MEDUSA: A Low-Cost, 16-Channel Neuromodulation Platform with Arbitrary Waveform Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. MEDUSA System Architecture
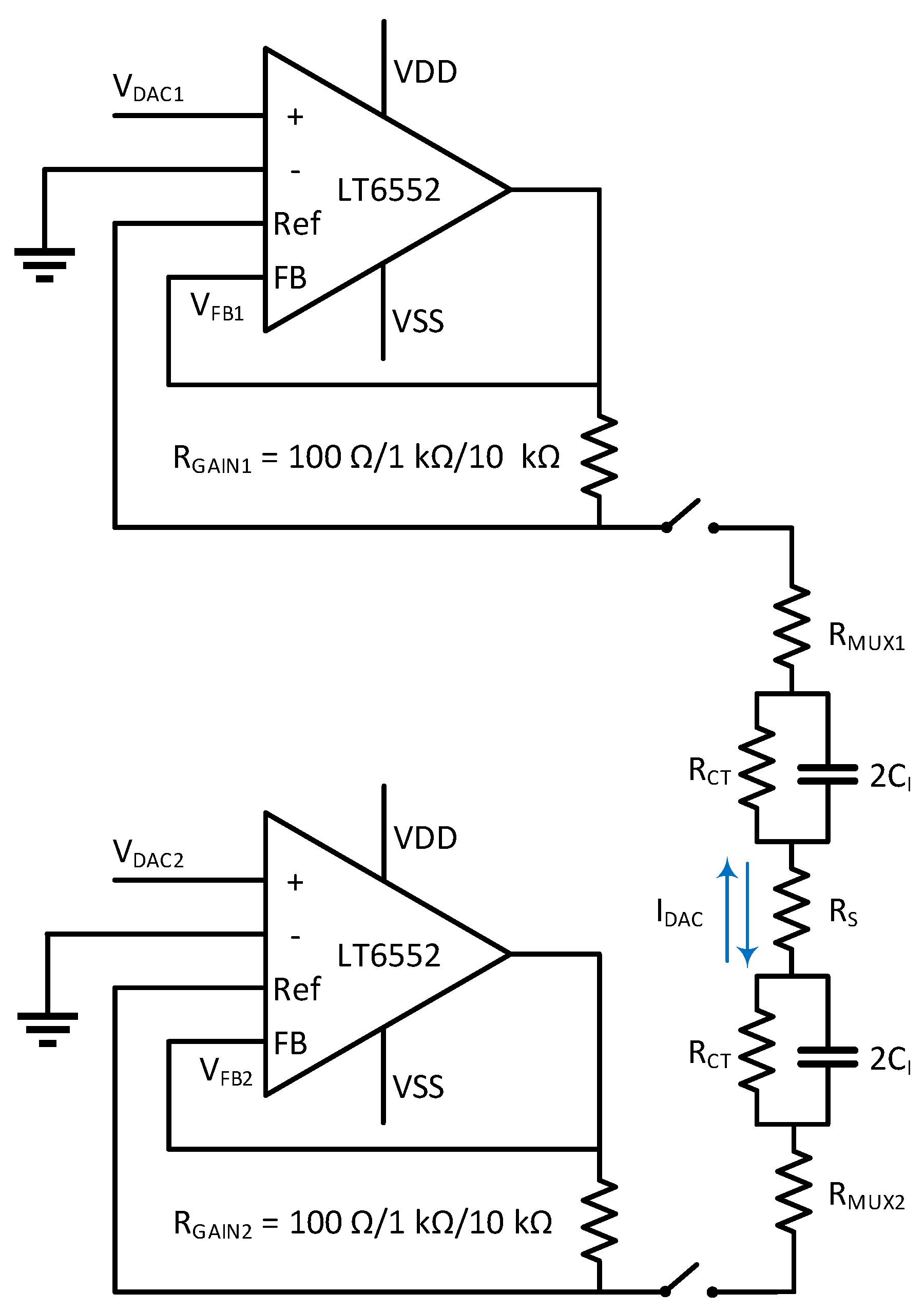
2.2. Bipolar Current Source
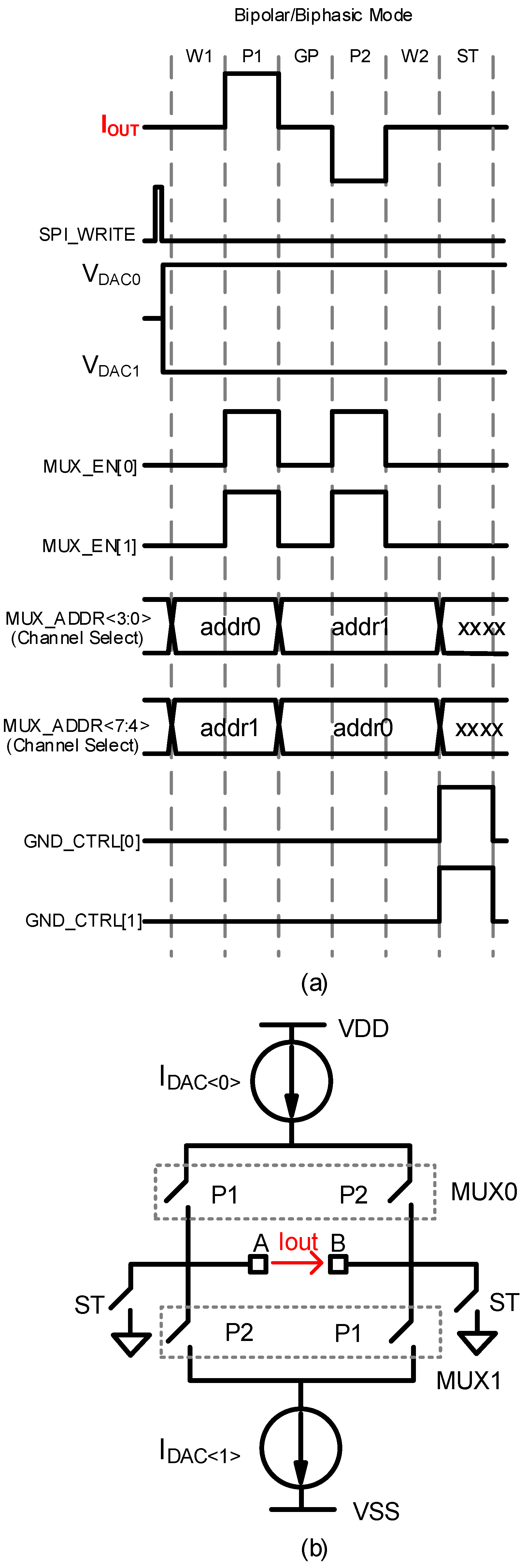
2.3. Digital Timing Control
3. Results
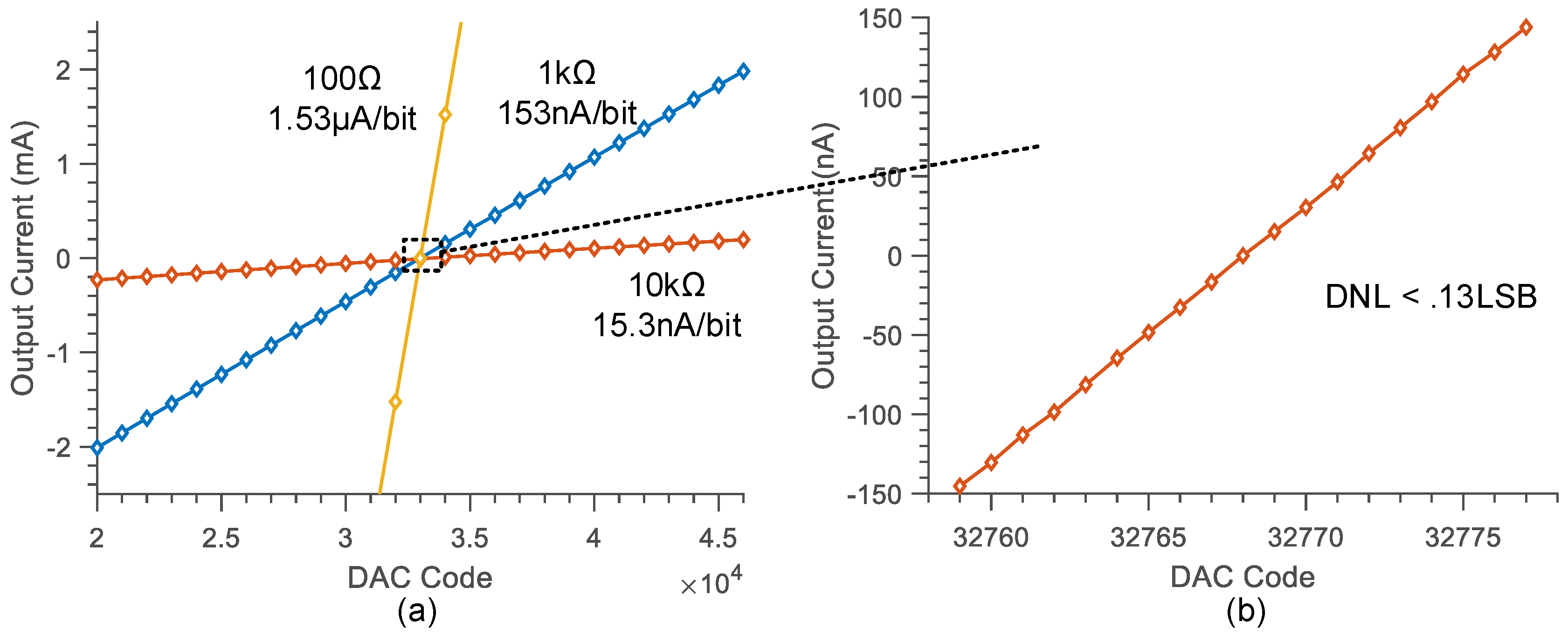
3.1. System Transfer Function
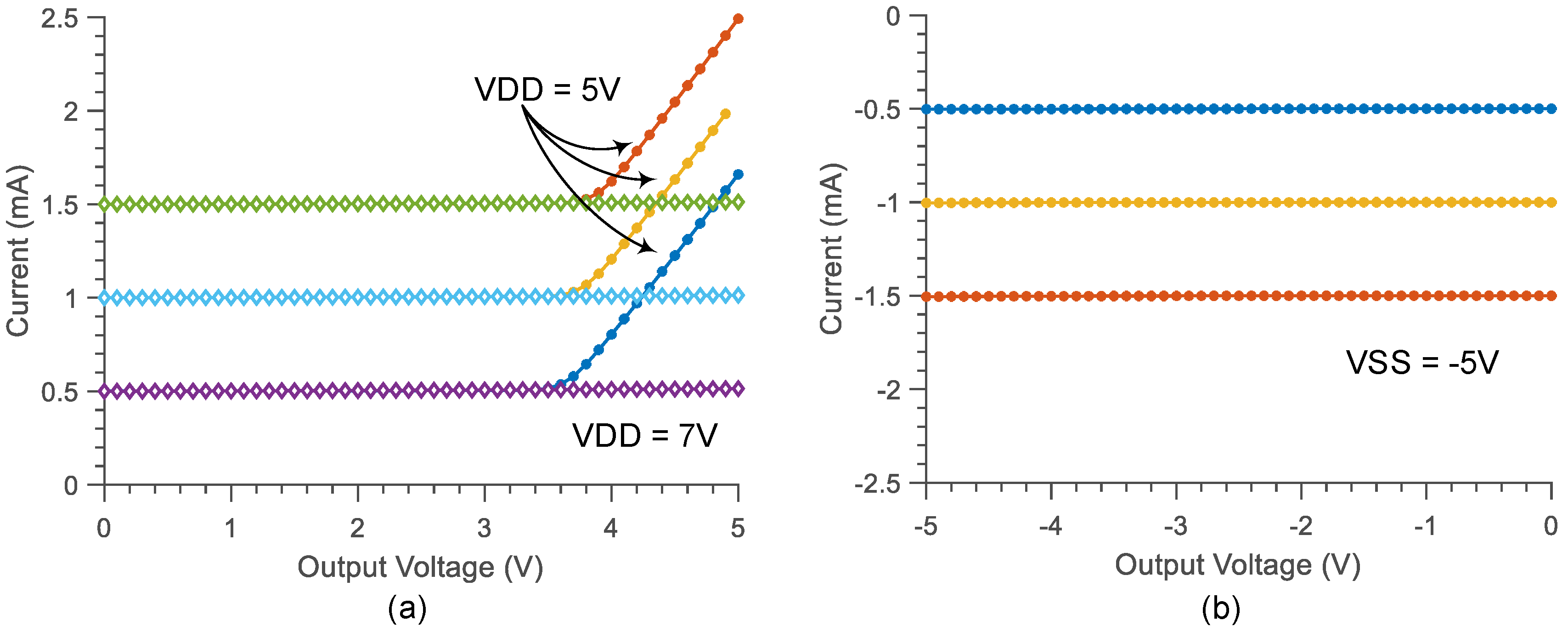
3.2. Compliance Voltage
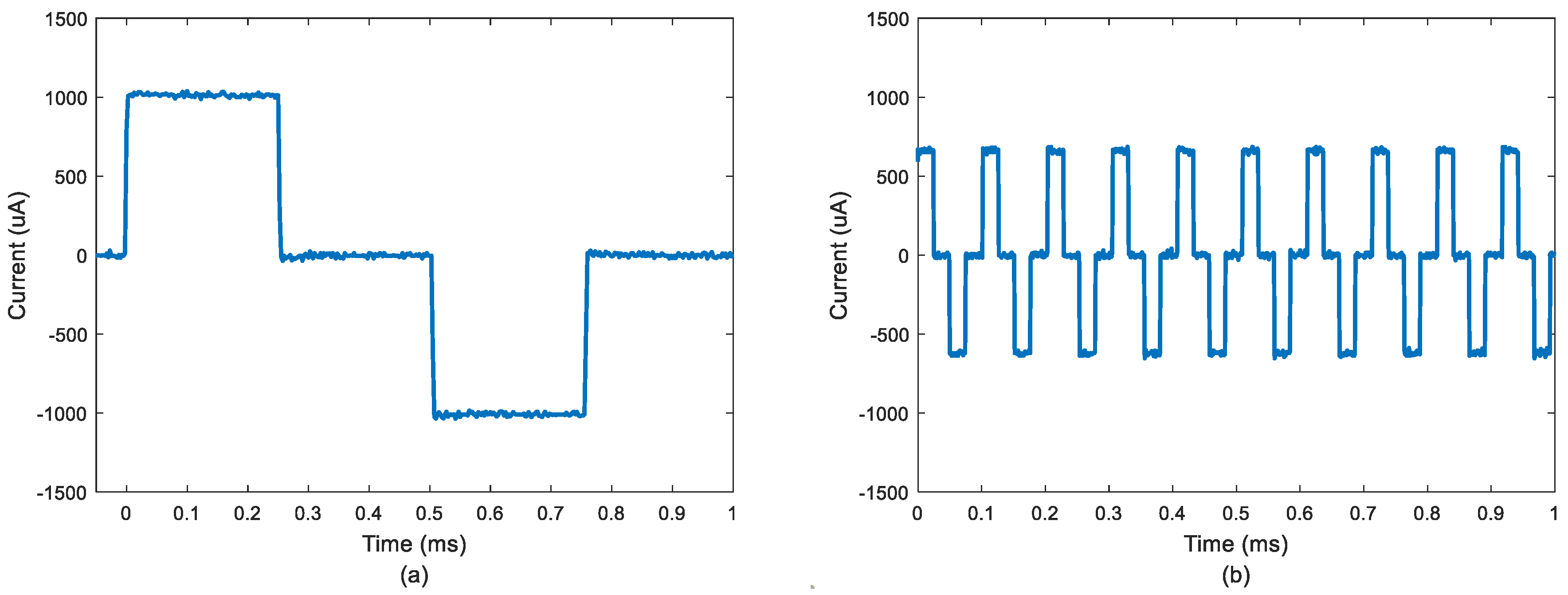
3.3. Arbitrary Waveform Generation
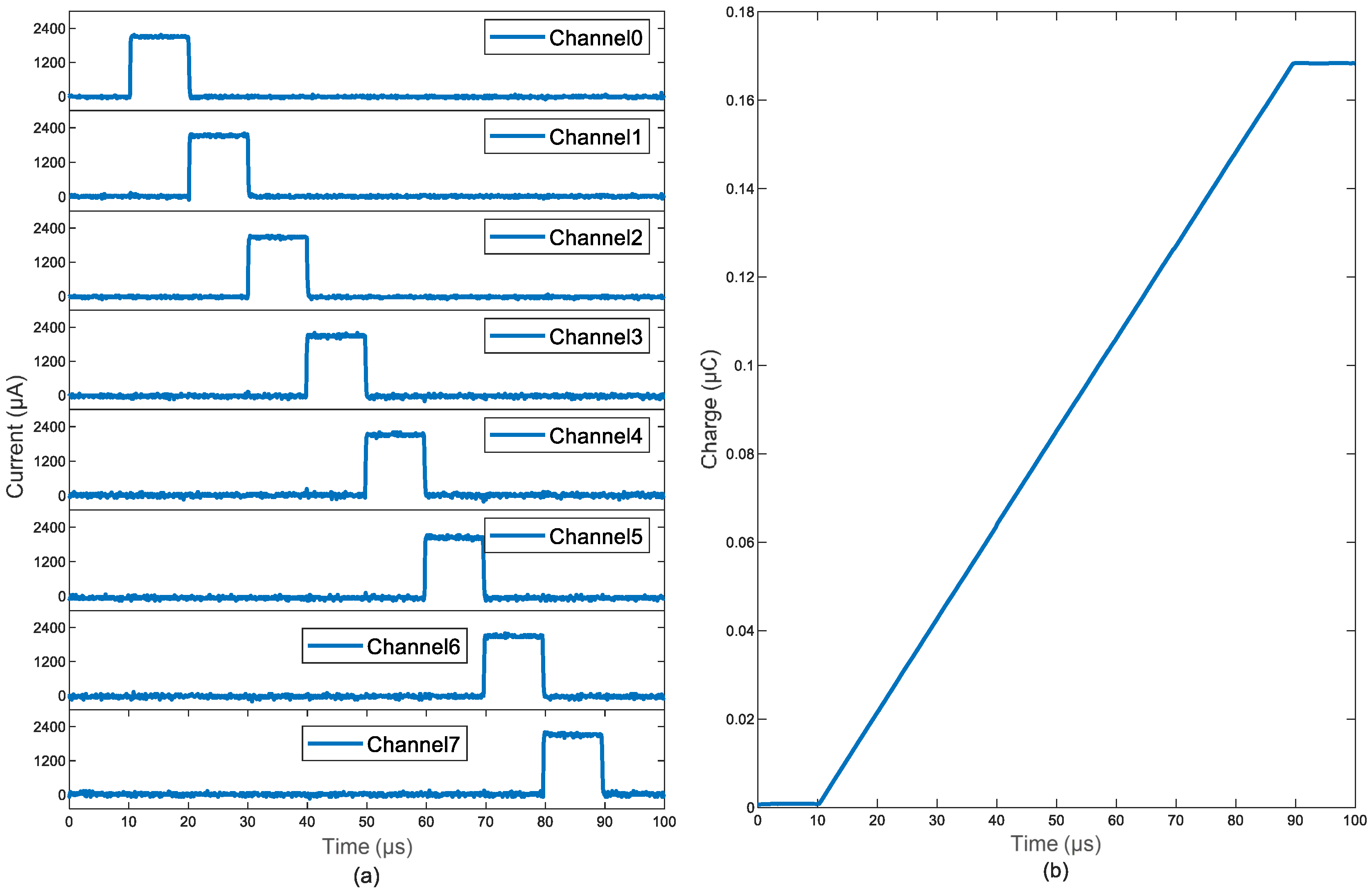
3.4. Charge-Balancing In Vitro
3.5. Selective Stimulation Protocols
3.5.1. Temporal Interference
3.5.2. Intersectional Short Pulse
3.6. Comparison with State-of-the-Art
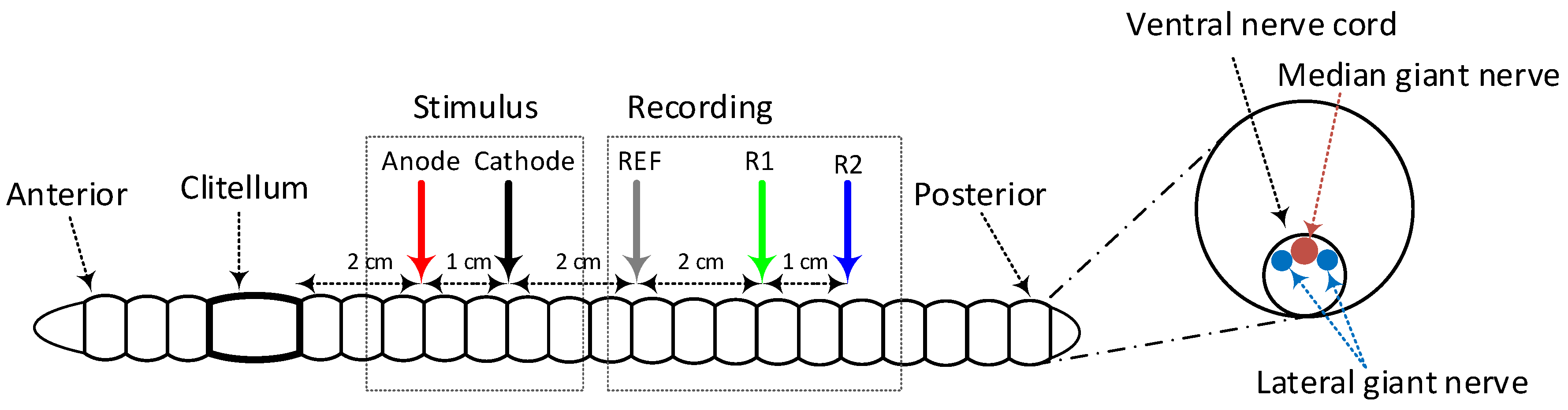
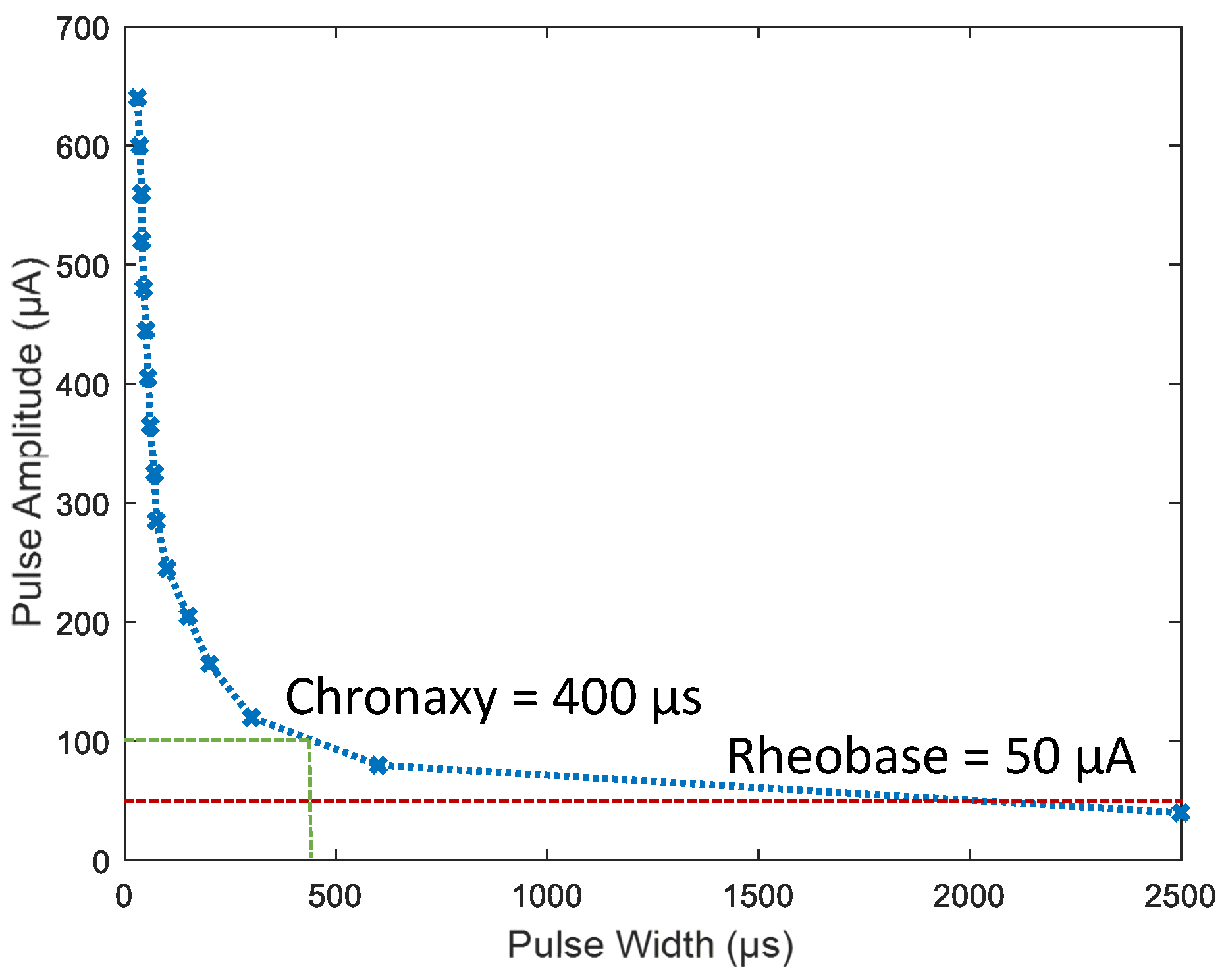
3.7. In Vivo Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plachta, D.T.T.T.; Gierthmuehlen, M.; Cota, O.; Espinosa, N.; Boeser, F.; Herrera, T.C.; Stieglitz, T.; Zentner, J. Blood pressure control with selective vagal nerve stimulation and minimal side effects. J. Neural Eng. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Koopman, F.A.; Chavan, S.S.; Miljko, S.; Grazio, S.; Sokolovic, S.; Schuurman, P.R.; Mehta, A.D.; Levine, Y.A.; Faltys, M.; Zitnik, R.; et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2016, 113, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Nava, A.; Christo, P.J.; Williams, K. Review of Recent Advances in Peripheral Nerve Stimulation (PNS). Curr. Pain Headache Rep. 2016, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A.L.; Pollak, P.; Hoffmann, D.; Gervason, C.; Hommel, M.; Perret, J.E.; de Rougemont, J.; Gao, D.M. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991, 337, 403–406. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Stanslaski, S.; Afshar, P.; Cong, P.; Giftakis, J.; Stypulkowski, P.; Carlson, D.; Linde, D.; Ullestad, D.; Avestruz, A.T.; Denison, T. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 410–421. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Lee, H.M.; Howell, B.; Grill, W.M.; Ghovanloo, M. Stimulation Efficiency with Decaying Exponential Waveforms in a Wirelessly Powered Switched-Capacitor Discharge Stimulation System. IEEE Trans. Biomed. Eng. 2018. [Google Scholar] [CrossRef]
- Johnson, B.C.; Gambini, S.; Izyumin, I.; Moin, A.; Zhou, A.; Alexandrov, G.; Santacruz, S.R.; Rabaey, J.M.; Carmena, J.M.; Muller, R. An implantable 700 μW 64-channel neuromodulation IC for simultaneous recording and stimulation with rapid artifact recovery. In Proceedings of the 2017 IEEE Symposium on VLSI Circuits, Kyoto, Japan, 5–8 June 2017; pp. C48–C49. [Google Scholar] [CrossRef]
- Taschwer, A.; Butz, N.; Kohler, M.; Rossbach, D.; Manoli, Y. A Charge Balanced Neural Stimulator with 3.3 v to 49 v Supply Compliance and Arbitrary Programmable Current Pulse Shapes. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference, BioCAS, Cleveland, OH, USA, 17–19 October 2018; pp. 1–3. [Google Scholar] [CrossRef]
- Zhou, A.; Santacruz, S.R.; Johnson, B.C.; Alexandrov, G.; Moin, A.; Burghardt, F.L.; Rabaey, J.M.; Carmena, J.M.; Muller, R. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates. Nat. Biomed. Eng. 2018, 3, 15–26. [Google Scholar] [CrossRef]
- Xu, J.; Guo, H.; Nguyen, A.T.; Lim, H.; Yang, Z. A bidirectional neuromodulation technology for nerve recording and stimulation. Micromachines 