Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study
Abstract
1. Introduction
2. Results
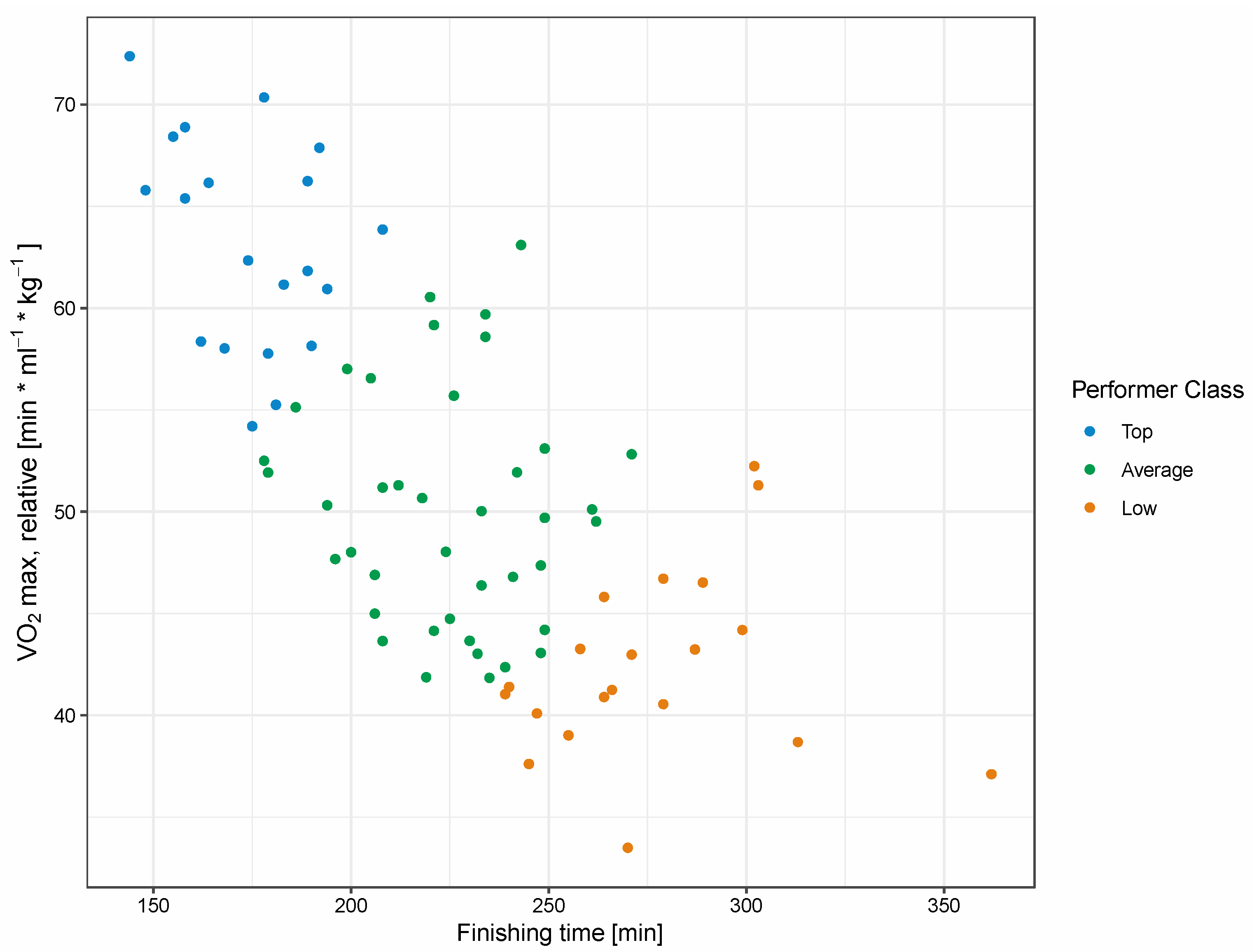
2.1. Characteristics of Study Participants
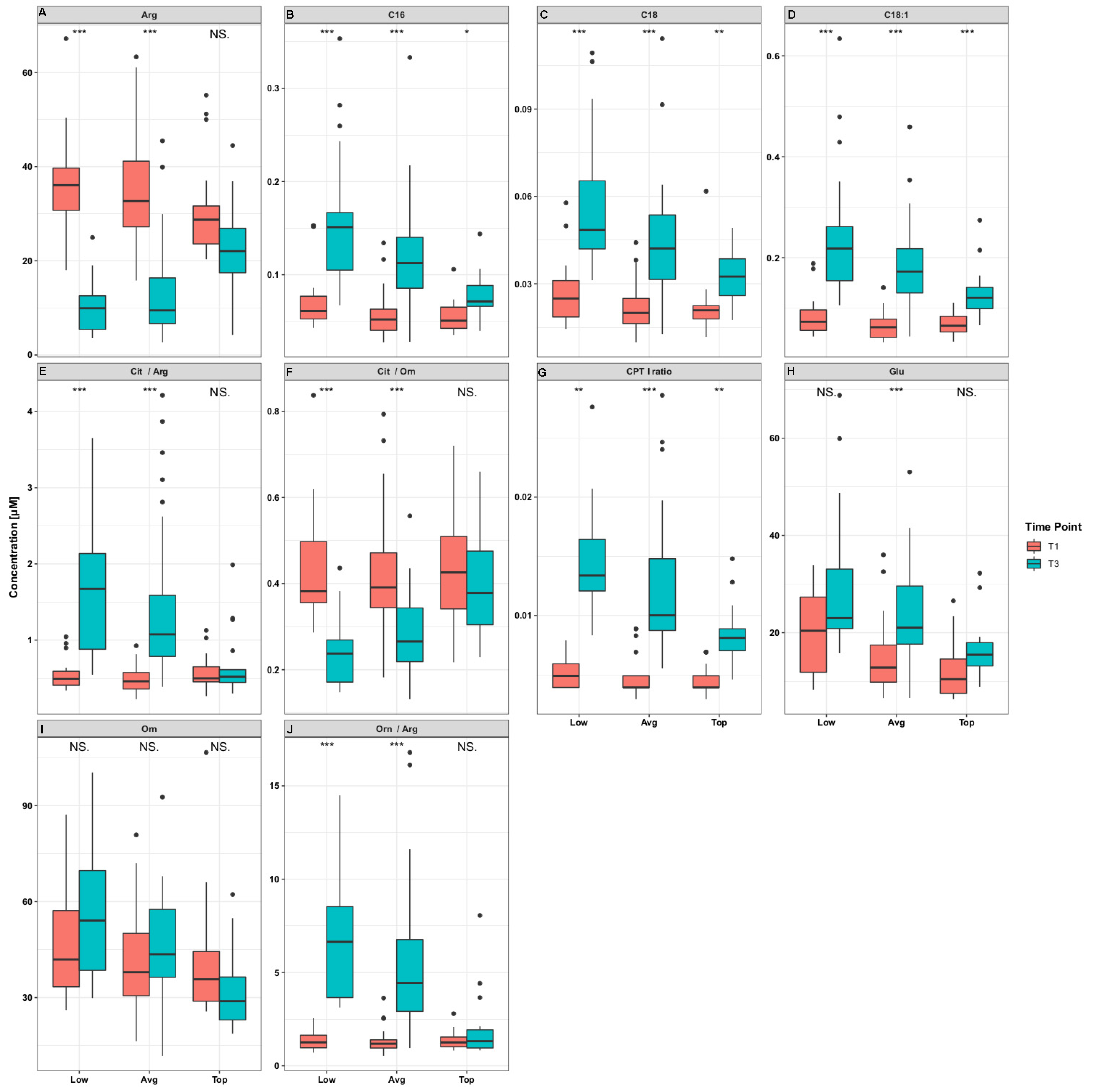
2.2. General Concentration Trends at Different Periods of Exercise
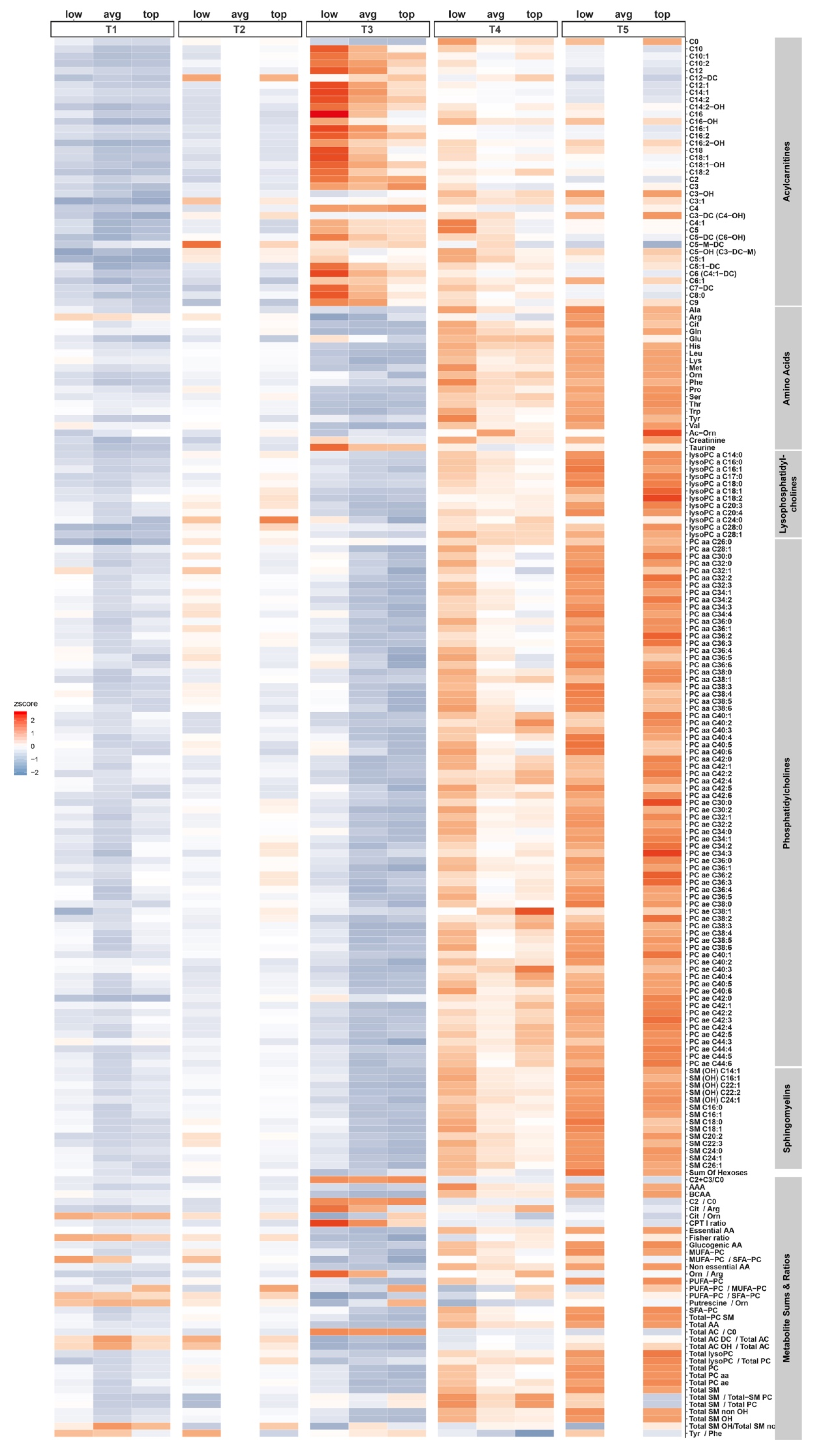
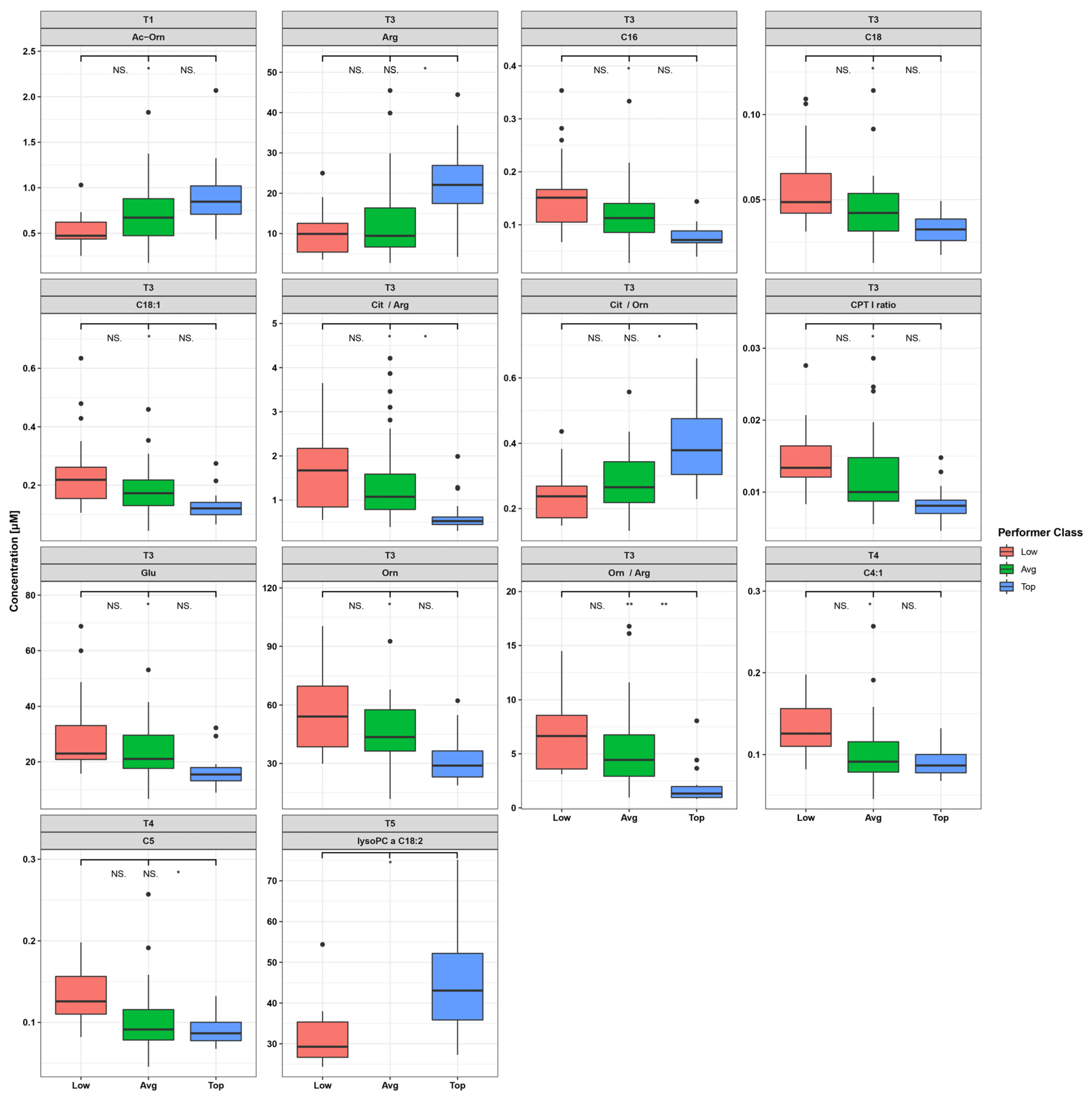
2.3. Differences between Performer Groups
3. Discussion
3.1. Metabolic Changes in Different Exercise Periods and Performance Groups
3.2. Acylcarnitines
3.3. Amino Acids, Arginine Metabolism- and Urea Cycle Related-Metabolites, and Biogenic Amines
3.4. Sphingolipids and Phospholipids
3.5. Limitations of the Study
4. Materials and Methods
4.1. Subjects and Procedures
4.1.1. Recruitment of Subjects
4.1.2. Separation of Subjects into Different Performance Groups
4.1.3. Blood Samples
4.1.4. Sample Handling and Metabolite Quantification
4.2. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef]
- Heydenreich, J.; Kayser, B.; Schutz, Y.; Melzer, K. Total Energy Expenditure, Energy Intake, and Body Composition in Endurance Athletes Across the Training Season: A Systematic Review. Sport. Med. Open 2017, 3, 8. [Google Scholar] [CrossRef]
