Enhanced Photoresponsivity of All-Inorganic (CsPbBr3) Perovskite Nanosheets Photodetector with Carbon Nanodots (CDs)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
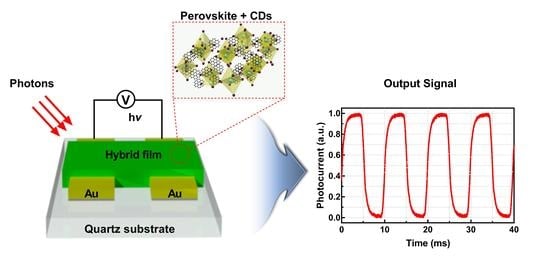
3.1. Device Structure
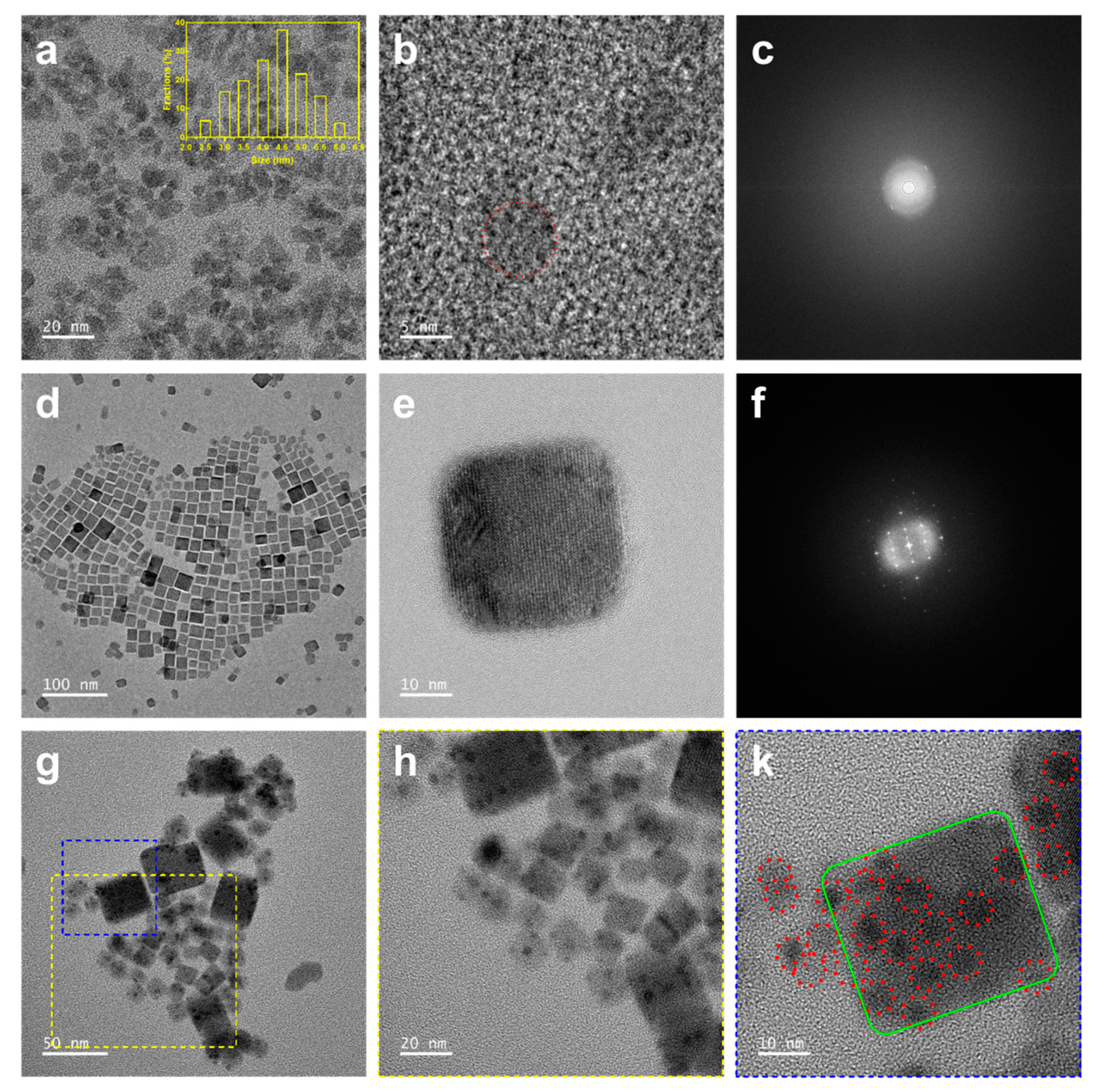
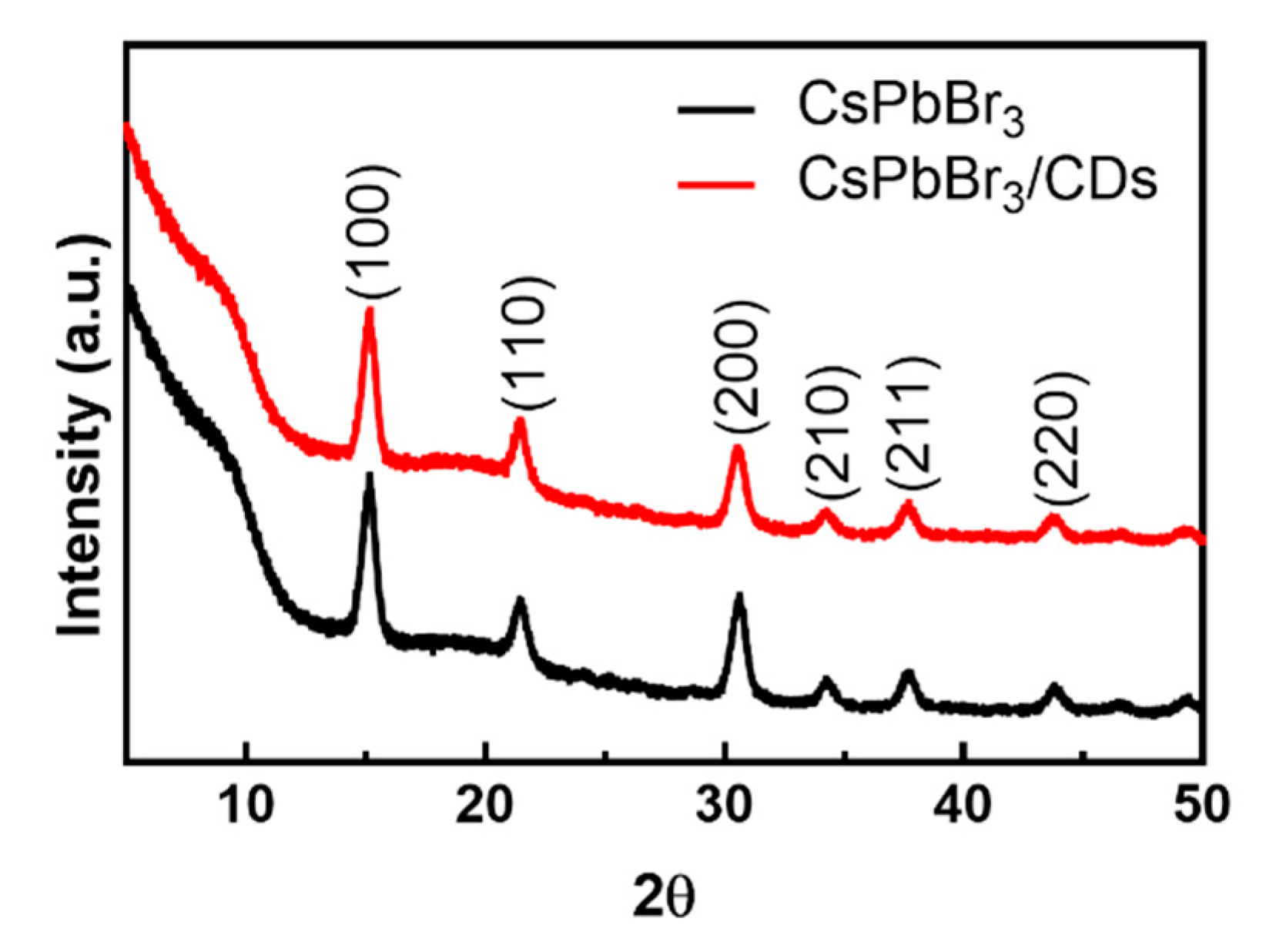
3.2. Characterization of CDs, CsPbBr3, and CsPbBr3/CD Composite Solutions
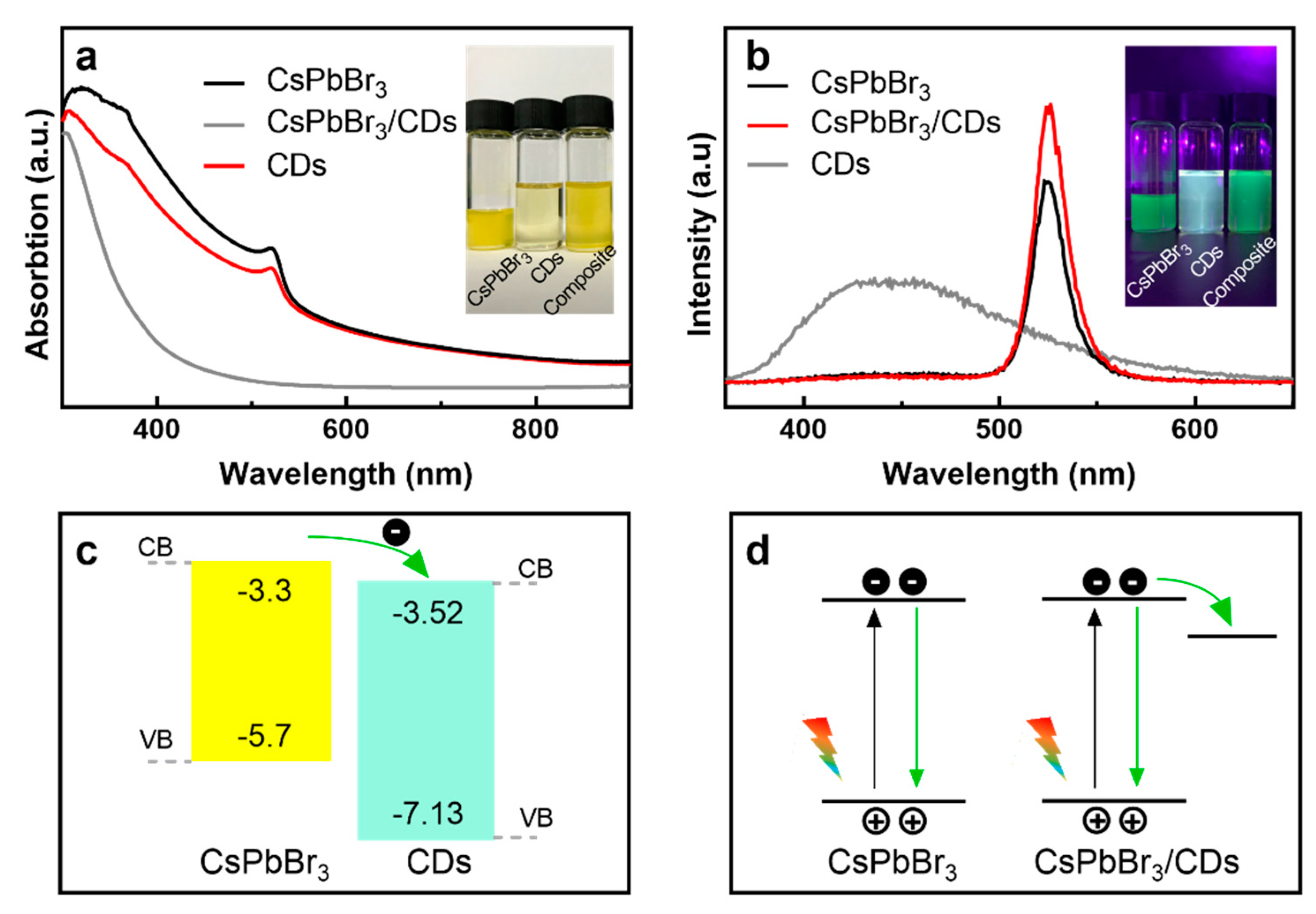
3.3. Optical characterization of the CDs, CsPbBr3, and CsPbBr3/CD Composite Solutions
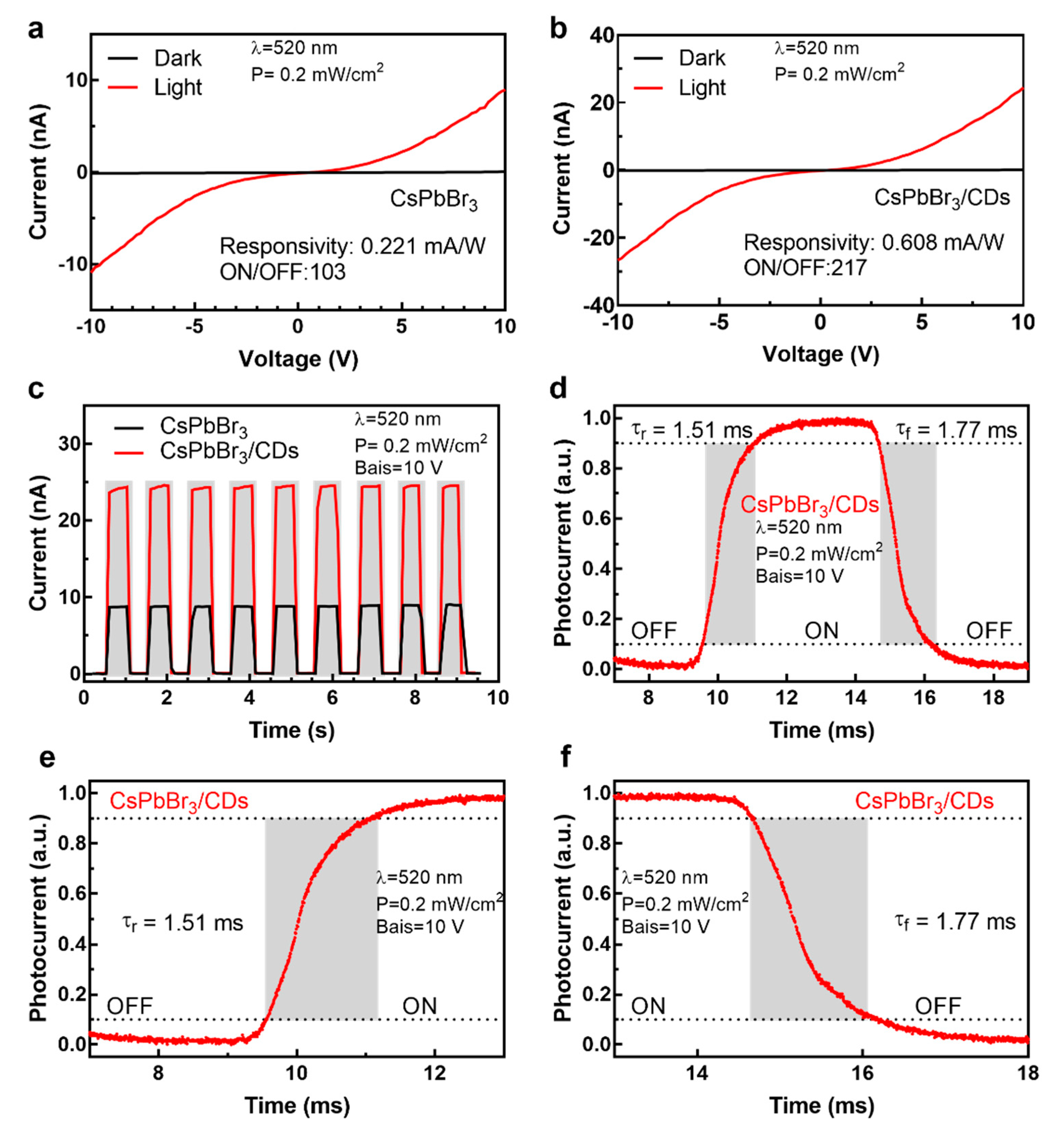
3.4. Performances of Photodetectors Based on CsPbBr3 and CsPbBr3/CD Composite Film
3.5. Working Principle of the CsPbBr3/CD Composite Photodetector
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deng, H.; Yang, X.; Dong, D.; Li, B.; Yang, D.; Yuan, S.; Qiao, K.; Cheng, Y.B.; Tang, J.; Song, H. Flexible and semitransparent organolead triiodide perovskite network photodetector arrays with high stability. Nano Lett. 2015, 15, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, X.; Liang, L.; Bao, J.; Li, S.; Yang, W.; Xie, Y. High-Performance Flexible Broadband Photodetector Based on Organolead Halide Perovskite. Adv. Funct. Mater. 2014, 24, 7373–7380. [Google Scholar] [CrossRef]
- Horváth, E.; Spina, M.; Szekrényes, Z.; Kamarás, K.; Gaal, R.; Gachet, D.; Forró, L. Nanowires of Methylammonium Lead Iodide (CH3NH3PbI3) prepared by low temperature solution-mediated crystallization. Nano Lett. 2014, 14, 6761–6766. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.M.; Kim, D.H.; Park, K.; Park, J.; Lee, J.W.; Song, J.K. Ultrasound synthesis of lead halide perovskite nanocrystals. J. Mater. Chem. C 2016, 4, 10625–10629. [Google Scholar] [CrossRef]