2018, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- FallahRad, M.; Zannou, A.L.; Khadka, N.; Prescott, S.A.; Ratté, S.; Zhang, T.; Esteller, R.; Hershey, B.; Bikson, M. Electrophysiology equipment for reliable study of kHz electrical stimulation. J. Physiol. 2019, 597, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.; Wilson, M.A.; Schiller, J.; Newman, J.P. Stimjim: Open source hardware for precise electrical stimulation. bioRxiv 2019, 757716. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Rivadeneyra, A.; Lugli, P. Low-Cost Bio-Impedance Analysis System for the Evaluation of Fruit Ripeness. In Proceedings of the IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Avery, J.; Dowrick, T.; Faulkner, M.; Goren, N.; Holder, D. A versatile and reproducible multi-frequency electrical impedance tomography system. Sensors 2017, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Nick, C.; Thielemann, C.; Schlaak, H.F. PEDOT:PSS coated gold nanopillar microelectrodes for neural interfaces. In Proceedings of the 2014 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, 3M-NANO, Taipei, Taiwan, 27–31 October 2014. [Google Scholar] [CrossRef]
- Shannon, K.M.; Gage, G.J.; Jankovic, A.; Wilson, W.J.; Marzullo, T.C. Portable conduction velocity experiments using earthworms for the college and high school neuroscience teaching laboratory. Am. J. Physiol. Adv. Physiol. Educ. 2014, 38, 62–70. [Google Scholar] [CrossRef]
- Follmann, R.; Rosa, E.; Stein, W. Dynamics of signal propagation and collision in axons. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2015, 92, 032707. [Google Scholar] [CrossRef] [PubMed]
- Kladt, N.; Hanslik, U.; Heinzel, H.G. Teaching basic neurophysiology using intact earthworms. J. Undergrad. Neurosci. Educ. 2010, 9, 20. [Google Scholar]
- Yun, S.; Koh, C.S.; Jeong, J.; Seo, J.; Ahn, S.H.; Choi, G.J.; Shim, S.; Shin, J.; Jung, H.H.; Chang, J.W.; et al. Remote-controlled fully implantable neural stimulator for freely moving small animal. Electronics 2019, 8, 706. [Google Scholar] [CrossRef]
- Johnson, B.C.; Shen, K.; Piech, D.; Ghanbari, M.M.; Li, K.Y.; Neely, R.; Carmena, J.M.; Maharbiz, M.M.; Muller, R. StimDust: A 6.5mm3, wireless ultrasonic peripheral nerve stimulator with 82% peak chip efficiency. In Proceedings of the 2018 IEEE Custom Integrated Circuits Conference, CICC 2018, San