- Fry, R.W.; Morton, A.R.; Keast, D. Periodisation and the prevention of overtraining. Can. J. Sport Sci. 1992, 17, 241–248. [Google Scholar]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Carneiro, G.; Radcenco, A.L.; Evaristo, J.; Monnerat, G. Novel strategies for clinical investigation and biomarker discovery: A guide to applied metabolomics. Horm. Mol. Biol. Clin. Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Margolis, L.M.; Murphy, N.E.; Carrigan, C.T.; Castellani, J.W.; Madslien, E.H.; Teien, H.K.; Martini, S.; Montain, S.J.; Pasiakos, S.M. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Sha, W. Identification of a select metabolite panel for measuring metabolic perturbation in response to heavy exertion. Metabolomics 2018, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Gillitt, N.D.; Pappan, K.L.; Lila, M.A. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J. Proteome Res. 2013, 12, 4577–4584. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef]
- Howe, C.C.F.; Alshehri, A.; Muggeridge, D.; Mullen, A.B.; Boyd, M.; Spendiff, O.; Moir, H.J.; Watson, D.G. Untargeted Metabolomics Profiling of an 80.5 km Simulated Treadmill Ultramarathon. Metabolites 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Stander, Z.; Luies, L.; Mienie, L.J.; Keane, K.M.; Howatson, G.; Clifford, T.; Stevenson, E.J.; Loots, D.T.J.M. The altered human serum metabolome induced by a marathon. Metabolomics 2018, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 2019, 21, 51–67. [Google Scholar] [CrossRef]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Fluck, D.; Bonne, T.C.; Burgi, S.; Christensen, P.M.; Toigo, M.; Lundby, C. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J. Appl. Physiol. 2013, 115, 785–793. [Google Scholar] [CrossRef]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Lehmann, R.; Zhao, X.; Weigert, C.; Simon, P.; Fehrenbach, E.; Fritsche, J.; Machann, J.; Schick, F.; Wang, J.; Hoene, M.; et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE 2010, 5, e11519. [Google Scholar] [CrossRef]
- Sahlin, K.; Harris, R.C. Control of lipid oxidation during exercise: Role of energy state and mitochondrial factors. Acta Physiol. 2008, 194, 283–291. [Google Scholar] [CrossRef]
