Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome
Abstract
1. Introduction
2. Results
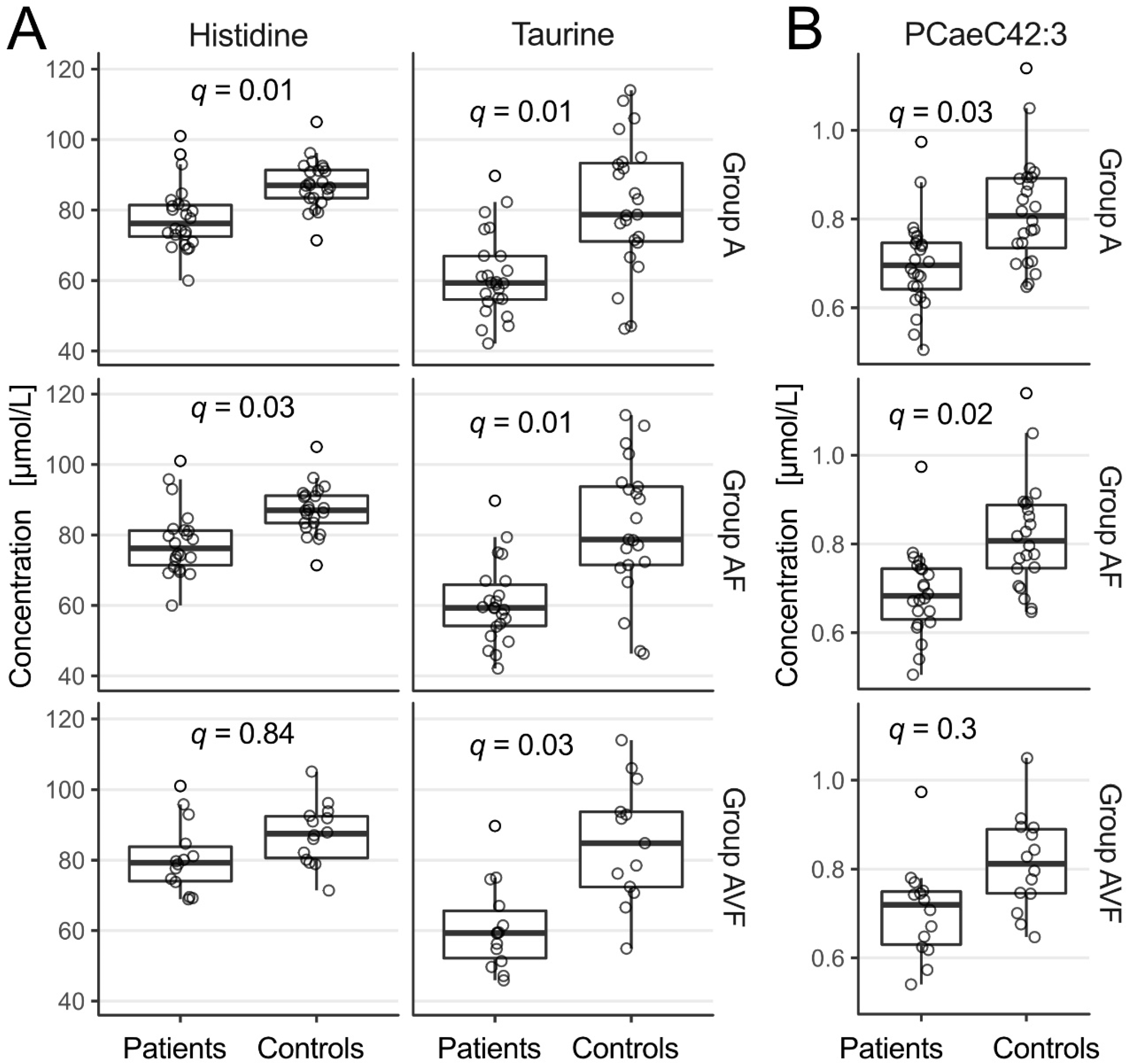
2.1. Amino Acids
2.2. Phospholipids and Acylcarnitines
2.3. Univariate Correlation of Routine Biochemical and Clinical Findings with Metabolomic Parameters
2.4. Multiple Regression Analysis of Metabolomics Parameters with Respect to Group Assignment
3. Discussion
3.1. Main Findings
3.2. Inflammation, Oxidative Stress, Endothelial Function
3.3. Myocardial Function
3.4. Limitations, Confounders
4. Materials and Methods
4.1. Study Design, Inclusion and Exclusion Criteria
4.2. Outcome
4.3. Routine Examination
Sample Preparation and Metabolomics Analysis
4.4. Statistics
4.5. Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marfan, B.J.A. An instance of congenital deformity of all four limbs, more prominent distally, characterised by lengthening of the bones with some increase in gracility. Bull. Mem. Soc. Med. Hosp. Paris 1896, 13, 220–226. [Google Scholar]
- Groth, K.A.; Hove, H.; Kyhl, K.; Folkestad, L.; Gaustadnes, M.; Vejlstrup, N.; Stochholm, K.; Østergaard, J.R.; Andersen, N.H.; Gravholt, C.H. Prevalence, incidence, and age at diagnosis in Marfan syndrome. Orphanet J. Rare Dis. 2015, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Hayward, C.; Keston, M.; Brock, D.J.; Dietz, H.C. Fibrillin (FBN1) mutations in Marfan syndrome. Hum. Mutat. 1992, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.C. TGF-beta in the pathogenesis and prevention of disease: A matter of aneurysmic proportions. J. Clin. Investig. 2010, 120, 403–407. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; Backer, J.D.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.F.; Devos, D.; Segers, P.; Matthys, D.; François, K.; Gillebert, T.C.; De Paepe, A.M.; De Sutter, J. Primary impairment of left ventricular function in Marfan syndrome. Int. J. Cardiol. 2006, 112, 353–358. [Google Scholar] [CrossRef]
- Rybczynski, M.; Koschyk, D.H.; Aydin, M.A.; Robinson, P.N.; Brinken, T.; Franzen, O.; Berger, J.; Hofmann, T.; Meinertz, T.; von Kodolitsch, Y. Tissue Doppler imaging identifies myocardial dysfunction in adults with Marfan syndrome. Clin. Cardiol. 2007, 30, 19–24. [Google Scholar] [CrossRef]
- Das, B.B.; Taylor, A.L.; Yetman, A.T. Left ventricular diastolic dysfunction in children and young adults with Marfan syndrome. Pediatr. Cardiol. 2006, 27, 256–258. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Moggridge, J.C.; Bijnens, B.H.; Kapetanakis, V.; Alpendurada, F.; Mullen, M.J.; Saha, S.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Biventricular and atrial diastolic function assessment using conventional echocardiography and tissue-Doppler imaging in adults with Marfan syndrome. Eur. J. Echocardiogr. 2009, 10, 947–955. [Google Scholar] [CrossRef][Green Version]