Diego, CA, USA, 8–11 April 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Lee, B.; Koripalli, M.K.; Jia, Y.; Acosta, J.; Sendi, M.S.E.; Choi, Y.; Ghovanloo, M. An Implantable Peripheral Nerve Recording and Stimulation System for Experiments on Freely Moving Animal Subjects. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Piech, D.K.; Johnson, B.C.; Shen, K.; Ghanbari, M.M.; Li, K.Y.; Neely, R.M.; Kay, J.E.; Carmena, J.M.; Maharbiz, M.M. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 2020, 4, 207–222. [Google Scholar] [CrossRef]
- Freeman, D.K.; O’Brien, J.M.; Kumar, P.; Daniels, B.; Irion, R.A.; Shraytah, L.; Ingersoll, B.K.; Magyar, A.P.; Czarnecki, A.; Wheeler, J.; et al. A sub-millimeter, inductively powered neural stimulator. Front. Neurosci. 2017, 11, 659. [Google Scholar] [CrossRef] [PubMed]






| Electrical Specifications | |||
|---|---|---|---|
| # of Current Sources | 8 | ||
| # of Output Channels | 16 | ||
| Current Resolution | Range 1 (R = 10 k) | 15.3 | nA |
| Range 2 (R = 1 k) | 153 | ||
| Range 3 (R = 100 ) | 1530 | ||
| Current Range | Range 1 (R = 10 k) | ±0.5 | mA |
| Range 2 (R = 1 k) | ±3 | ||
