Abstract
“Seta e Ciliegia” and “Narguilé” fragrances were mixed to form a binary blend with chemically stable, non-volatile, odourless, simple bases of different lipophilicity widely used in skin care and hair care formulations, such as caprylic-capric triglyceride, glycerine, paraffin, dimethicone, isopropyl myristate and butylene glycol, with the objective to verify how the olfactory performance of fragrances can be influenced by skin or hair care ingredients. The semiquantitative approach applied in this study aims in providing a practical solution to appropriately combine a fragrance with cosmetic ingredients. Pure fragrance and binary blends were analysed by solid phase microextraction gas chromatography tandem mass spectrometry (SPME-GC/MS), based on the assumption that the solid phase microextraction is able to extract volatile compounds, mimicking the ability of the nose to capture similar volatile compounds. Fifty-seven and forty-four compounds were identified by SPME-GC/MS in pure fragrances “Seta e Ciliegia” and “Narguilé”, respectively. Once mixed with the bases, the analysis of the blends revealed that a qualitative modification in the chromatograms could occur according to the characteristics of the bases. In general, for both fragrances, blends with glycerin and butylene glycol, which are the most hydrophilic bases among the ones tested, were able to release most of the peaks, that were thus still present in the chromatograms. Differently, in the blends with caprylic-capric triglyceride, most of the peaks are lost. Blends with paraffine, dimethicone and isopropyl myristate showed an intermediate behaviour. These results were thus compared with the sensory evaluation made by an experienced perfumer, capable of assessing the different olfactory performances of pure fragrances and their different binary blends. The evaluation made by the perfumer fitted well with the analytical results, and in the blends where most of the peaks were revealed in the chromatogram, the perfumer found a similar olfactory profile for example with glycerin, butylene glycol, while a modification of the olfactory profile was highlighted when several peaks were not still present in the chromatogram, as it was the case with caprylic-capric triglyceride. Interestingly, when the most typical peaks of a fragrance were still observed in the blend, even if some of them were lost, the olfactory performance was not lost, as was the case of paraffin and isopropyl myristate. In the case of dimethicone, its high volatility was considered responsible for a certain decrease in the fragrance “volume”. The results achieved with this investigation can be used to hypothesize that the different compounds of a fragrance, characterized for the first time by different volatility and solubility, could be differently retained by the bases: the more lipophilic are strongly retained by the lipophilic bases with a consequently reduced volatility that limits the possibility of being appreciated by the nose and that corresponds to disappearance or a percentage reduction from the chromatogram. Therefore, in a more accurate and helpful view for a formulator, we could come to the conclusion that based on the results achieved by our investigation, the inclusion of a less lipophilic base can be more appropriate to exalt more lipophilic fragrances.
1. Introduction
Fragrances are volatile organic compounds with characteristic odours. They can be made up of very complex mixtures or individual chemicals [1]. They have been used for thousands of years for beneficial purposes, for physical and mental health. Nowadays, the importance of the sense of smell in the human species does not only relate to food, hygiene or beauty, but also plays a significant role in lived experiences [2]. An odour has the sensational ability to evoke a past experience that is dormant in our minds with extreme clarity, allowing us to materialise our memories, travel through time and bring back the moment when that particular scent struck us [3,4].
Each fragrance is composed of different odorous compounds that form together a typical scent characterized by an olfactory pyramid [5]. The concept of the olfactory pyramid, coined by Aimé Guerlain in 1889 who applied this concept during the creation of his fragrances, is the classification of the olfactory notes that make up a fragrance. Each fragrance is subdivided according to its constituent notes’ volatility, intensity and persistence. The olfactory pyramid well represents these characteristics by dividing the notes in top, heart and base notes. The top notes are those which are picked up initially and are fresh and not very persistent. These constitute the most volatile part and indicate the first impression of a fragrance. The most common top notes are citrus notes such as lemon, orange, bergamot and marine [5]. After a few minutes, the heart notes begin to be perceived. These form the structure of the fragrance, usually making up 40–80% of the blend and giving both body and continuity to the top notes. The heart notes can be fruity (peach) or floral (rose, violet, jasmine). Finally, we have the base notes, which are singled out last because they evaporate more slowly and, thanks to their long permanence, characterise the fragrance. The base notes are woody (cedar, sandalwood, patchouli) [6,7], spicy (pepper and cinnamon), musky, ambery (balsam of Peru, amber, benzoin) gourmand (caramel, coffee, cocoa) or leathery.
A fragrance must have specific molecular properties to provoke sensory reactions [8,9]. It must have a certain degree of lipophilicity, a high vapour pressure to be transmitted to the olfactory system, a certain solubility in water to penetrate the mucus layer and it must be at a sufficient concentration to interact with the various olfactory receptors specific to each odour and thus provoke membrane depolarisation and the activation of action potentials [10]. The fragrance within any product must be stable and long-lasting to increase consumer appeal. Marketing companies have realised that fragrance is a key element as it influences both the purchase and consumer approval of the product [11]. However, the main ingredients of fragrances are labile and volatile. In fact, even one note may change during formulation: an example is the oxidation of limonene at room temperature.
