Abstract
Application of plant-derived colorants in products, i.e., cosmetics or food, apart from imparting the desired color without harming the environment, may provide other benefits. Valuable ingredients in cosmetic formulations include antioxidants showing an advantageous effect on the skin by neutralizing free radicals that accelerate the aging process and cause skin defects. Antioxidant activity can be determined by chemical-based methods. The aim of this study was to determine the antioxidant activity of plant-derived colorants (purple and red colorant) by two methods: CUPRAC and DPPH free-radical scavenging activity. Antioxidant activity evaluation using both methods for colorants samples was also performed after 5, 15, 30, and 60 min of exposure to UVC irradiation. The results obtained by CUPRAC method were for purple and red colorant unexposed samples as follows: 6.87 ± 0.09 and 4.48 ± 0.14 mg/100 mg colorant expressed as caffeic acid equivalent, respectively. UVC treatment did not affect the results of the antioxidant activity for red colorant and for the purple one only a slight influence was observed. DPPH free-radical scavenging activity for unexposed samples was 70.06 ± 7.74% DPPH/100 mg colorant for the red colorant and 96.11 ± 3.80% DPPH/100 mg colorant for the purple one.
1. Introduction
As cosmetic, food, or textile production and consumption are inseparable from the sense of sight, the appropriate color of products is required, which is usually related to the use of colorants. The application of plant-derived ones in various daily-use products such as cosmetics or food, apart from giving the desired color without harming the environment, may provide other benefits and give new properties [1]. For example, in cosmetic products, such essential ingredients include antioxidants, which have an advantageous effect on the skin by neutralizing free radicals that accelerate the aging process and cause damage to the skin. Natural antioxidants applications in skincare cosmetic formulations are the subject of many papers; a review article by Hoang et al. introduces their recent applications, challenges, and perspectives [2], many others concern the characterization of cosmetic formulations containing antioxidants of plant origin such as extracts obtained from Castanea sativa shells [3], red dragon fruit [4] or grape pomace, Pinus pinaster wood chips, Acacia dealbata flowers, and Lentinus edodes [5]. Many reports have established that various plant components containing vitamins, flavonoids, or other polyphenolic compounds show antioxidant properties but can also be a source of effective natural dyes for cosmetic, food, or textile application. Moreover, the antioxidant activity of numerous plant extracts, including those showing coloring potential, have been documented; among others, the following were tested (usually by DPPH free radical scavenging activity or CUPRAC method): dragon fruit [6], black chokeberry, black-thorn and strawberry [7], avocado [8], radish [9], black carrot [10], red currant, black currant, raspberry, blackberry and elderberry [11], purple corn [12], and Kalanchoe daigremontiana [13]. Numerous studies prove that plant-derived colorants may comprise promising ingredients in natural cosmetics with both coloring and antioxidant potential. When discussing antioxidant application in cosmetics, it is also worth mentioning that new sources of these compounds are sought not only among plant extracts but also among plant by-products from the industry, for example avocado, grape, opuntia, wine, and brewery by-products, which, aside from their antioxidant properties, sometimes also show dyeing ability [14,15,16,17,18,19]. Such an approach meets the current requirements of sustainable development and leads to the creation of clean-label products, for instance, cosmetics. Numerous studies concern the application of plant-derived substances in cosmetic formulation. As an example, Hennessey-Ramos et al. in their research applied a colorant extracted from plant waste (avocado seeds) as an ingredient in liquid-soap formulation. Extracts were previously examined for antioxidant properties using the DPPH free-radical scavenging activity, whereby water extract was characterized by the highest antioxidant activity. Furthermore, applied colorant was stable in liquid-soap formulation during one month of storage [8]. Another study indicated that the betacyanin pigment obtained from red dragon fruit can successfully be applied in lipstick formulation, both as an antioxidant, characterized by high antioxidant activity confirmed by DPPH free radical scavenging activity, and as a colorant, leading to the creation of a product with acceptable quality and low lead content [4].
