae. Besides DHPR and RyR1, other proteins participate in EC coupling: triadin and junction in the SR membrane, and calsequestrin-1 (CASQ1), the Ca2+-binding protein resident in the SR lumen. The ATP needed for muscle contraction is primarily generated within the mitochondria during aerobic respiration. In fast-twitch fibers, most mitochondria are located on the Z-line side of the triad, closely associated with the terminal SR cisternae via small (~8–10 nm) electron dense bridges termed “tethers”. As a result of this structural linkage, the average minimal distance between the RyR1 (site of Ca2+ release during EC coupling) and the outer membrane of the adjacent mitochondrion is ~130 nm. Right: Myoplasmic Ca2+ overload is the result of: (i) excessive SR Ca2+ release, due to gain-of-function point mutations (e.g., Y522S) in RyR1 linked to muscle disorders such as malignant hyperthermia (MH) and central core disease (CCD), that enhance channel opening probability; (ii) enhanced Ca2+ influx via STIM1/Orai1-dependent store-operated Ca2+ entry (SOCE), as the result of reduced SR Ca2+ content (SR depletion), stretch-activated Ca2+ channels (SAC), transient receptor potential canonical channels (TRPC), and microtears. These Ca2+ influx mechanisms are upregulated in Duchenne muscular dystrophy (DMD) and MH (SOCE). The resulting increase in Ca2+ influx into the myoplasm promotes mitochondrial Ca2+ uptake through the mitochondrial Ca2+ uniporter (MCU), ultimately leading to mitochondrial Ca2+ overload that increases electron transport chain activity and excessive production of reactive oxygen and nitrogen species (ROS/RNS), which underlie oxidative stress. In turn, increased ROS/RNS levels oxidize/nytrosylate both RyR1, which further enhances SR Ca2+ release channel opening, and the sarco/endoplasmic Ca2+ ATPase (SERCA), which reduces SR Ca2+ reuptake. The resulting accumulation of Ca2+ in the myoplasm, together with increased oxidative stress, triggers a series of intracellular signaling pathways (e.g., calpains activation, reduced protein synthesis, and increased protein degradation) that lead to: (i) myofibrillar disarray, (ii) sarcolemmal rupture, (iii) structural alterations (e.g., contractures, cores), and (iv) mitochondrial damage. These alterations, together with increased apoptosis, triggered by mitochondrial Ca2+ overload via the activation of the Bax/Bak/AIF pathway, drive loss of muscle mass and atrophy. Left: EC uncoupling, due to the reduction of DHPR expression during aging or as the result of RyR1 loss-of-function point mutations (e.g., I4897T) linked to myopathies such as CCD, reduces electricallyevoked SR Ca2+ release that contributes to reduced muscle specific force production, disrupted mitochondrial structure/function, mitochondrial damage, and fiber structural alterations (e.g., formation of cores and myofibrillar disarray). The two pictures showing fiber and mitochondrial damage are modified from Michelucci et al., 2017 Oxid Med Cell Longev. 2017; 2017: 6936897, and Michelucci et al., 2017 Oxid Med Cell Longev. 2017; 2017: 6792694, respectively.
1.2. Store-Operated Ca2+ Entry in Skeletal Muscle
DHPR/RyR1-mediated EC coupling and muscle contraction has long been known to persist in the absence of extracellular Ca2+ [26] and extracellular Ca2+ does not play a critical role in mechanical EC coupling in skeletal muscle [4,27,28,29]. Over the past two decades, however, a growing body of evidence indicates that influx of external Ca2+ into muscle fibers plays an important role both in muscle development/growth and in the maintenance of Ca2+ release and force generation during repetitive stimulation. Indeed, between the late 1990s and early 2000s, a robust store-operated Ca2+ entry (SOCE) pathway was unequivocally identified in both skeletal myotubes [30] and adult muscle fibers [31]. SOCE, a Ca2+ influx pathway activated by depletion of intracellular Ca2+ stores, is among the most important Ca2+ influx pathways in non-excitable cells [32,33,34]. SOCE is coordinated by a functional interaction between stromal-interacting molecule-1 (STIM1), the ER/SR luminal Ca2+ sensor [35,36,37], and ORAI1, the Ca2+ permeable channel of the plasma membrane [38,39,40]. Coupling of STIM1 and ORAI1 during SR Ca2+ depletion also serves as the primary molecular machinery of SOCE in skeletal muscle [41]. Although still not fully understood, intensive research over the past two decades has revealed several important aspects of the molecular mechanism and functional role of SOCE in skeletal muscle. First, SOCE plays a role in refilling SR Ca2+ stores, needed to sustain Ca2+ release and force generation during repetitive, high-frequency stimulation [42,43,44,45,46]. SOCE also plays a role in fatigue-resistant type I specification during postnatal development [47,48]. Together, these effects of SOCE serve to mitigate muscle susceptibility to fatigue during prolonged activity. Second, acute exercise drives the formation of new junctions within the I band of the sarcomere between SR-stacks and extension of T-tubule. These exercise-induced SR-T-tubule junctions are structurally distinct from triads as they lack RyR1 and DHPR proteins, but contain co-localized STIM1 and ORAI1 proteins that mediate SOCE, and thus, are referred to as “Ca2+ entry units” (CEUs) [44,45,46,49]. CEUs are also present, though fewer in number, in resting (non-exercised) muscle. Limited co-localization of STIM1 and ORAI1 at the triad [42,47] together with the presence of some CEUs in close proximity of the triad under resting (non-exercised) conditions [44] provides the machinery needed for the rapid activation of SOCE shown in skeletal muscle [50,51,52,53].
Exercise-induced assembly of additional CEUs, which requires remodeling of SR and of T-tubules at the I band, likely reflects the increased need to replenish intracellular SR Ca2+ stores during repetitive stimulation. Interestingly, we found that a key prerequisite for exercise-induced assembly of CEUs and increased SOCE function involves elongation of the T-tubule (containing Orai1) and association with stacks of SR membranes (containing STIM1) within the I band of the sarcomere. Assembly of CEUs following exercise provides a greater surface area for increased STIM1/Orai1 coupling needed to enhance Ca2+ influx during sustained muscle activity [44,45,46].
Besides being important in the physiology of adult muscle fibers, SOCE is also critical for the correct development and maturation of skeletal muscle tissue [54,55]. This is demonstrated by the fact that normal muscle development and fiber type specification are altered by early developmental muscle-specific ablation of STIM1 [56], ORAI1 [48], and muscle-specific expression of a dominant-negative ORAI1 [42]. Moreover, a growing body of evidence indicates that loss- and gain-of-function mutations in both STIM1 and ORAI1 are linked to multiple human diseases in which myopathy is a prominent clinical manifestation [57,58]. In addition, dysfunctional or enhanced STIM1/ORAI1-mediated SOCE is also implicated in the pathogenesis of other muscle disorders including muscular dystrophy [59,60,61], malignant hyperthermia [62], and sarcopenia [63,64]. Together, these findings demonstrate that a tight regulation of STIM1/ORAI1-dependent SOCE is critical for optimal muscle performance and that aberrant SOCE function contributes to muscle disease.
2. Mitochondria in Skeletal Muscle
2.1. Mitochondrial ATP Production and ROS Generation
In addition to Ca2+, muscle contraction also requires energy from ATP [65]. ATP hydrolysis is the universal biochemical reaction that provides energy to support a wide range of cellular processes including biosynthesis, active ion transport across membranes, and crossbridge cycling during muscle contraction. ATP is produced from multiple sources in skeletal muscle fibers. Rapid ATP generation occurs in the cytoplasm during hydrolysis of phosphocreatine and glycolysis. However, these anaerobic metabolic pathways provide only a small fraction of total ATP produced in skeletal muscle. The majority of ATP is generated within the mitochondria during a series of reactions that require molecular oxygen (O2) in a process referred to as aerobic respiration [66,67,68]. The transfer of electrons from NADH and FADH2, through a series to reactions to molecular O2 as the final electron acceptor, produces an electrochemical proton gradient across the inner mitochondrial membrane that is used to drive ATP generation by the F1/F0 ATP synthase. Under normal conditions, only ~1% of electrons that flow through the electron transport chain “slip” to molecular O2 to produce superoxide anions (O2•−). Either spontaneously or in a reaction catalyzed by superoxide dismutases (SODs), O2•− is converted to hydrogen peroxide (H2O2). Together, O2•−, H2O2, and hydroxyl radical (HO•) are collectively referred to as reactive oxygen species (ROS). Besides mitochondria, other cellular sources of ROS include extra-mitochondrial enzymes such as NADPH oxidases or xanthine oxidase. While for many years ROS produced as a byproduct of the mitochondrial metabolism were only considered pathogenic, recent evidence indicates that low physiological levels of mitochondrial ROS play an important role in cellular redox signaling pathways that regulate multiple cellular functions [69,70]. However, excessive ROS generation, often due to mitochondrial dysfunction, can lead to destructive or pathogenic levels of oxidative stress that contribute to muscle dysfunction in muscle disease (detailed in Section 3, Section 4 and Section 5 below).
