Abstract
Cardiometabolic diseases (CMD) represent a growing socioeconomic burden and concern for healthcare systems worldwide. Improving patients’ metabolic phenotyping in clinical practice will enable clinicians to better tailor prevention and treatment strategy to individual needs. Recently, elevated levels of specific lipid species, known as ceramides, were shown to predict cardiometabolic outcomes beyond traditional biomarkers such as cholesterol. Preliminary data showed that physical activity, a potent, low-cost, and patient-empowering means to reduce CMD-related burden, influences ceramide levels. While a single bout of physical exercise increases circulating and muscular ceramide levels, regular exercise reduces ceramide content. Additionally, several ceramide species have been reported to be negatively associated with cardiorespiratory fitness, which is a potent health marker reflecting training level. Thus, regular exercise could optimize cardiometabolic health, partly by reversing altered ceramide profiles. This short review provides an overview of ceramide metabolism and its role in cardiometabolic health and diseases, before presenting the effects of exercise on ceramides in humans.
1. Introduction
According to the Global Burden of Diseases, Injuries and Risk Factor Study, the rate of mortality from noncommunicable diseases (NCD) has increased from 1990 to 2019, to account for 74% of total mortality [1,2]. Strikingly, about one-half of NCD-related deaths are still attributable to cardiometabolic diseases (CMD), encompassing cardiovascular diseases (CVD), type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD) [3]. Whereas age-standardized death rates for CMD have decreased since 1990 in high-income countries, absolute number of CMD-related deaths are still increasing in these regions, mainly due to population aging [4]. Simultaneously, about two thirds of all CMD-related deaths are now occurring in low- and middle-income countries [5]. To combat the growing burden of CMD worldwide, it is urgent to improve patients’ stratification with respect to cardiometabolic health decline and CMD onset, which will enable the development of more precise and personalized prevention and treatment strategies [5].
Blood lipid analysis has been part of cardiometabolic risk stratification strategy since the middle of the last century [6]. Despite technological advances in mass-spectrometry and bioinformatics, clinical lipid analysis is still mostly restricted to total triglycerides, cholesterol, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) [7,8]. Although these biomarkers provide an acceptable risk assessment, growing evidence shows that lower-abundant specific sphingolipid species, known as ceramides, predict cardiometabolic outcomes more precisely in patients with and without coronary artery disease [9,10,11,12]. As ceramides could eventually replace cholesterol in clinical practice [13], it is important to determine whether and how physical activity, as a powerful prevention and treatment strategy, can influence ceramide levels.
Regular physical activity is, indeed, essential to maintain general health [14,15,16,17], prevent and treat CVD [18,19,20,21], insulin resistance and T2DM [22,23,24] as well as NAFLD [25]. Adding to its appeal, physical activity is a simple, low-cost and patient-empowering means to reduce CMD-related burden in low-income, middle-income, and high-income countries [26]. Exercise, as a subset of physical activity that is structured in order to improve or maintain physical fitness [27], not only mitigates traditional risk factors for CVD but also directly improves cardiometabolic health. For instance, exercise optimizes vascular endothelial function, stimulates secretion of cardioprotective myokines, but also acts through other mechanisms not fully elucidated yet, which might include changes in ceramide metabolism [19]. This short review provides an overview on ceramide metabolism and its role in human cardiometabolic health and diseases before presenting the effects of physical exercise on ceramides.
2. Ceramide Metabolism
Ceramides are synthesized through three major metabolic pathways: the de novo pathway that mainly results from the combination of serine and palmitoyl-CoA in the endoplasmic reticulum, the salvage pathway, which consists of the breakdown of sphingomyelins or glycosphingolipids in lysosomes, and the sphingomyelinase pathway, where sphingomyelins are converted into ceramides in cell membrane (Figure 1) [28,29]. Due to the specific subcellular localization of the enzymes controlling ceramides metabolism, the latter is highly compartmentalized [29].
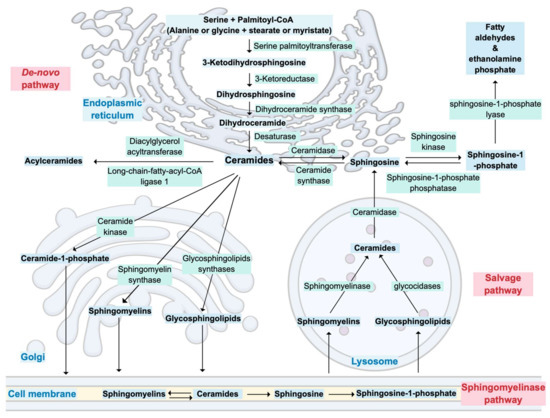
Figure 1.