Under the influence of light, plants are able to synthesize and accumulate secondary metabolites, such as phenolic compounds, most of which show antioxidant properties and thus play beneficial roles in human health [20]. Therefore, for various plants, the effect of a sunlight component, i.e., ultraviolet radiation on secondary metabolites production, antioxidant activity, and total phenolic content, was examined [21,22,23,24]. Many studies confirmed that UVC irradiation affects the content of antioxidants and other bioactive compounds in fresh fruits and vegetables, but an experiment by Papoutsis et al. investigated the effects of UVC radiation on dried plants. The tested material was dried lemon pomace powder, which was treated with UVC radiation in order to increase the phenolic content, and thus antioxidant activity, before its extraction and application. As expected, the UVC treatment positively affects the quality of the dried lemon pomace powder. The total flavonoid content, total phenolic content, and antioxidant capacity were significantly higher after the application of UVC radiation, which was confirmed using the CUPRAC and FRAP methods. The results indicated that UVC treatment can beneficially influence the content of bioactive substances extracted from dried lemon pomace [25]. As several studies have shown, the treatment of UVC irradiation can contribute to increasing the number of antioxidants in plants and plant extracts and thus enhance the antioxidant activity [26,27,28,29,30,31,32,33,34].
UV light, ionizing radiation, air pollution, and smoking are just a few examples of factors causing free-radical formation damaging the DNA structure; they contribute to the generation of skin defects and affect the aging process. This is why it is so important to provide the skin and other susceptible natural systems with substances capable of scavenging free radicals. Such antioxidant-rich raw materials are numerous plant components containing various compounds among other vegetable dyes including anthocyanins, carotenoids, betalains, flavonols, and quinones with documented antioxidant properties. All these substances are of increasing interest as they represent a high potential for preventing the aging process and increasing skin health, as confirmed in literature [35].
Due to the high content of various biologically active compounds, plant-derived colorants are also characterized by antioxidant properties, thus the aim of the study was to determine the antioxidant activity by the CUPRAC method and the DPPH free radical scavenging activity of two plant-derived colorants consisting of a mixture of various plant extracts. Antioxidant activity evaluation by both methods for colorants samples was also performed after 5, 15, 30, and 60 min of exposure to UVC irradiation.
2. Materials and Methods
2.1. Materials
Two commercially available colorants for food applications consisting only of fruit and vegetable concentrates obtained by the physical manufacturing process conducted with water were used in the examination: red and purple. Both colorants were in liquid form and were obtained from EXBERRY by GNT (GNT Group B.V., Mierlo, The Netherlands). Moreover, in determining the antioxidant activity, different reagents were used; neocuproine, caffeic acid, DPPH, and trolox were purchased from Sigma (Poznań, Poland), whereas ethanol, copper chloride, and ammonium acetate from Chemland (Stargard, Poland). All reagents were of analytical grade.
2.2. Sample Preparation
The analyzed samples of red and purple colorants were prepared by dissolving appropriate amounts of colorants in distilled water resulting 0.1% solutions. Next, samples were irradiated at a distance of 5 cm from the UV lamp (ULTRAVIOL NBV 15, Ultra-Viol, Zgierz, Poland) emitting mainly UVC at wavelength 254 nm and intensity of radiation 21.5 W/m2 during 5, 15, 30, and 60 min.
2.3. Determination of Antioxidant Capacity
2.3.1. Determination of Antioxidant Activity by CUPRAC (Cupric Reducing Antioxidant Capacity) Method
To examine colorants’ antioxidant activity by the CUPRAC method, the following reagents were used: neocuproine ethanol solution (0.0075 M), copper chloride solution (0.01 M), ammonium acetate buffer (1.0 M, pH = 7.0), and ethanolic solution of caffeic acid as standard (0.048 mg/mL). Absorbance was measured with the UV-Vis double-beam spectrophotometer Shimadzu UV-1601 (Shimadzu, Kyoto, Japan) in plastic cuvettes [36]. Before measurements, a calibration curve was prepared. The method of making the standard curve and calibration curve is included in the Supplementary Materials (Figure S1).
