Effect of Annealing Ambient on SnO2 Thin Film Transistors Fabricated via An Ethanol-based Sol-gel Route
Abstract
1. Introduction
2. Materials and Methods
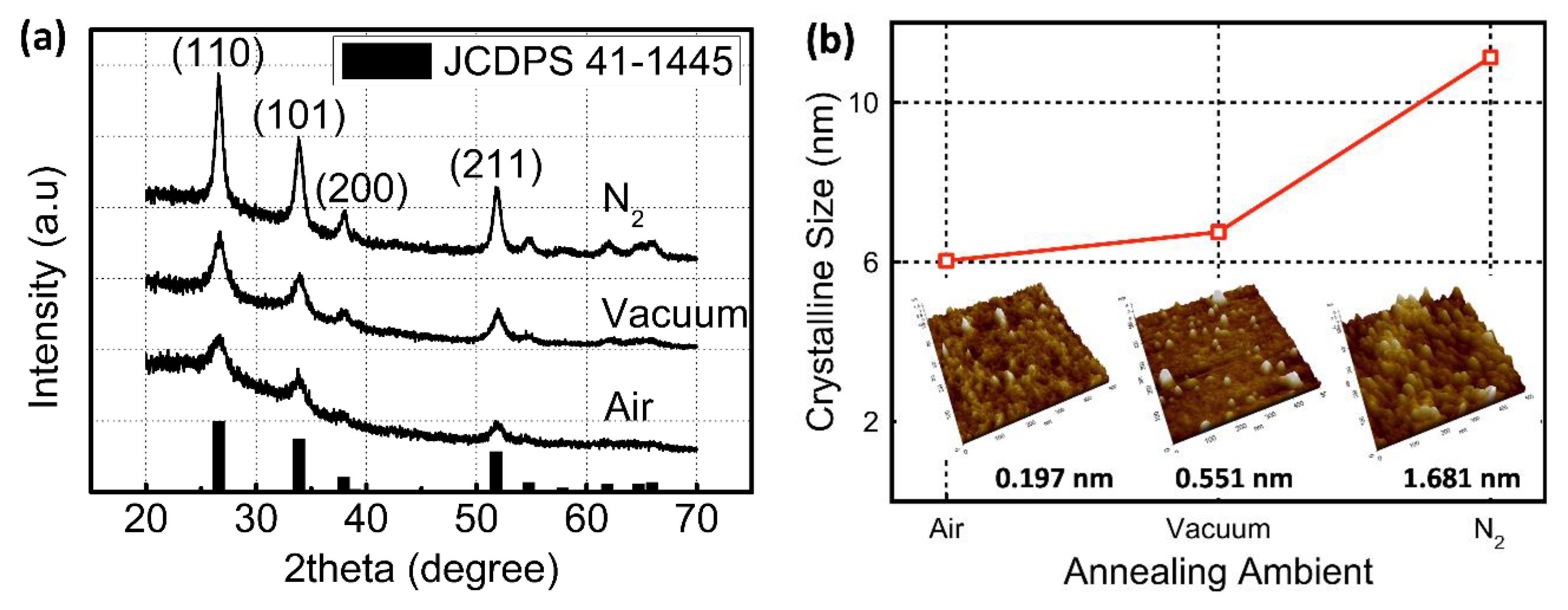
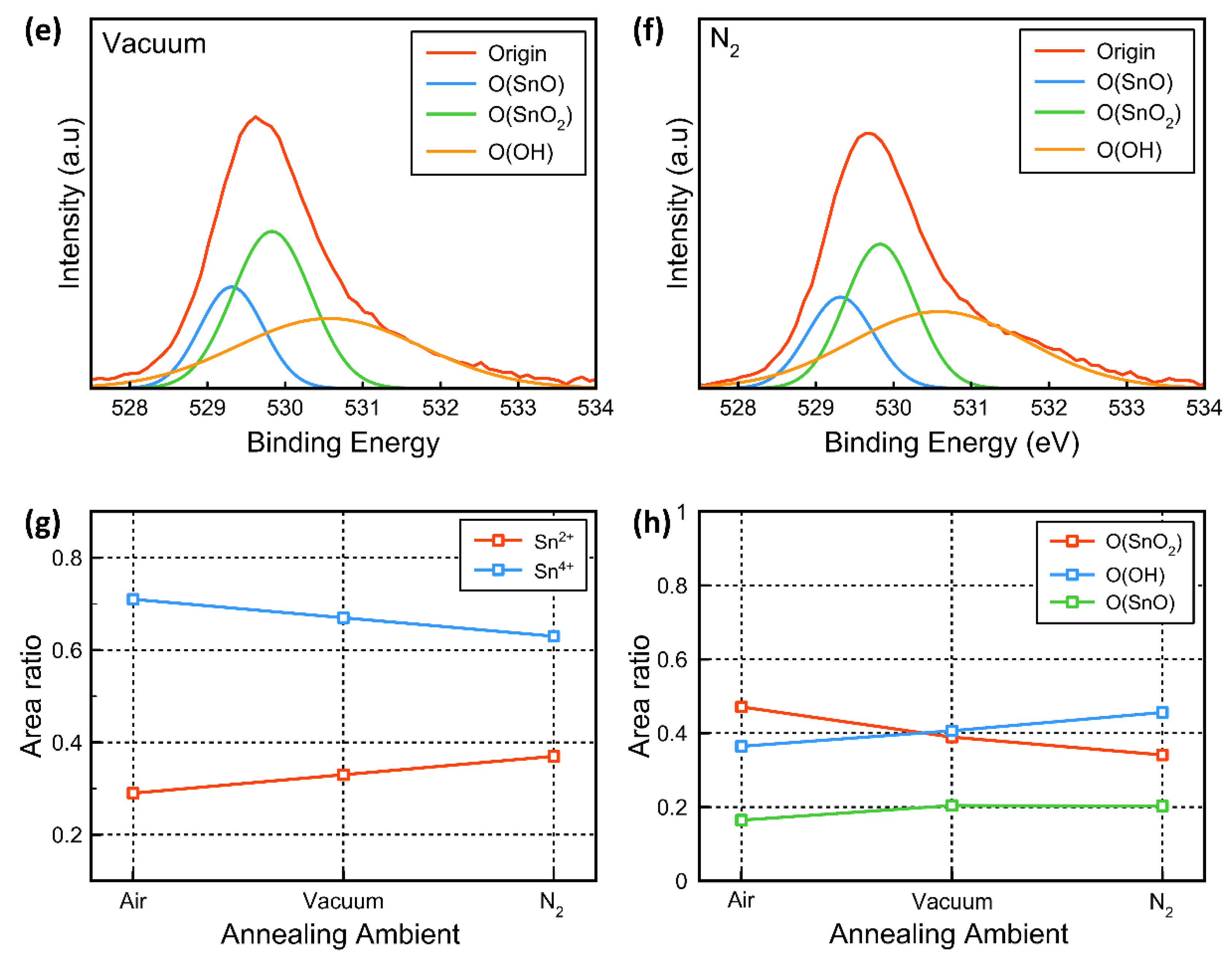
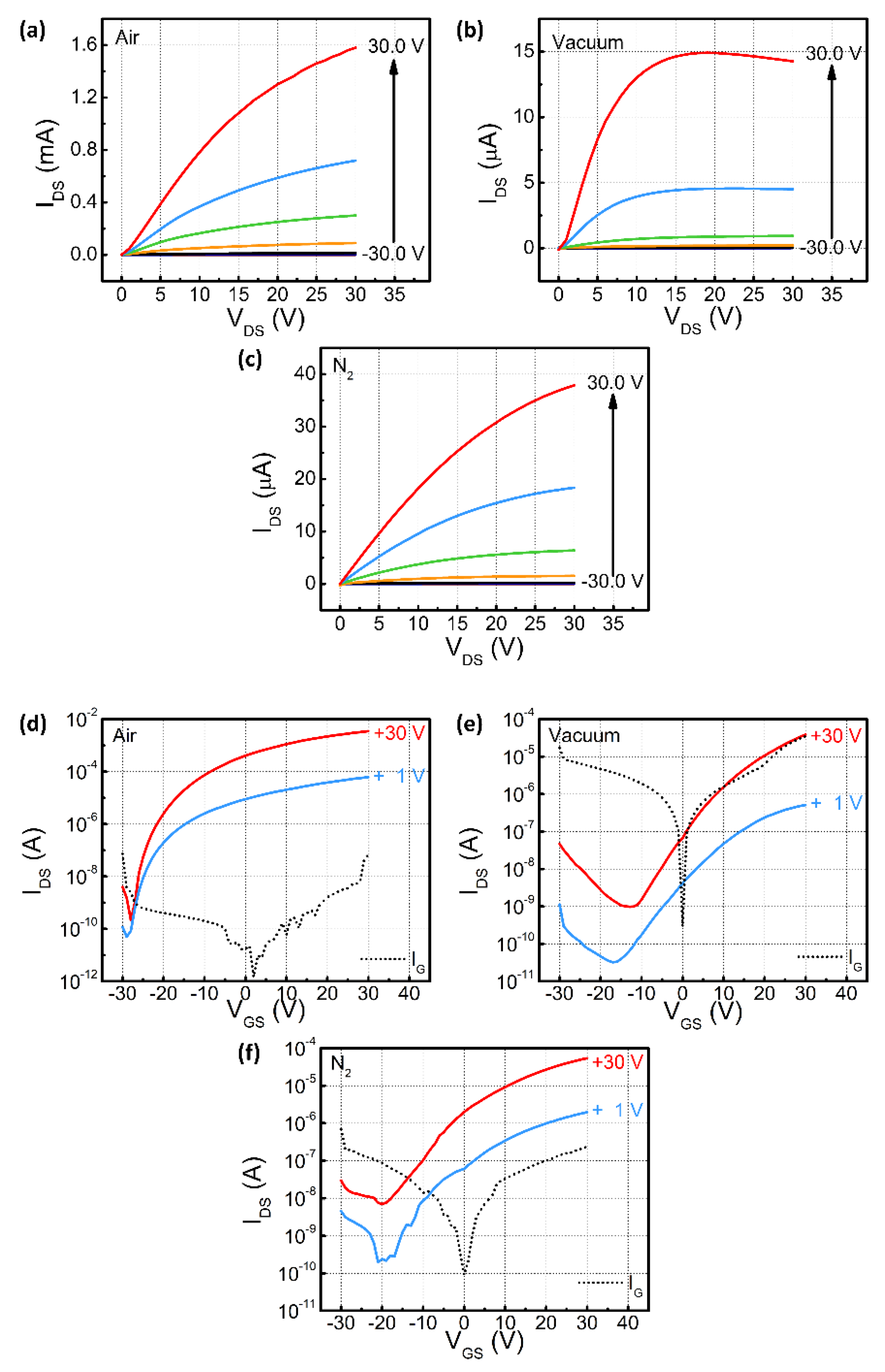
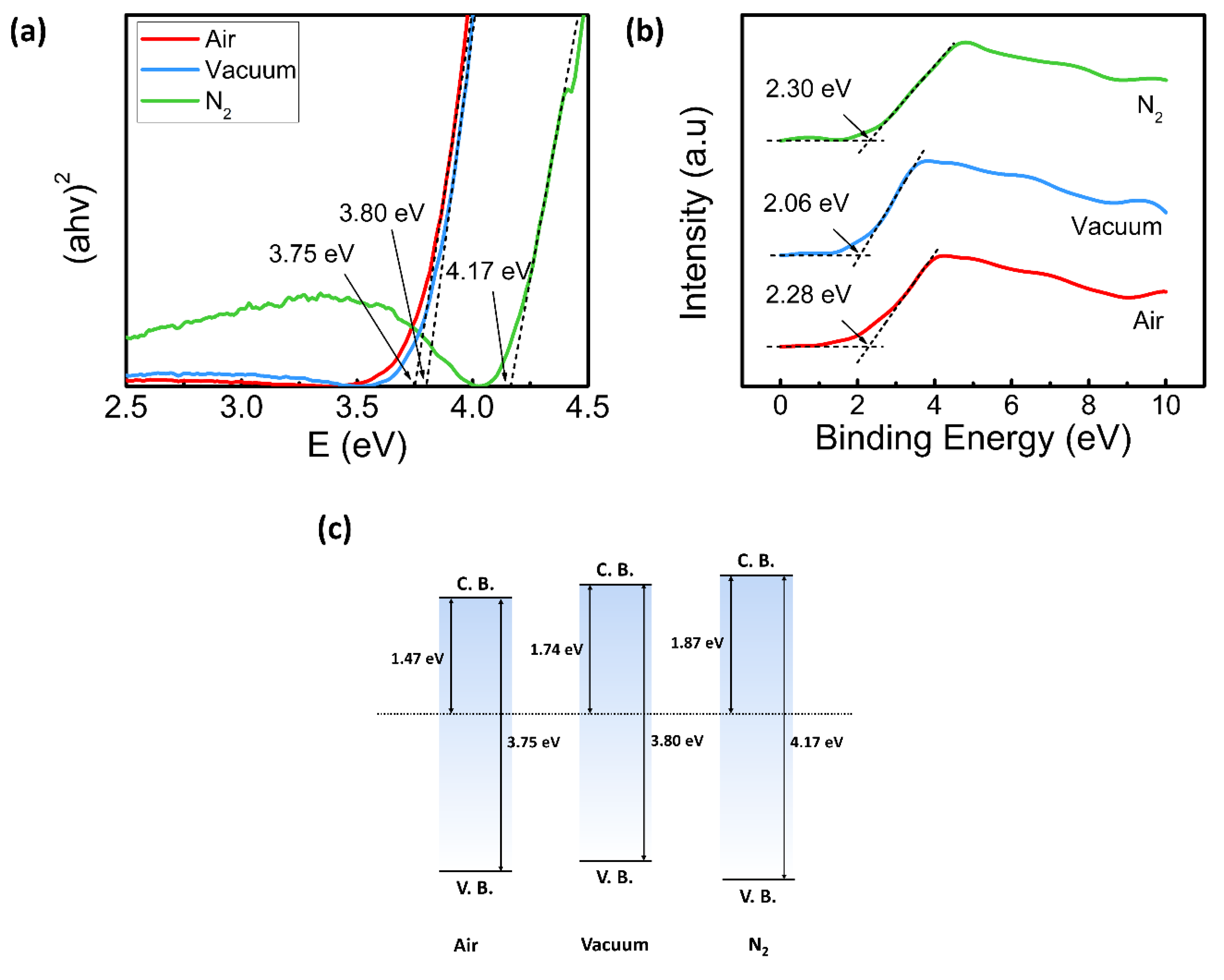
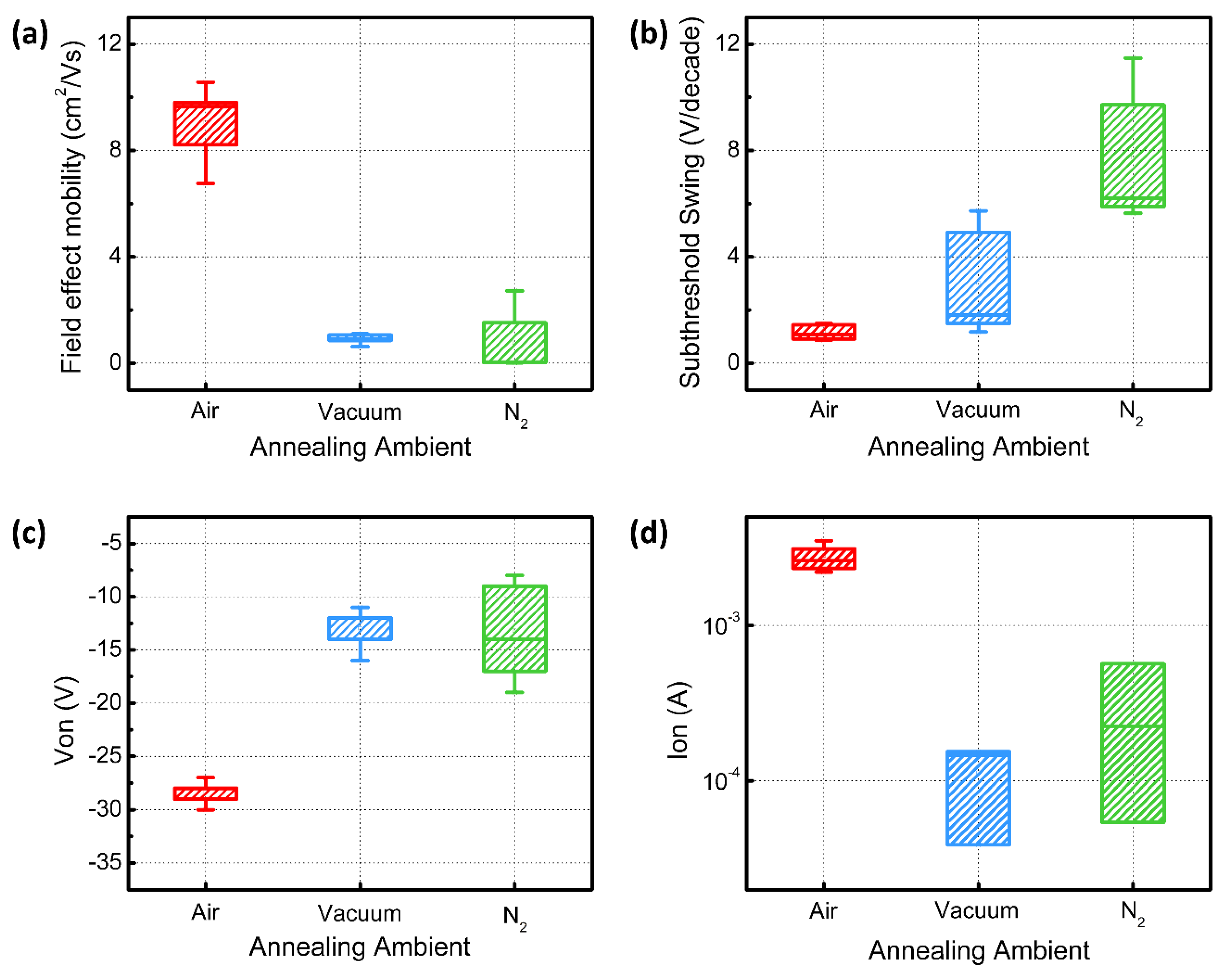
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, H.; Sano, M.; Abe, K.; Aiba, T.; Den, T.; Kumomi, H.; Nomura, K.; Kamiya, T.; Hosono, H. High-mobility thin-film transistor with amorphous InGaZnO4 channel fabricated by room temperature rf-magnetron sputtering. Appl. Phys. Lett. 2006, 89, 112123. [Google Scholar] [CrossRef]
- Tomai, S.; Nishimura, M.; Itose, M.; Matuura, M.; Kasami, M.; Matsuzaki, S.; Kawashima, H.; Utsuno, F.; Yano, K. High-Performance Thin Film Transistor with Amorphous In2O3–SnO2–ZnO Channel Layer. Jpn. J. Appl. Phys. 2012, 51, 3. [Google Scholar] [CrossRef]