- Siopi, A.; Deda, O.; Manou, V.; Kosmidis, I.; Komninou, D.; Raikos, N.; Theodoridis, G.A.; Mougios, V. Comparison of the Serum Metabolic Fingerprint of Different Exercise Modes in Men with and without Metabolic Syndrome. Metabolites 2019, 9, 116. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Ishii, N.; Mizuno, A.; Takamatsu, K. Enhancement of fat metabolism by repeated bouts of moderate endurance exercise. J. Appl. Physiol. 2007, 102, 2158–2164. [Google Scholar] [CrossRef]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, K.; Ra, S.-G.; Ohmori, H. Exercise-induced changes in amino acid levels in skeletal muscle and plasma. J. Phys. Fit. Sport. Med. 2013, 2, 301–310. [Google Scholar] [CrossRef]
- Henriksson, J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J. Exp. Biol. 1991, 160, 149–165. [Google Scholar] [PubMed]
- Felder, T.K.; Ring-Dimitriou, S.; Auer, S.; Soyal, S.M.; Kedenko, L.; Rinnerthaler, M.; Cadamuro, J.; Haschke-Becher, E.; Aigner, E.; Paulweber, B.; et al. Specific circulating phospholipids, acylcarnitines, amino acids and biogenic amines are aerobic exercise markers. J. Sci. Med. Sport. 2017, 20, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002, 22, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Arginine metabolism in vascular biology and disease. Vasc. Med. 2005, 10 (Suppl. 1), S83–S87. [Google Scholar] [CrossRef]