The present study originates from the observation made in our laboratories where we found that the olfactory performance of a fragrance, inserted into a cosmetic product for “skin care” or “hair care”, is strongly influenced by the characteristics of the cosmetic formulation in which the fragrance is mixed. By doing this study, we tried to find a reasonable answer to this observation. If we look at a fragrance on its own, it is made up of a highly complex mix of different odorous compounds. When a fragrance is used in a cosmetic formulation, it may contain compounds such as solvents, emollients and active ingredients, with different properties and activities that could change the “olfactory performance” of the fragrance. Some compounds are odourless, while some others may also possess unpleasant odours (for example, surfactants or some active ingredient) or characteristics odour, that the formulator tries to mask with the fragrance. A part from incompatibility issues (well known in the cosmetic and perfumer fragrance practises), that can lead with time to the modification of the odour, colour, and density of the cosmetic products, it can frequently occur that the olfactory performance of one fragrance can remain unmodified, or strongly change according to the formulation in which it is added. This phenomenon has been frequently observed in our laboratories, with the consequence that a selected fragrance that perfectly matched the design of the formulation cannot be used because once added into the formulation, it completely changed. The capacity of a single fragrance to keep all of its original qualities even after being included in a cosmetic product has been called “olfactory performance” in this study. Consequently, based on what we observed in our laboratory, we developed our working hypothesis from a formulator’s perspective. We demonstrated why the cosmetic formulation, which is made up of ingredients that can be either lipophilic or hydrophilic, has the potential to change the “volatility” of the fragrance, which is a complex mix of many different molecules, and therefore its ability to be “released” from the formulation and express itself olfactorily. To verify our hypothesis, we decided to choose two different fragrances “Seta e Ciliegia” and “Narguilé”, and mix each of them with chemically stable, non-volatile, odourless, simple bases of different lipophilicity widely used in skin care and hair care formulations, such as isopropyl myristate, paraffin, caprylic-capric triglyceride, dimethicone, butylene glycol and glycerine. Pure fragrances and binary blends were then analysed, after solid-phase microextraction (SPME), by gas chromatography-tandem mass spectrometry (GC-MS). The SPME was chosen because it can “mimic” what happens when the different components of a fragrance, according to their different volatilities, are released to hit the olfactory centres [12,13]. The peaks of the different chromatograms, combined with the mass fragmentations, made it possible to identify the different components of the fragrance. These components are precisely those that make up the fragrance and characterise it from an olfactory point of view. By direct comparison of the chromatograms of the pure fragrance with those of the blends, it was possible to observe that some peaks present in the chromatogram of the pure fragrance were no longer present in the binary blends. We then tried to explain these results in light of the volatility and solubility of the components. The data collected in this work can help us to deduce that the different components of the fragrance, characterised by different volatility and solubility, could be differently retained by the bases. We then compared the results of the SPME/GC-MS analysis with the sensory evaluation made by an experienced perfumer, capable of assessing the different olfactory performance of the pure fragrance and that when inserted in the different bases during the preparation of a cosmetic formulation.
2. Materials and Methods
2.1. Materials
Fragrances “Seta e Ciliegia” (SC) and “Narguilé” (N) were kindly provided by Muller & Koster (Milan, Italy). The following cosmetic bases were used: dimethicone (DMT) (Acesil 350), glycerine (GLY), paraffin (PFF), tricaprylate caprate (TCC), isopropyl myristate (IPM) and butylene glycol (BGLY), all supplied by ACEF SpA (Piacenza, Italy).
2.2. Solid-Phase Microextraction Tandem Mass Gas Chromatography (SPME/GC-MS)
Pure fragrances and bases, and binary blends were analysed by SPME/GC-MS. Binary blends of each fragrance with one base per time were prepared by mixing 1 g of each cosmetic base and 0.1 g of fragrance. The blending was assured by gently mixing the two ingredients with a glass rod in a glass vial. For the analysis, 0.2 g of each binary mix was placed in a stopped glass vial conditioned at 30 °C in the water bath for 15 min. The solid-phase microextraction (SPME) was performed by using a 65 μm blue divinylbenzene/polydimethylsiloxane (DVB/PDMS) fiber (SUPELCO, Bellafonte, Italy) that was exposed to the headspace above the sample blend for 2 min at 30 °C on a water bath. In addition, the empty capped vial was used as the blank control.
Samples were analysed on an Agilent 6890 Network (Milan, Italy) gas chromatograph (GC) with an Agilent 5977B mass selective detector (MS). The column used was a 30 m × 0.25 mm, 0.25 μm film thickness HP5-MS. Helium was the carrier gas, flow-controlled at 1.0 mL/min. The analytes were desorbed in the injection port of the GC using an inlet temperature set at 250 °C in the split mode in a 10:1 ratio. The GC analysis began with an initial oven temperature of 40 °C for 5 min, followed by a ramp of 25 °C/min to 300 °C, and ending with a 0.5 min hold. The single quadrupole mass analyser was operated in electron ionization (EI) mode and scanned over a mass range of m/z 50–550 in full scan mode.
The identification of the volatile components was performed by comparing their mass spectra with the retention times (RT) listed in the NIST05 (The National Institute of Standards and Technology, NIST, 2008, U.S. Department of Commerce. US) library. The chromatograms were analysed using MSD ChemStation software (Agilent version G1701DA D.01.00).
The compounds were confirmed by comparing their retention indices (RI) calculated by the use of a series of n-alkane standards (C6–C19) (Accu. Standard, New Haven, CT, USA) with related literature data (National Institute of Standards and Technology, 2011). The relative content of each compound was determined by using a peak normalization procedure based on the total ion flow chromatogram.
Only peaks with an area % >0.01 are considered and the analyses were carried out comparing relative peak areas.
2.3. Olfactory Evaluation by the Experienced Perfumer
An experienced perfumer assessed how the fragrance is perceived in different blends. The evaluation was conducted by comparing two mouillettes (fragrance papers) one soaked with the pure fragrance oil and the other with the one blend per time. The evaluation was carried out in an environment with no external stimuli and white walls, because the sight of a certain colour or object can lead to emotions that unconsciously give an incorrect olfactory evaluation. The evaluation was carried out at room temperature, ranging between 18 and −20 °C, using air conditioning if necessary to reach the right temperature, before the evaluation. In this way, we avoided higher and lower temperatures that could cause important smelling deviations from the standard olfactive profile of the fragrance. The olfactive evaluation was performed in the early morning, before breakfast time, thus avoiding stress stimuli coming from everyday life. Our nose, like our body, becomes tired during the day and undergoes a physiological drop in performance. Early morning is the right time to perform this kind of analysis; a rested nose could smell olfactive nuances, hardly noticeable after a few hours of normal daily activity. Following the mouillettes assessment, the olfactory evaluation was also performed, inhaling the sample directly from the bottle and confirming, in most instances, the mouillettes impression.
3. Results
3.1. Seta e Ciliegia
SPME/GC-MS of “Seta e Ciliegia” Fragrance
Figure 1 shows the total ion chromatogram (TIC) for the pure fragrance “Seta e Ciliegia”. Table 1 reports the fifty-seven compounds that were detected, together with their retention time (RT) and relative peak areas (%).
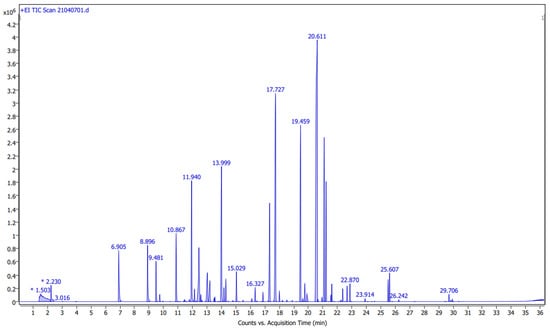
Figure 1.
Total ion chromatogram for the pure fragrance “Seta e Ciliegia”.