2.2. Mitochondrial Ca2+ Uptake and Ca2+ Microdomains in Skeletal Muscle
The hypothesis that mitochondria regulate intracellular Ca2+ homeostasiswas proposed in early studies that showed robust Ca2+ uptake by purified rat kidney mitochondria [71]. The driving force for accumulation of Ca2+ within the mitochondrial matrix is provided by the large negative electrochemical gradient (>−180 mV) across the mitochondrial inner membrane generated by electron transfer chain dependent H+ pumping during oxidative phosphorylation. Nonetheless, the affinity of mitochondria for Ca2+ uptake is relatively low, with a Ca2+ concentration required for half-maximal Ca2+ transport of ~30 µM [72]. This concentration is considerably higher than that measured within the muscle fibers not only at rest (~100 nM), but even at the peak of the global myoplasmic Ca2+ transient (1–3 µM) during activation of skeletal muscle contraction [73,74]. These observations raised questions regarding the potential physiological relevance of mitochondrial Ca2+ uptake in skeletal muscle. However, with the development of mitochondria-targeted Ca2+ sensitive probes [75], mitochondrial Ca2+ uptake during cytoplasmic Ca2+ oscillations was confirmed in multiple cell types (e.g., fibroblasts, neurons, endothelial cells) including skeletal muscle fibers [76,77]. The increase in cytosolic Ca2+ promotes the opening of the mitochondrial Ca2+ uniporter (MCU), a 40-kDa protein that functions as a highly Ca2+-selective channel within the mitochondrial inner membrane, thus enabling Ca2+ flux down the high electrochemical gradient from the cytoplasm to the mitochondrial matrix [78,79]. The entry of Ca2+ within the mitochondrial matrix stimulates the activity of several enzymes of the tricarboxylic acid (TCA) cycle and electron transport chain including pyruvate dehydrogenase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and the ATP synthetic activity of the F1/F0 ATPase [80]. As a result, mitochondria both shape the myoplasmic Ca2+ transient [81] and are energetically coupled to the metabolic needs of muscle through Ca2+-mediated regulation of mitochondrial TCA and electron transport chain activity. The essential role of Ca2+ in regulating mitochondrial function in skeletal muscle is further highlighted by the severe clinical muscle phenotypes (e.g., severe muscle weakness, fatigue, lethargy) observed in patients with loss-of-function mutations in MICU1, a modulatory subunit of the MCU complex that acts as a gatekeeper for mitochondrial Ca2+ entry [82,83,84].
How mitochondria in skeletal muscle are able to take up Ca2+ ions when the peak myoplasmic Ca2+ level is an order of magnitude lower than the concentration for half-maximal Ca2+ transport by MCU, is still debated. This apparent paradox is explained by a “Ca2+ microdomain” that results from the close positioning of mitochondria relative to sites of Ca2+ release (i.e., triads or CRUs in skeletal muscle). The concept of a Ca2+ microdomain suggests that mitochondrial Ca2+ uptake is driven by a high local concentration of Ca2+ around mitochondria that is much higher than that observed in the bulk myoplasm [85]. In skeletal fibers, mitochondria can be divided into three main classes: (a) longitudinal mitochondria, which form in rows between myofibrils (found mostly during maturation and in oxidative, slow-twitch fibers); (b) subsarcolemmal mitochondria, located in clusters under the surface membrane in proximity of capillaries (hence, also more frequently observed in more vascularized slow-twitch fibers); and (c) transversal mitochondria, which form in two transverse rows on both sides of Z-lines encircling myofibrils at the I band. The first description of a population of inter-myofibrillar mitochondria positioned within the I band of the sarcomere on both sides of the Z-line was made from elegant electron microscopy (EM) studies conducted by Ogata and Yamasaki [86,87,88,89]. Subsequent quantitative analyses of slow- and fast-twitch muscle fibers showed that mitochondria are preferentially oriented in transverse double rows with a sarcomeric periodicity of ~2 µm, consistent with their close apposition to triads [90].
More recently, EM studies in fast-twitch fibers from adult mice showed that:(a) mitochondrial positioning closely follows maturation of the EC coupling system, changing from predominantly longitudinal to transversal positioning during postnatal development; (b) in adult muscle fibers, most mitochondria are located on the Z-line side of the triad, closely associated with the terminal SR cisternae via small (~8–10 nm) electron dense bridges termed “tethers” (Figure 1). The tethers anchor the mitochondrial outer membrane to the SR, thus limiting/preventing mitochondrial movement from sites of Ca2+ release. As a result of this structural linkage, the average minimal distance between the RyR1 foot (site of Ca2+ release during EC coupling) and the outer membrane of the adjacent mitochondrion is only ~130 nm [91], thus creating a tightly coupled Ca2+ signaling SR-mitochondrial “nanodomain” (Figure 1). The CRU–mitochondrial nanodomain could represent the fundamental structural feature required to create the high Ca2+ microdomain needed to drive mitochondrial Ca2+ uptake via MCU [92] (Figure 1). Consistent with a critical role of this association for Ca2+ signaling between the two organelles, the frequency of osmotic shock-induced Ca2+ sparks is reduced three-fold during postnatal development, in direct linear correspondence with an increase in mitochondrion–CRU pairing [77]. Moreover, a reduction in CRU/mitochondria tethering was shown to contribute to impaired Ca2+ signaling, an increase in mitochondrial-dependent oxidative stress, and reduced muscle performance during aging [93]. These findings are discussed in greater detail in Section 4.
Precise regulation of Ca2+ signaling and ROS production is a fundamental pre-requisite for the correct function of skeletal muscle. Consistent with this, multiple pathological conditions in muscle are linked to dysregulation of Ca2+ homeostasis and/or Ca2+-dependent oxidative stress. In the following three sections, we discuss recent literature regarding how impaired Ca2+ handling, dysfunctional Ca2+-dependent production of ROS/RNS, and structural/functional alterations in CRUs–mitochondria association could contribute to loss of muscle function, increased damage following muscle rupture, and reduced muscle mass and contractile function in inherited muscle diseases, sarcopenia, and disuse atrophy.
3. Altered Ca2+ Handling and Mitochondrial ROS Production in Inherited Forms of Skeletal Muscle Disease
3.1. Muscular Dystrophy
Muscular dystrophies (MDs) comprise a heterogeneous group of muscle diseases characterized by weakness, muscle wasting, and progressive muscle degeneration that can ultimately lead to an impairment of mobility and premature death. The most common MD is the Duchenne muscular dystrophy (DMD), a currently incurable inherited X-linked recessive muscle disorder that affects 1 in 3500 male births. DMD is caused by loss-of-function mutations in the gene encoding dystrophin, a 427 kDa structural protein located at the cytoplasmic face of the sarcolemma [94]. Dystrophin links actin filaments and microtubules of the cytoskeleton to the extracellular matrix through a group of proteins collectively known as the dystrophin-glycoprotein complex (DGC). Disruption of the DGC, due to the loss of both dystrophin and sarcoglycan proteins, results in a reduction in sarcolemma integrity/stability, which results in microtears in the sarcolemma during mechanical stress. Until they are repaired, microtears promote non-specific influx of Ca2+ (and other ions) across the membrane followed by pathological myoplasmic Ca2+ overload that triggers an array of intracellular mechanisms that lead to myofiber degeneration and death including: dysregulation of cytosolicCa2+ homeostasis, mitochondrial Ca2+ overload/damage, increased mitochondrial ROS production and oxidative stress, and activation of the Ca2+-dependent proteases [95,96] (Figure 1). While disruption of sarcolemmal integrity is a common feature among the different MDs, a comprehensive understanding of the mechanisms responsible for aberrant Ca2+ handling and increased oxidative stress that underlie the dystrophic phenotype are far from being fully understood. Currently, two main pathomechanisms are hypothesized to be responsible for abnormalities in myoplasmic Ca2+ levels in DMD: (1) excessive transmembrane Ca2+ influx and (2) enhanced SR Ca2+ leak through oxidized RyR1 Ca2+ release channels (Figure 1). Besides a non-selective Ca2+ influx through sarcolemmal microtears, a number of studies provide evidence for the involvement of more specific entry pathways through Ca2+-permeable channels in the plasma membrane. Early studies reported that a significant portion of the increased membrane permeability to external Ca2+ is mediated by stretch-activated Ca2+ channels [97]. Indeed, inhibition of these channels prevents the increased stretch-induced rise in intracellular Ca2+ observed in muscle fibers from mdx mice. Subsequent studies provided evidence for a modulatory role of enhanced SOCE in DMD. Prior to the identification of STIM1 and ORAI1 in SOCE, several studies reported that store-dependent Ca2+-permeable TRPC channel activity is upregulated in muscle fibers of mdx mice, and thus, contributes to increased levels of myoplasmic Ca2+ [98,99,100]. Subsequent studies found that increased STIM1/ORAI1-dependent SOCE also contributes to the enhanced Ca2+ influx observed in DMD (Figure 1), as both the expression and activity of STIM1/ORAI1-mediated SOCE are markedly enhanced in mouse models of MD [59,60] and the dystrophic phenotype of mdx is reduced by inhibiting Orai1-dependent SOCE [101].
