Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review
Abstract
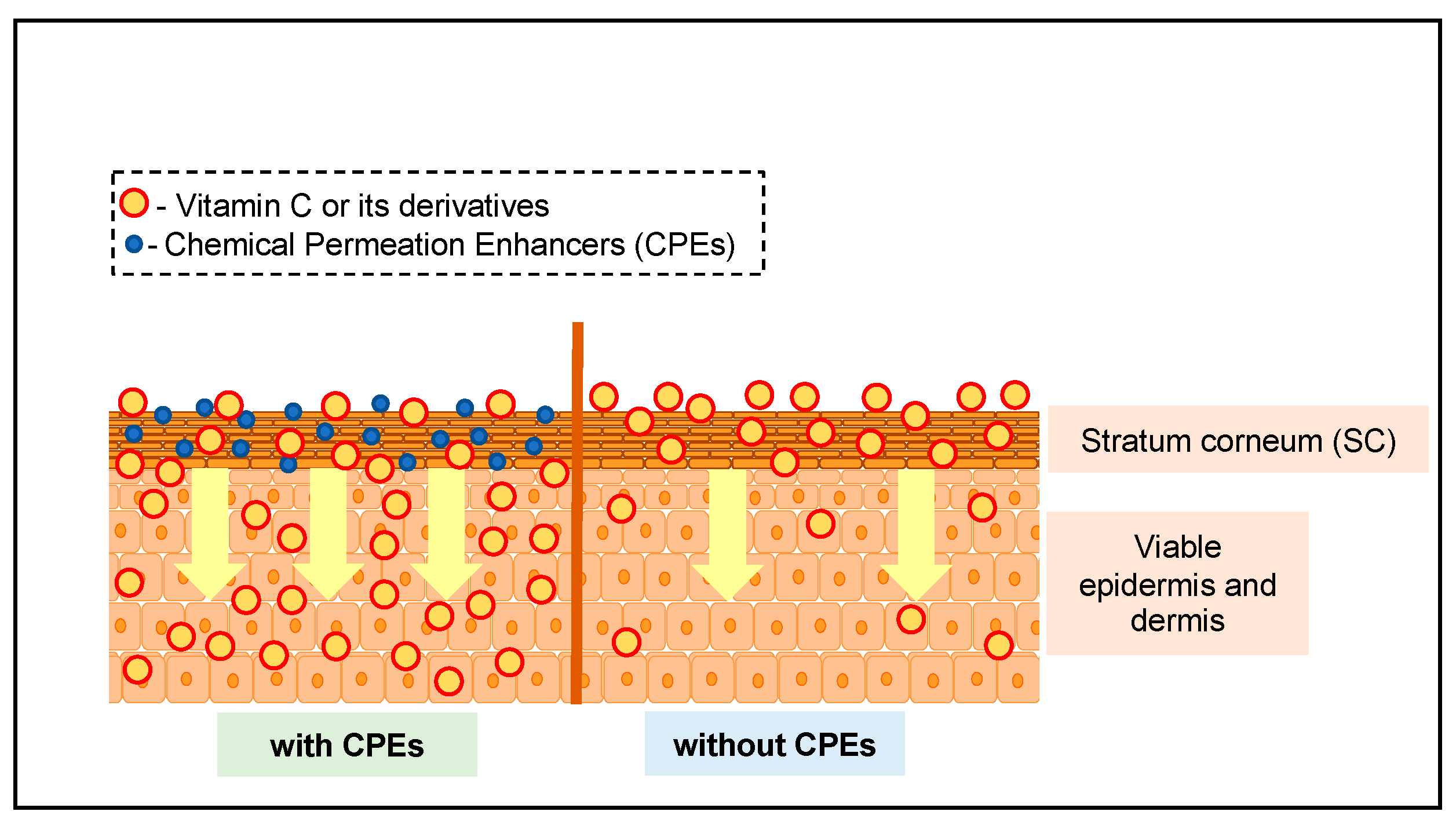
:1. Introduction
2. Materials and Methods
2.1. Data Gathering Procedure
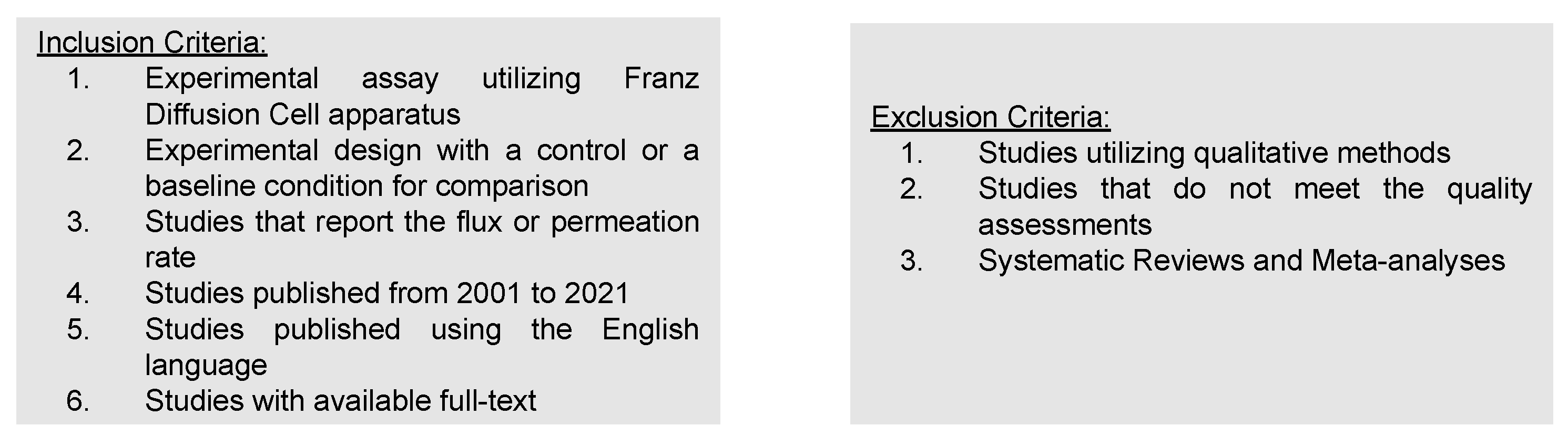
2.2. Selection and Inclusion Criteria
2.3. Quality Assessment
2.4. Data Analysis
3. Results
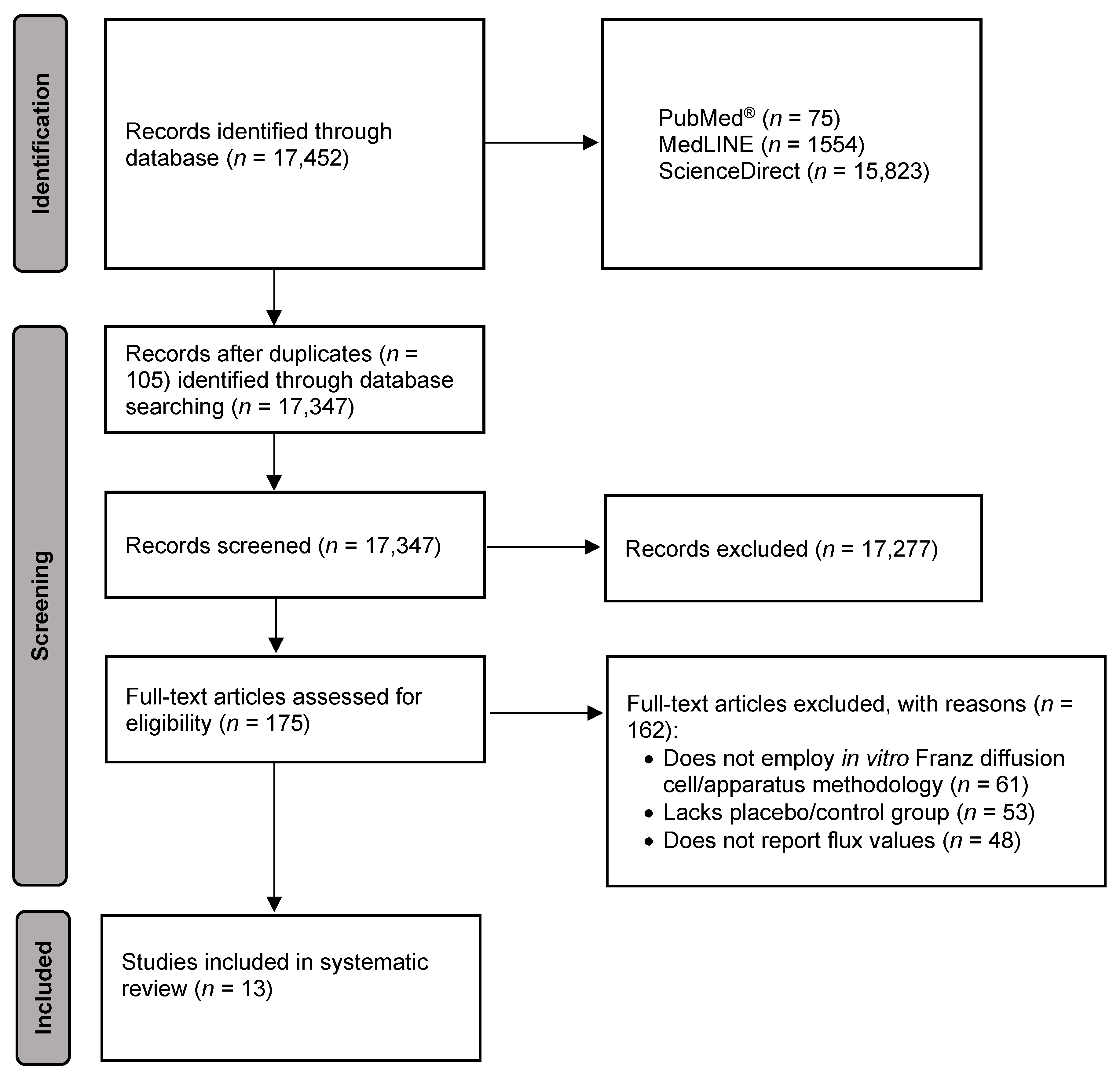
3.1. Study Selection
3.2. Study Characteristics
3.3. Chemical Permeation Enhancers Utilized for VC
4. Discussion
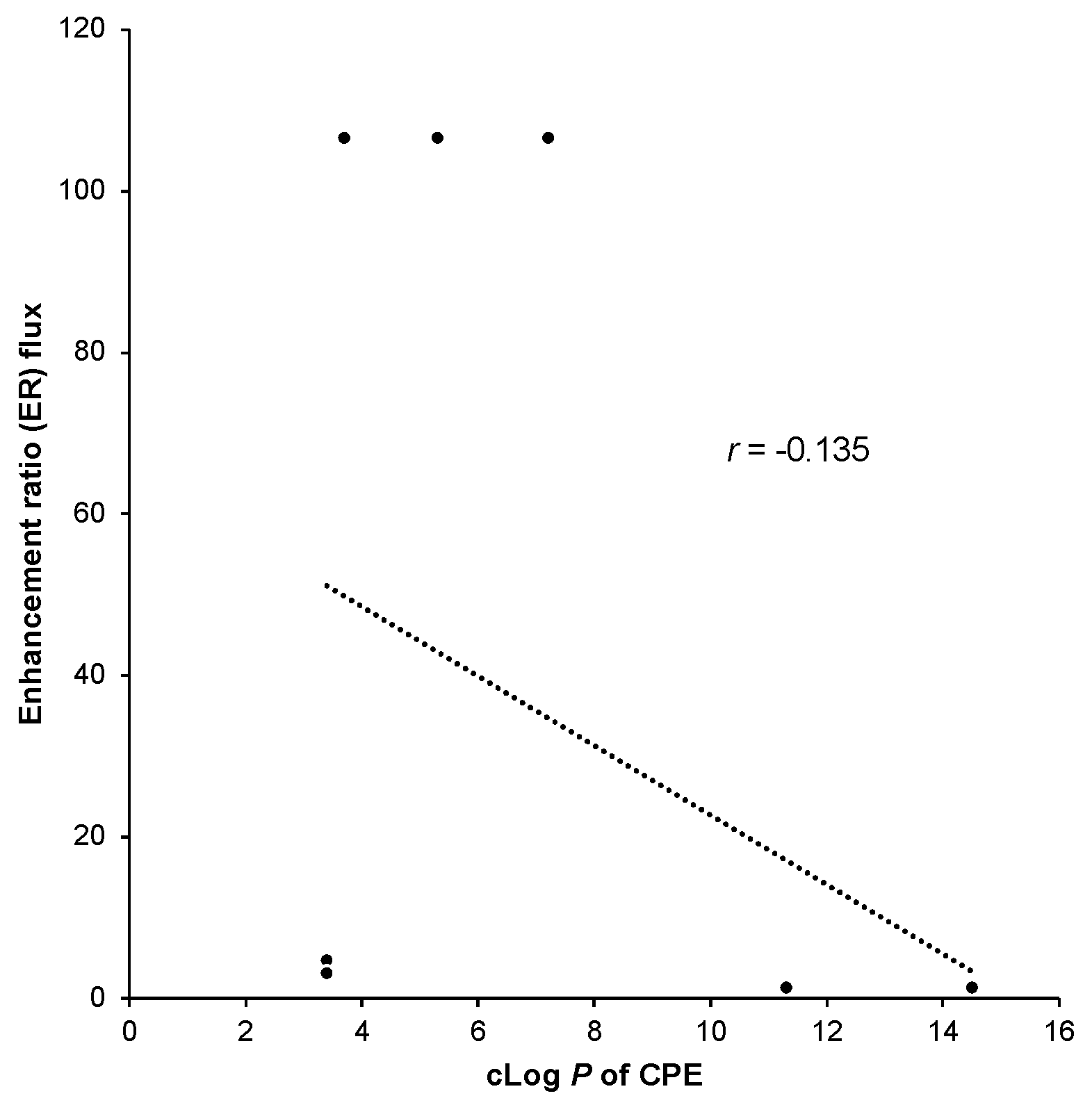
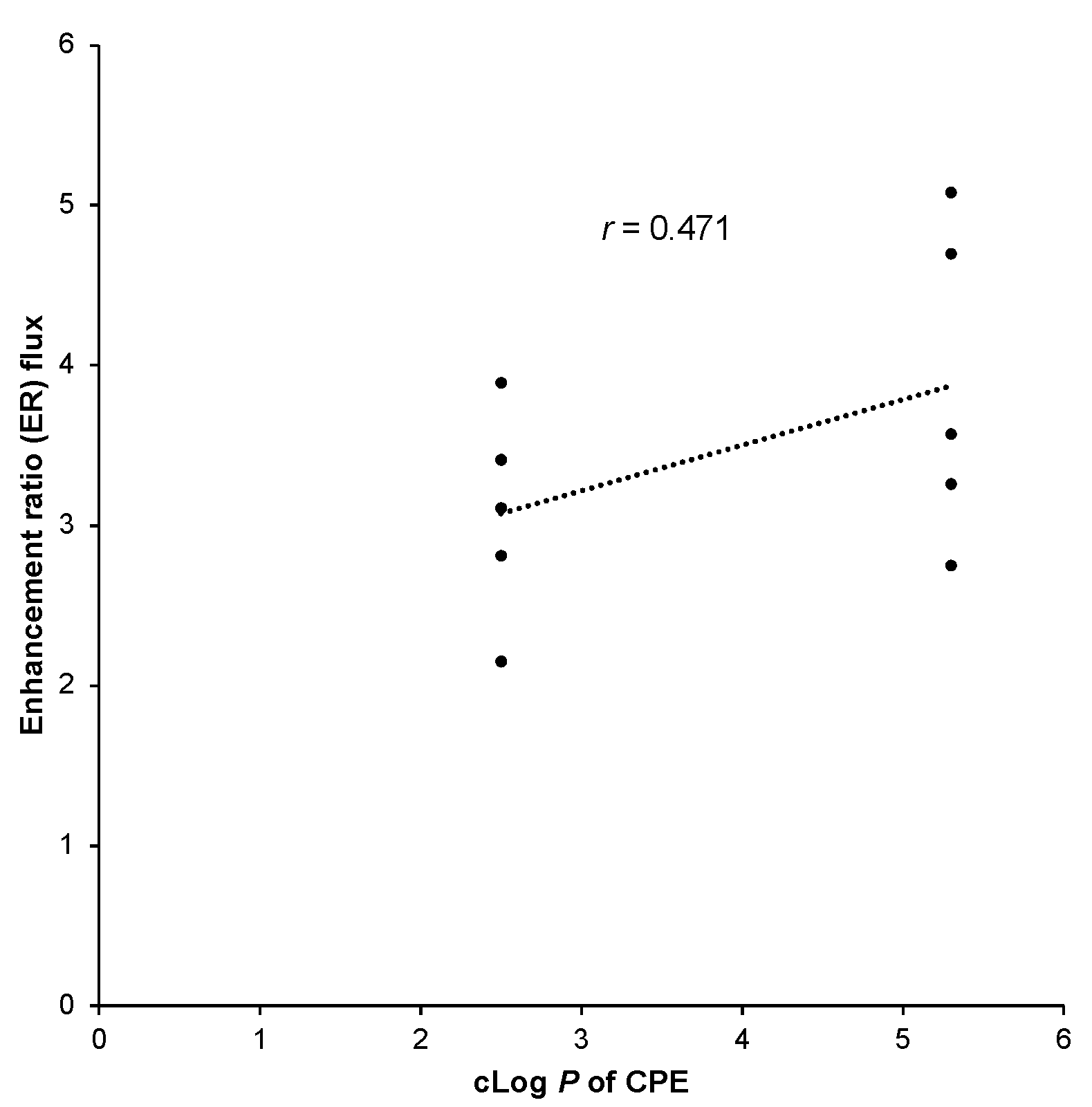
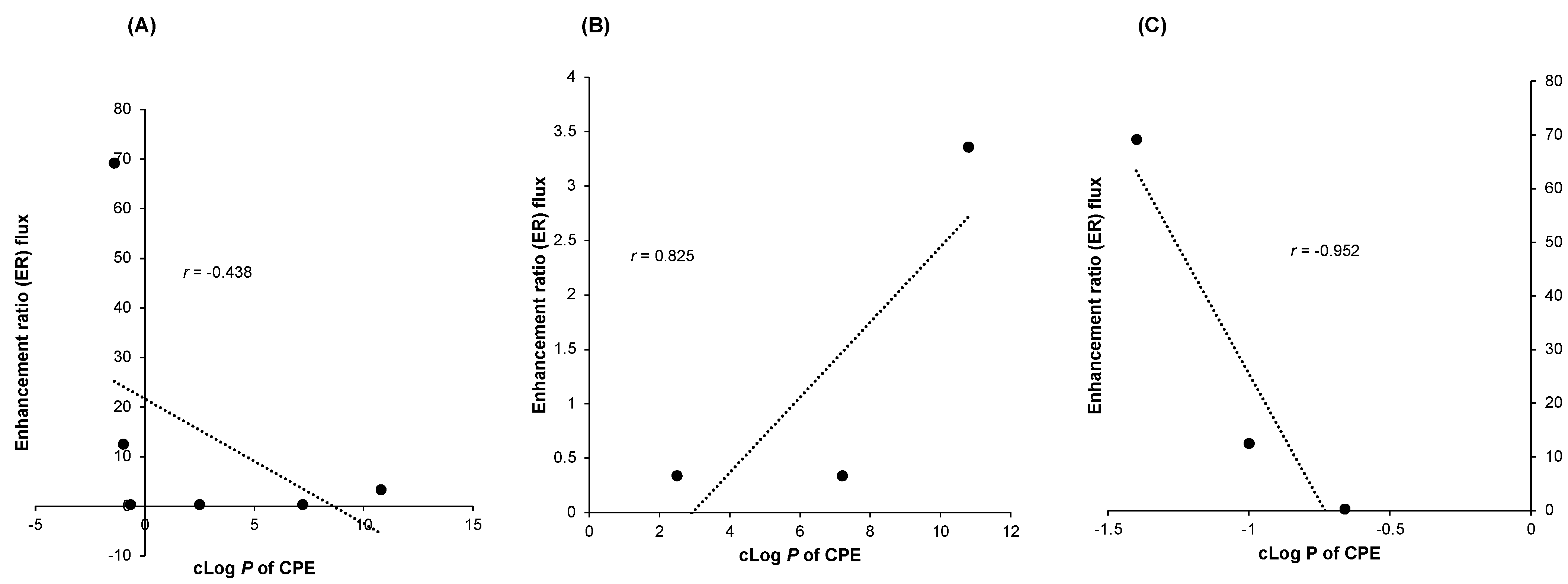
4.1. cLog P and Enhancement Ratio of CPEs
4.2. Factors Affecting the Permeation Enhancing Capacity of CPEs for VC
4.2.1. Solubility
4.2.2. Interaction with Stratum Corneum of Different Types of CPE
Non-Ionic Surfactants
Phospholipids
Amino Acids
Terpenes
Polyols
Esters
Fatty Acids
Colloids and Polymers
4.2.3. Permeation Characteristics of Topically Applied VC and VC Derivatives
4.3. Safety of CPEs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguntibeju, O.O. The biochemical, physiological and therapeutic roles of ascorbic acid. Afr. J. Biotechnol. 2021, 269, 13685–13688. [Google Scholar]
- Farris, P.K. Cosmetical vitamins: Vitamin C. In Cosmeceuticals. Procedures in Cosmetic Dermatology, 2nd ed.; Draelos, Z.D., Dover, J.S., Alam, M., Eds.; Saunders Elsevier: New York, NY, USA, 2009; pp. 51–56. [Google Scholar]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 67, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Khemis, A.; Cabou, J.; Dubois, J.; Ortonne, J.P. A randomized controlled study to evaluate the depigmenting activity of l-ascorbic acid plus phytic acid–serum vs. placebo on solar lentigines. J. Cosmet. Dermatol. 