Table 1.
Retention time (RT) and relative peak areas (%) of the analytes in the pure fragrance “Seta e Ciliegia” and its blends with cosmetic bases.
The pure bases chromatograms are characterized by the almost total absence of ions, confirming the absence of flavoured compounds that would interfere with the characterization of the blends, if no exception is made for the dimethicone with the presence of 3 different ions with retention times (RT) of 6.11, 11.76, and 16.61 min, that correspond to hexamethyl cyclotrisiloxane, octamethyl cyclotrisiloxane and decamethyl cyclotetrasiloxane (all slightly volatile colourless and odourless liquids), respectively. For the paraffin, the ion at RT 16.60 min corresponded to 1-octanol; for isopropyl myristate, the ion at RT of 32.44 min corresponded to the isopropyl myristate, a yellow oily and odourless liquid, insoluble in water; for the butylene glycol with a large band at 7.61 min, corresponding to the butylene glycol, a colourless, water-soluble liquid, practically odourless. Based on these results, we may conclude that these bases should not affect olfactory performance.
Then, the binary blends were analysed (Table 1). By comparing the blend’s chromatogram to that of pure fragrance, we saw that the TTC blend had the most “loss of components” compared to the other blends. Here we want to specify that for “loss of compounds” we intend that these compounds are not appreciable anymore in the chromatogram or the corresponding peak is under its limit of detection. Among the compounds lost in the TTC blend there are dimethyl silanediol; β-myrcene; 1,3-dimethyl-2-(1-methylethyl)-cyclopentene; 4-hexen-1-ol, acetate; eucalyptol; isoterpinolene; tetrahydrolinalool; diihydrocitronellal; verdone; citronellal; 6,6,-dimethoxy-2,5,5-trimethyl-2-hexene; decanal; 2,4,6-trimethyl-1,3-dioxane; 1,2,3-benzothiadiazole; α-linalool; p-menthan-7-ol, trans; 10-undecenal; 2,4-dimethyl-1,3-cyclopentanedione; piperonal; 1H-inden-1-one, 2,3-dihydro-3,4,7-trimethyl-; allyl cyclohexanepropionate; 2-phenoxyethyl isobutyrate; amberonne (isomer 2); amberonne (isomer 3) and hexyl salicylate. For many other compounds we could assess a certain different on the relative area % of the peak in the blend, with the peak in the chromatogram of the pure fragrance. We must consider that this study does not intend to provide a quantitative evaluation in the change of peak’s area percentage, and that all the evaluations have been carried out in absence of internal standards. Thus, an evaluation of changes in peak area percentages simply acquires a semi-quantitative meaning and is the only reason to proceed with a coarse assessment of possible differences between the blend and pure fragrance chromatograms. So that, we found a relevant increase in the area % of some compounds (ethyl acetate; 3-methoxy-3-methylbutanol; p-cymene; dipropylene glycol and dihydromycenol) while for some others there was a relevant decrease (butyric acid 2-methyl ethyl ester; α-pinene; β-pinene; limonene; γ-terpinene; ethyl linalool; p-methylbenzyl acetate; linalyl acetate; vertenex; dimethylbenzyl carbinyl acetate; neryl acetate; α-damascone; and geranyl acetate).
According to our idea, the TCC may significantly alter the “olfactory performance” of pure fragrance since the chemicals of pure fragrance are released differently once on a cosmetic basis.
Differently from TTC, the blend with GLY exhibited an olfactory performance very similar to the pure fragrance, with the loss of dimethyl silanediol and 10-undecane. Few compounds are also missed for BGLY blend (dimethyl silanediol, eucalyptol, isoterpinolene, tetrahydrolinalool, dihydrocitronellal, citronellal, 2,4,6-trimethyl-1,3-dioxane, p-menthan-7-ol, trans- and 10-undecanal).
In the blend with PFF we did notdetect compounds such as β-myrcene, cyclopentene 1,3-dimethyl-2-(-1-methylethyl), 4-hexenel-1-ol, acetate, eucalyptol, γ-terpinene, isoterpinolene, dihydrocitronellal, verdone, citronellal, α-linalool, 1H-inden-1-one, 2,3-dihydro-3,4,7-trimethyl, allyl cyclohexanepropionate and amberonne (isomer 3), while the blend with DMT lost dimethyl silanediol, β-myrcene, cyclopentene 1,3-dimethyl-2-(-1-methylethyl), 4-hexenel-1-ol, acetate; eucalyptol, isoterpinolene, dihydrocitronellal, citronellal, α-linalool, and amberonne (isomer 3).
The blend with IPM lost butyric acid 2-methyl ethyl ester; β-myrcene; cyclopenene, 1,3-dimethyl-2-(1-methylethyl)-; 4-hexen-1-ol, acetate; eucalyptol; γ-terpinene; isoterpinolene; tetrahydrolinalool; diihydrocitronellal; verdone; citronellal; 1,2,3-benzothiadiazole, α-linalool, 10-undecanal, piperonal, 1H-Inden-1-one, 2,3-dihydro-3,4,7-trimethyl-, allyl cyclohexanepropionate, 2-phenoxyethyl isobutyrate, amberonne isomer 2 and 3 and hexyl salicylate.
Perfume is made up of notes that are blended together in a harmonious manner. Based on its persistence, every note can be placed in the olfactory pyramid. Perfumers use it to create a three-tiered visual depiction of their notes [14,15]. The top notes of a perfume are those that are initially noticeable after application and are light, fresh, volatile, and not particularly long-lasting. Citrus and sea notes fall within this area. Midway up the pyramid are the “heart notes,” which are heard immediately after the “top” notes and have a medium degree of volatility and persistence to them. Perfume character and individuality are enhanced by the presence of fruity, flowery, and green notes at this level. A pyramid’s base notes, followed by the heart notes, are the longest-lasting ones. For example, musky, woody, spicy, sweet, and oriental tones may all be included at this level.
According to the olfactory evaluation of the experienced perfumer, the fragrance “Seta e Ciliegia” presents a fruity note with strong floral nuances, marine nuances, and a musky base with sweet hints.