Sample Analysis
Samples were prepared according to above-described method. Instead of caffeic acid, 0.1 mL of 0.1% colorant solution was added, and the volumetric flask was refilled with distilled water. The measurements were made on unexposed and irradiated samples for 5, 15, 30, and 60 min, respectively. Samples were placed in darkness for 0.5 h, and then the absorbance was performed at a wavelength of λ = 450 nm against the blank (copper (II) chloride, neocuproine solution, ammonium acetate buffer, and distilled water). Three independent measurements were made for each sample.
2.3.2. Determination of Antioxidant Activity by DPPH Assay
To study the colorants’ antioxidant activity by the DPPH assay, the following reagents were used: 0.012% 2,2′-diphenyl-1-picrylhydrazyl (DPPH) ethanolic solution and 0.1 mM 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) ethanolic solution. Absorbance was measured with the same UV-Vis double-beam spectrophotometer in plastic cuvettes [36]. Before measurements, a calibration curve was prepared. The procedure for making the standard curve and calibration curve is included in the Supplementary Materials (Figure S2).
Sample Analysis
Samples were prepared according to above method, and then, instead of trolox solution, 0.1 mL of colorant solution was pipetted. Measurements were made on unexposed and irradiated samples for 5, 15, 30, and 60 min, respectively. Three measurements for each sample were performed. Before absorbance measurements at a wavelength of λ = 517 nm, samples were stored in darkness for 15 min. The mixture of ethanol and DPPH solution was prepared as a blank test, and ethanol was used as a reference. The blank test was measured separately for all samples [36].
3. Results
3.1. Determination of Antioxidant Activity by CUPRAC Method
Using this method, which is based on SET (single-electron transfer) mechanism, a copper (II) complex is reduced to a copper (I) complex under the influence of antioxidants present in the examined sample. Copper (I) forms a yellow-orange complex with neocuproine (2,9-dimethyl-1,10-phenanthroline), with the highest absorbance at wavelength 450 nm. The absorbance of this colored complex is measured spectrophotometrically. The copper-neocuproine complex’s redox potential, which equals 0.6 V, is higher than the standard one of −0.16 V, which contributes to the efficiency and rate of polyphenol oxidation. The CUPRAC method is appropriate for examining the antioxidant activity in plant-derived samples, both hydrophobic and hydrophilic antioxidants. The antioxidant activity is expressed as the amount of caffeic acid equivalent in the sample. The linearity of the method was from 0.24 to 2.88 mg/L. The linear regression equation of the calibration curve was obtained as follows: y = (0.3375 ± 0.0111)x + (0.2159 ± 0.0193) with r2 = 0.9992. The calculated LOD and LOQ were 0.12 mg/L and 0.28 mg/L, respectively.
The antioxidant activity expressed as a caffeic acid equivalent was calculated in both tested colorants: red and purple after 0, 5, 15, 30, and 60 min of exposure to UVC irradiation. The results of the antioxidant activity from CUPRAC method obtained for red colorant after 0, 5, 15, 30, and 60 min exposure to UVC irradiation were as follows: 4.48 ± 0.14; 4.12 ± 0.29; 4.31 ± 0.25; 4.06 ± 0.21, and 4.16 ± 0.35 mg/100 mg colorant expressed as caffeic acid, respectively. On the other hand, the results obtained for purple colorant after 0, 5, 15, 30, and 60 min exposure to UVC irradiation were as follows: 6.87 ± 0.09; 7.11 ± 0.21; 8.23 ± 0.74; 7.40 ± 0.25; 6.80 ± 0.45 mg/100 mg colorant expressed as caffeic acid, respectively (Figure 1).
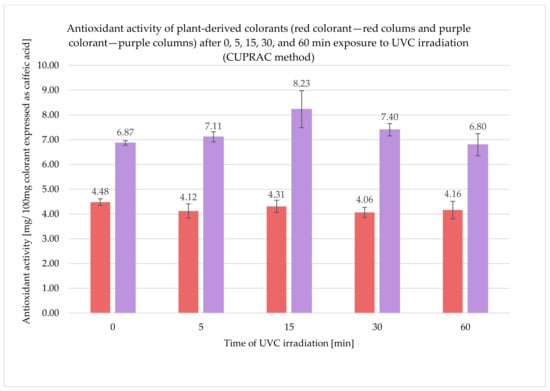
Figure 1.