- Ebata, K.; Tomai, S.; Tsuruma, Y.; Iitsuka, T.; Matsuzaki, S.; Yano, K. High-Mobility Thin-Film Transistors with Polycrystalline In–Ga–O Channel Fabricated by DC Magnetron Sputtering. Appl. Phys. Express 2012, 5, 011102. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare earth elements: Critical resources for high technology, U.S. Geol. Surv. Fact Sheet 2002, 87, 2. [Google Scholar]
- Sun, J.; Lu, A.; Wang, L.; Hu, Y.; Wan, Q. High-mobility transparent thin-film transistors with an Sb-doped SnO2 nanocrystal channel fabricated at room temperature. Nanotechnology 2009, 20, 335204. [Google Scholar] [CrossRef]
- Ju, S.; Chen, P.; Zhou, C.; Ha, Y.-G.; Facchetti, A.; Marks, T.J.; Kim, S.K.; Mohammadi, S.; Janes, D.B. 1∕f noise of SnO2 nanowire transistors. Appl. Phys. Lett. 2008, 92, 243120. [Google Scholar] [CrossRef]
- Ellmer, K. Resistivity of polycrystalline zinc oxide films: Current status and physical limit. J. Phys. D Appl. Phys. 2001, 34, 3097–3108. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Pimentel, A.; Pereira, L.; Gonçalves, G.; Martins, R. Amorphous IZO TTFTs with saturation mobilities exceeding 100 cm2/Vs. Phys. Stat. Sol. (RRL) 2007, 1, R34–R36. [Google Scholar] [CrossRef]