| Range 3 (R = 100 ) | ±5 | ||
| Compliance | ±5 mA | ±5 | V |
| Timing Specifications | |||
| Pulse Mode | |||
| Wait Period (W1) | Min: 0 Max: 65,535 Resolution: 1 | s | |
| Pulse (P1) | |||
| Interphase Gap (GP) | |||
| Pulse (P2) | |||
| Wait Period (W2) | |||
| Shorting Period (ST) | |||
| Rise Time | 120 | ns | |
| Arbitrary Waveform Mode | |||
| DAC Update Rate | 2 | s | |
| MEDUSA (This Work) | CS580 Voltage Controlled Current Source | Model 2000 Analog Stimulus Isolator | SYS-A395 Linear Stimulus Isolator | StimJim | |
|---|---|---|---|---|---|
| Output Type | Current | Current | Current/Voltage | Current | Current/Voltage |
| # of Current Sources | 8 | 1 | 1 | 1 | 2 |
| # of Output Channels | 16 | 1 | 1 | 1 | 2 |
| Required Input Source | Opal Kelly FPGA | Function Generator | Function Generator | Function Generator | No |
| Source Polarity | Bipolar | Bipolar | Bipolar | Bipolar | Bipolar |
| Analog Bandwidth | 3 MHz | 200 kHz | 40 kHz | 10 kHz | - |
| Maximum Current | ±5 mA | ±100 mA | ±5 mA | ±10 mA | ±1.36 mA |
| Current Resolution | 15.3 nA to 1530 nA | 100 fA to 10 A | - | - | 0.1 A |
| Rise Time | 120 ns | 2.8 s | <10 s | 26 s | 2 s to 6 s |