2011, 10, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; An, H.S.; Bae, J.S.; Kim, J.Y.; Choi, C.H.; Kim, J.Y.; Choi, S.Y. Effects of palmitoyl-KVK-L-ascorbic acid on skin wrinkles and pigmentation. Arch. Dermatol. Res. 2017, 309, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Telang, P.S. VC in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Discov. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Atkinson, J.P.; Maibach, H.I. Chemical penetration enhancers: Classification and mode of action. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–27. [Google Scholar]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. 2019, 1, 100025. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Hossain, A.S.M.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical delivery of 3-O-ethyl l-ascorbic acid from complex solvent systems. Sci. Pharm. 2020, 88, 19. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.U.; Khan, H.M.S.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of lascorbic acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- Davaran, S.; Hanaee, J.; Rashidi, M.R.; Valiolah, F.; Hashemi, M. Influence of poly (ethylene glycol)-α-cyclodextrin complexes on stabilization and transdermal permeation of ascorbic acid. J. Biomed. Mater. Res. 2006, 3, 590–594. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, Y.C.; Gao, M.Y.; Fang, Y.P. In vitro evaluation of permeation ability and in vivo whitening of ascorbic acid 2-glucoside in microemulsion. Int. J. Pharm. Sci. 2016, 3, 114–118. [Google Scholar]
- Lee, S.K.; Woo, R.S.; Choi, S.U.; Lee, J.H.; Choi, Y.W. The Effect of Limonene on Skin Permeation and Localization of Ascorbic Acid. J. Pharm. Investig. 2006, 36, 305–308. [Google Scholar]