- Dehuff, N.L.; Kettenring, E.S.; Hong, D.; Chiang, H.Q.; Wager, J.F.; Hoffman, R.L.; Park, C.-H.; Keszler, D. Transparent thin-film transistors with zinc indium oxide channel layer. J. Appl. Phys. 2005, 97, 64505. [Google Scholar] [CrossRef]
- Wang, L.; Yoon, M.-H.; Lu, G.; Yang, Y.; Facchetti, A.; Marks, T.J. High-performance transparent inorganic–organic hybrid thin-film n-type transistors. Nat. Mater. 2006, 5, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kitsomboonloha, R.; Swisher, S.L.; Park, E.S.; Kang, H.; Subramanian, V. Transparent high-performance thin film transistors from solution-processed SnO2/ZrO2 gel-like precursors. Adv. Mater. 2013, 25, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Pattanasattayavong, P.; Thomas, S.; Adamopoulos, G.; McLachlan, M.A.; Anthopoulos, T.D. p-channel thin-film transistors based on spraycoated Cu2O Films. Appl. Phys. Lett. 2013, 102, 163505. [Google Scholar] [CrossRef]
- Jang, J.; Kang, H.; Chakravarthula, H.C.N.; Subramanian, V. Fully inkjet-printed transparent oxide thin film transistors using a fugitive wettability switch. Adv. Electron. Mater. 2015, 1, 1500086. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.H.; Woo, C.H.; Lee, J.Y.; Cho, H.K. Effect of annealing temperature on the electrical performances of solution-processed InGaZnO thin film transistors. Thin Solid Films 2011, 519, 5146–5149. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Jo, K.-M.; Kim, S.-Y.; Lee, J.-H.; Kim, J.-J.; Heo, Y.-W. Effects of Post-Annealing Treatments on the Transfer Characteristics of Amorphous Indium-Gallium-Zinc Oxide Thin Film Transistors. J. Nanoelectron. Optoelectron. 2011, 6, 310–314. [Google Scholar] [CrossRef]
- D.M., P.; Mannam, R.; Rao, M.R.; Dasgupta, N.; Priyadarshini, D.; Ramanjaneyulu, M. Effect of annealing ambient on SnO2 thin film transistors. Appl. Surf. Sci. 2017, 418, 414–417. [Google Scholar]