Results of antioxidant activity of plant-derived colorants (red and purple) after 0, 5, 15, 30, and 60 min exposure to UVC irradiation (CUPRAC method).
3.2. Determination of Antioxidant Activity by DPPH Assay
The antioxidant activity of food products or plant-derived raw materials is commonly examined by this method, in which a stable, purple ethanolic solution of DPPH radical is used, indicating the maximum absorption at a wavelength equals 517 nm. This method is based on spectrophotometric measurements: the absorbance decrease of the examined sample containing antioxidants is a consequence of the color change from purple to yellow because of the DPPH reduction. The measured antioxidant activity of the colorant samples was expressed as a percentage of reduction of the DPPH radical. In this method, trolox was used as the standard. The calibration curve was prepared in the range 0.01–0.10 mmol trolox/L. The linear regression equation of the calibration curve was obtained as follows: y = (730.2 ± 39.13)x + (0.72 ± 2.375), with r2 value 0.9985. The calculated LOD and LOQ were 0.0061 mmol trolox/L and 0.0147 mmol trolox/L, respectively.
Antioxidant activity expressed as a percentage of the reduction of the DPPH radical for both tested colorants was calculated, and the results for the unexposed samples were as follows: for the red colorant 70.06 ± 7.74% DPPH/100 mg colorant and for the purple colorant 96.11 ± 3.80% DPPH/100 mg colorant (Figure 2). After 5, 15, 30, and 60 min exposure to UVC irradiation, the results for the red colorant were: 65.48 ± 10.97, 71.51 ± 3.71, 69.58 ± 7.07, and 67.41 ± 2.30% DPPH/100 mg colorant and for purple were 79.59 ± 15.46, 87.67 ± 4.91, 77.30 ± 6.37 and 86.82 ± 4.17% DPPH/100 mg colorant, respectively.
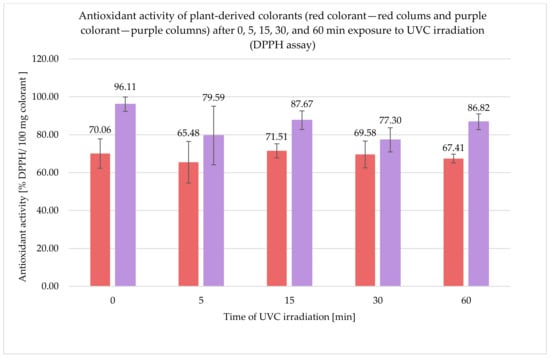
Figure 2.
Results of antioxidant activity of plant-derived colorants (red and purple) after 0, 5, 15, 30, and 60 min exposure to UVC irradiation (DPPH assay).
4. Discussion
Examination of the antioxidant activity using the CUPRAC method showed that the purple colorant was characterized by a significantly greater antioxidant activity when compared to the red one. UVC treatment did not affect the antioxidant activity of the red colorant, and the results for the unexposed and irradiated samples were similar; for the purple colorant after 15 min of UVC irradiation, the antioxidant activity slightly increased and subsequently, after 30 and 60 min, decreased, and the results were similar to the initial value. The results of the antioxidant activity determined using the DPPH assay were also greater for the purple colorant than for the red one. UVC irradiation did not significantly affect the antioxidant activity of the red colorant, but, for purple colorant, a decrease in the antioxidant activity was observed.
According to research conducted by Comert et al., there is a relationship between antioxidant activity and the color of fruits and vegetables. Fruits and vegetables can also be classified based on total antioxidant capacity (TAC), which provides information about the general concentration of bioactive ingredients, such as phenolic compounds, carotenoids, and many more, some of which are pigmented [37]. A study indicated that a color’s hue can be related to TAC in fruits and vegetables. Red-, blue-, and magenta-colored ones were characterized by the highest antioxidant properties. However, it is worth focusing on researching fruits, vegetables, and other plants as sources of dyes responsible for listed colors to ensure the highest possible content of antioxidants in further product formulation. Nevertheless, there is still an insufficient amount of research focusing on investigating the relationship between TAC and color [37].