Based on the SPME-GC/MS analysis, the perfumer found 44 compounds in the “Seta e Ciliegia” fragrance that give the odour rich and deep characteristics. Table 2 explains the relationship between each compounds and its related note. The fragrance composition is a succession of notes in harmony with each other. Many of the compounds in Table 2 have a fruity note that gives the fragrance a distinct character and good persistence in the air. The flowery and fruity notes work together harmoniously to create a sunny and velvety character. Top notes are mostly fruity ones, with a predominance of fresh esperidate notes [16] (p-cymene, d-limonene [17], citronellal, γ-terpinene, dihydromyrcenol, 6,6-dimethoxy-2,5,5-trimethyl-2-hexene). The floral notes are predominant and mainly present among the hearth notes together with fruity notes, giving intensity and seduction. In the fragrance “Seta e Ciliegia”, the perfumer also traced the note of green in the compound ligustral, 3-hexenel-1-ol, acetate (z), dihydrocitronellal and hexyl isobutyrate. The notes just described belong to the heart notes in the olfactory pyramid; they are more powerful and stronger than the top notes. Among the compounds listed in Table 2, α-damascone, 2-phenozyethyl isobutyrate and piperonal have a gourmand facet. They have a sweet and sensuous flavour evocative of the sweet world thanks to the gourmand notes. Persistent and slowly evaporating, these sounds are part of the olfactory pyramid’s base notes. An olfactory pyramid for the chemicals from Table 2 is shown in Figure 2.

Table 2.
List of odour compounds identified by the perfumer in the “Seta e Ciliegia” fragrance according to the compounds previously identified by SPME-GC/MS.
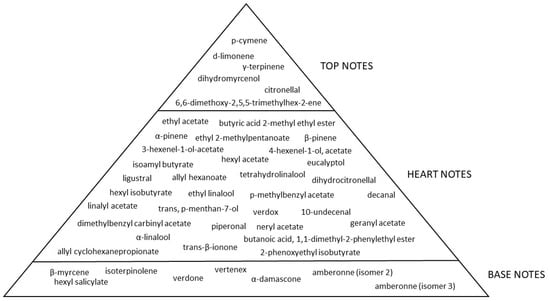
Figure 2.
Olfactory pyramid of “Seta e Ciliegia” pure fragrance.
The next step in the present study was trying to combine the analytical results with the perfumer’s evaluation and assess if the analytical evaluation can support the sensation perceived by the perfumer.
According to the perfumer, starting from the TTC blend, the “Seta e Ciliegia” fragrance is less perceptible and more ethereal; a slight base odour is perceived, which covers the composition and slows down the physiological process of diffusion.
Correlating this evaluation with the results shown in Table 1, we can consider the loss of several compounds and the decrease in the % area of many others compounds. Finally, we could attest a dominant presence of the following odorous compounds: ethyl acetate (area % 1.89), butyric acid 2-methyl ethyl ester (area % 1.05), 3-hexenel-1-ol, acetate (z) (area 4.41%), p-cymene (area % 7.37), dipropylene glycol (s,p) (area % 5.36), dipropylene glycol (p,p) (area % 3.55), dihydromyrcenol (area % 8.30), ethyl linalool (area % 2.95), p-methylbenzyl acetate (area % 9.91), linalyl acetate (area % 3.79), verdox (area % 23.99), vertenex (area % 3.64) and dimethylbenzyl carbinyl acetate (area % 2.25). These, in fact, present a high % area which confirms the olfactory sensation of the perfumer. Table 3 allows us to identify the compounds mainly responsible for the olfactory performance of the blend TCC-fragrance according to the perfumer’s evaluation.

Table 3.
Description of the olfactory performance of the blend TTC-fragrance (“Seta & Ciliegia”) according to the compounds identified by GC-MS analysis.
The fragrance in the blend with GLY is in line with its olfactory profile of the pure fragrance. The “heavy” and gourmand notes were more highly appreciated at the expense of the juicy and floral ones, due to the presence of α-damascone, 2-phenozyethyl isobutyrate and piperonal. Actually, the α-damascone has the highest % area (0.57%) compared to the other blends. This compound gives the blend a soft and at the same time “heavy” facet, slightly masking the floral notes of the other molecules. Nonetheless, the fragrance is in good line with the olfactory profile of the pure fragrance because only two compounds (dimenthyl silanediol and 10-undecenal) have been lost. In addition to α-damascone there is a dominant presence of butyric acid 2-methyl ethyl ester (area % 1.78), β-pinene (area % 1.99), 3-hexenel-1-ol, acetate, (z) (area % 4.99), p-cymene (area % 1.63), dihydromyrcenol (area % 9.00), ethyl linalool (area % 5.92), p methylbenzyl acetate (area % 13.34), linalyl acetate (area % 7.34), verdox (area % 24.35), vertenex (area % 6.55), dimethyl carbinyl acetate (area % 4.80), butanoic acid, 1,1, -diìmethyl-2-phenylethyl ester (area % 1.05). For many compounds, the percentage is not so high if compared with other in the same blend, but is high if compared to the same compound in the other blends and their presence can justify the sweet and gourmand notes: ligustral (area % 0.57), allyl hexanoate (area % 0.74), tetrahydrolinalool (area % 0.07), decanal (area % 0.40), piperonal (area % 0.19), neryl acetate (area % 0.41), α-damascone (area % 0.57), geranyl acetate (area % 0.58), allyl cyclohexanepropionate (area % 0.12), trans-β-ionone (area % 0.93), 2-phenoxyethyl isobutyrate (area % 0.08) and hexyl salicylate (area % 0.09). The loss of flowery and juicy notes can be justified by the low percentage of many compounds with notes from the heart level of the olfactory pyramid. Table 4 highlights the compounds that better contribute to the olfactory performance of the blend according to the perfumer’s evaluation.

Table 4.
Description of the olfactory performance of the blend GLY-fragrance (“Seta & Ciliegia”) according to the compounds identified by GC-MS analysis.
The perfumer appreciated how the fragrance spread when he looked at the blend with PFF. The aroma is only slightly kept, but it still has the same organoleptic profile and excellent intensity as when it was first made.
The following compounds, in particular responsible for the floral and fruity notes, 3-hexen-1-ol, acetate (area % 3.81), p-cymene (area % 6.75), dihydromyrcenol (area % 11.24), ethyl linalool (area % 6.11), p-methylbenzyl acetate (area % 14.94), linalyl acetate (area % 4.17), verdox (area % 16.78), vertenex (area % 2.94), and dimethylbenzyl carbinyl acetate (area % 3.54), are the ones with the highest area % in this blend, but also in the pure fragrance. This element may support the preservation of the olfactory profile of this blend in comparison to the pure fragrance.