- Yun, Y.; Choi, A.; Hahm, S.G.; Chung, J.W.; Lee, Y.U.; Jung, J.Y.; Kim, J.-Y.; Park, J.-I.; Lee, S.; Jang, J. Enhanced Performance of Thiophene-Rich Heteroacene, Dibenzothiopheno [6,5-b:6’,5’-f] Thieno[3,2-b]Thiophene Thin-Film Transistor With MoOx Hole Injection Layers. IEEE Electron Device Lett. 2017, 38, 649–652. [Google Scholar] [CrossRef]
- Jang, B.; Kim, T.; Lee, S.; Lee, W.-Y.; Kang, H.; Cho, C.S.; Jang, J. High performance ultrathin SnO2 thin film transistors by sol-gel method. IEEE Electron Device Lett. 2018, 39, 1179–1182. [Google Scholar] [CrossRef]
- Jang, B.; Kim, T.; Lee, S.; Lee, W.-Y.; Jang, J. Schottky Nature of Au/SnO2 Ultrathin Film Diode Prepared by Sol-gel Method. IEEE Electron Device Lett. 2018, 39, 1732–1735. [Google Scholar] [CrossRef]
- Gupta, N.; Tyagi, B.P. On-Current Modeling of Polycrystalline Silicon Thin-Film Transistors. Phys. Scr. 2005, 72, 339–342. [Google Scholar] [CrossRef]