- Kiotsekoglou, A.; Saha, S.; Moggridge, J.C.; Kapetanakis, V.; Govindan, M.; Alpendurada, F.; Mullen, M.J.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Impaired biventricular deformation in Marfan syndrome: A strain and strain rate study in adult unoperated patients. Echocardiography 2011, 28, 416–430. [Google Scholar] [CrossRef]
- Mas-Stachurska, A.; Siegert, A.M.; Batlle, M.; Del Blanco, D.G.; Meirelles, T.; Rubies, C.; Bonorino, F.; Serra-Peinado, C.; Bijnens, B.; Baudin, J.; et al. Cardiovascular benefits of moderate exercise training in Marfan syndrome: Insights from an animal model. J. Am. Heart Assoc. 2017, 6, e006438. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.J.; Petrashevskaya, N.; Marshall, S.; Krawczyk, M.; Talan, M. Cardiac remodeling in the mouse model of Marfan syndrome develops into two distinctive phenotypes. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H290–H299. [Google Scholar] [CrossRef] [PubMed]
- Agg, B.; Benke, K.; Szilveszter, B.; Pólos, M.; Daróczi, L.; Odler, B.; Nagy, Z.B.; Tarr, F.; Merkely, B.; Szabolcs, Z. Possible extracardiac predictors of aortic dissection in Marfan syndrome. BMC Cardiovasc. Disord. 2014, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, O.; Del Blanco, D.G.; González-Santamaría, J.; Habashi, J.P.; Calderon, J.F.; Sandoval, P.; Bedja, D.; Guinea-Viniegra, J.; Lopez-Cabrera, M.; Rosell-Garcia, T.; et al. Elevated expression levels of lysyl oxidases protect against aortic aneurysm progression in Marfan syndrome. J. Mol. Cell. Cardiol. 2015, 85, 48–57. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Guo, G.; Booms, P.; Halushka, M.; Dietz, H.C.; Ney, A.; Stricker, S.; Hecht, J.; Mundlos, S.; Robinson, P.N. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation 2006, 114, 1855–1862. [Google Scholar] [CrossRef]
- Guo, G.; Gehle, P.; Doelken, S.; Martin-Ventura, J.L.; von Kodolitsch, Y.; Hetzer, R.; Robinson, P.N. Induction of macrophage chemotaxis by aortic extracts from patients with Marfan syndrome is related to elastin binding protein. PLoS ONE 2011, 6, e20138. [Google Scholar] [CrossRef]
- He, R.; Guo, D.C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678. [Google Scholar] [CrossRef]
- Radonic, T.; de Witte, P.; Groenink, M.; de Waard, V.; Lutter, R.; van Eijk, M.; Jansen, M.; Timmermans, J.; Kempers, M.; Scholte, A.J.; et al. Inflammation aggravates disease severity in Marfan syndrome patients. PLoS ONE 2012, 7, e32963. [Google Scholar] [CrossRef]
- Oller, J.; Méndez-Barbero, N.; Ruiz, E.J.; Villahoz, S.; Renard, M.; Canelas, L.I.; Briones, A.M.; Alberca, R.; Lozano-Vidal, N.; Hurlé, M.A.; et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 2017, 23, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cheng, M.L.; Liu, M.H. Amino acid-based metabolic panel provides robust prognostic value additive to B-natriuretic peptide and traditional risk factors in heart failure. Dis. Markers 2018, 2018, 3784589. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Shimada-Takaura, K.; Jong, C.J.; Ito, T.; Takahashi, K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids 2016, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Dubowy, K.O.; Zlamy, M.; Karall, D.; Adam, M.G.; Entenmann, A.; Keller, M.A.; Koch, J.; Komazec, I.O.; Geiger, R.; et al. Targeted metabolomic analysis of serum phospholipid and acylcarnitine in the adult Fontan patient with a dominant left ventricle. Ther. Adv. Chronic Dis. 2020, 11, 2040622320916031. [Google Scholar] [CrossRef]
- Michel, M.; Dubowy, K.O.; Entenmann, A.; Karall, D.; Adam, M.G.; Zlamy, M.; Komazec, I.O.; Geiger, R.; Niederwanger, C.; Salvador, C.; et al. Targeted metabolomic analysis of serum amino acids in the adult Fontan patient with a dominant left ventricle. Sci. Rep. 2020, 10, 8930. [Google Scholar] [CrossRef] [PubMed]
- Cedars, A.; Manlhiot, C.; Ko, J.M.; Bottiglieri, T.; Arning, E.; Weingarten, A.; Opotowsky, A.; Kutty, S. Metabolomic profiling of adults with congenital heart disease. Metabolites 2021, 11, 525. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Yoss, E.B.; Spannhake, E.W.; Flynn, J.T.; Fish, J.E.; Peters, S.P. Arachidonic acid metabolism in normal human alveolar macrophages: Stimulus specificity for mediator release and phospholipid metabolism, and pharmacologic modulation in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 1990, 2, 69–80. [Google Scholar] [CrossRef]
- Chen, G.; Nan, C.; Tian, J.; Jean-Charles, P.; Li, Y.; Weissbach, H.; Huang, X.P. Protective effects of taurine against oxidative stress in the heart of MsrA knockout mice. J. Cell. Biochem. 2012, 113, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Doppler, C.; Arnhard, K.; Dumfarth, J.; Heinz, K.; Messner, B.; Stern, C.; Koal, T.; Klavins, K.; Danzl, K.; Pitterl, F.; et al. Metabolomic profiling of ascending thoracic aortic aneurysms and dissections—Implications for pathophysiology and biomarker discovery. PLoS ONE 2017, 12, e0176727. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Becatti, M.; Attanasio, M.; Lucarini, L.; Nassi, N.; Evangelisti, L.; Porciani, M.C.; Nassi, P.; Gensini, G.F.; Abbate, R.; et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int. J. Cardiol. 2010, 145, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Visser, M.; Paulus, W.J.; Vermeulen, M.A.; Richir, M.C.; Davids, M.; Wisselink, W.; de Mol, B.A.; van Leeuwen, P.A. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur. J. Heart Fail. 2010, 12, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.; Nielsen, C.; Alex, R.; Cooper, K.; Farney, M.; Gaufin, D.; Cui, J.Z.; van Breemen, C.; Broderick, T.L.; Vallejo-Elias, J.; et al. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J. Appl. Physiol. 2017, 123, 147–160. [Google Scholar] [CrossRef]
- Jud, P.; Hafner, F.; Verheyen, N.; Meinitzer, A.; Gary, T.; Brodmann, M.; Seinost, G.; Hackl, G. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci. Rep. 2018, 8, 14197. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Mügge, A.; Kienke, S.; Brandes, R.; Dwenger, A.; Frölich, J.C. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis 1995, 117, 273–284. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Bode-Böger, S.M.; Frölich, J.C.; Ritz, E.; Haller, H.; Fliser, D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 2003, 107, 1891–1895. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Cooke, J.P. Does ADMA cause endothelial dysfunction? Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Ito, T.; Schaffer, S.W. The ubiquitin-proteasome system and autophagy are defective in the taurine-deficient heart. Amino Acids 2015, 47, 2609–2622. [Google Scholar] [CrossRef]
- Gehle, P.; Robinson, P.N.; Heinzel, F.; Edelmann, F.; Yigitbasi, M.; Berger, F.; Falk, V.; Pieske, B.; Wellnhofer, E. NT-proBNP and diastolic left ventricular function in patients with Marfan syndrome. Int. J. Cardiol. Heart Vasc. 2016, 12, 15–20. [Google Scholar] [CrossRef]
- van der Palen, R.L.; Barker, A.J.; Bollache, E.; Garcia, J.; Rose, M.J.; van Ooij, P.; Young, L.T.; Roest, A.A.; Markl, M.; Robinson, J.D.; et al. Altered aortic 3D hemodynamics and geometry in pediatric Marfan syndrome patients. J. Cardiovasc. Magn. Reson. 2017, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Karur, G.R.; Pagano, J.J.; Bradley, T.; Lam, C.Z.; Seed, M.; Yoo, S.J.; Grosse-Wortmann, L. Diffuse myocardial fibrosis in children and adolescents with Marfan syndrome and Loeys-Dietz syndrome. J. Am. Coll. Cardiol. 2018, 72, 2279–2281. [Google Scholar] [CrossRef]