- Jang, D.M.; Park, K.; Kim, D.H.; Park, J.; Shojaei, F.; Kang, H.S.; Ahn, J.-P.; Lee, J.W.; Song, J.K. Reversible Halide Exchange Reaction of Organometal Trihalide Perovskite Colloidal Nanocrystals for Full-Range Band Gap Tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Adinolfi, V.; Peng, W.; Walters, G.; Bakr, O.M.; Sargent, E.H. The Electrical and Optical Properties of Organometal Halide Perovskites Relevant to Optoelectronic Performance. Adv. Mater. 2018, 30, 1–13. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Kamminga, M.E.; Fang, H.-H.; Loi, M.A.; ten Brink, G.H.; Blake, G.R.; Palstra, T.T.M.; ten Elshof, J.E. Micropatterned 2D hybrid perovskite thin films with enhanced photoluminescence lifetimes. ACS Appl. Mater. Interfaces 2018, 10, 12878–12885. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.E.; Mohamad, D.K.; Wong-Stringer, M.; Smith, A.; Lidzey, D.G. Spray-cast multilayer perovskite solar cells with an active-area of 1.5 cm2. Sci. Rep. 2017, 7, 7962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xu, K.; Luo, X.; Lv, W.; Peng, Y.; Wang, Y.; Lu, F.; Xu, S. Ultrasensitivity broadband photodetectors based on perovskite: Research on film crystallization and electrode optimization. Org. Electron. Phys. Mater. Appl. 2017, 46, 35–43. [Google Scholar] [CrossRef]
- Li, S.; Tong, S.; Yang, J.; Xia, H.; Zhang, C.; Zhang, C.; Shen, J.; Xiao, S.; He, J.; Gao, Y.; et al. High-performance formamidinium-based perovskite photodetectors fabricated via doctor-blading deposition in ambient condition. Org. Electron. Phys. Mater. Appl. 2017, 47, 102–107. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, X.; Huang, L.; Xu, X.; Wang, L.; Wang, J.; Shang, Q.; Lee, S.-T.; Jie, J. Aligned Single-Crystalline Perovskite Microwire Arrays for High-Performance Flexible Image Sensors with Long-Term Stability. Adv. Mater. 2016, 28, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz Conductivity within Colloidal CsPbBr3 Perovskite Nanocrystals: Remarkably High Carrier Mobilities and Large Diffusion Lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Zhang, L.; Yin, Z.; Wang, D.; Meng, J.; Jiang, Q.; Wang, Y.; You, J. A high-performance photodetector based on an inorganic perovskite-ZnO heterostructure. J. Mater. Chem. C 2017, 5, 6115–6122. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Chen, J.; Wang, Y.; Cao, F.; Wei, Y.; Wu, Y.; Wang, L.; Zhu, Y.; Sun, Z.; et al. Constructing Fast Carrier Tracks into Flexible Perovskite Photodetectors to Greatly Improve Responsivity. ACS Nano 2017, 11, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Li, J.; Xue, J.; Dong, Y.; Li, X.; Zeng, H. Monolayer and Few-Layer All-Inorganic Perovskites as a New Family of Two-Dimensional Semiconductors for Printable Optoelectronic Devices. Adv. Mater. 2016, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Dong, Y.; Song, J.; Zou, Y.; Li, X.; Zeng, H.; Li, J.; Xue, J.; Xu, L. Improving All-Inorganic Perovskite Photodetectors by Preferred Orientation and Plasmonic Effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Kwak, D.-H.; Lim, D.-H.; Ra, H.-S.; Ramasamy, P.; Lee, J.-S. High performance hybrid graphene–CsPbBr 3−x I x perovskite nanocrystal photodetector. RSC Adv. 2016, 6, 65252–65256. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Yu, D.; Huo, C.; Ji, J.; Li, X.; Zhang, S.; Zou, Y.; Zhu, G.; Wang, Y.; et al. Boosting Two-Dimensional MoS2/CsPbBr3 Photodetectors via Enhanced Light Absorbance and Interfacial Carrier Separation. ACS Appl. Mater. Interfaces 2018, 10, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Xu, C.; Wu, L.; Wang, J.; Ren, J.; Qu, X. Non-Enzymatic-Browning-Reaction: A Versatile Route for Production of Nitrogen-Doped Carbon Dots with Tunable Multicolor Luminescent Display. Sci. Rep. 2015, 4, 3564. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Pan, C.; Sun, Q.; Song, W.; Fang, L.; Chen, Q.; Banks, C.E. A carbon quantum dot decorated RuO2 network: Outstanding supercapacitances under ultrafast charge and discharge. Energy Environ. Sci. 2013, 6, 3665. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Yang, S.T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A Biocompatible Fluorescent Ink Based on Water-Soluble Luminescent Carbon Nanodots. Angew. Chem. Int. Ed. 2012, 51, 12215–12218. [Google Scholar] [CrossRef]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.Y. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, Y.; Zhang, X.; Duan, D.; Fan, C. Nitrogen-doped carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced visible light driven photocatalytic activity and stability. J. Colloid Interface Sci. 2017, 491, 238–245. [Google Scholar] [CrossRef]
- Do, S.; Kwon, W.; Rhee, S.-W. Soft-template synthesis of nitrogen-doped carbon nanodots: Tunable visible-light photoluminescence and phosphor-based light-emitting diodes. J. Mater. Chem. C 2014, 2, 4221. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H.; et al. Color-Switchable Electroluminescence of Carbon Dot Light-Emitting Diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Liu, C.; Ma, D. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502. [Google Scholar] [CrossRef]