In addition to sarcolemmal Ca2+ influx, increased SR Ca2+ leak through RyR1 channels represents an alternative proposed mechanism for altered Ca2+ handling in DMD. A series of studies from Marks and colleagues reported that loss of dystrophin (in mdx mice) or the DGC (in Sgcb-/- mice) is associated with hypernitrosylation of specific cysteine residues in RyR1, which lead to FKBP12 dissociation, destabilization of the RyR1 channel closed state, and increased RyR1-dependent SR Ca2+ leak [102,103]. The subsequent pathogenic rise in myoplasmic Ca2+ leads to mitochondrial Ca2+ overload and uncontrolled ROS/RNS production that further oxidizes/nitrosylates RyR1 in a destructive feed-forward cycle of Ca2+ leak and ROS/RNS production (Figure 1). The importance of increased oxidative stress as a key pathomechanism of muscle degeneration in DMD is supported by improvement of the dystrophic phenotype in mdx mice following treatment with N-actetylcysteine (NAC), a potent antioxidant [95]. Interestingly, an interplay between altered Ca2+ signaling and excessive mitochondrial ROS production was shown to be a key pathomechanism not only in MDs, but even in other muscle diseases such as malignant hyperthermia (MH) and central core disease (CCD) (see Section 3.2 for more details) (Figure 1). In fact, we previously reported that pre-treatment of mice with NAC for two months was able of reducing either anesthetic-triggered lethal hyperthermic episodes in mice lacking CASQ1 (CASQ1-null) [104] or mitochondrial damage and formation of cores in both heterozygous RYR1-Y522S [105] and CASQ1-null [106] mice.
A reduction in oxidative stress normalizes SR Ca2+ release by reducing the opening probability of destabilized RyR1 channels, thus interrupting the destructive feed-forward cycle of Ca2+ leak and ROS/RNS production and ameliorating the dystrophic phenotype. However, it remains unclear whether or not increased ROS/RNS levels modify proteins that mediate SOCE to augment Ca2+ entry and signaling. Likewise, it is unknown if inhibiting Ca2+ entry reduces oxidative stress. Future studies using mouse models to specifically inhibit SOCE in skeletal muscle will be needed to answer this important questionand, thus, validate SOCE as a potential therapeutic target to treat this incurable disease.
3.2. Malignant Hyperthermia and Central Core Disease
Aberrant Ca2+ handling and increased oxidative stress are pathomechanisms of the myopathic phenotype of two overlapping muscle disorders: MH susceptibility and CCD. MH is a potentially lethal inherited pharmacogenetic disorder characterized by a life-threatening hyperthermic reaction in susceptible individuals exposed to volatile/halogenated anesthetics (halothane, isofluorane, etc.) and/or succinylcholine, compounds commonly used during surgical procedures [107]. CCD, one of the most common inherited human congenital myopathies, is characterized by hypotonia, proximal muscle weakness, and delayed attainment of motor milestones [108,109]. An association between MH and CCD exists as muscle biopsies of some MH patients exhibit cores [110,111], amorphous central areas of muscle fibers lacking glycolytic/oxidative enzymes and mitochondria upon histological analysis [112]. In addition, CCD patients are at risk for hyperthermic episodes during exposure to MH-triggering agents (e.g., anesthetics) and muscle biopsies from some CCD patients exhibit increased susceptibility to contractures during in vitro caffeine-halothane contracture testing [113,114,115].
MH and CCD are inexorably linked to one another as most cases for both conditions are linked to mutations in the RYR1 gene [116,117]. Association of gain-of-function mutations in the RYR1 gene with MH (and some individuals with CCD) indicates that these disorders result, at least in part, from defective RyR1 function and Ca2+ regulation in skeletal muscle. MH-related mutations in RyR1 destabilize the SR Ca2+ release channel closed state, resulting in an increased susceptibility to opening in response to activators, SR Ca2+ leak, and mitochondrial Ca2+ uptake and subsequent ROS/RNS production [118]. In turn, RyR1 oxidative modifications (e.g., S-nitrosylation and S-gluthationylation) further destabilize the channel, and thus, further enhance RyR1 Ca2+ leak. This destructive feed-forward mechanisms of increased RyR1 Ca2+ leak and oxidative stress enhances RyR1 sensitivity to activation in MH that eventually leads to mitochondrial damage and the development of central cores in CCD [119] (Figure 1).
RYR1-Y522S knock-in mice [120] exhibit age-dependent development of central cores that occurs in conjunction with a mild myopathy, characterized by muscle weakness and mitochondrial damage, which together mirror key functional and structural abnormalities observed in human CCD patients [105,119,121]. MacLennan and colleagues proposed that the formation of cores in the center of the fiber, due to the Ca2+-dependent disruption of mitochondria, may represent a protective compensative response to chronic elevations in myoplasmic Ca2+ designed to protect the fiber from further Ca2+-induced damage [122]. However, it is worth to pointing out that in addition to RYR1 gain-of-function mutations that enhance Ca2+ leak, some mutations in RyR1 linked to CCD reduce voltage-dependent SR Ca2+ release during EC coupling, a phenomenon referred to as “EC uncoupling” [123,124]. For instance, the I4897T CCD mutation in RyR1 (mouse RyR1 numbering) reduces the release of Ca2+ from the SR following skeletal muscle excitation (Figure 1). Individuals with this mutation exhibit muscle weakness with the presence of ultrastructural changes in muscle including disruption of the myofibrils, “Z-line streaming”, sarcomeric disorganization, predominance of fiber type I, and the lack of mitochondria in small areas of the fibers [125]. Interestingly, the reduction in SR Ca2+ release as the result of the I4897T mutation correlates with an increased mitochondrial damage-dependent oxidative stress, a feature that closely resembles that also observed in aged muscles (see Section 4 for more details). However, the structural abnormalities observed in I4897T mice are less severe than those observed in RYR1-Y522S mice, in line with excessive Ca2+ leak, larger degree of store depletion and higher oxidative stress displayed by the latter.
The critical pathogenic role of increased oxidative stress in the mitochondrial damage and formation of cores in CCD has been the subject of significant attention. Treatment of mice with an antioxidant (NAC) reduces mitochondrial damage and the development of cores in RYR1-Y522S mice, but not MHS [105,119]. Whether or not other mechanisms that control Ca2+ homeostasis in muscle are also involved in MH and CCD pathophysiology remains poorly understood. The reason might lie in the fact that for many years it was assumed that sustained elevations in myoplasmic Ca2+ are solely the consequence of increased RyR1-mediated SR Ca2+ leak/release. However, excessive RyR1 Ca2+ leak can lead to local SR depletion that activates SOCE to further enhance resting Ca2+ levels (see [126] for detailed discussion) (Figure 1). In support of this idea, muscle fibers/myotubes from RYR1-Y522S were shown to exhibit reduced SR Ca2+ content [118,127] and an increased rate of SOCE [128], suggesting increased STIM1 and ORAI1 expression/function. Consistent with this, increased SOCE activity was postulated to be an important mechanism for aberrant cytosolic Ca2+ dynamics in muscle biopsies of MH patients [61,62]. Nevertheless, further studies will be required to determine whether SOCE contributes to the pathogenesis of MH and CCD, and thus, if this pathway represents a potential therapeutic target.