- Pakpayat, N.; Nielloud, F.; Fortuné, R.; Tourne-Peteilh, C.; Villarreal, A.; Grillo, I.; Bataille, B. Formulation of ascorbic acid microemulsions with alkyl polyglycosides. Eur. J. Pharm. Biopharm. 2009, 72, 444–452. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Choi, Y.W. Skin permeation enhancement of ascorbyl palmitate by liposomal hydrogel (lipogel) formulation and electrical assistance. Biol. Pharm. Bull. 2007, 30, 393–396. [Google Scholar] [CrossRef]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef]
- Hikima, T.; Tamura, Y.; Yamawaki, Y.; Yamamoto, M.; Tojo, K. Skin accumulation and penetration of a hydrophilic compound by a novel gemini surfactant, sodium dilauramidoglutamide lysine. Int. J. Pharm. 2013, 443, 288–292. [Google Scholar] [CrossRef]
- Valgimigli, L.; Gabbanini, S.; Berlini, E.; Lucchi, E.; Beltramini, C.; Bertarelli, Y.L. Lemon (Citrus limon, Burm. f.) essential oil enhances the trans-epidermal release of lipid-(A, E) and water-(B6, C) soluble vitamins from topical emulsions in reconstructed human epidermis. Int. J. Cosmet. Sci. 2012, 34, 347–356. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Huang, J.; Xia, N.; Li, T.; Xia, Q. Self-double-emulsifying drug delivery system incorporated in natural hydrogels: A new way for topical application of vitamin C. J. Microencapsul. 2018, 35, 90–101. [Google Scholar] [CrossRef]
- Wathoni, N.; Panji Luhur, M. Effect of iontophoresis and propylene glycol on the in vitro diffusion of ethyl vitamin c cream. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 31–34. [Google Scholar]