- Xie, C.; Nie, B.; Zeng, L.; Liang, F.-X.; Wang, M.-Z.; Luo, L.; Feng, M.; Yu, Y.; Wu, C.-Y.; Wu, Y.; et al. Core–Shell Heterojunction of Silicon Nanowire Arrays and Carbon Quantum Dots for Photovoltaic Devices and Self-Driven Photodetectors. ACS Nano 2014, 8, 4015–4022. [Google Scholar] [CrossRef]
- Guo, D.-Y.; Shan, C.-X.; Qu, S.-N.; Shen, D.-Z. Highly Sensitive Ultraviolet Photodetectors Fabricated from ZnO Quantum Dots/Carbon Nanodots Hybrid Films. Sci. Rep. 2015, 4, 7469. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, M.L.; Yu, C.W.; Wan, T.C.; Chen, S.H.; Chang, C.Y.; Hsu, T.Y. Dual functional photo-response for p-Si/SiO2/n-InGaZnO graphene nanocomposites photodiodes. Nanotechnology 2018, 29, 505202. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kang, C.C.; Ma, Y.C.; Chou, Y.C.; Ye, J.H.; Huang, R.T.; Siao, C.Z.; Lin, Y.C.; Chang, Y.H.; Shen, J.L.; et al. P-GaN/n-ZnO nanorods: The use of graphene nanosheets composites to increase charge separation in self-powered visible-blind UV photodetectors. Nanotechnology 2018, 29. [Google Scholar] [CrossRef]
- Dai, M.K.; Lian, J.T.; Lin, T.Y.; Chen, Y.F. High-performance transparent and flexible inorganic thin film transistors: A facile integration of graphene nanosheets and amorphous InGaZnO. J. Mater. Chem. C 2013, 1, 5064–5071. [Google Scholar] [CrossRef]