4. Role of Altered Ca2+ Handling and Mitochondrial ROS Production in Loss of Muscle Mass and Reduced Contractility in Sarcopenia
Sarcopenia, age-related skeletal muscle decline, is a major national health problem. Sarcopenia is characterized by loss of muscle mass, lowered strength, increased susceptibility to fatigue, and reduced velocity of contraction [129,130]. The loss of muscle mass during aging is primarily due to reduced fiber number and size [131,132], degeneration of neuromuscular junctions as the result of stem cell depletion [133], and loss of motor units [134]. However, age-related muscle atrophy alone is not sufficient to account for the massive decline of muscle function and weakness observed during aging. Indeed, while muscle atrophy certainly contributes to muscle weakness in aging, the decline in muscle strength and increase in susceptibility to fatigue occur even before development of atrophy, consistent with a reduction in intrinsic muscle specific force production that is independent of muscle size or neuromuscular function.
Dysregulation in Ca2+ handling and increased oxidative stress are two mechanisms proposed to contribute to age-dependent reduction in muscle specific force. In seminal studies conducted by Delbono and colleagues, age-dependent decline in intrinsic muscle force production was shown to involve a marked reduction in DHPR α1-subunit expression that results in a functional uncoupling of DHPR and RyR1 proteins in CRUs [135,136] (Figure 1). A reduction of both voltage sensor function and L-type Ca2+ current activity with aging was associated to an impaired voltage-dependent SR Ca2+ release and specific force production [137,138]. Boncompagni et al. (2006) proposed a slightly different explanation for the inefficient delivery of Ca2+ ions to the contractile elements in aged fibers [139]. This study found a progressive reduction in the number of CRUs in muscle biopsies from sedentary seniors (loss of about 40–50% of CRUs compared to muscles from young adults) as the cause of specific force loss in aging muscle. These findings were subsequently supported by studies in mice [93] and human muscle biopsies [140] that quantified mitochondrial association with CRUs: a significant reduction of both the total number/density of CRUs, mitochondria, and CRU–mitochondrial pairs in muscles from aged mice/humans. These structural alterations were accompanied by parallel reductions in SR Ca2+ release during EC coupling, impaired mitochondrial Ca2+ uptake, and increased levels of oxidative stress [93]. Similar results were observed in human biopsies where total mitochondrial number and CRU–mitochondrial pairs are higher in well-trained seniors who exercised regularly for the past 30 years compared to age-matched healthy sedentary seniors [140]. Interestingly, these age-dependent structural and functional alterations were almost prevented by regular endurance exercise when mice housed in cages with voluntary exercise wheels [141].Specifically, in this study, long-term exercise prevented and/or corrected age-dependent uncoupling of mitochondria from the EC coupling apparatus, thus preserving SR Ca2+ release during EC coupling, mitochondrial Ca2+ uptake, and reduced levels of oxidative stress. Thus, the correct positioning of mitochondria with respect to the SR is essential not only for correct Ca2+ handling, but also for proper maintenance of physiological levels of ROS/RNS. It is interesting to note that oxidative stress occurs both as a result of mitochondrial Ca2+ overload (as observed in DMD, MH, and CCD) (Figure 1) and when mitochondrial Ca2+ uptake is reduced, consistent with correct Ca2+ signaling being required for proper control of the redox state. Future studies will be required to elucidate the molecular mechanisms underlying the complex Ca2+-dependent regulation of mitochondrial ROS production.
A third mechanism proposed to contribute to an age-dependent reduction in intrinsic muscle specific force generation involves altered SR Ca2+ release function due to oxidative stress-dependent modification in RyR1, likely due to altered mitochondrial function. In line with this idea, Marks and colleagues reported an age-dependent increase in RyR1 oxidation/nitrosylation, FKBP12 dissociation, and SR Ca2+ leak [142]. As a result of this increased SR Ca2+ leak, peak electrically evoked Ca2+ release and muscle specific force production were reduced in muscle from aged mice [142].
Beside impaired EC coupling due to DHPR/RyR1 uncoupling, reduced CRUs and CRU–mitochondrial association, altered mitochondria structure/function, and increased nitrosylation-dependent RyR1 Ca2+ leak, a reduction in STIM1/ORAI1-mediated SOCE activity is also proposed tocontribute to age-dependent reduction in muscle specific force productionandincreasedsusceptibility to fatigue in aged muscle. In support of this idea, muscles from 2-year-old mice exhibit reduced SOCE and increased susceptibility to fatigue during high-frequency stimulation [143,144]. We recently reported that extensor digitorum longus muscles from 2-year-old mice exhibited an accelerated decline in force generation during high-frequency stimulation compared to that of muscles from 4-month-old mice [64]. Consistent with a reduced role for Ca2+ entry in aged muscle, removal of Ca2+ from the extracellular medium during repetitive high-frequency stimulation reduced contractility of muscles from young, but not aged, mice. These data are in line with findings of Thornton and colleagues who reported an inability of aged muscle to recover Ca2+ ions from the extracellular space via SOCE [63].
An age-dependent reduction in SOCE activity could lead to impaired SR Ca2+ refilling during prolonged muscle activity, and thus, lead to reduced Ca2+ availability within the SR to support force production during sustained activity. This idea is consistent with the fact that pharmacological and/or genetic inhibition of SOCE in skeletal muscle of young mice results in a reduction in force generation and increased susceptibility to fatigue [44,45,63,143]. However, the relative role of STIM1/ORAI1-mediated SOCE in age-related decline in muscle contractility remains controversial as other groups reported that: (1) the role of SOCE is marginal and/or absent in the maintenance of force generation during high-frequency repetitive stimulation [145] and (2) despite a significant reduction of STIM1 expression (~40%), SOCE activity is unaltered in muscle fibers from aged mice [146]. Clearly, additional studies are needed to fully elucidate the role of SOCE in muscle dysfunction during aging.
5. Altered Mitochondrial Function and Ca2+ Homeostasis in Muscle Atrophy
Skeletal muscle atrophy reflects the loss of muscle mass as a result of muscle disuse (e.g., bed rest, limb immobilization or unloading, space flight, mechanical ventilation, or denervation) and certain pathological conditions (cancer, diabetes, sepsis) [147]. Muscle atrophy, commonly accompanied by a loss of muscle strength, results from a net effect of decreased protein synthesis and/or increased protein degradation, with proteolysis often being a dominant contributing factor [148]. Among the multitude of proteolytic systems identified, the calpain and caspase-3 systems are Ca2+-dependent signaling pathways involved in the dynamic process of protein degradation [149] (Figure 1). Specifically, Ca2+ alloserstically activates calpainand caspase-12 to activate caspase-3, which cleaves cytoskeletal proteins to disrupt myofilaments and facilitate protein degradation. In this regard, dysregulation in myoplasmic Ca2+ levels promotes atrophy in various muscle disuse animal models and pathological conditions [150,151]. For example, a ~20–30%increase in resting myoplasmic Ca2+ was observed in soleus muscle fibers following hindlimb unloading and reloading in rats [152]. In an in vitro model of cancer cachexia, exposure to proteolysis-inducing factor triggered a transient increase in myoplasmic Ca2+ level through activation of Ca2+ release via inositol 1,4,5 trisphosphate receptors, that was proposed to activate downstream caspase-mediated proteolytic pathways [153,154]. However, further studies are needed to establish a direct causative link between myoplasmicCa2+ activation of calpain-mediated downstream proteolytic pathways in muscle atrophy during cancer cachexia.
Beyond Ca2+ dysregulation, increased oxidative stress also promotes muscle atrophy, as administration of antioxidants can prevent or alleviate atrophic responses [155]. Of note, increased SR Ca2+ leak due to oxidative stress represents an important proposed mechanism for skeletal muscle atrophy. Specifically, Matecki et al. [156] reported that long-term mechanical ventilation results in S-nitrosylation and RyR1 Ser-2844 that leads to reduced Ca2+ spark activity during electrical stimulation of the diaphragm, which was mitigated by antioxidant treatment with Trolox to prevent RyR1 oxidation, SR Ca2+ leak and diaphragm weakness. Diaphragm dysfunction was also prevented by treatment with S107, a small molecule that stabilizes the association of FKBP12 with RyR1. Similarly, S107 administration attenuated RyR1 hyper-nitrosylation and skeletal muscle atrophy in chronically hypoxic animals due to exposure to high altitude [157]. Taken together, these studies suggest that increased oxidative stress, RyR1 post-translational modification and SR Ca2+ leak contribute to certain forms of muscle atrophy and weakness.