The development of cosmetic products should also focus on such a selection of components to ensure skin protection and to prevent damage caused by free radicals. Moreover, the preparation of cosmetic formulations preceded by an antioxidant activity examination of particular ingredients contributes to its effectiveness. As the intensive search for plant-based cosmetic ingredients with a strong emphasis on natural antioxidants is observed, many plant extracts are evaluated for their valuable properties and beneficial influence on the skin. Simultaneously, new plants that are a source of active compounds such as antioxidants are constantly sought. For instance, in studies conducted by Mota et al., Araucaria angustifolia seed extracts were used to prepare various types of cosmetic formulations: W/O emulsion, O/W emulsion, and nonionic gel. Previously, extracts were tested for antioxidant activity using DPPH and ABTS (2,2′-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) assay. Due to the fact that different factors, such as storage conditions or type of formulation and its pH values, determine antioxidant activity, this parameter examination was also performed for finished cosmetic formulations. The antioxidant activity of the final products was confirmed [38,39].
It should be also emphasized that one human cell is exposed to more than 100 oxidative hits a day from hydroxyl radicals and other reactive species [40]. Oxidative stress contributes to the initiation of the mechanism causing changes in proteins, lipids, or inflammation and the immunosuppression process. Permanent modification of genetic material resulting from these “oxidative damage” incidents represents the first step of carcinogenesis involved in mutagenesis and aging. DNA alterations caused by radicals are removed by specific and non-specific repair mechanisms. However, a failure to repair DNA damage could result in mutations such as base substitution and deletion, also leading to carcinogenesis. Botanical compounds with anti-carcinogenic and anti-mutagenic properties can use multiple mechanisms to maintain homeostasis and balance interrupted by reactive oxygen species (ROS) [41]. Those compounds are able to stimulate immune and anti-inflammatory responses, changes in gene expression, the detoxification or modulation of antioxidants. Skin-protection mechanisms include the production of antioxidants and other compounds able to absorb UV radiation [42]. Human skin, because of the presence of antioxidant enzymes such as superoxide dismutase, glutathione, or catalase, can protect its cells from free radicals and, as a consequence, from damage they induce. However, antioxidant compounds such as vitamins A, C, and E, and other molecules, which skin possesses, influence the process of its ageing by protecting sensitive biological molecules from oxidizing by free radicals or by reducing the creation of free radicals and quenching formed ones. Nevertheless, different factors, such as environmental ones, for instance, UV irradiation or the natural process of ageing of organisms, can decrease their amount [43,44]. Thus, antioxidants contribute to strengthening the endogenous skin capacity and supporting the neutralization of the ROS-induced process formed under the influence of external factors [41]. To protect the skin, it becomes necessary to apply antioxidants topically onto the skin and provide them with the correct diet. When using plant-derived ingredients in cosmetics with antioxidant properties, one can expect that oxidative damage in human skin can also be reduced [44].
The plant-derived colorants tested in this study may have the potential to impart antioxidant properties or enhance the effect of other antioxidants present in skincare formulations, and, in the same manner, they may have the potential to impart color or enhance the effect of other colorants in cosmetic products. The individual ingredients of the cosmetic formulation and the finished cosmetic product should have confirmed the effectiveness, including the antioxidant properties to be a valuable product for the skin, maintaining it in healthy conditions. This should be taken into account when creating and researching natural skincare cosmetic products based on plant extracts. Confirmation of these properties can be the determination of antioxidant activity, defined as the ability to reduce or inhibit compounds with high oxidation–reduction potential through the use of chemical-based methods such as CUPRAC or DPPH free-radical scavenging activity [45]. However, as various methods provide different results related to the antioxidant activity of plant extracts due to the influence of many factors such as the presence of other components in tested samples, antioxidant distribution or complexity of mechanism, thus multiple assay methods should be considered simultaneously to evaluate this parameter objectively [46]. Numerous results of various methods of antioxidant capacity evaluation in common fruits are summarized and presented in a review paper by Lu et al., which confirms the complex nature of the study. As vegetable raw materials are rich in many active compounds, chemical methods of antioxidant capacity determination should be still developed [46].