- Rozman, B.; Gosenca, M.; Gasperlin, M.; Padois, K.; Falson, F. Dual influence of colloidal silica on skin deposition of vitamins C and E simultaneously incorporated in topical microemulsions. Eur. J. Pharm. Biopharm. 2010, 36, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Rozman, B.; Gasperlin, M.; Tinois-Tessoneaud, E.; Pirot, F.; Falson, F. Simultaneous absorption of vitamins C and E from topical microemulsions using reconstructed human epidermis as a skin model. Eur. J. Pharm. Biopharm. 2009, 72, 69–75. [Google Scholar] [CrossRef]
- Rozman, B.; Zvonar, A.; Falson, F.; Gasperlin, M. Temperature-sensitive microemulsion gel: An effective topical delivery system for simultaneous delivery of vitamins C and E. AAPS PharmSciTech 2009, 10, 54–61. [Google Scholar] [CrossRef]
- Arce, F.V., Jr.; Asano, N.; Yamashita, K.; Oda, A.; Uchida, T.; Sano, T.; Todo, H.; Sugibayashi, K. Effect of layered application on the skin permeation of a cosmetic active component, rhododendrol. J. Toxicol. Sci. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Bergamante, V.; Maffei, P.; Borgia, S.L.; Calabrese, V. Biserni, S.; Ronchi, C. Solubility and Transdermal Permeation Properties of a Dehydroepiandrosterone Cyclodextrin Complex from Hydrophilic and Lipophilic Vehicles. Drug Deliv. 2008, 12, 275–280. [Google Scholar] [CrossRef]
- Pereira, R.; Silva, S.G.; Pinheiro, M.; Reis, S.; Vale, M. Current Status of Amino Acid-Based Permeation Enhancers in Transdermal Drug Delivery. Membr. J. 2021, 11, 343. [Google Scholar] [CrossRef]
- Ita, K. Chemical permeation enhancers. In Transdermal Drug Delivery; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 63–96. [Google Scholar]
- Ibrahim, S.A.; Li, S.K. Efficiency of fatty acids as chemical penetration enhancers: Mechanisms and structure enhancement relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Olabisi, A.O. The Chemistry of L-Ascorbic acid Derivatives in the Asymmetric Synthesis of C2-and C3-Substituted Aldono-γ-Lactones; Wichita State University: Wichita, KS, USA, 2005. [Google Scholar]