In addition to oxidative stress-induced changes in RyR1 Ca2+ leak, oxidation of SERCA can also contribute to skeletal muscle atrophy and weakness in both humans and animals [158,159,160]. Qaisar et al. [159] reported that increased oxidative stress leads to reduced SERCA activity and mitochondrial dysfunction in SOD1-deficient mice. Consistent with this, CDN1163, an allosteric SERCA activator, restored SERCA activity, attenuated loss of muscle mass, and alleviated mitochondrial ROS production and oxidative damage [158]. Moreover, an increase of sarcolipin, a protein that regulates SERCA activity and muscle thermogenesis, coincided with reduced SERCA expressions and activity, as well as increased CASQ1 expression, lipid peroxidation, and mitochondrial ROS production in skeletal muscle of asthmatic patients [159]. Increased sarcolipin transcription and protein expression, along with increased phosphorylated Ca2+/calmodulin-dependent protein kinase II, were observed in atrophied muscles of mice following hindlimb immobilization [160]. Although measurements of myoplasmic, mitochondrial, and SR Ca2+ levels are needed, these studies suggest that increased oxidative stress signaling leading to alterations in Ca2+ homeostasis plays a significant role in certain forms of muscle atrophy and weakness.
Mitochondrial dysfunction is an additional key feature in the development and progression of skeletal muscle disuse atrophy (Figure 1). Mitochondrial alterations during disuse atrophy include reduced volume, disrupted morphology and dynamics (fusion and fission), and dysfunction including defective respiratory activity, reduced mitochondrial protein levels, and increased mitochondrial ROS production [161,162,163,164]. A critical role of increased oxidative stress due to mitochondrial dysfunction in muscle atrophy was demonstrated by amelioration of mitochondrial ROS production, muscle oxidative damage, and protease activation during disuse atrophy following treatment with the mitochondrial-targeted antioxidant peptide, SS-31 [165]. As a consequence of damage, mitochondria release apoptosis inducing factor (AIF) and cytochrome C into the cytosol, which activates caspase-3 to trigger myonuclear apoptosis and nuclear DNA fragmentation [166,167]. Indeed, denervation-induced muscle atrophy is attenuated in a double knockout model of Bax and Bak, which prevent the release of AIF and apoptosis. Together, these studies indicate that mitochondrial dysfunction leading to increased oxidative stress and activation of apoptotic pathways are important components of the signaling cascade that underlies disuse muscle atrophy.
A potential mechanism for mitochondrial dysfunction in muscle atrophy is that enhanced mitochondrial Ca2+ uptake could promote mitochondrial ROS generation that subsequently activates downstream muscle atrophy signaling pathways (Figure 1). In contrast to this idea, mitochondrial ROS production is increased in the absence of detectable changes in either myoplasmic Ca2+ concentration or mitochondrial Ca2+ uptake in flexor digitorum brevis fibers during denervation-induced muscle atrophy [168]. Electrical stimulation was able to promote mitochondrial Ca2+ uptake and reduce mitochondrial ROS production. Importantly, these effects were abolished by Ru360, an inhibitor of mitochondrial Ca2+ uptake, consistent with mitochondrial Ca2+ uptake being required to mitigate mitochondrial ROS production during denervation. As one possible mechanism, mitochondrial Ca2+ uptake could result in partial depolarization of the mitochondrial membrane potential, which would reduce mitochondrial ROS production by Complex I [169]. Consistent with these results, Mammucari et al. [170] reported that muscle atrophy following denervation was reduced by overexpression of MCU to enhance mitochondrial Ca2+ uptake. Additional work is needed to assess the relative impact of mitochondrial Ca2+ uptake and mitochondrial ROS production in other models of muscle atrophy (e.g., disuse atrophy).
6. Concluding Remarks and Future Directions
Over the past several years, substantial progress was made in elucidating exciting new insights into the role of impaired Ca2+ homeostasis and increased oxidative stress in the loss of muscle mass, increase in muscle damage, and reduction in muscle contractility that are characteristic of sarcopenia, disuse atrophy, and a wide range of muscle disorders including DMD, CCD, and heat/exercise-induced rhabdomyolysis. Nevertheless, precise molecular mechanisms are yet to be fully understood and a considerable number of unresolved issues and open questions remain to be addressed.
This review provides a comprehensive overview of the literature supporting a role for dysfunctional Ca2+ handling and Ca2+-dependent mitochondrial ROS/RNS production in the decline of muscle function during aging, muscle atrophy, and the pathogenesis of several genetically inherited muscle disorders. These advances identify several signaling pathways and molecular mechanisms that represent potential new targets for the development of more effective therapies to treat a wide range of debilitating human myopathies.
Funding
This work was supported by the following grants: (1) GGP19231 from Italian Telethon ONLUS to F.P.; (2) AR059646 from the National Institutes of Health USA to R.T.D. and a subcontract to F.P.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Abbreviations
| AIF | apoptosis inducing factor |
| ATP | adenosine triphosphate |
| Ca2+ | calcium |
| CCD | central core disease |
| CEU | Ca2+ entry unit |
| CRU | Ca2+ release unit |
| CASQ1 | calsequestrin-1 |
| DGC | dystrophin-glycoprotein complex |
| DMD | Duchenne muscular dystrophy |
| DHPR | dihydropyridine receptor |
| EC coupling | excitation-contraction coupling |
| ER | endoplasmic reticulum |
| MCU | mitochondrial Ca2+ uniporter |
| MD | musclular dystrophy |
| MH | malignant hyperthermia |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RyR1 | ryanodine receptor type-1 |
| SERCA | sarco/endoplasmic Ca2+ ATPase |
| SOCE | store-operated Ca2+ entry |
| SR | sarcoplasmic reticulum |
| STIM1 | stromal-interacting molecule 1 |
| TA | tubular aggregate |
| TAM | tubular aggregate myopathy |
| TCA | tricarboxylic acid |
| T-tubule | transverse tubule |
References
- Franzini-Armstrong, C. Structure of sarcoplasmic reticulum. Fed. Proc. 1980, 39, 2403–2409. [Google Scholar] [PubMed]
- Franzini-Armstrong, C. Freeze-fracture of frog slow tonic fibers. Structure of surface and internal membranes. Tissue Cell 1984, 16, 647–664. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Jorgensen, A.O. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 1994, 56, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.F.; Chandler, W.K. Voltage dependent charge movement in skeletal muscle: A possible step in excitation-contraction coupling. Nature 1973, 242, 244–246. [Google Scholar] [CrossRef]
- Smith, J.S.; Imagawa, T.; Ma, J.; Fill, M.; Campbell, K.P.; Coronado, R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J. Gen. Physiol. 1988, 92, 1–26. [Google Scholar] [CrossRef]
- Schneider, M.F. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 1994, 56, 463–484. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Protasi, F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 1997, 77, 699–729. [Google Scholar] [CrossRef]
- Rios, E.; Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987, 325, 717–720. [Google Scholar] [CrossRef]
- Knudson, C.M.; Chaudhari, N.; Sharp, A.H.; Powell, J.A.; Beam, K.G.; Campbell, K.P. Specific absence of the α1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J. Biol. Chem. 1989, 264, 1345–1348. [Google Scholar] [CrossRef]
- Adams, B.A.; Tanabe, T.; Mikami, A.; Numa, S.; Beam, K.G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature 1990, 346, 569–572. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Canato, M.; Scorzeto, M.; Giacomello, M.; Protasi, F.; Reggiani, C.; Stienen, G.J.M. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA 2010, 107, 22326–22331. [Google Scholar] [CrossRef] [PubMed]
- Ziman, A.P.; Ward, C.W.; Rodney, G.G.; Lederer, W.J.; Bloch, R.J. Quantitative measurement of Ca2+ in the sarcoplasmic reticulum lumen of mammalian skeletal muscle. Biophys. J. 2010, 99, 2705–2714. [Google Scholar] [CrossRef]