5. Conclusions
The antioxidant activity of plant-derived colorants was confirmed using DPPH and CUPRAC assays. The addition of natural colorants of plant origin may beneficially influence the quality and effectiveness of cosmetic products, thus following the current dominant trend in the cosmetic industry which draws inspiration from the richness of nature and aims to create cosmetic formulations with an application of plant-derived ingredients with minimal impact on the environment. Therefore, they can be valuable cosmetic ingredients and potentially act as effective dyes and antioxidants, but further research is required to create appropriate cosmetic formulations containing plant-derived colorants with antioxidant properties. Conducting extensive studies is necessary, as those colorants are more complicated in application, because they tend to be more variable in the shade, less stable under different conditions, and may introduce undesirable odors to formulations in comparison to synthetic ones [47].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics9040081/s1, Figure S1: Calibration curve for determination of antioxidant activity by CUPRAC method; Figure S2: Calibration curve for determination of antioxidant activity by DPPH method.
Author Contributions
Conceptualization, M.K., P.B. and A.S.; methodology, M.K.; software, P.B.; validation, M.K., P.B. and A.S.; formal analysis, M.K.; investigation, P.B.; resources, M.K., P.B. and A.S.; data curation, P.B. and M.K.; writing—original draft preparation, P.B. and A.S.; writing—review and editing, A.S. and M.G.; visualization, P.B.; supervision, A.S. and M.G.; project administration, A.S.; funding acquisition, M.K., P.B. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-derived colorants for food, cosmetic and textile industries: A review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural antioxidants from plant extracts in skincare cosmetics: Recent Applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and stability of a formulation containing antioxidants-enriched Castanea sativa shells extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Lwin, T.; Myint, C.Y.; Win, H.H.; Oo, W.W.; Chit, K. Formulation and evaluation of lipstick with betacyanin pigment of Hylocereus polyrhizus (Red Dragon Fruit). J. Cosmet. Dermatol. 2020, 10, 212–224. [Google Scholar] [CrossRef]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-care products formulated with natural antioxidant extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Thaiudom, S.; Oonsivilai, R.; Thaiwong, N. Production of colorant powder from dragon fruit (Hylocerecus polyrhizus) peel: Bioactivity, heavy metal contamination, antimutagenicity, and antioxidation aspects. J. Food Process. Preserv. 2021, 45, 1. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J.; García-Viguera, C. Anthocyanin-based natural colorants: A new source of antiradical activity for foodstuff. J. Agric. Food Chem. 2000, 48, 1588–1592. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Guayabo, G.T. Evaluation of a colorant and oil extracted from avocado waste as functional components of a liquid soap formulation. Rev. Fac. Nac. Agron. Medellin 2019, 72, 8855–8862. [Google Scholar] [CrossRef]
- Singh, B.K.; Koley, T.K.; Karmakar, P.; Tripathi, A.; Singh, B.; Singh, M. Pigmented radish (Raphanus sativus): Genetic variability, heritability and interrelationships of total phenolics, anthocyanins and antioxidant activity. Indian J. Agric. Sci. 2017, 87, 1600–1606. [Google Scholar]
- Suzme, S.; Boyacioglu, D.; Toydemir, G.; Capanoglu, E. Effect of industrial juice concentrate processing on phenolic profile and antioxidant capacity of black carrots. Int. J. Food Sci. Technol. 2014, 49, 819–829. [Google Scholar] [CrossRef]
- Cserjési, P.; Bélafi-Bakó, K.; Csanádi, Z.; Beszédes, S.; Hodúr, C. Simultaneous recovery of pectin and colorants from solid agro-wastes formed in processing of colorful berries. Prog. Agri. Eng. Sci. 2011, 7, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Puertas Mejía, M.A.; Tobón Gallego, J.; Arango, V. Kalanchoe daigremontiana Raym.-Hamet. & H. and its potential use as a source of natural antioxidants and colorants. Rev. Cuba. Plantas Med. 2014, 19, 61–68. [Google Scholar]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.R.F.; Achour, L. Seeds of Opuntia spp. as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Techol. 2004, 92, 201–208. [Google Scholar] [CrossRef]