- Moribe, K.; Limwikrant, W.; Higashi, K.; Yamamoto, K. Drug Nanoparticle Formulation Using Ascorbic Acid Derivatives. J. Drug Deliv. 2011, 2011, 138929. [Google Scholar] [CrossRef]

| Active Ingredient | Author and Year | Chemical Permeation Enhancer | Membrane |
|---|---|---|---|
| Vitamin C | [13] | Polyoxyethylene 20 (POE-20) | Rabbit skin |
| Polyoxyethylene 80 (POE-80) | |||
| Ascorbic acid 2-glucoside | [15] | Isopropyl myristate | Mouse skin |
| Sorbitan monolaurate/Span® 20 | |||
| Polyoxyethylene 80 (POE-80)/Tween® 80 | |||
| Vitamin C | [16] | Limonene | Rat skin |
| Vitamin C | [17] | Decylglucoside | Porcine skin |
| Sorbitan monolaurate | |||
| Ascorbyl palmitate | [18] | Dimyristoylphosphatydilcholine | Rat skin |
| Dicetylphosphate | |||
| Vitamin C | [19] | Cholesterol | Porcine skin |
| Ascorbic acid 2-glucoside | [20] | Sodium lauramide glutamine | TESTSKINTM (LSE-high) |
| Sodium dilauramidoglutamide lysine | |||
| Vitamin C | [21] | Lemon essential oil | SkinEthicTM (RHE) |
| Vitamin C | [22] | Oleic acid | Porcine skin |
| Polyglycerol polyricinoleate | |||
| Tween® 80 | |||
| Vitamin C | [23] | Propylene glycol | Porcine skin |
| Vitamin C | [24] | Tween® 40 | Porcine skin |
| Isopropyl myristate | |||
| Colloidal silica | |||
| Vitamin C | [25] | Polyoxyethylene 40 (POE-40)/Tween® 40 | EpiSkinTM (RHE) |
| Isopropyl myristate | |||
| Colloidal silica | |||
| ‘Vitamin C | [26] | Carbomer (Carbopol 974) | Porcine skin |
| Polyoxyethylene 40 (POE-40)/Tween® 40 | |||
| Isopropyl myristate |
| CPE Category | CPE |
|---|---|
| Amino acid | Sodium lauramide glutamine (LG) Sodium dilauramidoglutamide lysine (DLGL) |