- Haralambie, G.; Berg, A. Serum urea and amino nitrogen changes with exercise duration. Eur. J. Appl. Physiol. Occup. Physiol. 1976, 36, 39–48. [Google Scholar] [CrossRef]
- Riccioni, G.; Scotti, L.; Guagnano, M.T.; Bosco, G.; Bucciarelli, V.; Di Ilio, E.; Speranza, L.; Martini, F.; Bucciarelli, T. Physical exercise reduces synthesis of ADMA, SDMA, and L-Arg. Front. Biosci. 2015, 7, 417–422. [Google Scholar] [CrossRef]
- Metz, R.; Salter, J.M.; Brunet, G. Effect of pyruvate and other substrates on urea synthesis in rat liver slices. Metabolism 1968, 17, 158–167. [Google Scholar] [CrossRef]
- Leibowitz, A.; Klin, Y.; Gruenbaum, B.F.; Gruenbaum, S.E.; Kuts, R.; Dubilet, M.; Ohayon, S.; Boyko, M.; Sheiner, E.; Shapira, Y.; et al. Effects of strong physical exercise on blood glutamate and its metabolite 2-ketoglutarate levels in healthy volunteers. Acta Neurobiol. Exp. 2012, 72, 385–396. [Google Scholar]
- Makhro, A.; Haider, T.; Wang, J.; Bogdanov, N.; Steffen, P.; Wagner, C.; Meyer, T.; Gassmann, M.; Hecksteden, A.; Kaestner, L.; et al. Comparing the impact of an acute exercise bout on plasma amino acid composition, intraerythrocytic Ca(2+) handling, and red cell function in athletes and untrained subjects. Cell Calcium. 2016, 60, 235–244. [Google Scholar] [CrossRef][Green Version]
- Newsom, S.A.; Brozinick, J.T.; Kiseljak-Vassiliades, K.; Strauss, A.N.; Bacon, S.D.; Kerege, A.A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J. Appl. Physiol. 2016, 120, 1355–1363. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Brook, M.S.; Smith, K.; Atherton, P.J. Stable isotope tracers and exercise physiology: Past, present and future. J. Physiol. 2017, 595, 2873–2882. [Google Scholar] [CrossRef]
- Grabs, V.; Nieman, D.C.; Haller, B.; Halle, M.; Scherr, J. The effects of oral hydrolytic enzymes and flavonoids on inflammatory markers and coagulation after marathon running: Study protocol for a randomized, double-blind, placebo-controlled trial. BMC Sports Sci. Med. Rehabil. 2014, 6, 8. [Google Scholar] [CrossRef]
- Grabs, V.; Kersten, A.; Haller, B.; Braun, S.; Nieman, D.C.; Halle, M.; Scherr, J. Rutoside and Hydrolytic Enzymes Do Not Attenuate Marathon-Induced Inflammation. Med. Sci. Sports Exerc. 2017, 49, 387–395. [Google Scholar] [CrossRef] [PubMed]
- (CHMP), C.f.M.P.f.H.U. Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 29 February 2020).