In the DMT fragrance blend, the perfumer found a slight decrease in the “volume” of the composition due to the silicone nature of the base. In fact, there is a greater evaporation tendency of the different notes and the diffusivity increases exponentially (the situation is very similar in presence of paraffin).Analysing the chromatogram of the blend, it is possible to note the presence of the characteristic peaks of dimethicone: hexamethyl cyclotrisiloxane, octamethyl cyclotrisiloxane and decamethyl cyclotrisiloxane. These volatile compounds, which come from the family of silicones, are released together with the odorous compounds favouring the release of the latter. The slight decrease in the “volume” of the composition is confirmed by the decrease in the area % for most of the molecules. In particular, for the following compounds, characterized by fruity and floral notes, 3-hexen-1-ol, acetate, (z), p-cymene, dihydromyrcenol, ethyl linalool, p-methylbenzyl acetate, linalyl acetate, verdox, vertenex, dimethylbenzyl carbinyl acetate, the % areas are the highest, as in the pure fragrance. The decrease in the area % of the following compounds with floral and fruity notes, butyric acid 2-methyl ethyl ester, ethyl 2-methylpentanoate, 3-hexenl-1-ol, acetate, isoamyl butyrate, γ-terpinene, ligustral, allyl hexanoate, linalyl acetate, verdox, vertenex, dimethylbenzyl carbinyl acetate, neryl acetate, α-damascone, geranyl acetate, trans-β-ionone, butanoic acid, 1,1, -dimethyl-2-phenylethyl ester, hexyl salicylate, confirmed the reduction in the “volume” of the composition.
In the blend with IPM, the olfactory profile is maintained, and the floral and fresh-fruity part is in line with one of the pure fragrances. These typical notes can be ascribed to the following compounds: ethyl acetate (area % 1.80), 3-hexen-1-ol, acetate, (z), dihydromyrcenol (area % 7.16), ethyl linalool (area % 2.43), p-methylbenzyl acetate (area % 10.12), linalyl acetate (area % 3.3), verdox (area % 21.90), vertenex (area % 3.25), dimethylbenzyl carbiny acetate (area % 2.45). The % areas of these compounds are the highest in this blend, like in the pure fragrance, supporting the evidence that the olfactory profile is maintained in this blend as supported by the perfumer. The olfactory notes are well balanced;nonetheless, a slight solvent note is present. A slight base note is present, due to the presence of the characteristic isopropyl-myristate peak that may have veiled the facets of the fragrance.
Lastly, the perfumer thought that BGLY was an excellent solvent because he liked the fragrance as a whole. The fruity part is well balanced and gives space to the delicate musky base of the composition.
Again, this can be explained by the fact that the area % of the typical molecules of the pure fragrance are similar in the blend. Actually, the compounds with the most abundant peaks confer fruity facets and green colour nuances, making it possible to perceive the fragrance in its entirety. The typical fruity and floral notes, present in the heart level of the olfactory pyramid, depend to the presence of 3-hexen-1-1ol, acetate, (z), p-methylbenzyl acetate, linalyl acetate, verdox, vertenex, and dimethylbenzyl carbinyl acetate.
The base notes are confirmed by the presence of high percentage for α-pinene (area % 1.60), β-pinene (area % 2.92), β-myrcene (area % 0.03), vertenex (area % 6.91) that in this blend have the highest areas. The important % areas for linalyl acetate (area % 8.32), verdox (area % 32.06) and vertenex (area % 6.91) confirm and highlight the fruity note characteristic of the pure fragrance with a reduction in the area of molecules with a citrus note like p-cymene (area % 1.45) and dihydromyrcenol (area % 2.06).
3.2. Narguilé
SPME/GC-MS of “Narguilé” Fragrance
Figure 3 shows the TIC for the pure fragrance “Narguilé”. Forty-four compounds were detected and they are listed with their RT and relative peak areas (%) in Table 5.
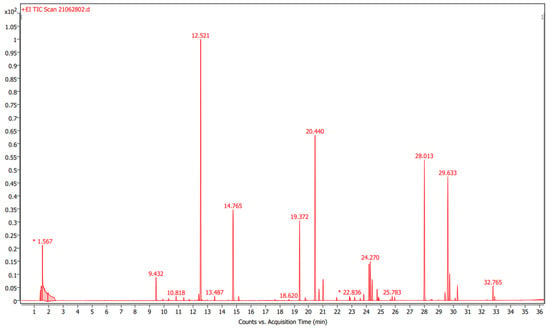
Figure 3.
Total ion chromatogram for the pure fragrance “Narguilé”.

Table 5.
Retention time (RT) and relative peak areas (%) of the analytes in the pure fragrance “Narguilé” and its blends with cosmetic bases.
Because the pure bases are the same as those used in the fragrance “Seta e Ciliegia,” we may conclude that the bases should not affect the olfactory performance.
Binary blends were thus analysed. The blend with TCC lost a lot of compounds: camphene; β-myrcene; α-phellandrene; ethyl maltol; 3-phenylpropanol; α-terpinyl acetate; β-patchoulene; β-damascone; 2-fluoro-α-methylbenzyl alcohol; caryophyllene; 10s, 11s-himachala-3(12),4-diene; γ-patchoulene; isopropyl isopropylfhosphonofluoridate; cashmeran; δ-guaiene; p-cyanophenyl p-(2-methylbutoxy benzoate); amberonne (isomer 1); ethenone, 1-[1,6-dimethyl-4(4-methyl-3-pentenyl)-3-cyclohexen-1-yl)-; tonalid and 4-tert-octylphenol, TMS derivative.
Few compounds are lost for GLY, such as p-cymene; phenylethyl alcohol; ethyl maltol; 3-phenylpropanol; α-terpinyl acetate; vanillin and tonalid.
The blend with PFF lost several compounds such as camphene; β-pinene; β-myrcene; α-phellandrene; γ-terpinene; α-terpinyl acetate; β-patchoulene; β-damascone; caryophyllene; seychellene; α-patchoulene; 10s, 11s-himachala-3(12),4-diene; γ-patchouelene; isopropyl isopropylphosphonofluoridate; cashmeran; δ-guaiene; amberonne (isomer 1); ethenone, 1-[1,6-dimethyl-4(4-methyl-3-pentenyl)-3-cyclohexen-1-yl)-; galaxolide and; tonalid. The relative % of the areas of α-pinene, p-cymene, d-limonene, linalyl acetate, verdox, vertenex, α-guaiene and amberonne are far lower than those of the pure fragrance. On the contrary, an increase in the relative % area of phenylethyl alcohol, ethyl maltol, 3-phenylpropanol, cinnamaldehyde, cinnamic alcohol, vanillin, coumarin and diethyl phthalate is observed.