| Colloid | Colloidal silica |
| Ester | Isopropyl myristate (IPM) |
| Fatty acid | Oleic acid |
| Non-ionic surfactants | Polyoxyethylene 20 (POE-20) Polyoxyethylene 80 (POE-80) Decyl glucoside Sorbitan monolaurate/Span® 20 Polyoxyethylene 40 (POE-40)/Tween® 40 |
| Phospholipid | Dimyristoylphosphatidylcholine (DMPC) Dicetyl phosphate (DCP) Cholesterol |
| Polyol | Polyglycerol polyricinoleate (PGPR) Propylene glycol |
| Polymer | Carbomer (Carbopol 974) |
| Terpene | Limonene Lemon essential oil |
| Active Ingredient | Chemical Permeation Enhancer/s (CPE) | * cLog P of CPE | ER | Reference |
|---|---|---|---|---|
| Vitamin C | Cholesterol | 8.7 | 4.35 | [19] |
| Vitamin C | Polyglycerol polyricinoleate (8%) | 3 | 2.739 | [22] |
| Tween® 80 (8%) | 5.3 | |||
| Oleic acid | 6.5 | |||
| Vitamin C | Tween® 40 (13.29%) | 2.5 | 0.87 | [24] |
| Isopropyl myristate (22.15%) | 7.2 | |||
| Colloidal silica (10%) | −0.66 | |||
| Tween® 40 (13.29%) | 2.5 | 1.819 | ||
| Isopropyl myristate (53.16%) | 7.2 | |||
| Colloidal silica (10%) | −0.66 | |||
| Vitamin C | Carbomer (Carbopol 974, 2.47%) | 1.2 | 0.198 | [26] |
| Polyoxyethylene 40 (POE-40)/Tween® 40 (14.42%) | 2.5 | |||
| Isopropyl myristate (24.03%) | 7.2 | |||
| Vitamin C | Decyl glucoside (13.5%) | 2.4 | 2.01 | [17] |
| Sorbitan monolaurate (31.5%) | 3.7 | |||
| Decyl glucoside (6%) | 2.4 | 6.222 | ||
| Sorbitan monolaurate (14%) | 3.7 | |||
| Decyl glucoside (11.4%) | 2.4 | 2.653 | ||
| Sorbitan monolaurate (45.6%) | 3.7 | |||
| Vitamin C | Propylene glycol (6%) | −0.9 | 2.429 | [23] |
| Active Ingredient | Chemical Permeation Enhancer/s (CPE) | * cLog P of CPE | ER | Reference |
|---|---|---|---|---|
| Ascorbyl palmitate | Dimyristoylphosphatidylcholine | 11.3 | 1.64 | [18] |