| Active Charge Balance | Yes | - | - | - | Yes |
| Estimated Cost | $200 | $2795 | $1400 | $1869 | $202 |
| Weight | 0.3 lbs | 15 lbs | 2.53 lbs | 4 lbs | - |
| Dimension | 3.6 × 5.0 × 0.5 in3 | 8.3 × 3.5 × 13 in3 | 2.5 × 6.1 × 6.2 in3 | 6.5 × 4 × 3.5 in3 | 5.5 × 4.1 × 1.37 in3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tala, F.; Johnson, B.C. MEDUSA: A Low-Cost, 16-Channel Neuromodulation Platform with Arbitrary Waveform Generation. Electronics 2020, 9, 812. https://doi.org/10.3390/electronics9050812
Tala F, Johnson BC. MEDUSA: A Low-Cost, 16-Channel Neuromodulation Platform with Arbitrary Waveform Generation. Electronics. 2020; 9(5):812. https://doi.org/10.3390/electronics9050812
Chicago/Turabian StyleTala, Fnu, and Benjamin C. Johnson. 2020. "MEDUSA: A Low-Cost, 16-Channel Neuromodulation Platform with Arbitrary Waveform Generation" Electronics 9, no. 5: 812. https://doi.org/10.3390/electronics9050812
APA StyleTala, F., & Johnson, B. C. (2020). MEDUSA: A Low-Cost, 16-Channel Neuromodulation Platform with Arbitrary Waveform Generation. Electronics, 9(5), 812. https://doi.org/10.3390/electronics9050812