- Zukunft, S.; Prehn, C.; Rohring, C.; Moller, G.; Hrabe de Angelis, M.; Adamski, J.; Tokarz, J. High-throughput extraction and quantification method for targeted metabolomics in murine tissues. Metabolomics 2018, 14, 18. [Google Scholar] [CrossRef]
- Zukunft, S.; Sorgenfrei, M.; Prehn, C.; Möller, G.; Adamski, J. Targeted Metabolomics of Dried Blood Spot Extracts. Chromatographia 2013, 76. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Roy. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Performer | Low [n = 20] | Average [n = 40] | Top [n = 20] | p Value | Total [n = 80] | ||
|---|---|---|---|---|---|---|---|
| Anthropometry | Top vs. Low | Top vs. Avg | Avg vs. Low | ||||
| Age [year] | 50.5 ± 7.9 | 44.0 ± 9.2 | 32.8 ± 7.9 | 5.99 × 10−08 | 1.27 × 10−05 | 1.14 × 10−02 | 42.8 ± 10.8 |
| BMI [kg/m2] | 25.3 ± 2.9 | 23.4 ± 2.3 | 21.9 ± 1.3 | 2.17 × 10−05 | 1.12 × 10−02 | 1.16 × 10−02 | 23.5 ± 2.5 |
| Body fat Stander | 17.6 ± 3.7 | 14.0 ± 4.1 | 8.3 ± 1.7 | 1.90 × 10−12 | 8.50 × 10−08 | 1.68 × 10−03 | 13.5 ± 4.9 |
| Performance | |||||||
| Marathon time | 276.6 ± 29.6 | 224.6 ± 22.7 | 174.5 ± 17.0 | 5.96 × 0−16 | 7.65 × 10−13 | 1.06 × 10−07 | 225.1 ± 43.1 |
| VO2max, absolute [l/min] | 3.5 ± 0.7 | 3.7 ± 0.4 | 4.3 ±0.4 | 8.52 × 10−06 | 1.43 × 10−07 | 3.35 × 10−01 | 3.8 ± 0.5 |
| VO2max, relative [mL × min−1 × kg−1] | 41.8 ± 5.5 | 50.0 ± 5.7 | 63.3 ± 5.2 | 6.28 × 10−16 | 2.60 × 10−11 | 1.20 × 10−06 | 51.2 ± 9.4 |
| Training history | |||||||
| Training kilometers 2013 [km/year] | 831.5 ± 471.9 | 1713.3 ± 1082.5 | 2420.5 ± 1128.8 | 1.91 × 10−05 | 4.13 × 10−02 | 4.19 × 10−03 | 1694.8 ± 1125.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schader, J.F.; Haid, M.; Cecil, A.; Schoenfeld, J.; Halle, M.; Pfeufer, A.; Prehn, C.; Adamski, J.; Nieman, D.C.; Scherr, J. Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites 2020, 10, 87. https://doi.org/10.3390/metabo10030087
Schader JF, Haid M, Cecil A, Schoenfeld J, Halle M, Pfeufer A, Prehn C, Adamski J, Nieman DC, Scherr J. Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites. 2020; 10(3):87. https://doi.org/10.3390/metabo10030087
Chicago/Turabian StyleSchader, Jana F., Mark Haid, Alexander Cecil, Julia Schoenfeld, Martin Halle, Arne Pfeufer, Cornelia Prehn, Jerzy Adamski, David C. Nieman, and Johannes Scherr. 2020. "Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study" Metabolites 10, no. 3: 87. https://doi.org/10.3390/metabo10030087
APA StyleSchader, J. F., Haid, M., Cecil, A., Schoenfeld, J., Halle, M., Pfeufer, A., Prehn, C., Adamski, J., Nieman, D. C., & Scherr, J. (2020). Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites, 10(3), 87. https://doi.org/10.3390/metabo10030087