Overview of ceramide metabolism and its cellular compartmentalization.
In the Golgi apparatus, ceramides are transformed into various complex sphingolipids such as ceramide-1-phosphate, sphingomyelin or glycosphingolipids [28,29] Ceramides can also be acylated to form acylceramides, which accumulate in intracellular lipid droplets [29,30]. Finally, the only ‘exit’ from ceramide metabolism results in the production of fatty aldehydes and ethanolamine phosphate by the action of a sphingosine-1-phosphate (S1P) lyase on sphingoid base phosphates [29].
Less frequently, substrates other than serine and palmitoyl-CoA, such as alanine or glycine plus stearate or myristate, can also enter the de novo pathway [29]. This leads to the formation of structurally different sphingoid bases, namely deoxysphingoid and 1-deoxymethyl sphingoid bases [29]. Distinct isoforms of ceramide synthases also add variations in the acyl groups of sphingoid bases, which further increases diversity and complexity of ceramides structures [29]. Additionally, hydroxylases and desaturases can modify acyl groups; thus, ceramides should be regarded as a family of related but structurally distinct lipid species [29]. These structural variations are at the origin of the distinct biological functions attributed to the different ceramide species [29].
3. Ceramides in Cardiometabolic Health and Diseases
Ceramides and their derivatives are involved in many, if not all, essential cellular processes such as cell growth, cell adhesion and migration, senescence, apoptosis, inflammation, immune responses, and angiogenesis [28,29]. They are also part of cell membrane, where they influence vesicular transport, membrane receptors, and exosome secretion [29]. Thus, ceramides are implicated in both intracellular and cell-to-cell signaling [31]. Ceramides synthesis and accumulation are influenced by multiple factors, which include excessive supply of substrates, systemic inflammation, oxidative stress and the microbiome [31]. In obesity, levels of Tumor Necrosis Factor-Alpha (TNF-α) are elevated, and Toll-like receptors (TLRs) are stimulated by excess of fatty acids [32]. Both TNF-α and TLRs can stimulate ceramide synthesis enzymes [32]. Ceramides will then activate inflammasomes (specifically the NLRP3 inflammasome in adipocytes), which lead to an increase in pro-inflammatory cytokine secretion [32]. This inflammatory response can be modulated by the adipokine adiponectin through activation of the ceramidase activity of the adiponectin receptors [33]. Therefore, the many roles played by ceramides in cardiometabolic health and diseases are associated with this connection between excessive supply of lipids and inflammation [32].
Changes in ceramide levels have been observed in diverse pathological processes such as NAFLD [34,35], obesity [36,37], insulin resistance [38,39], vascular inflammation, and atherosclerosis (Figure 2) [40,41,42]. Patients suffering from NAFLD display elevated levels of circulating and liver ceramides [34]. Hepatocellular ceramides stimulate fatty acid uptake, by increasing the translocation of fatty acid transporters to the plasma membrane, and facilitate their transformation into intracellular triglycerides [34]. Ceramides also decrease glucose uptake, by inhibiting Akt/protein kinase B (PKB) activity, which inhibits translocation of glucose transporters (GLUT4) to the cell membrane and reduce hepatic gluconeogenesis [34,39,43]. This enables hepatocytes to favor fatty acids over glucose as a preferred energy source and leads to NAFLD [34].
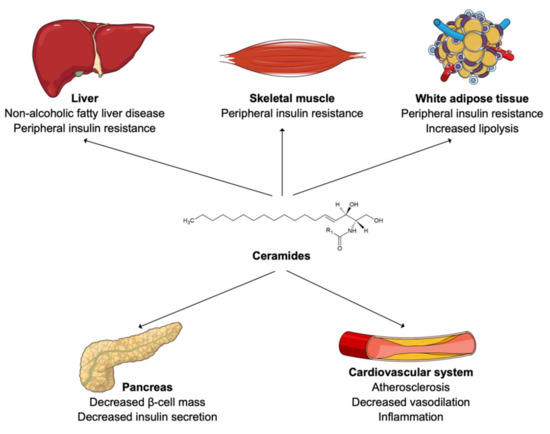
Figure 2.
Ceramides in human cardiometabolic diseases.
Elevated circulating ceramide levels have been reported in obese patients with T2DM [44,45]. Furthermore, LDL were shown to be enriched in ceramides in lean patients with T2DM compared to healthy individuals [46]. In fact, an accumulation of ceramides in muscle cells and adipocytes also inhibits Akt/PKB, reduces GLUT4 translocation to the membrane, and ultimately decreases glucose uptake [43,47,48]. In adipocytes, ceramides also slow down fatty acid oxidation by inhibiting specific hormone-sensitive lipases [49]. This results in peripheral insulin resistance [42]. In addition, ceramide accumulation is believed to induce β-cell dysfunction and reduce insulin secretion [50,51]. Looking at species level, long-chain ceramides, such as Cer (18:1;2/16:0) or Cer (18:1;2/18:0), have been shown to correlate the most with insulin resistance and NAFLD [52,53]. Treatment with the glucagon-like peptide-1 receptor agonist liraglutide was recently shown to reduce ceramide levels in patient with T2DM [54]. The authors of this study then hypothesized that the cardiovascular benefit of this treatment observed within patients with T2DM might be partially due to this reduction in ceramide content [54].
From a cardiovascular perspective, ceramides located at the surface of LDL drive their transcytosis through the endothelium and uptake into macrophages, which results in foam cell formation, vascular inflammation, and atherosclerosis [40,41,42]. Thoracic adipose tissue of obese individuals secretes a particular ceramide species, named Cer (18:1;2/16:0), which acts on endothelial cells to reduce vasodilatation, induce inflammation, oxidative stress, and finally increase risk of cardiovascular death [55]. Again, circulating long-chain ceramides, such as Cer (18:1;2/16:0), Cer (18:1;2/18:0) and Cer (18:1;2/24:1), seem to be the species most frequently associated with cardiovascular morbidity and mortality [10,31,56]. Statin therapy showed conflicting results on ceramide levels. A recent study reported no reduction in absolute ceramide content within LDL particles, or even an increase of its relative concentration as other lipid species composing LDL, such as lysophospholipids, were reduced [57]. The authors of this study hypothesized that ceramides might be responsible for residual cardiovascular risk on statin therapy [57]. Conversely, other studies found a decrease in circulating ceramide levels on simvastatin or rosuvastatin therapy [58,59] as well as on proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors [60]. Ezetimibe therapy did not lead to a reduction in circulating ceramides [59]. Thus, larger population trials are necessary to unravel effects of lipid-lowering drugs on ceramide levels [61].
4. Ceramide Measurement in Clinical Research and Practice
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the technique of choice for ceramide measurement as it offers a high sensitivity to detect low abundant species and a large breadth of coverage with respect to chemical diversity [62,63]. Currently, hurdles remain in the translation of lipidomic output, obtained by LC-MS/MS, to clinical practice [64]. First, harmonization of data quality assessment is necessary to allow for measurements comparison between laboratories using different technologies and methodologies [64]. Several ring trials are currently ongoing to optimize and standardize this analytical aspect [64]. The next step forward is the establishment of biological reference intervals for the species, which are amenable to be used in clinical practice. Establishing these reference values through large-scale population studies, while taking variables such as age, sex, physical activity, diet, medication, and circadian rhythm into consideration, is required to distinguish physiological from pathological variations [64,65].
Nevertheless, plasma ceramides are already measured in daily practice in some clinical facilities, such as at the Mayo Clinic (Rochester, Minnesota, USA) [66,67]. Concretely, the Coronary Event Risk Test 1 (CERT1) is calculated based on concentrations of Cer (18:1;2/16:0), Cer (18:1;2/18:0), Cer (18:1/24:1) and on the ratios Cer (18:1;2/16:0) to Cer (18:1;2/24:0), Cer (18:1;2/18:0) to Cer (18:1;2/24:0) and Cer (18:1;2/24:1) to Cer (18:1;2/24:0) [9,10,11,12,56,68,69,70,71,72,73]. One or two points are attributed for each result above the median or the third quartile, respectively [66]. This results in a 12-point scale categorizing the risk of myocardial infarction, acute coronary syndromes, and mortality within 1 to 5 years [66]. This score was improved by adding some specific phosphatidylcholine (PC) species to it, resulting in the Coronary Event Risk Test 2 (CERT2) [12]. This score consists of the ratios Cer (18:1;2/24:1) to Cer (18:1;2/24:0), Cer (18:1;2/16:0) to PC (16:0/22:5), Cer (18:1;2/16:0) to PC (14:0/22:6) and the concentration of PC 16:0/16:0 [12]. CERT2 was shown to efficiently predict residual cardiovascular events in patients with coronary artery disease (CAD) [12], to identify high-risk patients among patients with stable CAD [74] and to predict the risk of cardiovascular death in patients with acute coronary syndrome, independently of established risk factors [75]. Both CERT1 and CERT2 were shown to be superior to the Systematic COronary Risk Evaluation (SCORE) with regard to prediction of cardiovascular events, cardiovascular mortality, and overall mortality in patients with CVD, with CERT2 displaying better results than CERT1 [76]. Predictive performance of CERT2 was further improved once combined with SCORE [76]. As SCORE was recently updated to SCORE2, it might be worthful to repeat these comparisons and likely to integrate ceramide and phosphatidylcholine items in the next version of SCORE [77].
Overall, circulating ceramides can predict cardiovascular outcomes beyond traditional cardiovascular risk factors in healthy individuals and patients with CAD (Figure 3) [9,10,12,78,79,80,81]. On a mechanistic level, however, ceramide metabolism, along with its role in cardiometabolic health, is not as deeply understood as that of cholesterol [13]. Therefore, further mechanistic, epidemiological, and interventional studies are required to advance this field of research [13]. Conversely to CVD, no ceramides-based risk assessment tool is available for insulin resistance, type 2 diabetes and NAFLD yet, although circulating ceramides have been associated with these pathologies in clinical cohort studies [34,44,82,83]. Finally, ceramide metabolism might represent a future therapeutic target, as exemplified by studies in rodents demonstrating that inhibition of ceramide synthesis reduces incidence of hypertension, type 2 diabetes mellitus, NAFLD, atherosclerosis and heart failure [49,61].
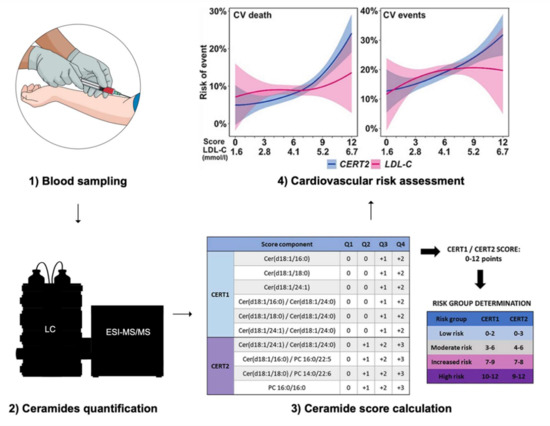
Figure 3.
Cardiovascular risk assessment using ceramides-based scores. Abbreviations: LC = liquid chromatography, ESI-MS/MS = electrospray ionization tandem-mass spectrometry, CV = cardiovascular, LDL-C = low-density lipoprotein cholesterol, CERT1 = Ceramide Test Score 1, CERT2 = Ceramide Test Score 2. The figures illustrating the stages 3 and 4 are reproduced, without modification, under the terms of the Creative Commons Attribution License from Hilvo et al. [84].
5. Exercise-A Modulator of Ceramide Levels
Physical activity and exercise represent cost-effective means to prevent and treat NCD [15,26,85,86,87]. While physical activity notoriously mitigates traditional cardiometabolic risk factors, mechanisms through which exercise directly improves cardiometabolic health remain poorly understood [19]. Preliminary data suggests that exercise could influence ceramide metabolism and thereby optimize human health.
Circulating ceramide levels were increased following a single session of moderate-intensity continuous training (MICT) in endurance athletes, sedentary obese individuals and patients with T2DM [88]. Likewise, muscle ceramide levels increased after three hours of cycling [89]. Conversely, a several-week period of MICT lowered circulating ceramide levels in patients suffering from obesity or T2DM [90,91]. Regular MICT or high-intensity interval training (HIIT) also lead to a reduction in muscle ceramides [91,92,93].
Cardiorespiratory fitness (CRF), defined as the peak oxygen uptake (VO2peak), is inversely associated with incidence of cancer, cardiometabolic diseases and all-cause mortality [94,95,96,97,98,99]. CRF has been shown to be a better predictor of morbidity and mortality than physical activity itself [100,101,102]. Thus, the American Heart Association even recommends assessing CRF as a vital sign in clinical practice [97]. Strikingly, several ceramide species were reported to be negatively associated with CRF: Cer (18:0;2/22:1) [103], Cer (18:0;2/24:1) [103], Cer (18:1;2/14:0) [103], Cer (18:1;2/16:0) [103,104,105], Cer (18:1;2/18:0) [105,106], Cer (18:1;2/20:0) [105,106], Cer (18:1;2/22:1) [103] and Cer (18:1;2/24:1) [105]. The three cardiometabolically deleterious ceramide species clinically used in the ceramide-phospholipid score (Cer (18:1;2/16:0), Cer (18:1;2/18:0) and Cer (18:1;2/24:1)) were notably reported to be negatively associated with CRF [12,79]. Finally, some glycosphingolipids have also been negatively associated with CRF, such as HexCer (18:1;2/16:0) [103,107], HexCer (18:1;2/18:0) [103,105], LacCer (18:1;2/18:1) [103] and LacCer (18:1;2/22:0) [103].
To summarize, acute bouts of physical activity tend to elevate both circulating and muscle ceramide levels, while regular exercise leads to a reduction of ceramides in the circulation and myocytes (Figure 4). It has been hypothesized that the increase in ceramide could inhibit insulin action, promote fatty acid oxidation and thereby favor lipids as an energy source during exercise [28]. The effect of regular exercise on ceramides is also supported by the reported negative associations between ceramides and CRF. Therefore, it can be hypothesized that regular exercise, leading to an improvement of CRF, optimizes cardiometabolic health, partly by reversing altered ceramide profiles.
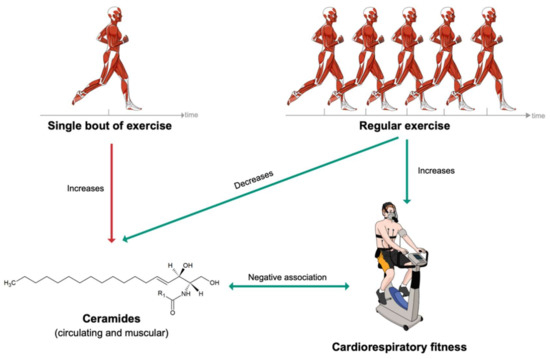
Figure 4.
Short- and long-term effects of exercise on ceramide levels.
6. Conclusions
Upgrading patients’ metabolic stratification in clinical practice has the potential to improve personalization of CMD prevention and treatment. Specific ceramide species were shown to predict cardiometabolic outcomes beyond classical biomarkers such as cholesterol. While an acute bout of physical exercise tends to increase circulating and muscular ceramide levels, regular exercise leads to a reduction of ceramide levels. This is also reflected by the fact that CRF, a powerful marker reflecting cardiometabolic health and training status, has been negatively associated with several ceramide species. Preventive and therapeutic effectiveness of a well-conducted exercise intervention to mitigate ceramides profile remains to be investigated.
Author Contributions
Conceptualization, J.C. and N.W.; resources, A.S.-T.; writing—original draft preparation, J.C. and N.W.; writing—review and editing, J.C., H.G.-A., N.W., L.S., H.H., F.C., C.S. J.I. and A.S.-T.; visualization, J.C.; supervision, J.I. and A.S.-T.; project administration, J.C.; funding acquisition, A.S.-T. All authors have read and agreed to the published version of the manuscript.
Funding
The publication fee was funded by the University Basel Open Access Publication Fund.
Acknowledgments
We acknowledge the use of the Mind the Graph platform (www.mindthegraph.com (accessed on 27 September 2021)) to create the graphical abstract and Figure 1, Figure 2, Figure 3 and Figure 4.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.B.R.; Foreman, K.J.; GBD 2019 DALYs and HALE Collaborator. Global, regional, and national disability-adjusted life years (dalys) for diseases and injuries and healthy life expectancy (hale), 1990 to 2019: Quantifying the epidemiological transition. In GBD Compare Data Visualization; Institute for Health Metrics and Evaluation, University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Hunter, D.J.; Reddy, K.S. Noncommunicable diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef] [Green Version]
- Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [CrossRef]
- Benziger, C.P.; Roth, G.A.; Moran, A.E. The global burden of disease study and the preventable burden of ncd. Glob. Heart 2016, 11, 393–397. [Google Scholar] [CrossRef]
- Morrison, L.M.; Hall, L.; Chaney, A.L. Cholesterol metabolism and its relationship to atherosclerosis, coronary artery disease, and arteriosclerosis. Am. J. Med. 1948, 4, 616. [Google Scholar] [CrossRef]
- Kuijpers, P. History in medicine: The story of cholesterol, lipids and cardiology. J. Cradiol. Pract. 2021, 19, 1–5. [Google Scholar]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; Marz, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond ldl-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population-based finrisk 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [Green Version]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2019, 41, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, S.A. Could ceramides become the new cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Thompson, A.M.; Blair, S.N.; Sallis, J.F.; Powell, K.E.; Bull, F.C.; Bauman, A.E. Sport and exercise as contributors to the health of nations. Lancet 2012, 380, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Kohl, H.W., III; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 esc guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary behavior, exercise, and cardiovascular health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Slentz, C.A.; Bateman, L.A.; Willis, L.H.; Granville, E.O.; Piner, L.W.; Samsa, G.P.; Setji, T.L.; Muehlbauer, M.J.; Huffman, K.M.; Bales, C.W.; et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia 2016, 59, 2088–2098. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (nafld): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The pure study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid metabolism and signaling in skeletal muscle: From physiology to physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Senkal, C.E.; Salama, M.F.; Snider, A.J.; Allopenna, J.J.; Rana, N.A.; Koller, A.; Hannun, Y.A.; Obeid, L.M. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017, 25, 686–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte ceramides—The nexus of inflammation and metabolic disease. Front. Immunol. 2020, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front. Endocrinol. 2020, 11, 763. [Google Scholar] [CrossRef]
- Poss, A.M.; Summers, S.A. Too much of a good thing? An evolutionary theory to explain the role of ceramides in nafld. Front. Endocrinol. 2020, 11, 505. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Heras, V.; Castellano, J.M.; Fernandois, D.; Velasco, I.; Rodríguez-Vazquez, E.; Roa, J.; Vazquez, M.J.; Ruiz-Pino, F.; Rubio, M.; Pineda, R.; et al. Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 2020, 32, 951–966.e958. [Google Scholar] [CrossRef]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E423–E435. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.S.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, X.; Xing, S.; Bian, F.; Yao, W.; Bai, X.; Zheng, T.; Wu, G.; Jin, S. Endogenous ceramide contributes to the transcytosis of oxldl across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med. Cell Longev. 2014, 2014, 823071. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Wang, P.; Zhang, S.-Y.; Dong, Y.; Zeng, G.; Yan, Y.; Sun, L.; Wu, Q.; Liu, H.; et al. Adipocyte hypoxia-inducible factor 2α suppresses atherosclerosis by promoting adipose ceramide catabolism. Cell Metab. 2019, 30, 937–951.e5. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Chavez, J.A.; Knotts, T.A.; Wang, L.-P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, R.N.; Yu, C.; Hoofnagle, A.; Hari, N.; Jensen, P.N.; Fretts, A.M.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; et al. Circulating sphingolipids, insulin, homa-ir, and homa-b: The strong heart family study. Diabetes 2018, 67, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides contained in ldl are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zierath, J.R. The path to insulin resistance: Paved with ceramides? Cell Metab. 2007, 5, 161–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, P.T.; Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; Summers, S.A.; Drummond, M.J. Influence of exercise training on skeletal muscle insulin resistance in aging: Spotlight on muscle ceramides. Int. J. Mol. Sci. 2020, 21, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, B.; Tippetts, T.S.; Monibas, R.M.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Kowluru, A.; Kowluru, R.A. Racking up ceramide-induced islet β-cell dysfunction. Biochem. Pharmacol. 2018, 154, 161–169. [Google Scholar] [CrossRef]
- Lang, F.; Ullrich, S.; Gulbins, E. Ceramide formation as a target in beta-cell survival and function. Expert Opin. Ther. Targets 2011, 15, 1061–1071. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced cers6-dependent c16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A c18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Zobel, E.H.; Wretlind, A.; Ripa, R.S.; Rotbain Curovic, V.; von Scholten, B.J.; Suvitaival, T.; Hansen, T.W.; Kjær, A.; Legido-Quigley, C.; Rossing, P. Ceramides and phospholipids are downregulated with liraglutide treatment: Results from the liraflame randomized controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e002395. [Google Scholar] [CrossRef] [PubMed]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J. Am. Coll. Cardiol. 2021, 77, 2494–2513. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the predimed trial (prevención con dieta mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Thérond, P.; Meikle, P.J. Ldl subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense ldl[s]. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.W.K.; Ooi, E.M.M.; Watts, G.F.; Chan, D.C.; Weir, J.M.; Meikle, P.J.; Barrett, P.H.R. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2335–E2340. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and pcsk9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Svatikova, A.; Meeusen, J.W.; Kludtke, E.L.; Kopecky, S.L. Effect of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma ceramide levels. Am. J. Cardiol. 2020, 128, 163–167. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Burla, B.; Muralidharan, S.; Wenk, M.R.; Torta, F. Sphingolipid analysis in clinical research. In Clinical Metabolomics: Methods and Protocols; Giera, M., Ed.; Springer: New York, NY, USA, 2018; pp. 135–162. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhang, H.; Yau, L.F.; Mi, J.-N.; Lee, S.; Lee, K.C.; Hu, P.; Liu, L.; Jiang, Z.-H. Improved sphingolipidomic approach based on ultra-high performance liquid chromatography and multiple mass spectrometries with application to cellular neurotoxicity. Anal. Chem. 2014, 86, 5688–5696. [Google Scholar] [CrossRef]
- Burla, B.; Arita, M.; Arita, M.; Bendt, A.K.; Cazenave-Gassiot, A.; Dennis, E.A.; Ekroos, K.; Han, X.; Ikeda, K.; Liebisch, G.; et al. Ms-based lipidomics of human blood plasma: A community-initiated position paper to develop accepted guidelines1. J. Lipid Res. 2018, 59, 2001–2017. [Google Scholar] [CrossRef] [Green Version]
- Carrard, J.; Gallart-Ayala, H.; Infanger, D.; Teav, T.; Wagner, J.; Knaier, R.; Colledge, F.; Streese, L.; Königstein, K.; Hinrichs, T.; et al. Metabolic view on human healthspan: A lipidome-wide association study. Metabolites 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ceramides: A Class of Lipids with Links to Heart Disease. Available online: https://www.mayoclinic.org/medical-professionals/cardiovascular-diseases/news/ceramides-a-class-of-lipids-with-links-to-heart-disease/mac-20429577 (accessed on 1 September 2021).
- Mi-Heart Ceramides. Be in the Know. Now. Available online: https://news.mayocliniclabs.com/ceramides-miheart/#form-row-right (accessed on 1 September 2021).
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anroedh, S.; Hilvo, M.; Akkerhuis, K.M.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Peterson, L.R.; Jiang, X.; Chen, L.; Goldberg, A.C.; Farmer, M.S.; Ory, D.S.; Schaffer, J.E. Alterations in plasma triglycerides and ceramides: Links with cardiac function in humans with type 2 diabetes. J. Lipid Res. 2020, 61, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Duncan, M.; Xanthakis, V.; Peterson, L.R.; Mitchell, G.F.; McManus, D.; Cheng, S.; Vasan, R.S. Association of circulating ceramides with cardiac structure and function in the community: The framingham heart study. J. Am. Heart Assoc. 2019, 8, e013050. [Google Scholar] [CrossRef]
- Hilvo, M.; Lääperi, M.; Jylhä, A.; Kleber, M.E.; Hurme, R.; Scharnagl, H.; März, W.; Sinisalo, J.; Laaksonen, R. Prior myocardial infarction, coronary artery disease extent, diabetes mellitus, and cert2 score for risk stratification in stable coronary artery disease. Eur. J. Prev. Cardiol. 2021, zwab122. [Google Scholar] [CrossRef]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur J. Prev. Cardiol. 2020, zwaa143. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Mündlein, A.; Laaksonen, R.; Lääperi, M.; Jylhä, A.; Fraunberger, P.; Drexel, H. Comparison of recent ceramide-based coronary risk prediction scores in cardiovascular disease patients. Eur. J. Prev. Cardiol. 2021, zwab112. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. Score2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Fang, Z.; Li, S.; Xu, M.; Zhang, J.; Han, D.; Hu, W.; Yan, L.; Wang, Y.; Fan, L.; et al. Circulating ceramide: A new cardiometabolic biomarker in patients with comorbid acute coronary syndrome and type 2 diabetes mellitus. Front. Physiol. 2020, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Wallentin, L.; Lakic, T.G.; Held, C.; Kauhanen, D.; Jylhä, A.; Lindbäck, J.; Siegbahn, A.; Granger, C.B.; Koenig, W.; et al. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J. Am. Heart Assoc. 2020, 9, e015258. [Google Scholar] [CrossRef]
- Mantovani, A.; Dugo, C. Ceramides and risk of major adverse cardiovascular events: A meta-analysis of longitudinal studies. J. Clin. Lipidol. 2020, 14, 176–185. [Google Scholar] [CrossRef]
- Poss, A.M.; Holland, W.L.; Summers, S.A. Risky lipids: Refining the ceramide score that measures cardiovascular health. Eur. Heart J. 2019, 41, 381–382. [Google Scholar] [CrossRef]
- Thorens, B.; Rodriguez, A.; Cruciani-Guglielmacci, C.; Wigger, L.; Ibberson, M.; Magnan, C. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—A step towards precision medicine. Mol. Metab. 2019, 27, S147–S154. [Google Scholar] [CrossRef] [PubMed]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and ceramide scores: Clinical applications for cardiometabolic risk stratification. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; Van Mechelen, W.; Pratt, M. Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Merkur, S.; Sassi, F.; McDaid, D. Promoting Health, Preventing Disease: Is there an Economic Case? WHO: Copenhagen, Denmark, 2013; p. 72. [Google Scholar]
- Gojanovic, B. Physical activity is an opportunity for the health of nations: What should we do next? Praxis 2018, 107, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helge, J.W.; Dobrzyn, A.; Saltin, B.; Gorski, J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp. Physiol. 2004, 89, 119–127. [Google Scholar] [CrossRef]
- Kasumov, T.; Solomon, T.P.J.; Hwang, C.; Huang, H.; Haus, J.M.; Zhang, R.; Kirwan, J.P. Improved insulin sensitivity after exercise training is linked to reduced plasma c14:0 ceramide in obesity and type 2 diabetes. Obesity 2015, 23, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Meikle, P.J.; Mellett, N.A.; Ranasinghe, A.M.; Barker, T.A.; Wagenmakers, A.J.M.; Shaw, C.S. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int. J. Obes. 2017, 41, 1745–1754. [Google Scholar] [CrossRef]
- Bruce, C.R.; Thrush, A.B.; Mertz, V.A.; Bezaire, V.; Chabowski, A.; Heigenhauser, G.J.; Dyck, D.J. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E99–E107. [Google Scholar] [CrossRef] [Green Version]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef] [Green Version]
- Robsahm, T.E.; Falk, R.S.; Heir, T.; Sandvik, L.; Vos, L.; Erikssen, J.E.; Tretli, S. Measured cardiorespiratory fitness and self-reported physical activity: Associations with cancer risk and death in a long-term prospective cohort study. Cancer Med. 2016, 5, 2136–2144. [Google Scholar] [CrossRef] [Green Version]
- Gander, J.C.; Sui, X.; Hébert, J.R.; Hazlett, L.J.; Cai, B.; Lavie, C.J.; Blair, S.N. Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clin. Proc. 2015, 90, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Farrell, S.W.; Finley, C.E.; Radford, N.B.; Haskell, W.L. Cardiorespiratory fitness, body mass index, and heart failure mortality in men. Circ. Heart Fail. 2013, 6, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the american heart association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Blaha, M.J.; Whelton, S.P.; Blumenthal, R.; Jones, S.R.; Keteyian, S.J.; Schairer, J.; Brawner, C.A.; Al-Mallah, M.H. Physical fitness and hypertension in a population at risk for cardiovascular disease: The henry ford exercise testing (fit) project. J. Am. Heart Assoc. 2014, 3, e001268. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, N.S.; Ruiz, J.R.; Hurtig-Wennlöf, A.; Ortega, F.B.; Sjöström, M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The european youth heart study. J. Pediatr. 2007, 150, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kaykha, A.; George, S.; Abella, J.; Zaheer, N.; Lear, S.; Yamazaki, T.; Froelicher, V. Fitness versus physical activity patterns in predicting mortality in men. Am. J. Med. 2004, 117, 912–918. [Google Scholar] [CrossRef]
- Lee, D.-C.; Sui, X.; Ortega, F.B.; Kim, Y.-S.; Church, T.S.; Winett, R.A.; Ekelund, U.; Katzmarzyk, P.T.; Blair, S.N. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br. J. Sports Med. 2011, 45, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Physical fitness and activity as separate heart disease risk factors: A meta-analysis. Med. Sci. Sports Exerc. 2001, 33, 754–761. [Google Scholar] [CrossRef]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular choreography of acute exercise. Cell 2020, 181, 1112.e1116–1130.e1116. [Google Scholar] [CrossRef]
- Nayor, M.; Shah, R.V.; Miller, P.E.; Blodgett, J.B.; Tanguay, M.; Pico, A.R.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Deik, A.; et al. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 2020, 142, 1905–1924. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Marzolini, S.; Mielke, M.M.; Andreazza, A.; Oh, P.I.; Vattem Venkata, S.L.; Haughey, N.J.; Lanctôt, K.L. Association between sphingolipids and cardiopulmonary fitness in coronary artery disease patients undertaking cardiac rehabilitation. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 75, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Yang, A.; Simonsick, E.M.; Chia, C.W.; Zoli, M.; Haughey, N.J.; Mielke, M.M.; Ferrucci, L.; Coen, P.M. Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54-96 years from the baltimore longitudinal study of aging. Aging Cell 2016, 15, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Contaifer, D.; Buckley, L.F.; Wohlford, G.; Kumar, N.G.; Morriss, J.M.; Ranasinghe, A.D.; Carbone, S.; Canada, J.M.; Trankle, C.; Abbate, A.; et al. Metabolic modulation predicts heart failure tests performance. PLoS ONE 2019, 14, e218153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).