The blend with DMT lost camphene; β-pinene; β-myrcene; α-phellandrene; β-patchouele; caryophyllene; 10s, 11s-himachala-3(12),4-diene; γ-patchouelene; δ-guaiene; p-cyanophenyl p(2-methylbutoxy) benzoate and 4-tert-octylphenol, TMS derivative.
The blend with IPM lost camphene; β-pinene; β-myrcene; α-phellandrene; γ-terpinene; 3-phenylpropanol; α-terpinyl acetate; β-patchoulene; β-damascone; 2-fluoro-α-methylbenzyl alcohol; caryophyllene; α-patchoulene; 10s, 11s-himachala-3(12),4-diene; γ-patchouelene; isopropyl isopropylphosphonofluoridate; cashmeran; δ-guaiene; p-cyanophenyl p-(2-methylbutoxy) benzoate; amberonne (isomer 1); ethenone, 1-[1,6-dimethyl-4(4-methyl-3-pentenyl)-3-cyclohexen-1-yl)-; tonalid; and 4-tert-octylphenol, TMS derivative. It was observed an important decrease in the relative % area of the peaks of some compounds such as α-pinene; d-limonene; linalool; linalyl acetate; verdox; cinnamic alcohol; vertenex; α-guaiene; seychellene and amberonne.
The blend with BGLY lost linalool; phenylethyl alcohol; ethyl maltol; 3-phenylpropanol; cinnamic alcohol; β-damascone; isopropyl isopropylphosphonofluoridate; p-cyanophenyl p-(2-methylbutoxy) benzoate and 4-tert-octylphenol, TMS derivative.
According to the olfactory evaluation of the perfumer, the fragrance “Narguilé” presents an oriental fragrance with woody and smoky tones in a vanilla body with amber reflexes.
Starting from the SPME-GC/MS analysis, the perfumer identified in the “Narguilé” fragrance 34 compounds that compose the fragrance. The correlations between each compound and the notes are in the Table 6. Only a few notes characterize the top and hearth notes, while most compounds of the fragrance “Narguilé” belong to the base notes in the olfactory pyramid giving the fragrance typical intensity and power of these notes: spicy, woody, herbaceous, citrusy, musk and sweet notes (Figure 4).

Table 6.
List of odour compounds identified by the perfumer in the “Narguilé” fragrance according to the compounds previously identified by SPME-GC/MS.
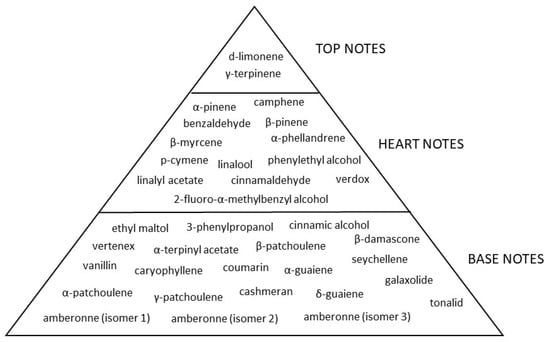
Figure 4.
Olfactory pyramid of “Narguilé” pure fragrance.
Once mixed with TCC, the fragrance “Narguilé” has a “dirty” note that changes the composition and makes the balance, characteristic of this fragrance, difficult to perceive. The sweet and fruity parts are more retained. The compounds such as α-pinene, d-limonene, linalool, linalyl acetate, verdox, vertenex, coumarin, α-guaiene, seychellene, amberonne (isomer 2 and 3) are in this blend the ones with the highest area % as well in the pure fragrance. Nevertheless, in the blend with TCC their area % is significantly decreased with the respect to the other blends (the ratio among the different compounds is not maintained); this is why the sweet and fruity parts are more evident with the respect to the pure fragrance.
For the blend with GLY, the perfumer described a well-balanced olfactory profile, with a good intensity and in line with the pure fragrance. This is confirmed by the percentage of the most important compounds that are very similar to those of the pure fragrance “Narguilé”: α-pinene (area % 1.07), d-limonene (area % 16.74), linalyl acetate (area % 5.65), verdox (area % 12.54), vertenex (area % 1.61), coumarin (area % 1.88), α-guaiene (area % 2.81), seychellene (area % 1.54), amberonne (isomer 2) (area % 10.80), amberonne (isomer 3) (area % 2.21), with the exception of linalool (area % 0.09).
For the blend with PFF, the perfume is in line with the one of pure fragrance, with a good diffusivity and a maintained olfactory profile. In spite of this, the percentages of the areas of the most typical compounds for this fragrance are significantly lower: α-pinene (area % 0.09), d-limonene (area % 3.26), linalool (area % 6.40), linalyl acetate (area % 1.96), verdox (area % 5.04), vertenex (area % 0.48), α-guaiene (area % 0.10), amberonne (isomer 2) (area % 3.01) and amberonne (isomer 3) (area % 0.45). With the exception of coumarin (area % 10.11), the typical fragrance is probably maintained thanks to the loss of 17 compounds.
When the pure fragrance is combined with DMT, the volatility of the notes increases, resulting in an exceptional intensity that reaches the nose quickly and a minor loss of the fragrance’s “volume”. The scenario is very similar to the fragrance “Seta e Ciliegia”. Also, in this case, the presence of the peaks of dimethicone favours the evaporation of the note. The decrease in the “volume” of the composition is confirmed by the decrease in the area % for most of the molecules (with the exception of coumarin), with a great effect for the most abundant: α-pinene (area % 0.16), d-limonene (area % 5.42), linalool (area % 6.14), linalyl acetate (area % 2.05), verdox (area % 6.04), vertenex (area % 0.63), α-guaiene (area % 0.46), seychellene (area % 0.32), amberonne (isomer 2) (area % 7.93), and amberonne (isomer 3) (area % 1.46).
In the blend with IPM, the perfumer could appreciate a maintained olfactory profile, but with a slight loss of the volume and a slight hint of the solvent, that covers the amber and woody scents of the composition. In fact, the chromatogram confirmed the presence of the isopropyl-myristate peak, typical of the base. The reduction in the “volume” is confirmed by the decrease of the area % of most of the molecules, with an important impact for the most important peaks (with the exception of coumarin): α-pinene (area % 0.19), d-limonene (area % 4.78), linalool (area % 3.32), linalyl acetate (area % 0.92), verdox (area % 4.97), vertenex (area % 0.36), α-guaiene (area % 0.03), seychellene (area % 0.05), amberonne (isomer 2) (area % 2.28), and amberonne (isomer 3) (area % 0.30). The reduction in the perception of the perfumer of amber and woody scents is confirmed by the loss or the reduction of most of the molecules at the base of the olfactory pyramid, with amber and woody notes: 3-phenylpropanol [27], cinnamic alcohol, vertenex, α-terpinyl acetate, β-patchoulene, β-damascone, vanillin, caryophyllene, α-guaiene, seychellene, α-patchoulene, γ-patchouelene, cashmeran, δ-guaiene, amberonne (isomers 1,2,3), galaxolide and tonalid [7].