| Dimyristoylphosphatidylcholine (90%) | 11.3 | 1.59 | ||
| Dicetyl phosphate (10%) | 14.5 | |||
| Dimyristoylphosphatidylcholine (80%) | 11.3 | 1.36 | ||
| Dicetyl phosphate (20%) | 14.5 | |||
| Vitamin C | Limonene (1%0 | 3.4 | 3.66 | [16] |
| Limonene (2%) | 3.4 | 4.73 | ||
| Limonene (5%) | 3.4 | 3.18 | ||
| Ascorbic acid 2-glucoside | Isopropyl myristate (40%) | 7.2 | 106.57 | [15] |
| Sorbitan monolaurate/Span 20 (24%) | 3.7 | |||
| Polyoxyethylene 80 (POE-80)/Tween® 80(36%) | 5.3 |
| Active Ingredient | Chemical Permeation Enhancer/s (CPE) | * cLog P of CPE | ER | Reference |
|---|---|---|---|---|
| Vitamin C | Polyoxyethylene 20 (POE-20) (1%) | 5.3 | 2.75 | [13] |
| Polyoxyethylene 20 (POE-20) (2%) | 5.3 | 3.26 | ||
| Polyoxyethylene 20 (POE-20) (3%) | 5.3 | 3.57 | ||
| Polyoxyethylene 20 (POE-20) (4%) | 5.3 | 4.70 | ||
| Polyoxyethylene 20 (POE-20) (5%) | 5.3 | 5.08 | ||
| Vitamin C | Polyoxyethylene 80 (POE-80) (1%) | 2.5 | 2.15 | |
| Polyoxyethylene 80 (POE-80) (2%) | 2.5 | 2.81 | ||
| Polyoxyethylene 80 (POE-80) (3%) | 2.5 | 3.11 | ||
| Polyoxyethylene 80 (POE-80) (4%) | 2.5 | 3.41 | ||
| Polyoxyethylene 80 (POE-80) (5%) | 2.5 | 3.89 |
| Active Ingredient | Chemical Permeation Enhancer/s (CPE) | * cLog P of CPE | ER | Reference |
|---|---|---|---|---|
| Ascorbic acid 2-glucoside | Sodium lauramide glutamine | −1.4 | 69.24 | [20] |
| Sodium dilauramidoglutamide lysine | −1 | 12.56 | ||
| Vitamin C | Polyoxyethylene 40 (POE-40)/Tween® 40 (13.29%) | 2.5 | 0.34 | [25] |
| Isopropyl myristate (22.15%) | 7.2 | |||
| Colloidal silica (10%) | −0.66 | |||
| Vitamin C | Lemon essential oil (10%) | 10.8 | 3.36 | [21] |
| Chemical Permeation Enhancer * | LD50 | GHS Classification |
|---|---|---|