- Fang, X.; Ding, J.; Yuan, N.; Sun, P.; Lv, M.; Ding, G.; Zhu, C. Graphene quantum dot incorporated perovskite films: Passivating grain boundaries and facilitating electron extraction. Phys. Chem. Chem. Phys. 2017, 19, 6057–6063. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.K.; Mitra, P.; Bera, R.; Das, S.; Pramanik, A.; Parta, A. An efficient charge separation and photocurrent generation in the carbon dot-zinc oxide nanoparticle composite. Nanoscale 2017, 9, 6791–6799. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.K.; Paramanik, B.; Bain, D.; Patra, A. Light Harvesting and White-Light Generation in a Composite of Carbon Dots and Dye-Encapsulated BSA-Protein-Capped Gold Nanoclusters. Chem. A Eur. J. 2016, 22, 11699–11705. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xin, X.; Zang, Z.; Tang, X.; Li, C.; Hu, W.; Zhou, M.; Du, J. Tunable photoluminescence of CsPbBr3perovskite quantum dots for light emitting diodes application. J. Solid State Chem. 2017, 255, 115–120. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. Carbon 2018, 4, 67. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Z.; Du, S.; Yuan, F.; Li, Z.; Li, Z.; Dai, Q.; Wang, H.; Luo, S.; Zhang, S.; et al. Solution-processed photodetectors based on organic–inorganic hybrid perovskite and nanocrystalline graphite. Nanotechnology 2016, 27, 175201. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of Luminescent Carbon Dots with Ultrahigh Quantum Yield and Inherent Folate Receptor-Positive Cancer Cell Targetability. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ka, I.; Gerlein, L.F.; Nechache, R.; Cloutier, S.G. High-performance nanotube-enhanced perovskite photodetectors. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, D.; Wang, X.; Huo, C.; Wu, Y.; Zhu, Z.; Zeng, H. Two-dimensional CsPbBr3/PCBM heterojunctions for sensitive, fast and flexible photodetectors boosted by charge transfer. Nanotechnology 2018, 29. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.F.; Li, S.; Lei, L.Z.; Ji, H.F.; Wu, D.; Xu, T.T.; Tian, Y.T.; Li, X.J. High-performance perovskite photodetectors based on solution-processed all-inorganic CsPbBr3 thin films. J. Mater. Chem. C 2017, 5, 8355–8360. [Google Scholar] [CrossRef]
- AlZoubi, T.; Qutaish, H.; Al-Shawwa, E.; Hamzawy, S. Enhanced UV-light detection based on ZnO nanowires/graphene oxide hybrid using cost-effective low temperature hydrothermal process. Opt. Mater. 2018, 77, 226–232. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, Y.; Zeng, H.; Cao, F.; Yu, D.; Li, X.; Gu, Y.; Song, J. Healing All-Inorganic Perovskite Films via Recyclable Dissolution-Recyrstallization for Compact and Smooth Carrier Channels of Optoelectronic Devices with High Stability. Adv. Funct. Mater. 2016, 26, 5903–5912. [Google Scholar] [CrossRef]
- Tang, X.; Zu, Z.; Shao, H.; Hu, W.; Zhou, M.; Deng, M.; Chen, W.; Zang, Z.; Zhu, T.; Xue, J. All-inorganic perovskite CsPb(Br/I)3 nanorods for optoelectronic application. Nanoscale 2016, 8, 15158–15161. [Google Scholar] [CrossRef] [PubMed]
- Jansen-van Vuuren, R.D.; Armin, A.; Pandey, A.K.; Burn, P.L.; Meredith, P. Organic Photodiodes: The Future of Full Color Detection and Image Sensing. Adv. Mater. 2016, 28, 4766–4802. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Du, S.; Zuo, Z.; Zhao, Y.; Cui, H.; Zhan, X. High Detectivity and Rapid Response in Perovskite CsPbBr3 Single-Crystal Photodetector. J. Phys. Chem. C 2017, 121, 4917–4923. [Google Scholar] [CrossRef]