In the blend with BGLY the note maintains a good intensity and an unchanged olfactory profile, with a perfect balance between the woody component and the gourmand one. This is confirmed by the areas % of most of the compounds, similar to those in the pure fragrance (Table 5). The situation is similar to the one with the blend with GLY. The highest percentages of the molecules giving a woody note to the fragrance are for α-pinene (area % 1.13), verdox (area % 8.04), α-guaiene (area % 2.43), α-patchoulene (area % 1.34), linalyl acetate (area % 3.40), verdox (area % 8.04), vertenex (area % 0.91), α-guaiene (area % 2.43), α-patchoulene (area % 1.34), amberonne isomer 2 (area % 9.71), amberonne isomer 3 (area % 2.00) and galaxolide (area % 1.74). d-limonene (area % 16.90) and linalyl acetate (area % 3.40) confer a perfect balance with the woody notes [7].
4. Discussion
The purpose of this study was to demonstrate and understand why a cosmetic product, either lipophilic or hydrophilic, can influence the “volatility” of the fragrance and, consequently, its capacity to be “released” and express its olfactory performance. To verify our purpose, we started by focusing on the affinities between the compounds of both fragrances and bases under study, and a relationship based on the polarity can be highlighted. In both of the fragrances used in this work, we found some similarities in the retention of the molecules between the bases with the same characteristics of polarity. The characteristics of the olfactory profiles of the blends resulted similar between GLY and BGLY which are polar, between PFF and DMT, with non-polar characteristics and between TCC and IPM with medium polarity. This is confirmed by the chromatograms and the behaviour of the compounds, which is very similar between GLY and BGLY, PFF and DMT, and TCC and IPM (Table 1 and Table 5).
The polarity of the bases determines how the compounds are retained, both for odorous and odourless compounds, and how the odorous molecules are included influences the final aroma of the mix. The distinction is obvious, particularly amongst bases with different polarities, such as PFF and DMT and GLY and BGLY. For compounds with intermediate chemical properties, the distinction is less obvious (with a non-polar structure but with some polar groups). The bases with middle characteristics retain most molecules.
For the fragrance “Seta & Ciliegia”, most of the molecules are non-polar and they are retained mostly by PFF and DMT. This means that the highest areas % of the peaks are given when the blend is made with polar bases, GLY and BGLY. The non-polar compounds are butyric acid 2-methyl ethyl ester, α-pinene, ethyl 2-methylpentanoate, β-pinene, β-myrcene, cyclopentene 1,3-dimethyl-2-(-1-methylethyl), 3-hexen-1-ol, acetate (z), hexyl acetate, 4-hexen-1-ol, acetate, d-limonene, eucalyptol, isoamyl butyrate, γ-terpinene, allyl hexanoate, isoterpinolene, verdone, hexyl isobutyrate, citronellal, 6,6-dimethoxy-2,5,5-trimethylhex-2-ene, 1,2,3-benzothiadiazole, α-linalool, linalyl acetate, verdox, vertenex, 1H-inden-1-one,2,3-dihydro-3,4,7-trimethyl, geranyl acetate, allyl cyclohexanepropionate, butanoic acid, 1,1,-dimethyl-2-phenylethyl ester, amberonne and hexyl salicylate.
Molecules with polar groups react in different way; they are largely retained from GLY and BGLY; thus the maximum areas percentages are provided by the peaks in blends with PFF and DMT. Dimethyl silanediol, e-methoxy-3-methyl butanol, dipropylene glycol (s,p), dipropylene glycol (p,p), dihydromycenol, 10-undecenal, and piperonal are examples. Some compounds, such as ligustral, tetrahydrolinalool, 3,4-dimethyl-3-cyclohexene-1-carboxaldehyde, dihydrocitronellal, ethyl linalool, p-methylbenzyl acetate, decanal, and p-methan-7-ol, trans, show intermediate behaviour. The behaviour of molecules with intermediate polarity is less predictable.
The situation is more evident for the fragrance “Narguilé”, which contained more polar molecules or groups, such as carbohydrazide, dipropylene glycole, linalool, phenylethyl alcohol, ethyl maltol, 3-phenylpropanol, cinnamaldehyde, cinnamic alcohol, vertenex, vanillin, coumarin and diethyl phthalate. These molecules are retained by GLY and BGLY, and the peaks with the highest areas % are present in the chromatograms of the blends with PFF and DMT paraffin and dimethicone. Non-polar molecules, such as α-pinene, camphene, β-pinene, β-myrcene, α-phellandrene, p-cymene, d-limonene, γ-terpinene, heptanoic acid ethyl ester, linalyl acetate, verdox, β-patchoulene, caryophyllene, α-guaiene, α-patchouelene, 10s, 11s-himachala-3(12),4-diene, γ-patchoulene, cashmeran, δ-guaiene, amberonne, ethanone, 1-(1,6-dimethyl-4-(4-methyl-3-pentenyl)-3-cyclohexen-1-yl)- and galaxolide, showed an opposite situation. For the molecules with an intermediate polarity, the behaviour is not easily explained.
GLY and BGLY produce the bases in which the fragrances can be fully appreciated, possibly due to the strong polarity that permits the release of the majority of the fragrance’s compounds. Oily fragrances consist mainly of non-polar molecules, which allows them to preserve their olfactory profile in bases with intermediate polarity.
5. Conclusions
The aim of this study was to provide for the first time an explanation of the olfactory performance of fragrances when mixed with the most used common cosmetic bases in binary blends. Through our investigation, we have achieved successfully our purpose of delivering formulators with valuable and practical guidance when operating with fragrance in a cosmetic formulation. We could assess that the lipophilicity of the formulation influences the olfactory performance of a fragrance in a blend. The SPME-GC/MS, mimicking the nose perception, confirms the evaluation made by the experienced perfumer on the olfactory characteristics of each blend with the respect to the reference pure fragrance. The following is a relatively basic and universal rule: lipophilic chemicals are less readily retained by less lipophilic bases, allowing for the preservation of the original fragrance’s olfactory signature. Although this rule cannot be implemented “precisely,” one may deduce a relatively general and practical rule: choose more lipophilic compounds for more hydrophilic bases and select more hydrophilic substances for more lipophilic bases.