| Carbomer (Carbopol 974) | >2000 mg/kg | Category 5 |
| Cholesterol | >2000 mg/kg | Category 5 |
| Colloidal silica | >4933 mg/kg | Category 5 |
| Decylglucoside | >2000 mg/kg | Category 5 |
| Dicetylphosphate | >2000 mg/kg | Category 5 |
| Dimyristoylphosphatydilcholine | >1250 mg/kg | Category 4 |
| Isopropyl myristate | 5000 mg/kg | Category 5 |
| Lemon essential oil | >10,000 mg/kg | Category 5 |
| Limonene | >5000 mg/kg | Category 5 |
| Oleic acid | >3000 mg/kg | Category 5 |
| Polyglycerol polyricinoleate | >18,700 g/kg | Category 5 |
| Polyoxyethylene 20 (POE-20) | > 2000 mg/kg | Category 5 |
| Polyoxyethylene 40 (POE-40)/Tween® 40 | >10,000 mg/kg | Category 5 |
| Polyoxyethylene 80 (POE-80) | >3000 mg/kg | Category 5 |
| Propylene glycol | 20,800 mg/kg | Category 5 |
| Sodium lauramide glutamine | >2000 mg/kg | Category 5 |
| Sodium dilauramidoglutamide lysine | 10,000 mg/kg | Category 5 |
| Sorbitan monolaurate/Span® 20 | >3000 mg/kg | Category 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F., Jr. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics 2022, 9, 85. https://doi.org/10.3390/cosmetics9040085
Liston LS, Rivas PL, Sakdiset P, See GL, Arce F Jr. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics. 2022; 9(4):85. https://doi.org/10.3390/cosmetics9040085
Chicago/Turabian StyleListon, Lord Sam, Precious Lorraine Rivas, Pajaree Sakdiset, Gerard Lee See, and Florencio Arce, Jr. 2022. "Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review" Cosmetics 9, no. 4: 85. https://doi.org/10.3390/cosmetics9040085
APA StyleListon, L. S., Rivas, P. L., Sakdiset, P., See, G. L., & Arce, F., Jr. (2022). Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics, 9(4), 85. https://doi.org/10.3390/cosmetics9040085