- Ahn, B.D.; Lim, J.H.; Cho, M.-H.; Park, J.-S.; Chung, K.-B. Thin film transistor behavior and the associated physical origin of water annealed In-Ga-Zn oxide semiconductor. J. Phys. D Appl. Phys. 2012, 45, 445307-1–415307-6. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Park, C.H. Rich Variety of Defects in ZnO via an Attractive Interaction between O Vacancies and Zn Interstitials: Origin of n -Type Doping. Phys. Rev. Lett. 2009, 102, 806403. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Raza, S.R.A.; Lee, H.S.; Jeon, P.J.; Pezeshki, A.; Min, S.W.; Kim, J.S.; Yoon, W.; Ju, S.Y.; Lee, K.; et al. Trap density probing on top-gate MoS2 nanosheet field effect transistors by photo-exited charge collection spectroscopy. Nanoscale 2015, 7, 5617. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Mo, Y.-G.; Kim, H.D.; Park, J.-S.; Jeong, J.K. Electronic transport properties of amorphous indium-gallium-zinc oxide semiconductor upon exposure to water. Appl. Phys. Lett. 2008, 92, 72104. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Ha, S.; Bae, J.-H.; Kang, I.-M.; Kim, K.; Lee, W.-Y.; Jang, J. Effect of Annealing Ambient on SnO2 Thin Film Transistors Fabricated via An Ethanol-based Sol-gel Route. Electronics 2019, 8, 955. https://doi.org/10.3390/electronics8090955
Lee H, Ha S, Bae J-H, Kang I-M, Kim K, Lee W-Y, Jang J. Effect of Annealing Ambient on SnO2 Thin Film Transistors Fabricated via An Ethanol-based Sol-gel Route. Electronics. 2019; 8(9):955. https://doi.org/10.3390/electronics8090955
Chicago/Turabian StyleLee, Hyunjae, Seunghyun Ha, Jin-Hyuk Bae, In-Man Kang, Kwangeun Kim, Won-Yong Lee, and Jaewon Jang. 2019. "Effect of Annealing Ambient on SnO2 Thin Film Transistors Fabricated via An Ethanol-based Sol-gel Route" Electronics 8, no. 9: 955. https://doi.org/10.3390/electronics8090955
APA StyleLee, H., Ha, S., Bae, J.-H., Kang, I.-M., Kim, K., Lee, W.-Y., & Jang, J. (2019). Effect of Annealing Ambient on SnO2 Thin Film Transistors Fabricated via An Ethanol-based Sol-gel Route. Electronics, 8(9), 955. https://doi.org/10.3390/electronics8090955