Author Contributions
Conceptualization, P.D.M. and M.R.G.; methodology, P.D.M., M.R.G.; L.D.N., S.A., L.I., D.V.P.; software, L.D.N., S.A. and M.R.G.; validation, P.D.M., M.R.G., L.I., S.A. and L.D.N.; formal analysis, L.D.N., S.A. and L.I.; investigation, P.D.M., M.R.G. and L.D.N.; resources. P.D.M. and R.C.; data curation, P.D.M., M.R.G., L.D.N. and L.I.; writing—original draft preparation, L.D.N.; writing—review and editing, P.D.M., M.R.G., L.D.N.; visualization, P.D.M., M.R.G., L.D.N.; supervision, P.D.M., R.C. and M.R.G.; project administration, P.D.M.; funding acquisition, P.D.M. and M.R.G. Authorship must be limited to those who have contributed substantially to work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska Curie grant agreement No. 777682 (CANCER), 734684 (CHARMED), 872391 (CONCRETE), 872860 (PRISAR2), 807281 (ACORN), 852985 (SIMICA), 952520 (BIOSAFETY), 101029908 (GELNANODEP).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herz, R.S. Aromatherapy facts and fictions: A scientific analysis of olfactory effects on mood, physiology and behavior. Int. J. Neurosci. 2009, 119, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Warrenburg, S. Effects of fragrance on emotions: Moods and physiology. Chem. Senses 2005, 30 (Suppl. S1), i248–i249. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Cotton, S. Every Molecules Tells a Story; Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Teixeira, M.A.; Rodriguez, O.; Gomes, P.; Mata, V.; Rodrigues, A. Perfume Engineering: Design, Performance and Classification; Butterwoth-Heinemann Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Brown, A.; Mettetal, A.; Hettiarachchi, D. Sandalwood—Perfumery. In Indian Sandalwood: A Compendium; Arunkumar, A.N., Joshi, G., Warrier, R.R., Karaba, N.N., Eds.; Springer Nature: Singapore, 2022; pp. 449–461. [Google Scholar]
- Anonis, D.P. Material Review Woody Notes in Perfumery—Vetiver and Derivatives. Part I. Perfum. Flavorist 2004, 29, 30–36. [Google Scholar]
- Richardson, A. Measurement of Fragrance Perception; RSC Publishing: London, UK, 1999. [Google Scholar]
- Zarzo, M. What is a Fresh Scent in Perfumery? Perceptual Freshness is Correlated with Substantivity. Sensors 2013, 13, 463–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, F.H.; Timmons, M.J.; Tallitsch, R.B. Anatomia Umana; EdiSES s.r.l.: Naples, Italy, 2008. [Google Scholar]
- Stora, T.; Escher, S.; Morris, A. The physicochemical basis of perfume performance in consumer products. Chimia 2001, 55, 406–412. [Google Scholar]
- Chisvert, A.; Lopez-Nogueroles, M.; Salvador, A. Essential oils: Analytical methods to control the quality of perfumes. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Villatoroa, C.; Vera, L.; Gygaxb, H. Comparative study of odours present in twin fragrances by GC-sniffing-ToFMS. Chem. Eng. 2016, 54, 133–138. [Google Scholar]
- Mata, V.; Gomes, P.; Rodrigues, A. Perfumery Ternary Diagrams (PTD): A new concept applied to the optimization of perfume compositions. Flavour Fragr. J. 2005, 20, 465–471. [Google Scholar] [CrossRef]
- Robert, R.; Calkin, J.S.J. Perfumery: Practice and Principles; Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Cohen-Letessier, A.; Mac-Mary, S.; Sainthillier, J.-M.; Humbert, P. Cosmetici maschili. EMC-Cosmetol. Med. E Med. Degli Inestetismi Cutanei 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Parmentier, M. Chemical composition and olfactory characterization of essential oils of fruits and seeds of African pear (Dacryodes edulis (G. Don) H. J. Lam) from Cameroon. Flavour Fragr. J. 2005, 20, 215–218. [Google Scholar] [CrossRef]
- Amenduni, A.; Massari, F.; Palmisani, J.; de Gennaro, G.; Brattoli, M.; Tutino, M. Chemical characterization of odor active volatile organic compounds emitted from perfumes by gc/ms-o. Environ. Eng. Manag. J. (EEMJ) 2016, 15, 1963–1969. [Google Scholar]
- Kvittingen, L.; Sjursnes, B.J.; Schmid, R. Limonene in Citrus: A String of Unchecked Literature Citings? J. Chem. Educ. 2021, 98, 3600–3607. [Google Scholar] [CrossRef]
- Frasnelli, J.; Albrecht, J.; Bryant, B.; Lundström, J. Perception of specific trigeminal chemosensory agonists. Neuroscience 2011, 189, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, R.; Ranjta, S.; Varadwaj, P.K. SMILES to Smell: Decoding the Structure–Odor Relationship of Chemical Compounds Using the Deep Neural Network Approach. J. Chem. Inf. Model. 2021, 61, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Junior, S.B.; Março, P.H.; Valderrama, P.; Damasceno, F.C.; Aranda, M.S.; Zini, C.A.; Caramão, E.B.; Melo, A.M.T.; Teixiera Filho, J.; Godoy, H.T. Analysis of volatile compounds in Capsicum spp. by headspace solid-phase microextraction and GC× GC-TOFMS. Anal. Methods 2015, 7, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Aldehydic, F.; Brechbill, G.O. Arranging Fine Perfume Compositions; Fragrance Books Inc.: Rahway, NJ, USA, 2006. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Stoyanova, A.S.; Georgiev, E.V.; Damianova, S.T. Composition, quality control and antimicrobial activity of the essential oil of cumin (Cuminum cyminum L.) seeds from Bulgaria that had been stored for up to 36° years. Int. J. Food Sci. Technol. 2005, 40, 305–310. [Google Scholar] [CrossRef]
- Jain, P.L.B.; Patel, S.R.; Desai, M.A. Patchouli oil: An overview on extraction method, composition and biological activities. J. Essent. Oil Res. 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vázquez, C. Volatile Flavor Constituents of Karanda (Carissa carandas L.) Fruit. J. Essent. Oil Res. 2004, 16, 432–434. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).