| Active Materials | POptical @ ʎ | R(AW−1) | τr/τf (ms) | Reference |
|---|---|---|---|---|
| CsPbBr3 nanosheets | 0.35 mWcm−2 @ 442 nm | 0.25 | 0.019/0.025 | [21] |
| CsPbBr3 QDs | 0.40 mWcm−2 @ 532 nm | 0.005 | 0.2/1.3 | [22] |
| CsPbBr3 micro-particles | 1.01 mWcm−2 @ 442 nm | 0.18 | 1.8/1.0 | [52] |
| Single crystal CsPbBr3 | 1 mW @ 450 nm | 0.028 | 90.7/57 | [55] |
| CsPb(Br/I)3 nanorods | 20 mw @ 450 nm | 0.18 | 680/660 | [53] |
| CsPbBr3/CD composite | 0.2 mWcm−2 @ 520 nm | 0.61 | 1.51/1.77 | Our work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algadi, H.; Mahata, C.; Woo, J.; Lee, M.; Kim, M.; Lee, T. Enhanced Photoresponsivity of All-Inorganic (CsPbBr3) Perovskite Nanosheets Photodetector with Carbon Nanodots (CDs). Electronics 2019, 8, 678. https://doi.org/10.3390/electronics8060678
Algadi H, Mahata C, Woo J, Lee M, Kim M, Lee T. Enhanced Photoresponsivity of All-Inorganic (CsPbBr3) Perovskite Nanosheets Photodetector with Carbon Nanodots (CDs). Electronics. 2019; 8(6):678. https://doi.org/10.3390/electronics8060678
Chicago/Turabian StyleAlgadi, Hassan, Chandreswar Mahata, Janghoon Woo, Minkyu Lee, Minsu Kim, and Taeyoon Lee. 2019. "Enhanced Photoresponsivity of All-Inorganic (CsPbBr3) Perovskite Nanosheets Photodetector with Carbon Nanodots (CDs)" Electronics 8, no. 6: 678. https://doi.org/10.3390/electronics8060678
APA StyleAlgadi, H., Mahata, C., Woo, J., Lee, M., Kim, M., & Lee, T. (2019). Enhanced Photoresponsivity of All-Inorganic (CsPbBr3) Perovskite Nanosheets Photodetector with Carbon Nanodots (CDs). Electronics, 8(6), 678. https://doi.org/10.3390/electronics8060678