- Sztretye, M.; Yi, J.; Figueroa, L.; Zhou, J.; Royer, L.; Ríos, E. D4cpv-calsequestrin: A sensitive ratiometric biosensor accurately targeted to the calcium store of skeletal muscle. J. Gen. Physiol. 2011, 138, 211–229. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Wong, P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1971, 68, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. BBA Biomembr. 1975, 389, 51–68. [Google Scholar] [CrossRef]
- Campbell, K.P.; MacLennan, D.H.; Jorgensen, A.O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye “Stains-all”. J. Biol. Chem. 1983, 258, 11267–11273. [Google Scholar] [CrossRef]
- Yano, K.; Zarain-Herzberg, A. Sarcoplasmic reticulum calsequestrins: Structural and functional properties. Mol. Cell. Biochem. 1994, 135, 61–70. [Google Scholar] [CrossRef]
- Ikemoto, N.; Ronjat, M.; Mészáros, L.G.; Koshita, M. Postulated Role of Calsequestrin in the Regulation of Calcium Release from Sarcoplasmic Reticulum. Biochemistry 1989, 28, 6764–6771. [Google Scholar] [CrossRef]
- Beard, N.A.; Sakowska, M.M.; Dulhunty, A.F.; Laver, D.R. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 2002, 82, 310–320. [Google Scholar] [CrossRef]
- Beard, N.A.; Casarotto, M.G.; Wei, L.; Varsányi, M.; Laver, D.R.; Dulhunty, A.F. Regulation of ryanodine receptors by calsequestrin: Effect of high luminal Ca2+ phosphorylation. Biophys. J. 2005, 88, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Yi, J.; Figueroa, L.; Zhou, J.; Royer, L.; Allen, P.; Brum, G.; Ríos, E. Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca 2+ release in skeletal muscle. J. Gen. Physiol. 2011, 138, 231–247. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Brandl, C.J.; Korczak, B.; Green, N.M. Amino-acid sequence of a Ca2++ Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 1985, 316, 696–700. [Google Scholar] [CrossRef]
- Brandl, C.J.; Green, N.M.; Korczak, B.; MacLennan, D.H. Two Ca2+ ATPase genes: Homologies and mechanistic implications of deduced amino acid sequences. Cell 1986, 44, 597–607. [Google Scholar] [CrossRef]
- Hasselbach, W. Relaxation and the sarcotubular calcium pump. Fed. Proc. 1964, 23, 909–912. [Google Scholar] [PubMed]
- Armstrong, C.M.; Bezanilla, F.M.; Horowicz, P. Twitches in the presence of ethylene glycol bis(β-aminoethyl ether)-N,N′-tetraacetic acid. BBA Bioenerg. 1972, 267, 605–608. [Google Scholar] [CrossRef]
- Chandler, W.K.; Rakowski, R.F.; Schneider, M.F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J. Physiol. 1976, 254, 285–316. [Google Scholar] [CrossRef]
- Ríos, E.; Pizarro, G.; Stefani, E. Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu. Rev. Physiol. 1992, 54, 109–133. [Google Scholar] [CrossRef]
- Ríos, E.; Karhanek, M.; Ma, J.; González, A. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 1993, 102, 449–481. [Google Scholar] [CrossRef]
- Hopf, F.W.; Reddy, P.; Hong, J.; Steinhardt, R.A. A capacitative calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds. J. Biol. Chem. 1996, 271, 22358–22367. [Google Scholar] [CrossRef]
- Kurebayashi, N.; Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 2001, 533, 185–199. [Google Scholar] [CrossRef]
- Putney, J.W. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B.; Putney, J.W. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Won, D.H.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store- depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Lyfenko, A.D.; Dirksen, R.T. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Wei-Lapierre, L.; Carrell, E.M.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013, 4, 1–2. [Google Scholar] [CrossRef]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Oláh, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 1–2, Erratum in 2018, 8, 17463. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; García-Castañeda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse tubule remodeling enhances orai1-dependent Ca2+ entry in skeletal muscle. Elife 2019, 8, e47576. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 2020, 152, e202012617. [Google Scholar] [CrossRef] [PubMed]
- Stiber, J.; Hawkins, A.; Zhang, Z.S.; Wang, S.; Burch, J.; Graham, V.; Ward, C.C.; Seth, M.; Finch, E.; Malouf, N.; et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 2008, 10, 688–697. [Google Scholar] [CrossRef]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016, 30, 4109–4119. [Google Scholar] [CrossRef]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Launikonis, B.S.; Ríos, E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 2007, 583, 81–97. [Google Scholar] [CrossRef]
- Edwards, J.N.; Murphy, R.M.; Cully, T.R.; von Wegner, F.; Friedrich, O.; Launikonis, B.S. Ultra-rapid activation and deactivation of store-operated Ca2+ entry in skeletal muscle. Cell Calcium 2010, 47, 458–467. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Choi, R.H.; Launikonis, B.S. Store-operated Ca2+ entry is activated by every action potential in skeletal muscle. Commun. Biol. 2018, 1, 31. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; König, S.; Jousset, H.; Bader, C.; Demaurex, N.; Bernheim, L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J. Biol. Chem. 2009, 284, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Kiviluoto, S.; Decuypere, J.P.; De Smedt, H.; Missiaen, L.; Parys, J.B.; Bultynck, G. STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet. Muscle 2011, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Finch, E.A.; Graham, V.; Zhang, Z.-S.; Ding, J.-D.; Burch, J.; Oh-hora, M.; Rosenberg, P. STIM1-Ca2+ Signaling Is Required for the Hypertrophic Growth of Skeletal Muscle in Mice. Mol. Cell. Biol. 2012, 32, 3009–3017. [Google Scholar] [CrossRef]
- Böhm, J.; Chevessier, F.; De Paula, A.M.; Koch, C.; Attarian, S.; Feger, C.; Hantaï, D.; Laforêt, P.; Ghorab, K.; Vallat, J.M.; et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am. J. Hum. Genet. 2013, 92, 271–278. [Google Scholar] [CrossRef]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca2+ channels. Hum. Mol. Genet. 2015, 24, 637–648. [Google Scholar] [CrossRef]
- Edwards, J.N.; Friedrich, O.; Cully, T.R.; Von Wegner, F.; Murphy, R.M.; Launikonis, B.S. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am. J. Physiol. Cell Physiol. 2010, 299, C42–C50. [Google Scholar] [CrossRef]
- Zhao, X.; Moloughney, J.G.; Zhang, S.; Komazaki, S.; Weisleder, N. Orai1 Mediates Exacerbated Ca2+ Entry in Dystrophic Skeletal Muscle. PLoS ONE 2012, 7, e49862. [Google Scholar] [CrossRef]
- Cully, T.R.; Choi, R.H.; Bjorksten, A.R.; George Stephenson, D.; Murphy, R.M.; Launikonis, B.S. Junctional membrane Ca2+ dynamics in human muscle fibers are altered by malignant hyperthermia causative RyR mutation. Proc. Natl. Acad. Sci. USA 2018, 115, 8215–8220. [Google Scholar] [CrossRef]
- Duke, A.M.; Hopkins, P.M.; Calaghan, S.C.; Halsall, J.P.; Steele, D.S. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem. 2010, 285, 25645–25653. [Google Scholar] [CrossRef]
- Thornton, A.M.; Zhao, X.; Weisleder, N.; Brotto, L.S.; Bougoin, S.; Nosek, T.M.; Reid, M.; Hardin, B.; Pan, Z.; Ma, J.; et al. Store-Operated Ca2+ Entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Albany. N. Y.) 2011, 3, 621–634. [Google Scholar] [CrossRef]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca2+ Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 601057. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Madeira, V.M.C. Overview of mitochondrial bioenergetics. Methods Mol. Biol. 2018, 1782, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453. [Google Scholar] [CrossRef]
- Deluca, H.F.; Engstrom, G.W. Calcium uptake by rat kidney mitochondria. Proc. Natl. Acad. Sci. USA 1961, 47, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Graziotti, P. Mechanisms for intracellular calcium regulation in heart: I. stopped-flow measurements of ca++ uptake by cardiac mitochondria. J. Gen. Physiol. 1973, 62, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Crompton, M. The regulation of intracellular calcium by mitochondria. Ann. N. Y. Acad. Sci. 1978, 307, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Sembrowich, W.L.; Quintinskie, J.J.; Li, G. Calcium uptake in mitochondria from different skeletal muscle types. J. Appl. Physiol. 1985, 59, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Simpson, A.W.M.; Brini, M.; Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 1992, 358, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Mongillo, M.; Magalhäes, P.J.; Pozzan, T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 2004, 166, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.E.; Boncompagni, S.; Wei, L.; Protasi, F.; Dirksen, R.T. Differential impact of mitochondrial positioning on mitochondrial Ca2+ uptake and ca 2+ spark suppression in skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 301, C1128–C1139. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Rossi, A.E.; Boncompagni, S.; Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef]