- Saura, M.; Zaragoza, C.; Herranz, B.; Griera, M.; Diez-Marqués, L.; Rodriguez-Puyol, D.; Rodriguez-Puyol, M. Nitric oxide regulates transforming growth factor-beta signaling in endothelial cells. Circ. Res. 2005, 97, 1115–1123. [Google Scholar] [CrossRef]
- Paglia, G.; Del Greco, F.M.; Sigurdsson, B.B.; Rainer, J.; Volani, C.; Hicks, A.A.; Pramstaller, P.P.; Smarason, S.V. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin. Chim. Acta 2018, 486, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Siskos, A.P.; Jain, P.; Römisch-Margl, W.; Bennett, M.; Achaintre, D.; Asad, Y.; Marney, L.; Richardson, L.; Koulman, A.; Griffin, J.L.; et al. Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal. Chem. 2017, 89, 656–665. [Google Scholar] [CrossRef]

| Patient # | Variant of FBN1 Point Mutation | Protein Phenotype | Predicted Protein Phenotype | Frequency in UMD-FBN1 Database | Pathogenicity |
|---|---|---|---|---|---|
| 1 | c.1754G > A | p.G585E | Missense, loss of function | 0 | class 4 |
| 2 | c.6453C > A | p.C2151Ter | Stop codon at p.2151 | 1 | class 5 |
| 3 | c.508delT | p.Y170Tfs*20 | Frameshift with stop codon at p.189 | 1 | class 5 |
| 4 | c.508delT | p.Y170Tfs*20 | Frameshift with stop codon at p.189 | 1 | class 5 |
| 5 | c.7801C > T | p.Q2601* | Missense, loss of function | 2 | class 5 |
| 6 | c.4172G > T | p.C1391F | Disulfide bond interruption | 1 | class 5 |
| 7 | |||||
| 8 | |||||
| 9 | c.732dupT | p.Q245STerfs*5 | Frameshift with stop codon at p.248 | 0 | class 4 |
| 10 | c.7801C > T | p.Q2601* | Missense, loss of function | 2 | class 5 |
| 11 | c.7801C > T | p.Q2601* | Missense, loss of function | 2 | class 5 |
| 12 | IVS14-2A > G | Intron 14 SNP | Invariant AG acceptor splice site mutation | 0 | class 5 |
| 13 | c.6453C > A | p.C2151Ter | Stop codon at p.2151 | 1 | class 5 |
| 14 | IVS45+3insCC | Intron 45 insert | Loss of exon 45 | 0 | class 4 |
| 15 | c.6313+5G > A | Intron 51 SNP | Splice error, exon 50 | 3 | class 4 |
| 16 | c.5840G > T | p.C1947F | Disulfide bond interruption | 1 | class 5 |
| 17 | c.6675T > A | p.Y2225* | Stop codon at p.2225 | 0 | class 4 |
| 18 | c.2638G > A | p.G880S | Missense, loss of function | 12 | class 4 |
| 19 | c.732dupT | p.Q245STerfs*5 | Frameshift with stop codon at p.248 | 0 | class 4 |
| 20 | c.732dupT | p.Q245STerfs*5 | Frameshift with stop codon at p.248 | 0 | class 4 |
| 21 | c.6313+5G > A | Intron 51 SNP | Splice error, exon 50 | 3 | class 4 |
| 22 | c.4202delC | p.T1402Terfs*11 | Frameshift with stop codon at p.1405 | 1 | class 4 |
| 23 | |||||
| 24 | c.4816G > A | p.D1606N | Splice error, exon 39 | 3 | class 4 |
| - | - | Group A | Group AF | Group AFV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | p | Patients | Controls | p | Patients | Controls | p | ||
| Total [n] | 24 | 24 | 22 | 22 | 14 | 14 | ||||
| Female sex [n] | 13 | 13 | 12 | 12 | 7 | 7 | ||||
| Age [ys] | 37 ± 13 | 37 ± 13 | 0.9 | 38 ± 13 | 38 ± 13 | 0.9 | 36 ± 11 | 36 ± 11 | 0.9 | |
| Bodyweight [kg] | 77 ± 13 | 74 ± 15 | 0.4 | 75 ± 13 | 74 ± 16 | 0.7 | 79 ± 12 | 72 ± 14 | 0.1 | |
| Height [cm] | 185 ± 9 | 174 ± 8 | 0.00 | 184 ± 9 | 174 ± 8 | 0.00 | 187 ± 9 | 174 ± 9 | 0.00 | |
| BMI [kg/m²] | 22 ± 3 | 24 ± 4 | 0.1 | 22 ± 3 | 24 ± 4 | 0.08 | 22 ± 3 | 23 ± 4 | 0.6 | |
| BP systolic [mm Hg] | 115 ± 10 | 122 ± 9 | 0.02 | 115 ± 10 | 121 ± 9 | 0.06 | 115 ± 11 | 118 ± 9 | 0.5 | |
| BP diastolic [mm Hg] | 81 ± 8 | 79 ± 8 | 0.3 | 81 ± 7 | 78 ± 8 | 0.2 | 80 ± 8 | 78 ± 9 | 0.6 | |
| HR at rest [1/min] | 57 ± 9 | 57 ± 10 | 56 ± 8 | |||||||
| SpO2 at rest [%] | 99 ± 1 | 99 ± 1 | 99 ± 1 | |||||||
| Cardiac manif. [n] | Aortic dilation | 22 | 22 | 13 | ||||||
| AR | 2 | 2 | 2 | |||||||
| MV prolapse | 17 | 15 | 10 | |||||||
| MR | 1 | 1 | 1 | |||||||
| Cardiac procedure [n] | Bentall | 3 | 3 | 2 | ||||||
| David | 12 | 12 | 6 | |||||||
| Stent graft thor. aorta | 1 | 1 | 1 | |||||||
| Repl. thor. aorta | 1 | 1 | 0 | |||||||
| MV reconstruction | 1 | 1 | 0 | |||||||
| PM implantation | 1 | 1 | 1 | |||||||
| EC manifestation [n] | Facial features | |||||||||
| Dolichocephaly | 15 | 13 | 8 | |||||||
| Enophthalmos | 3 | 3 | 2 | |||||||
| Retrognathia | 6 | 6 | 5 | |||||||
| High arched palate | 20 | 18 | 11 | |||||||
| Malocclusion | 1 | 1 | 1 | |||||||
| Crowding of teeth | 2 | 2 | 0 | |||||||
| Skeleton | ||||||||||
| Arachnodactyly | 21 | 19 | 11 | |||||||
| Steinberg positive | 20 | 18 | 11 | |||||||
| Murdoch positive | 19 | 18 | 11 | |||||||
| Joint hypermobility | 10 | 10 | 7 | |||||||
| Pectus carinatum | 7 | 6 | 3 | |||||||
| Pectus excavatum | 8 | 7 | 5 | |||||||
| Scoliosis | 17 | 16 | 10 | |||||||
| Kyphosis | 6 | 6 | 4 | |||||||
| Spinal disc herniation | 1 | 1 | 1 | |||||||
| Protrusio acetabuli | 9 | 9 | 5 | |||||||
| Coxarthrosis | 2 | 2 | 1 | |||||||
| Hindfoot deformity | 11 | 10 | 6 | |||||||
| Hallux valgus | 6 | 6 | 3 | |||||||
| Skin | ||||||||||
| Striae | 21 | 21 | 13 | |||||||
| Eyes | ||||||||||
| Myopia | 11 | 11 | 8 | |||||||
| Astigmatismus | 3 | 3 | 2 | |||||||
| Cataracta senilis | 3 | 3 | 2 | |||||||
| Strabismus conv. | 1 | 1 | 0 | |||||||
| Subluxatio lentis | 4 | 3 | 3 | |||||||
| Ectopia lentis | 6 | 6 | 4 | |||||||
| Iridodonesis | 3 | 3 | 1 | |||||||
| Vitreous prolapse | 1 | 1 | 1 | |||||||
| Ablatio retinae | 2 | 2 | 1 | |||||||
| Chest | ||||||||||
| Pneumothorax | 2 | 2 | 1 | |||||||
| CNS | ||||||||||
| Tarlov cyst | 1 | 1 | 1 | |||||||
| EC procedures [n] | Hip prosthesis | 2 | 2 | 1 | ||||||
| Scoliosis surgery | 2 | 1 | 1 | |||||||
| Pigeon chest correction | 1 | 1 | 1 | |||||||
| Pectus excavatus surg. | 2 | 2 | 1 | |||||||
| Lens enucleation | 1 | 1 | 0 | |||||||
| Lentectomie | 1 | 1 | 1 | |||||||
| Phacoemulsification | 2 | 2 | 1 | |||||||
| Further disease [n] | Morbus Scheuermann | 1 | 1 | 1 | ||||||
| Spondyloarthrosis | 1 | 1 | 1 | |||||||
| Knee valgus | 3 | 3 | 2 | |||||||
| Baker cyst | 1 | 1 | 1 | |||||||
| Metatarsalgia | 2 | 2 | 2 | |||||||
| Lyme arthritis | 1 | 1 | 1 | |||||||
| Varicose veins | 1 | 1 | 0 | |||||||
| Cerebral infarction | 2 | 2 | 1 | |||||||
| Hyperopia | 1 | 1 | 1 | |||||||
| Exopthalmus | 1 | 1 | 1 | |||||||
| Further procedure [n] | Cesarean section | 2 | 2 | 2 | ||||||
| Cruciate ligament surg. | 2 | 2 | 2 | |||||||
| Meniscus surg. | 1 | 1 | 1 | |||||||
| Tooth regulation | 6 | 6 | 4 | |||||||
| Vein stripping | 1 | 1 | 0 | |||||||
| Orchidopexy | 1 | 1 | 1 | |||||||
| Dietary intake [n] | 0 | 0 | 0 | |||||||
| Medication | ||||||||||
| ACE inhibitor [n] | 1 | 1 | 1 | |||||||
| Betablocker [n] | 17 | 16 | 8 | |||||||
| Aldosterone ant. [n] | ||||||||||
| AT-II rec. ant. [n] | 15 | 15 | 8 | |||||||
| Statin | 2 | 2 | 0 | |||||||
| Echo/CMR | ||||||||||
| EF < 55% or FS < 27% | 2 | 0 | 0 | |||||||
| DD * | 10 | 8 | 5 | |||||||
| ≤mild AVR [n] | 14 | 14 | 14 | |||||||
| ≤mild AR [n] | 14 | 14 | 14 | |||||||
| Aortic root [mm] | 33 ± 5.7 | 37 ± 5.1 | 35 ± 4 | |||||||
| Aortic root [z-score] | 2.1 ± 1.7 | 2.1 ± 1.7 | 1.1 ± 1.3 | |||||||
| Asc. aorta [mm] | 30 ± 5.8 | 30 ± 5.6 | 30 ± 4.6 | |||||||
| Asc. aorta [z-score] | 1.4 ± 1.9 | 1.7 ± 1.8 | 1.4 ± 1.7 | |||||||
| ECG | ||||||||||
| Incomplete RBBB [n] | 9 | 9 | 7 | |||||||
| AVB grade I [n] | 1 | 1 | 1 | |||||||
| QTc prolongation [n] | 2 | 1 | 1 | |||||||
| Laboratory Value | Group A | p | Group AF | p | Group AFV | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | ||||
| CRP [mg/dL] | 0.29 ± 0.47 | 0.14 ± 0.19 | 0.159 | 0.35 ± 0.5 | 0.15 ± 0.19 | 0.215 | 0.44 ± 0.6 | 0.08 ± 0.07 | 0.08 |
| NT-proBNP [pg/mL] | 144 ± 103 | 37.4 ± 30.9 | 0.00 | 156.9 ± 107.4 | 24.9 ± 31.3 | 0.00 | 153.5 ± 99.1 | 30.5 ± 20.6 | 0.002 |
| Total cholesterol [mg/dL] | 192 ± 31 | 192.5 ± 32 | 195.9 ± 27.2 | ||||||
| HDL-C [mg/dL] | 55 ± 14 | 54.5 ± 14.2 | 54.43 ± 14.9 | ||||||
| Non-HDL-C [mg/dL] | 137 ± 32 | 138 ± 32.7 | 141.5 ± 22.4 | ||||||
| Triglycerides [mg/dL] | 129 ± 54 | 129.8 ± 55.7 | 132.6 ± 52.3 | ||||||
| Total protein [g/dL] | 7.2 ± 0.3 | 7.2 ± 0.3 | 7.1 ± 0.38 | ||||||
| Uric acid [mg/dL] | 5.1 ± 1.1 | 5 ± 1.2 | 5.2 ± 1.0 | ||||||
| Urea [mg/dL] | 29.0 ±6.7 | 28.8 ± 6.9 | 28.0 ± 6.5 | ||||||
| Creatinine [mg/dL] | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.17 | ||||||
| Total bilirubin [mg/dL] | 0.67 ± 0.5 | 0.6 ± 0.5 | 0.7 ± 0.6 | ||||||
| AST [U/L] | 20.9 ± 5.6 | 21.3 ± 5.6 | 20.6 ± 6.8 | ||||||
| ALT [U/L] | 19.8 ± 10.2 | 20 ± 10.8 | 21.9 ± 12.8 | ||||||
| gGT [U/L] | 28.1 ± 26.7 | 28.9 ± 28.3 | 26.86 ± 19.9 | ||||||
| Alkaline phosphatase [U/L] | 62.9 ± 15.3 | 64.4 ± 15 | 62.71 ± 12.6 | ||||||
| Leucocytes [1/nL] | 5.8 ± 1.2 | 5.8 ± 1.2 | 5.6 ± 1.3 | ||||||
| Thrombocytes [1000/nL] | 253 ± 64 | 258.3 ± 64.2 | 229.2 ± 44.4 | ||||||
| Hemoglobin [g/dL] | 140.9 ± 11.3 | 141.2 ± 11.6 | 143 ± 9.6 | ||||||
| Hematocrit [%] | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartenbach, L.; Karall, T.; Koch, J.; Keller, M.A.; Oberacher, H.; Scholl-Bürgi, S.; Karall, D.; Oemer, G.; Baumgartner, D.; Meinel, K.; et al. Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome. Metabolites 2021, 11, 805. https://doi.org/10.3390/metabo11120805
Bartenbach L, Karall T, Koch J, Keller MA, Oberacher H, Scholl-Bürgi S, Karall D, Oemer G, Baumgartner D, Meinel K, et al. Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome. Metabolites. 2021; 11(12):805. https://doi.org/10.3390/metabo11120805
Chicago/Turabian StyleBartenbach, Lisa, Thomas Karall, Jakob Koch, Markus Andreas Keller, Herbert Oberacher, Sabine Scholl-Bürgi, Daniela Karall, Gregor Oemer, Daniela Baumgartner, Katharina Meinel, and et al. 2021. "Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome" Metabolites 11, no. 12: 805. https://doi.org/10.3390/metabo11120805
APA StyleBartenbach, L., Karall, T., Koch, J., Keller, M. A., Oberacher, H., Scholl-Bürgi, S., Karall, D., Oemer, G., Baumgartner, D., Meinel, K., Aly, S., Odri-Komazec, I., Geiger, R., & Michel, M. (2021). Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome. Metabolites, 11(12), 805. https://doi.org/10.3390/metabo11120805