- Censi, R.; Vargas Peregrina, D.; Gigliobianco, M.R.; Lupidi, G.; Angeloni, C.; Pruccoli, L.; Tarozzi, A.; di Martino, P. New antioxidant ingredients from brewery by-products for cosmetic formulations. Cosmetics 2021, 8, 96. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Cetin, E.S. Induction of secondary metabolite production by UV-C radiation in Vitis vinifera L. Öküzgözü callus cultures. Biol. Res. 2014, 47, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Silvestre, K.E.; Santiz-Gómez, J.A.; Luján-Hidalgo, M.C.; Ruiz-Lau, N.; Sánchez-Roque, Y.; Gutiérrez-Miceli, F.A. Effect of UV-B radiation on flavonoids and phenols accumulation in tempisque (Sideroxylon capiri Pittier) callus. Plants 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.B. Ultraviolet-B-induced changes on phenolic compounds, antioxidant capacity and HPLC profile of in vitro-grown plant materials in Echium orientale L. Ind. Crops Prod. 2020, 153, 112584. [Google Scholar] [CrossRef]
- Nocchi, N.; Duarte, H.M.; Pereira, R.C.; Konno, T.U.P.; Soares, A.R. Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112021. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Pristijono, P.; Golding, J.B.; Bowyer, M.C.; Scarlett, C.J.; Stathopoulos, C.E. Enhancing the total phenolic content and antioxidants of lemon pomace aqueous extracts by applying UV-C irradiation to the dried powder. Foods 2016, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.A.; Gardea, A.A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007, 72, 197–202. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Du, W.X.; Woods, R.; Olson, D.; Breksa, A.P.; McHugh, T.H. Ultraviolet-B light treatment increases antioxidant capacity of carrot products. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef]
- Mandal, R.; Wiktor, A.; Mohammadi, X.; Pratap-Singh, A. Pulsed UV light irradiation processing of black tea infusions: Effect on color, phenolic content, and antioxidant capacity. Food Bioprocess. Technol. 2022, 15, 92–104. [Google Scholar] [CrossRef]
- Pataro, G.; Sinik, M.; Capitoli, M.M.; Donsì, G.; Ferrari, G. The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innov. Food Sci. Emerg. Technol. 2015, 30, 103–111. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Hassan, A.B.; Al Maiman, S.A.; Sir Elkhatim, K.A.; Elbadr, N.A.; Alsulaim, S.; Osman, M.A.; Ahmed, I.A.M. Effect of UV-C radiation treatment on microbial load and antioxidant capacity in hot pepper, fennel and coriander. LWT 2020, 134, 109946. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. Technol. 2009, 10, 512–516. [Google Scholar] [CrossRef]
- Islam, M.S.; Patras, A.; Pokharel, B.; Wu, Y.; Vergne, M.J.; Shade, L.; Xiao, H.; Sasges, M. UV-C Irradiation as an alternative disinfection technique: Study of its effect on polyphenols and antioxidant activity of apple juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Chen, C.-T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Adamiak, K.; Kurzawa, M.; Sionkowska, A. Physicochemical performance of collagen modified by Melissa officinalis extract. Cosmetics 2021, 8, 95. [Google Scholar] [CrossRef]
- Comert, E.D.; Mogol, B.A.; Gokmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Ziosi, P.; Manfredini, S.; Vertuani, S.; Ruscetta, V.; Sacchetti, G.; Radice, M.; Bruni, R. Evaluating essential oils in cosmetics: Antioxidant capacity and functionality. Cosmet. Toilet. 2010, 125, 32–40. [Google Scholar]
- Mota, G.; Arantes, A.; Sacchetti, G.; Spagnoletti, A.; Ziosi, P.; Scalambra, E.; Vertuani, S.; Manfredini, S. Antioxidant activity of cosmetic formulations based on novel extracts from seeds of Brazilian Araucaria angustifolia (Bertoll) Kuntze. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef] [Green Version]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a new light on skin aging, iron- and redox-homeostasis and emerging natural antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D.H. Antioxidants and skin aging: A review. Cosmet. Dermatol. 2009, 22, 563–570. [Google Scholar]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Marmion, D.M. Handbook of US Colorants: Foods, Drugs, Cosmetics, and Medical Devices, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1992; p. 120. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).