Pickering Emulsions: A Novel Tool for Cosmetic Formulators
Abstract
:1. Introduction
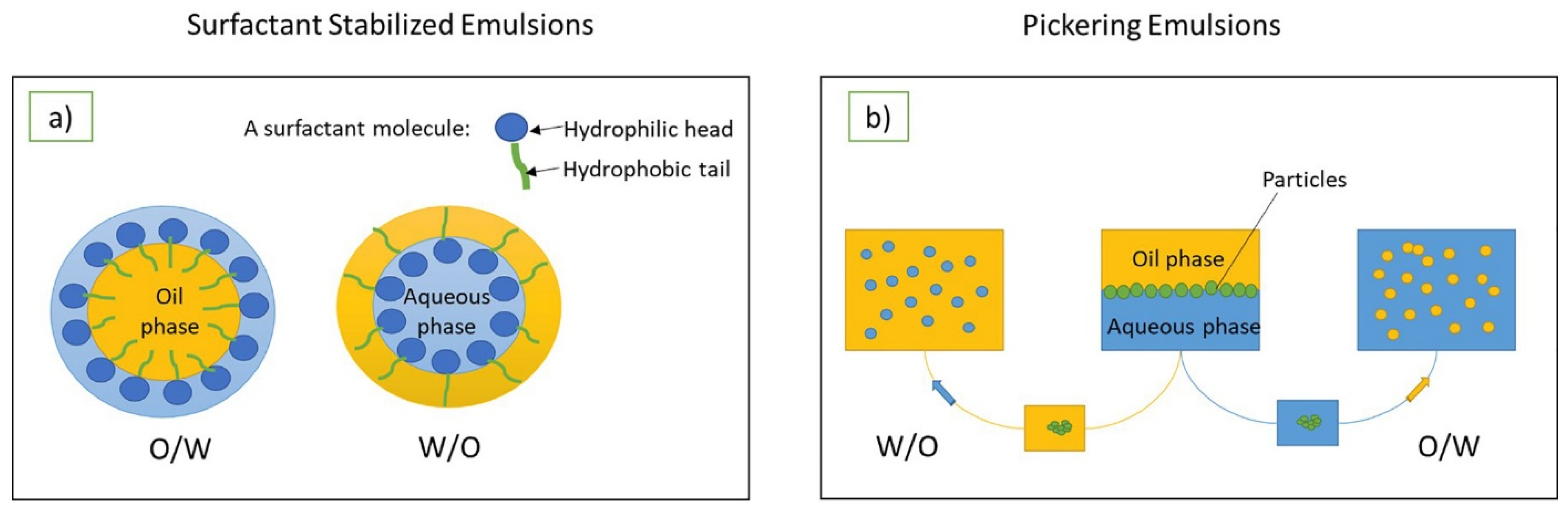
2. General Aspects of Pickering Emulsions
2.1. Preparation of Pickering Emulsions
2.1.1. Rotor-Stator Homogenization
2.1.2. High-Pressure Homogenization
2.1.3. Ultrasound-Assisted Emulsification
2.1.4. Membrane Emulsification
2.1.5. Microfluidic Technologies
2.2. Understanding the Stabilization of Emulsions by Colloidal Particles
2.3. Rheology of Pickering Emulsions
2.4. Destabilization Mechanisms of Emulsions
3. Particles in Cosmetic Pickering Emulsions
- Polymer particles of different nature (both natural and synthetic) are currently exploited mainly in skin care products, including anti-aging and moisturizing creams. For instance, chitosan particles, a copolymer of D-glucosamine and N-acetyl-D-glucosamine, are widely used due to their natural abundance, biocompatibility and biodegradability. On the other side, hyaluronic acid is also very common in the cosmetics industry due to its anti-wrinkle properties [56].
- Silver nanoparticles are very common in cosmetic products due to their antimicrobial and antifungal properties. In fact, silver nanoparticles are common in toothpastes, creams, soaps, lotions or deodorants [57].
- Gold nanoparticles are used to help with the delivery of different types of molecules through the skin. Their penetration into the stratum corneum shows a strong dependence on its physico-chemical properties, e.g., shape, size and chemical surface, and compatibility with the lipid domains existing within the skin. Moreover, the use of gold particles in cosmetics is gaining interest due to their antioxidant and antimicrobial properties, contributing to improvements in skin firmness and elasticity. This has pushed their incorporation into anti-aging creams, lotions and deodorants [58].
- Titanium oxide and zinc oxide nanoparticles are commonly incorporated in cosmetic products for providing UV filter properties due to their capacity for reflecting the UVB and UVA radiations, respectively. In fact, the combination of both oxides provides good protection against sun radiation, allowing to prepare transparent products with good spreadability and texture. Moreover, they reduce the skin irritation associated with most of the chemical UV filters. The mechanisms of these types of particles for providing UV protection are related to their ability to be deposited on the external surface of the stratum corneum [59].
- Silica nanoparticles, commonly in the diameter range of 5–100 nm, have received interest in the cosmetic industry mainly as a result of some of their specific characteristics, e.g., their pleasant sensorial properties and their capacity for delivery of lipophilic and lipophobic compounds. Furthermore, this type of material can be easily obtained in large amounts using low-cost processes and can be chemically modified to obtain specific characteristics. This has stimulated the use of silica nanoparticles in toothpastes, makeup products, hair styling, deodorants and skin care. On the other hand, silica nanoparticles can be used as emulsifiers, emollients and water barriers, having an adjuvant effect in sun protection products because they contribute to the enhancement of their spreadability, reducing the degradation of the products [60].
4. Pickering Emulsions in Cosmetics
5. