In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Products
2.2. Human Volunteers and Clinical Study Design
2.3. Wrinkle Morphometric Analysis and Quantitation
2.4. Human Skin Explant Experimentation and Histological Staining
2.5. Histological Image Processing and Marker Quantitation
2.6. Reconstitutive Skin Tissue Model for ELISA and RT-PCR Analysis
3. Statistical Analysis
4. Results
4.1. Demographics Data and Adverse Events
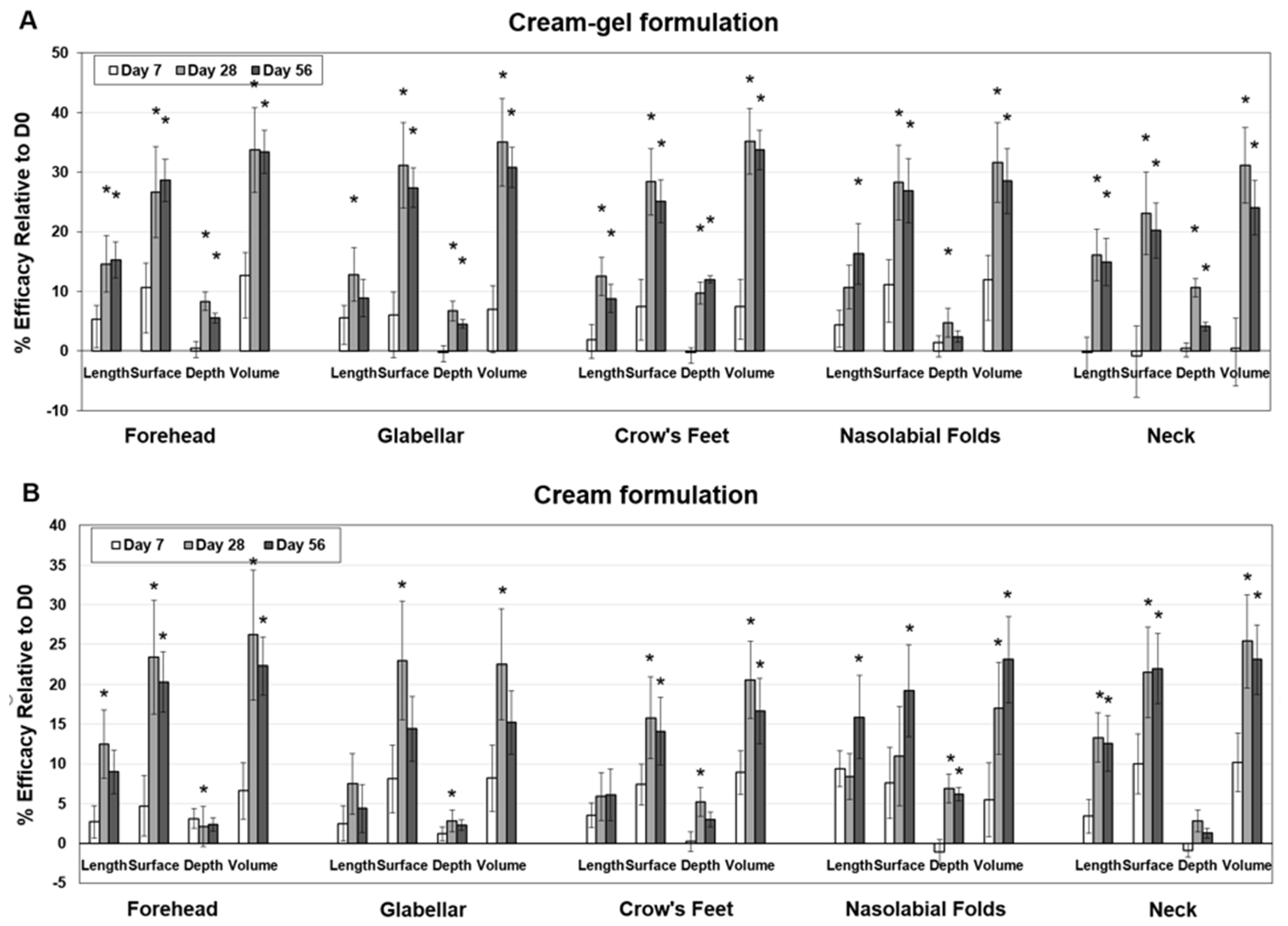
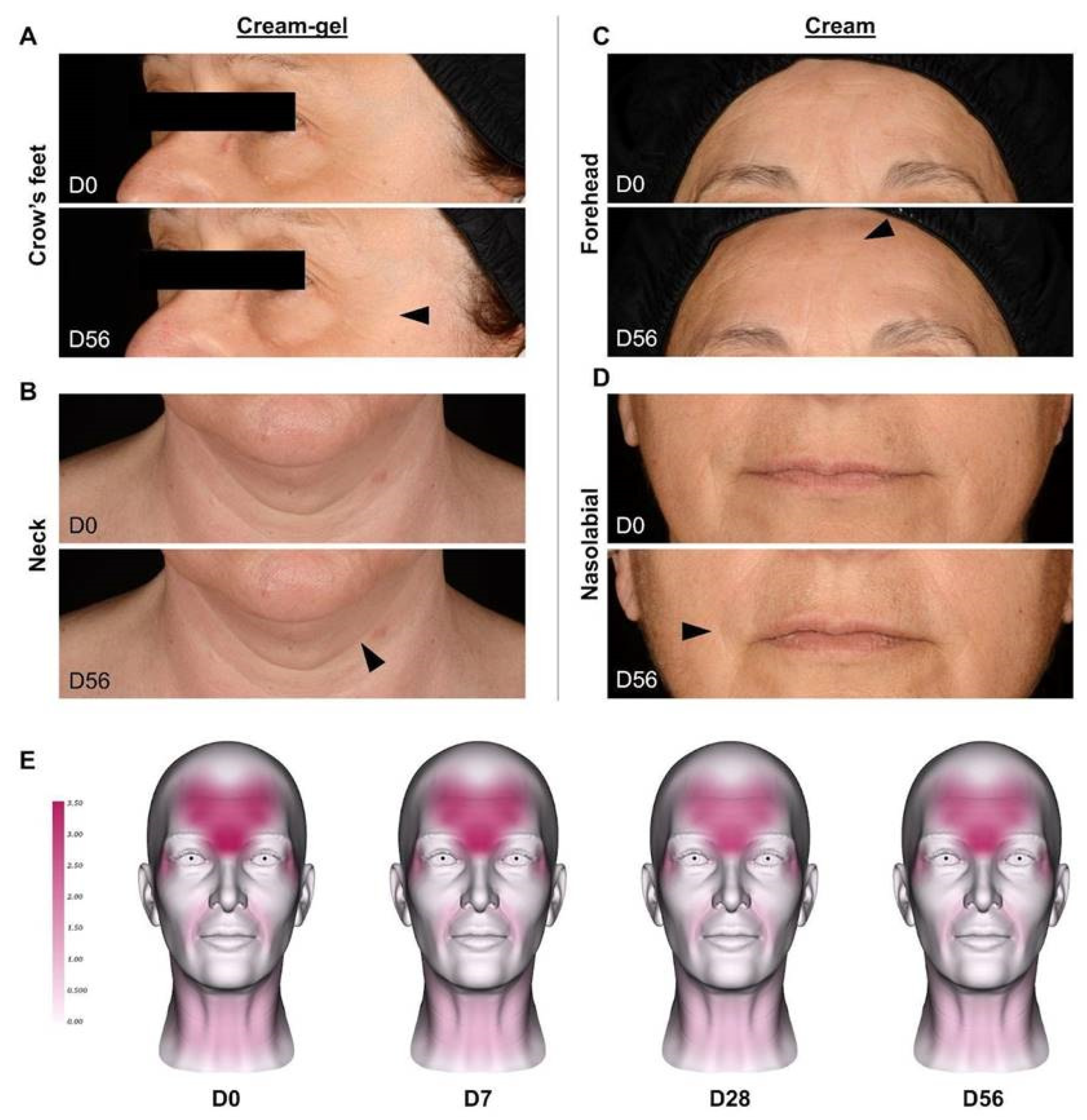
4.2. Clinical Reduction of Wrinkles after Cream-Gel Application
4.3. Clinical Reduction of Wrinkles after Cream Application
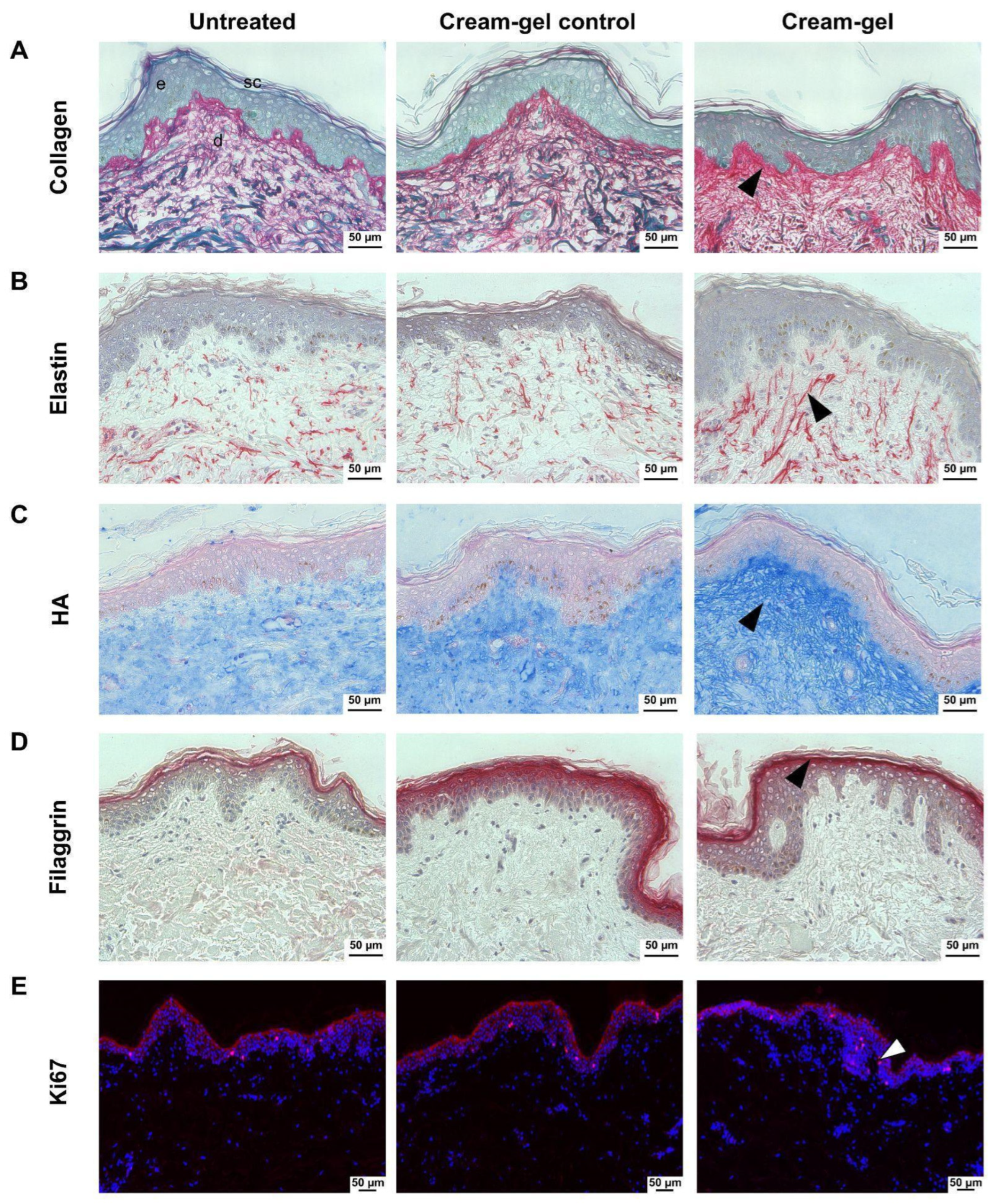
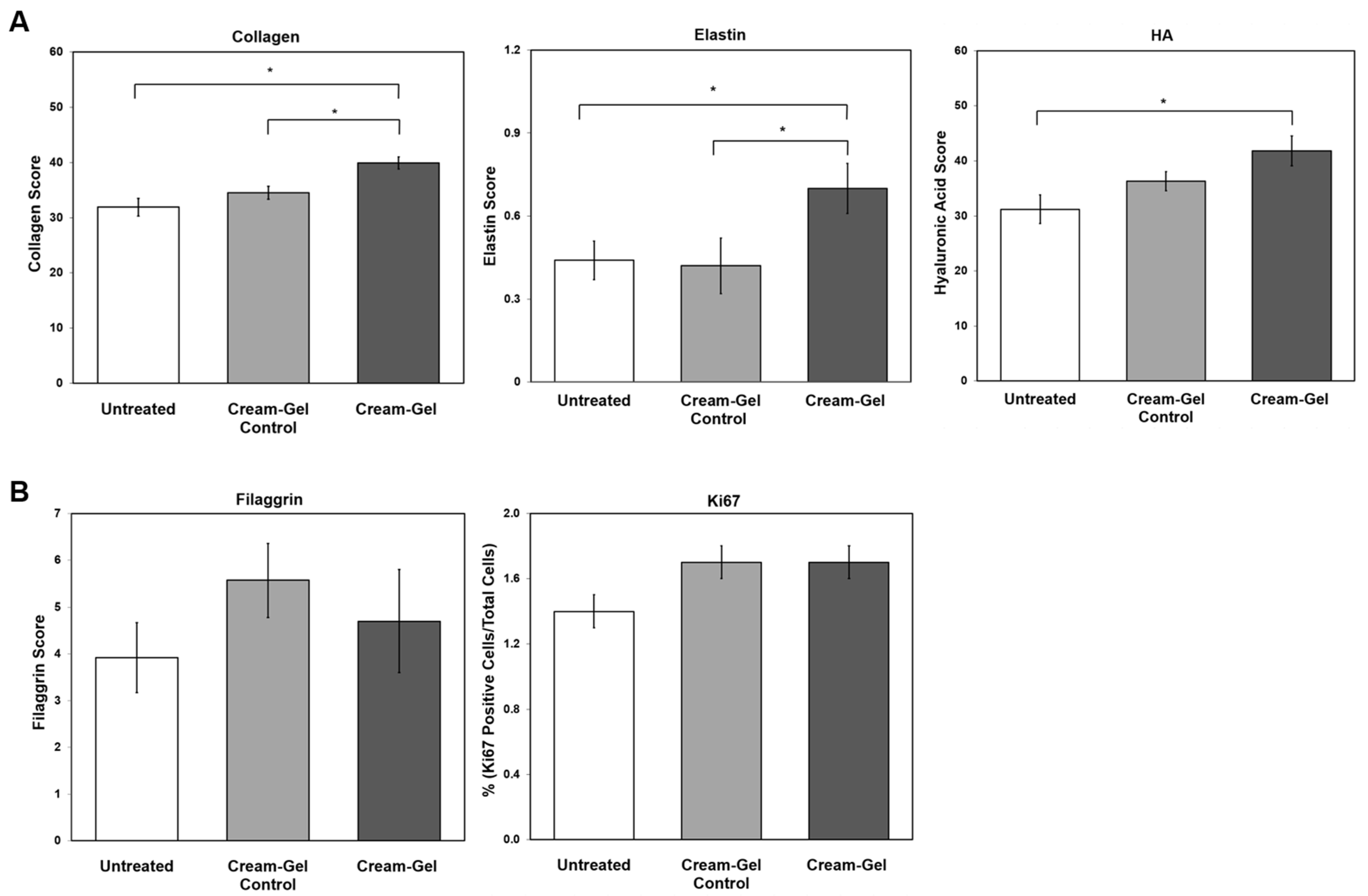
4.4. Histological Changes in Dermal ECM Components
4.5. Histological Examination of the Epidermis
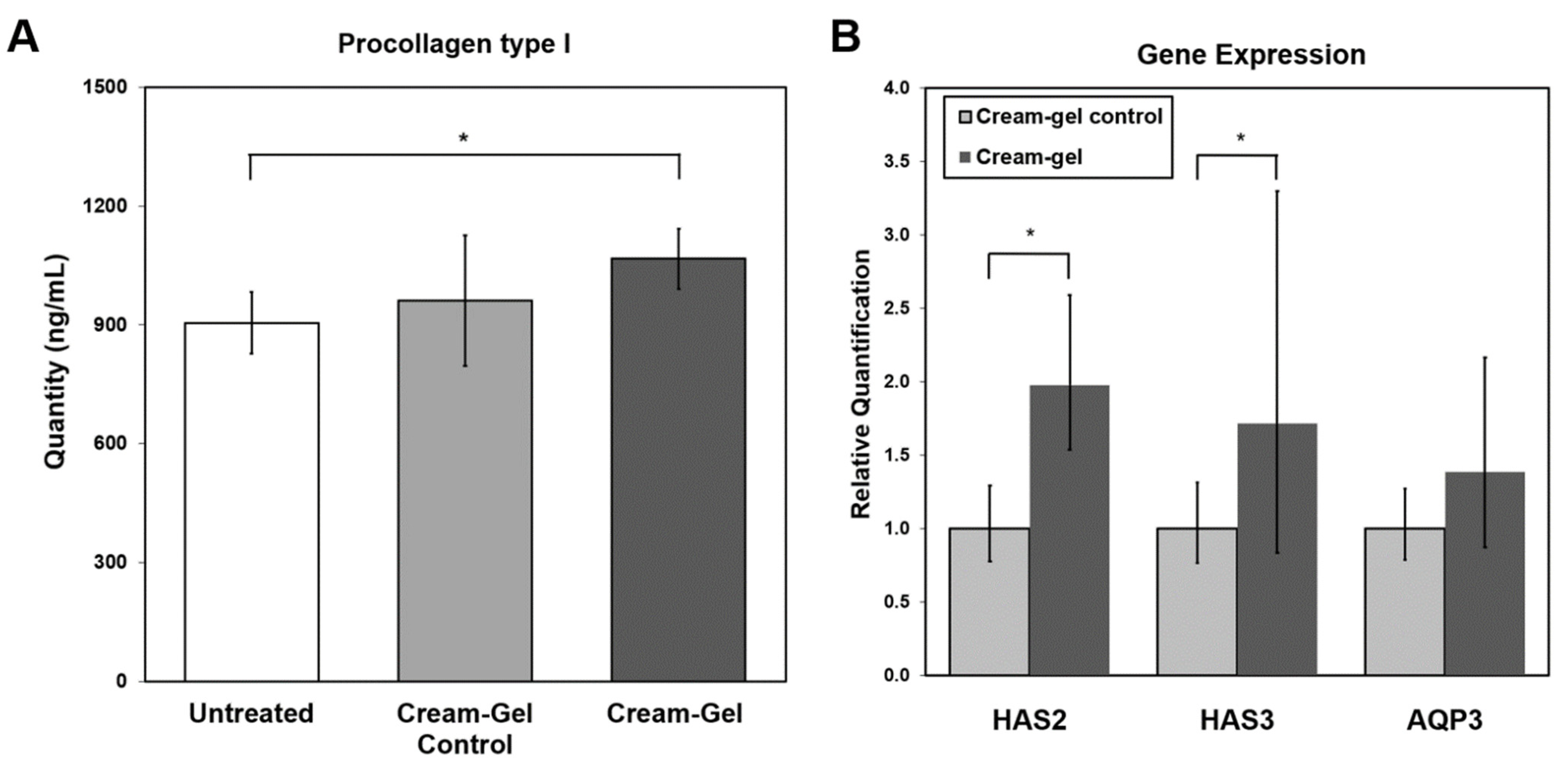
4.6. Protein Expression in Reconstitutive Skin Tissue Model
4.7. Hydration-Related Gene Expression Changes in Reconstitutive Skin Tissue Model
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Cream-Gel Ingredient List
Appendix A.2. Cream Ingredient List
References
- Wolff, E.; Pal, L.; Altun, T.; Madankumar, R.; Freeman, R.; Amin, H.; Harman, M.; Santoro, N.; Taylor, H.S. Skin wrinkles and rigidity in early postmenopausal women vary by race/ethnicity: Baseline characteristics of the skin ancillary study of the KEEPS trial. Fertil. Steril. 2011, 95, 658–662. [Google Scholar]
- Makrantonaki, E.; Bekou, V.; Zouboulis, C.C. Genetics and skin aging. Dermato-Endocrinol. 2012, 4, 280–284. [Google Scholar]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar]
- Choi, J.W.; Kwon, S.H.; Huh, C.H.; Park, K.C.; Youn, S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: A comprehensive and objective approach. Skin Res. Technol. 2013, 19, e349–e355. [Google Scholar]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7 (Suppl. S2), S12–S16. [Google Scholar]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar]
- Antonicelli, F.; Bellon, G.; Debelle, L.; Hornebeck, W. Elastin-elastases and inflamm-aging. Curr Top. Dev. Biol 2007, 79, 99–155. [Google Scholar]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar]
- Knott, A.; Reuschlein, K.; Mielke, H.; Wensorra, U.; Mummert, C.; Koop, U.; Kausch, M.; Kolbe, L.; Peters, N.; Stäb, F.; et al. Natural Arctium lappa fruit extract improves the clinical signs of aging skin. J. Cosmet. Dermatol. 2008, 7, 281–289. [Google Scholar]
- Park, S.J.; Lee, M.H.; Yun, J.M.; Kim, D.K.; Lee, Y.H. Effects of Zingiber mioga extract (FSH-ZM) on moisturizing, wrinkle improvement and whitening activity in skin cells. J. Korean Soc. Food Sci. Nutr. 2019, 48, 215–222. [Google Scholar]
- Boury-Jamot, M.; Daraspe, J.; Bonté, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J.M. Skin aquaporins: Function in hydration, wound healing, and skin epidermis homeostasis. In Aquaporins. Handbook of Experimental Pharmacology; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 205–217. [Google Scholar]
- McDaniel, D.H.; Dover, J.S.; Wortzman, M.; Nelson, D.B. In Vitro and in vivo evaluation of a moisture treatment cream containing three critical elements of natural skin moisturization. J Cosmet. Dermatol. 2020, 19, 1121–1128. [Google Scholar]
- Katekawa, E.; Caverzan, J.; Mussi, L.; Camargo-Junior, F.B.; Sufi, B.; Padovani, G.; Nazato, L.; Nogueira, C.; Magalhães, W.V.; Di Stasi, L.C. Novel topical skin hydration agent containing Anadenanthera colubrina polysaccharide-standardized herbal preparation. J. Cosmet. Dermatol. 2020, 19, 1691–1698. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Psychological and social implications of aging skin: Normal aging and the effects of cutaneous disease. In Textbook of Aging Skin, 1st ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 949–957. [Google Scholar]
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics; The Aesthetic Society: Garden Grove, CA, USA, 2019; Available online: https://www.surgery.org/sites/default/files/Aesthetic-Society_Stats2019Book_FINAL.pdf (accessed on 21 March 2022).
- Draelos, Z.D. Revisiting the skin health and beauty pyramid: A clinically based guide to selecting topical skincare products. J. Drugs Dermatol. 2021, 20, 695–699. [Google Scholar]
- Nolan, K.A.; Marmur, E.S. Over-the-counter topical skincare products: A review of the literature. J. Drugs Dermatol. 2012, 11, 220–224. [Google Scholar]
- Meunier, M.; Chapuis, E.; Lapierre, L.; Auriol, P.; Paulus, C.; Elbaum, B.; Don Simoni, E.; Sandré, J.; Auriol, D.; Scandolera, A.; et al. Mannose-6-phosphate complex and improvement in biomechanical properties of the skin. J. Cosmet. Dermatol. 2021, 20, 1598–1610. [Google Scholar]
- Makino, E.T.; Kadoya, K.; Chung, R.; Jiang, L.; Mikati, M.; Mehta, R.C. Efficacy and tolerability of a novel topical treatment for neck: A randomized, double-blind, regimen-controlled study. J. Drugs Dermatol. 2021, 20, 184–191. [Google Scholar]
- Farris, P.K.; Edison, B.L.; Weinkauf, R.L.; Green, B.A. A novel, volumizing cosmetic formulation significantly improves the appearance of target Glabellar lines, nasolabial folds, and crow’s feet in a double-blind, vehicle-controlled clinical trial. J. Drugs Dermatol. 2014, 13, 41–46. [Google Scholar]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.Y.; et al. Anti-wrinkle benefits of peptides complex stimulating skin basement membrane proteins expression. Int. J. Mol. Sci. 2019, 21, 73. [Google Scholar]
- Bhardwaj, V.; Fabijanic, K.I.; Cohen, A.; Mao, J.; Azadegan, C.; Pittet, J.C.; Bris, B.L. Holistic approach to visualize and quantify collagen organization at macro, micro, and nano-scale. Skin Res. Technol. 2022, 28, 419–426. [Google Scholar]
- Nguyen, T.Q.; Zahr, A.S.; Kononov, T.; Ablon, G. A randomized, double-blind, placebo-controlled clinical study investigating the efficacy and tolerability of a peptide serum targeting expression lines. J. Clin. Aesthet. Dermatol. 2021, 14, 5–14. [Google Scholar]
- Jung, Y.K.; Shin, D. Imperata cylindrica: A review of phytochemistry, pharmacology, and industrial applications. Molecule 2021, 26, 1454. [Google Scholar]
- Georgiev, V.; Slalov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar]
- Hameury, S.; Borderie, L.; Monneuse, J.M.; Skorski, G.; Pradines, D. Prediction of skin anti-aging clinical benefits of an association of ingredients from marine and maritime origins: Ex vivo evaluation using a label-free quantitative proteomic and customized data processing approach. J. Cosmet. Dermatol. 2019, 18, 355–370. [Google Scholar]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological insights into halophyte bioactive extract action on anti-inflammatory, pain relief and antibiotics-type mechanisms. Molecules 2021, 26, 3140. [Google Scholar]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar]
- Balaev, A.N.; Okhmanovich, K.A.; Osipov, V.N. A shortened, protecting group free, synthesis of the anti-wrinkle venom analogue Syn-Ake® exploiting an optimized Hofmann-type rearrangement. Tetrahedron Lett. 2014, 55, 5745–5747. [Google Scholar]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending anti-aging peptides. Cosmetics 2020, 7, 91. [Google Scholar]
- Bazin, R.; Doublet, E. Skin Aging Atlas. Caucasian Type; Editions Med’Com: Paris, France, 2007; Volume 1. [Google Scholar]
- Voegeli, R.; Rawlings, A.V.; Seroul, P.; Summers, B. A novel continuous colour mapping approach for visualization of facial skin hydration and transepidermal water loss for four ethnic groups. Int. J. Cosmet. Sci. 2015, 37, 595–605. [Google Scholar]
- Bhardwaj, V.; Sharma, K.; Maksimovic, S.; Fan, A.; Adams-Woodford, A.; Mao, J. Professional-grade TCA-lactic acid chemical peel: Elucidating mode of action to treat photoaging and hyperpigmentation. Front. Med. 2021, 8, 617068. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar]
- Takino, Y.; Okura, F.; Kitazawa, M.; Iwasaki, K.; Tagami, H. Zinc l-pyrrolidone carboxylate inhibits the UVA-induced production of matrix metalloproteinase-1 by in vitro cultured skin fibroblasts, whereas it enhances their collagen synthesis. Int. J. Cosmet. Sci. 2012, 34, 23–28. [Google Scholar]
- Mahoney, M.G.; Brennan, D.; Starcher, B.; Faryniarz, J.; Ramirez, J.; Parr, L.; Uitto, J. Extracellular matrix in cutaneous ageing: The effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp. Dermatol. 2009, 18, 205–211. [Google Scholar]
- Calve, S.; Isaac, J.; Gumucio, J.P.; Mendias, C.L. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am. J. Physiol. Cell Physiol. 2012, 303, C577–C588. [Google Scholar]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120 Pt B, 1682–1695. [Google Scholar]
- Caucanas, M.; Montastier, C.; Piérard, G.E.; Quatresooz, P. Dynamics of skin barrier repair following preconditioning by a biotechnology-driven extract from samphire (Crithmum maritimum) stem cells. J. Cosmet. Dermatol. 2011, 10, 288–293. [Google Scholar]
- Leite e Silva, V.R.; Schulman, M.A.; Ferelli, C.; Gimenis, J.M.; Ruas, G.W.; Baby, A.R.; Velasco, M.V.R.; Taqueda, M.E.; Kaneko, T.M. Hydrating effects of moisturizer active compounds incorporated into hydrogels: In Vivo assessment and comparison between devices. J. Cosmet. Dermatol. 2009, 8, 32–39. [Google Scholar]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Md, S.; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—A review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar]
- Rossetti, D.; Kielmanowicz, M.G.; Vigodman, S.; Hu, Y.P.; Chen, N.; Nkengne, A.; Oddos, T.; Fischer, D.; Seiberg, M.; Lin, C.B. A novel anti-ageing mechanism for retinol: Induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet Sci. 2011, 33, 62–69. [Google Scholar]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar]
- Olsson, M.; Broberg, A.; Jernås, M.; Carlsson, L.; Rudemo, M.; Suurküla, M.; Svensson, P.A.; Benson, M. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006, 61, 1132–1137. [Google Scholar]
| Inclusion Criteria | Cream-Gel | Cream |
|---|---|---|
| Caucasian | ✓ | ✓ |
| Female, age 35–60, extended to 65 (amended protocol) | ✓ | ✓ |
| Fitzpatrick skin type I to III | ✓ | ✓ |
| Combination oily to oily skin on face with no more than 5 subjects enrolled that are not of this type (e.g., dry combination) | ✓ | X |
| Normal to dry skin on face | X | ✓ |
| 50% of subjects with sensitive skin (self-assessed) | ✓ | ✓ |
| Presenting wrinkles and fine lines with grade ≥2 and ≤4 on the crow’s feet, forehead, nasolabial fold, neck and glabellar regions [34] | ✓ | ✓ |
| Exclusion Criteria | Cream-Gel | Cream |
|---|---|---|
| Known allergic or reactivity to cosmetic products | ✓ | ✓ |
| For subjects undergoing hormonal treatment, a change in treatment within the past 3 months or an expectation of a change in treatment | ✓ | ✓ |
| Any anti-allergy, antibiotic, anti-inflammatory, dermatological treatment or corticosteroid treatment within the past 2 weeks | ✓ | ✓ |
| Pregnant or breast-feeding. Not taking precautions to prevent pregnancy. | ✓ | ✓ |
| Application of skin care products to the face and neck within 24 h of the inclusion visit | ✓ | ✓ |
| Washing of the face with anything other than water on the inclusion visit | ✓ | ✓ |
| Application of anti-aging or anti-wrinkle products to the face and neck within 15 days of the inclusion visit | ✓ | ✓ |
| Having received injections on the face and neck within 6 months of the inclusion visit or the anticipation of having them during the study | ✓ | ✓ |
| Application of self-tanning products to the face and neck within 2 weeks of the inclusion visit | ✓ | ✓ |
| Cutaneous marks on the face and neck that could interfere with the visual assessment | ✓ | ✓ |
| Sun/Ultraviolet radiation exposure within 2 weeks of the inclusion visit or the expectation of such exposure during the study | ✓ | ✓ |
| Treatment with vitamin A or derivatives during 3 months prior to the inclusion visit | ✓ | ✓ |
| Retinoid based oral treatment within 6 months prior to the inclusion visit or topical treatment within 2 months of the inclusion visit | ✓ | ✓ |
| Carotene based treatment within 2 weeks of the inclusion visit | ✓ | ✓ |
| Previous aesthetic or dermatological surgery on the face and neck or the expectation of having such surgery during the study | ✓ | ✓ |
| Cream-Gel Study n = 30 | Cream Study n = 33 | |
|---|---|---|
| Age | ||
| Mean age | 57.1 years (100%) | 58.9 years (100%) |
| Age range | 45–65 years (100%) | 46–65 years (100%) |
| Phototype (caucasians) | ||
| Phototype I | 0 (0%) | 1 (3.0%) |
| Phototype II | 20 (66.7%) | 23 (69.7%) |
| Phototype III | 10 (33.7%) | 9 (27.3%) |
| Sensitivity by self-assessment | ||
| Sensitive skin | 22 (73.3%) | 23 (69.7%) |
| Non-sensitive skin | 8 (26.7%) | 10 (30.3%) |
| Skin characteristics | ||
| Normal | 0 (0%) | 5 (15.2%) |
| Dry skin/Dry combination | 4 (13.3%) | 28 (84.8%) |
| Oil skin/Oily combination | 26 (86.6%) | 0 (0%) |
| Clinical assessment of wrinkles | ||
| Facial and neck wrinkles between grade 2 and 4 | 30 (100%) | 33 (100%) |
| Forehead wrinkles | ||
| Length | 4.69 ± 0.17 mm | 4.44 ± 0.17 mm |
| Surface | 2.82 ± 0.18 mm2 | 2.60 ± 0.18 mm2 |
| Depth | 408.06 ± 9.18 mm | 351.19 ± 8.37 mm |
| Volume | 1.16 ± 0.08 mm3 | 0.93 ± 0.08 mm3 |
| Glabellar wrinkles | ||
| Length | 3.12 ± 0.11 mm | 3.03 ± 0.08 mm |
| Surface | 3.04 ± 0.22 mm2 | 3.39 ± 0.24 mm2 |
| Depth | 383.09 ± 8.99 mm | 397.44 ± 12.35 mm |
| Volume | 1.17 ± 0.09 mm3 | 1.29 ± 0.06 mm3 |
| Crow’s feet wrinkles | ||
| Length | 2.95 ± 0.08 mm | 2.87 ± 0.08 mm |
| Surface | 1.95 ± 0.11 mm2 | 1.84 ± 0.11 mm2 |
| Depth | 263.76 ± 6.07 mm | 261.36 ± 7.73 mm |
| Volume | 0.51 ± 0.03 mm3 | 0.47 ± 0.02 mm3 |
| Nasolabial wrinkles | ||
| Length | 1.92 ± 0.07 mm | 1.86 ± 0.05 mm |
| Surface | 0.96 ± 0.07 mm2 | 0.88 ± 0.05 mm2 |
| Depth | 740.00 ± 21.31 mm | 663.33 ± 21.42 mm |
| Volume | 0.69 ± 0.05 mm3 | 0.57 ± 0.02 mm3 |
| Neck wrinkles | ||
| Length | 2.74 ± 0.12 mm | 2.74 ± 0.10 mm |
| Surface | 0.93 ± 0.07 mm2 | 0.97 ± 0.06 mm2 |
| Depth | 454.59 ± 13.03 mm | 437.75 ± 11.39 mm |
| Volume | 0.41 ± 0.03 mm3 | 0.43 ± 0.04 mm3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, V.; Namkoong, J.; Tartar, O.; Diaz, I.; Mao, J.; Wu, J. In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies. Cosmetics 2022, 9, 80. https://doi.org/10.3390/cosmetics9040080
Bhardwaj V, Namkoong J, Tartar O, Diaz I, Mao J, Wu J. In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies. Cosmetics. 2022; 9(4):80. https://doi.org/10.3390/cosmetics9040080
Chicago/Turabian StyleBhardwaj, Vinay, Jin Namkoong, Océane Tartar, Isabel Diaz, Junhong Mao, and Joanna Wu. 2022. "In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies" Cosmetics 9, no. 4: 80. https://doi.org/10.3390/cosmetics9040080
APA StyleBhardwaj, V., Namkoong, J., Tartar, O., Diaz, I., Mao, J., & Wu, J. (2022). In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies. Cosmetics, 9(4), 80. https://doi.org/10.3390/cosmetics9040080