- Favaro, G.; Romanello, V.; Varanita, T.; Andrea Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 2019, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.V.; Szabadkai, G.; Sharpe, J.A.; Parry, D.A.; Torelli, S.; Childs, A.M.; Kriek, M.; Phadke, R.; Johnson, C.A.; Roberts, N.Y.; et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 2014, 46, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Smith, D.; Kamer, K.J.; Griffin, H.; Childs, A.M.; Pysden, K.; Titov, D.; Duff, J.; Pyle, A.; Taylor, R.W.; Yu-Wai-Man, P.; et al. Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Neurol. Genet. 2016, 2, e59. [Google Scholar] [CrossRef]
- Musa, S.; Eyaid, W.; Kamer, K.; Ali, R.; Al-Mureikhi, M.; Shahbeck, N.; Al Mesaifri, F.; Makhseed, N.; Mohamed, Z.; Alshehhi, W.A.; et al. A middle eastern founder mutation expands the genotypic and phenotypic spectrum of mitochondrial MICU1 deficiency: A report of 13 patients. JIMD Rep. 2019, 43, 79–83. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 1993, 262, 744–747. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y. Scanning electron-microscopic studies on the three-dimensional structure of mitochondria in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985, 241, 251–256. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y. High -resolution scanning electron-microscopic studies on the three-dimensional structure of mitochondria and sarcoplasmic reticulum in the different twitch muscle fibers of the frog. Cell Tissue Res. 1987, 250, 489–497. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y.; Shimada, Y.; Forbes, M.S.; Hikida, R.S. Ultra-high resolution scanning electron microscopic studies on the sarcoplasmic reticulum and mitochondria in various muscles: A review. Scanning Microsc. 1993, 7, 145–156. [Google Scholar] [PubMed]
- Ogata, T.; Yamasaki, Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat. Rec. 1997, 248, 214–223. [Google Scholar] [CrossRef]
- Vendelin, M.; Béraud, N.; Guerrero, K.; Andrienko, T.; Kuznetsov, A.V.; Olivares, J.; Kay, L.; Saks, V.A. Mitochondrial regular arrangement in muscle cells: A “crystal-like” pattern. Am. J. Physiol. Cell Physiol. 2005, 288, C757–C767. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol. Biol. Cell. 2009, 20, 1058–1067. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca2+ release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Whitehead, N.P.; Pham, C.; Gervasio, O.L.; Allen, D.G. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. 2008, 586, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.J.; Tidball, J.G. Calpain translocation during muscle fiber necrosis and regeneration in dystrophin-deficient mice. Exp. Cell Res. 1996, 226, 264–272. [Google Scholar] [CrossRef]
- Whitehead, N.P.; Yeung, E.W.; Allen, D.G. Muscle damage in mdx (dystrophic) mice: Role of calcium and reactive oxygen species. Clin. Exp. Pharmacol. Physiol. 2006, 33, 657–662. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Martin, D.; Van Schoor, M.C.; Debaix, H.; Gailly, P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 2002, 158, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Goonasekera, S.A.; Sargent, M.A.; Maillet, M.; Aronow, B.J.; Molkentin, J.D. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 19023–19028. [Google Scholar] [CrossRef] [PubMed]
- Gailly, P. TRP channels in normal and dystrophic skeletal muscle. Curr. Opin. Pharmacol. 2012, 12, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Goonasekera, S.A.; Davis, J.; Kwong, J.Q.; Accornero, F.; Wei-LaPierre, L.; Sargent, M.A.; Dirksen, R.T.; Molkentin, J.D. Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum. Mol. Genet. 2014, 23, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, A.M.; Reiken, S.; Carlson, C.; Mongillo, M.; Liu, X.; Rothman, L.; Matecki, S.; Lacampagne, A.; Marks, A.R. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 2009, 15, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Meli, A.C.; Reiken, S.; Betzenhauser, M.J.; Umanskaya, A.; Shiomi, T.; D’Armiento, J.; Marks, A.R. Leaky ryanodine receptors in β-sarcoglycan deficient mice: A potential common defect in muscular dystrophy. Skelet. Muscle 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Paolini, C.; Canato, M.; Wei-Lapierre, L.; Pietrangelo, L.; De Marco, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Antioxidants protect calsequestrin-1 knockout mice from halothane- and heat-induced sudden death. Anesthesiology 2015, 123, 603–617. [Google Scholar] [CrossRef]
- Michelucci, A.; De Marco, A.; Guarnier, F.A.; Protasi, F.; Boncompagni, S. Antioxidant treatment reduces formation of structural cores and improves muscle function in RYR1Y522S/WT mice. Oxid. Med. Cell. Longev. 2017, 2017, 6792694. [Google Scholar] [CrossRef] [PubMed]
- Paolini, C.; Quarta, M.; Wei-LaPierre, L.; Michelucci, A.; Nori, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Oxidative stress, mitochondrial damage, and cores in muscle from calsequestrin-1 knockout mice. Skelet. Muscle 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet J. Rare Dis. 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Shy, G.M.; Magee, K.R. A new congenital non-progressive myopathy. Brain 1956, 79, 610–621. [Google Scholar] [CrossRef]
- Jungbluth, H. Central core disease. Orphanet J. Rare Dis. 2007, 2, 25. [Google Scholar] [CrossRef]
- Quane, K.A.; Healy, J.M.S.; Keating, K.E.; Manning, B.M.; Couch, F.J.; Palmucci, L.M.; Doriguzzi, C.; Fagerlund, T.H.; Berg, K.; Ording, H.; et al. Mutations in the ryanodine receptor gene in central core disease and malignant hyperthermia. Nat. Genet. 1993, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Denborough, M.A.; Dennett, X.; Anderson, R.M. Central-Core Disease and Malignant Hyperpyrexia. Br. Med. J. 1973, 1, 272–273. [Google Scholar] [CrossRef][Green Version]
- Dubowitz, V.; Everson Pearse, A.G. OXidative enzymes and phosphorylase in central-core disease of muscle. Lancet 1960, 276, 23–24. [Google Scholar] [CrossRef]
- Eng, G.D.; Epstein, B.S.; Engel, W.K.; McKay, D.W.; McKay, R. Malignant Hyperthermia and Central Core Disease in a Child with Congenital Dislocating Hips: Case Presentation and Review. Arch. Neurol. 1978, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.P.; Harati, Y.; Butler, I.J.; Nelson, T.E.; Scott, C.I. Central core disease and malignant hyperthermia syndrome. Ann. Neurol. 1980, 7, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, A.; Paasuke, R.T.; Brownell, A.K.W. Central core disease: Clinical features in 13 patients. Medicine 1987, 66, 389–396. [Google Scholar] [CrossRef]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum. Mutat. 2006, 27, 830. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in malignant hypertheraiia and central core disease. Hum. Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Canato, M.; Capitanio, P.; Cancellara, L.; Leanza, L.; Raffaello, A.; Reane, D.V.; Marcucci, L.; Michelucci, A.; Protasi, F.; Reggiani, C. Excessive Accumulation of Ca2+ in Mitochondria of Y522S-RYR1 Knock-in Mice: A Link Between Leak From the Sarcoplasmic Reticulum and Altered Redox State. Front. Physiol. 2019, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-Nitrosylation Underlies Environmental Heat Stroke and Sudden Death in Y522S RyR1 Knockin Mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef]
- Chelu, M.G.; Goonasekera, S.A.; Durham, W.J.; Tang, W.; Lueck, J.D.; Riehl, J.; Pessah, I.N.; Zhang, P.; Bhattacharjee, M.B.; Dirksen, R.T.; et al. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 2006, 20, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Paolini, C.; Boncompagni, S.; Canato, M.; Reggiani, C.; Protasi, F. Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia. FASEB J. 2017, 31, 3649–3662. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Hamilton, S.L.; Dirksen, R.T.; Franzini-Armstrong, C.; Protasi, F. Characterization and temporal development of cores in a mouse model of malignant hyperthermia. Proc. Natl. Acad. Sci. USA 2009, 106, 21996–22001. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; MacLennan, D.H. Malignant hyperthermia and central core disease: Disorders of Ca2+ release channels. Am. J. Med. 1998, 104, 470–486. [Google Scholar] [CrossRef]
- Avila, G.; O’Brien, J.J.; Dirksen, R.T. Excitation-Contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Avila, G.; O’Connell, K.M.S.; Dirksen, R.T. The pore region of the skeletal muscle ryanodine receptor is a primary locus for excitation-contraction uncoupling in central core disease. J. Gen. Physiol. 2003, 121, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Loy, R.E.; Dirksen, R.T.; Franzini-Armstrong, C. The I4895T mutation in the type 1 ryanodine receptor induces fiber-type specific alterations in skeletal muscle that mimic premature aging. Aging Cell 2010, 9, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Figueroa, L.; Royer, L.; Pouvreau, S.; Lee, C.S.; Volpe, P.; Nori, A.; Zhou, J.; Meissner, G.; Hamilton, S.L.; et al. Altered Ca2+ concentration, permeability and buffering in the myofibre Ca2+ store of a mouse model of malignant hyperthermia. J. Physiol. 2013, 591, 4439–4457. [Google Scholar] [CrossRef] [PubMed]
- Yarotskyy, V.; Protasi, F.; Dirksen, R.T. Accelerated Activation of SOCE Current in Myotubes from Two Mouse Models of Anesthetic- and Heat-Induced Sudden Death. PLoS ONE 2013, 8, e77633. [Google Scholar] [CrossRef]
- Evans, W.J. What is sarcopenia? J. Gerontol. Ser. A Biol. Sci. Med Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef]
- Roubenoff, R.; Hughes, V.A. Sarcopenia: Current concepts. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M716–M724. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Human aging, muscle mass, and fiber type composition. J. Gerontol. Ser. A Biol. Sci. Med Sci. 1995, 50, 11–16. [Google Scholar]
- Frontera, W.R.; Suh, D.; Krivickas, L.S.; Hughes, V.A.; Goldstein, R.; Roubenoff, R. Skeletal muscle fiber quality in older men and women. Am. J. Physiol. Cell Physiol. 2000, 279, C611–C618. [Google Scholar] [CrossRef]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Delbono, O.; O’Rourke, K.S.; Ettinger, W.H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 1995, 148, 211–222. [Google Scholar] [CrossRef]
- Renganathan, M.; Messi, M.L.; Delbono, O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 1997, 157, 247–253. [Google Scholar] [CrossRef]
- Weisleder, N.; Brotto, M.; Komazaki, S.; Pan, Z.; Zhao, X.; Nosek, T.; Parness, J.; Takeshima, H.; Ma, J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J. Cell Biol. 2006, 174, 639–645. [Google Scholar] [CrossRef]
- Weisleder, N.; Ma, J. Altered Ca2+ sparks in aging skeletal and cardiac muscle. Ageing Res. Rev. 2008, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; D’Amelio, L.; Fulle, S.; Fanò, G.; Protasi, F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 995–1008. [Google Scholar] [CrossRef]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef]
- Pietrangelo, L.; Michelucci, A.; Ambrogini, P.; Sartini, S.; Guarnier, F.A.; Fusella, A.; Zamparo, I.; Mammucari, C.; Protasi, F.; Boncompagni, S. Muscle activity prevents the uncoupling of mitochondria from Ca 2+ Release Units induced by ageing and disuse. Arch. Biochem. Biophys. 2019, 663, 22–33. [Google Scholar] [CrossRef]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, D.; Nagaraj, R.Y.; Nosek, T.A.; Nishi, M.; Takeshima, H.; Cheng, H.; Ma, J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 2002, 4, 379–383. [Google Scholar] [CrossRef]
- Zhao, X.; Weisleder, N.; Thornton, A.; Oppong, Y.; Campbell, R.; Ma, J.; Brotto, M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008, 7, 561–568. [Google Scholar] [CrossRef]
- Payne, A.M.; Jimenez-Moreno, R.; Wang, Z.M.; Messi, M.L.; Delbono, O. Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging. Exp. Gerontol. 2009, 44, 261–273. [Google Scholar] [CrossRef][Green Version]
- Edwards, J.N.; Blackmore, D.G.; Gilbert, D.F.; Murphy, R.M.; Launikonis, B.S. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell 2011, 10, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Seider, M.J. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Talbert, E.E.; Smuder, A.J.; Min, K.; Kwon, O.S.; Powers, S.K. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J. Appl. Physiol. 2013, 114, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Andrianjafiniony, T.; Dupré-Aucouturier, S.; Pouvreau, S.; Desplanches, D.; Jacquemond, V. Altered myoplasmic Ca2+ handling in rat fast-twitch skeletal muscle fibres during disuse atrophy. Pflug. Arch. Eur. J. Physiol. 2010, 459, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, C.P.; Warren, G.L.; Armstrong, R.B. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J. Appl. Physiol. 1999, 87, 386–390. [Google Scholar] [CrossRef]
- Mirza, K.A.; Tisdale, M.J. Role of Ca2+ in proteolysis-inducing factor (PIF)-induced atrophy of skeletal muscle. Cell. Signal. 2012, 24, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Mechanism of activation of dsRNA-dependent protein kinase (PKR) in muscle atrophy. Cell. Signal. 2010, 22, 783–790. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef]
- Matecki, S.; Dridi, H.; Jung, B.; Saint, N.; Reiken, S.R.; Scheuermann, V.; Mrozek, S.; Santulli, G.; Umanskaya, A.; Petrof, B.J.; et al. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc. Natl. Acad. Sci. USA 2016, 113, 9069–9074. [Google Scholar] [CrossRef]
- Agrawal, A.; Rathor, R.; Kumar, R.; Suryakumar, G.; Singh, S.N.; Kumar, B. Redox modification of ryanodine receptor contributes to impaired Ca2+ homeostasis and exacerbates muscle atrophy under high altitude. Free. Radic. Biol. Med. 2020, 160, 643–656. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Qayum, M.; Muhammad, T. Reduced sarcoplasmic reticulum Ca2+ ATPase activity underlies skeletal muscle wasting in asthma. Life Sci. 2021, 273, 119296. [Google Scholar] [CrossRef]
- Tomiya, S.; Tamura, Y.; Kouzaki, K.; Kotani, T.; Wakabayashi, Y.; Noda, M.; Nakazato, K. Cast immobilization of hindlimb upregulates sarcolipin expression in atrophied skeletal muscles and increases thermogenesis in C57BL/6J mice. Am. J. Physiol.Regul. Integr. Comp. Physiol. 2019, 317, R649–R661. [Google Scholar] [CrossRef] [PubMed]
- Kavazis, A.N.; Talbert, E.E.; Smuder, A.J.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic. Biol. Med. 2009, 46, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Song, W.; Jang, Y.C.; Liu, Y.; Sabia, M.; Richardson, A.; Van Remmen, H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1159–R1168. [Google Scholar] [CrossRef]
- Abadi, A.; Glover, E.I.; Isfort, R.J.; Raha, S.; Safdar, A.; Yasuda, N.; Kaczor, J.J.; Melov, S.; Hubbard, A.; Qu, X.; et al. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS ONE 2009, 4, e6518. [Google Scholar] [CrossRef]
- Picard, M.; Azuelos, I.; Jung, B.; Giordano, C.; Matecki, S.; Hussain, S.; White, K.; Li, T.; Liang, F.; Benedetti, A.; et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J. Appl. Physiol. 2015, 118, 1161–1171. [Google Scholar] [CrossRef]
- Min, K.; Smuder, A.J.; Kwon, O.S.; Kavazis, A.N.; Szeto, H.H.; Powers, S.K. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J. Appl. Physiol. 2011, 111, 1459–1466. [Google Scholar] [CrossRef]
- Adhihetty, P.J.; Ljubicic, V.; Menzies, K.J.; Hood, D.A. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am. J. Physiol. Cell Physiol. 2005, 289, C994–C1001. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.F.N.; Vainshtein, A.; Carter, H.N.; Zhang, Y.; Hood, D.A. Denervation-induced mitochondrial dysfunction and autophagy in skeletal muscle of apoptosis-deficient animals. Am. J. Physiol. Cell Physiol. 2012, 303, C447–C454. [Google Scholar] [CrossRef]
- Karam, C.; Yi, J.; Xiao, Y.; Dhakal, K.; Zhang, L.; Li, X.; Manno, C.; Xu, J.; Li, K.; Cheng, H.; et al. Absence of physiological Ca2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skelet. Muscle 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Krysiewicz, S.; Mellinger, B.C. The role of imaging in the diagnostic evaluation of impotence. Am. J. Roentgenol. 1989, 153, 1133–1139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).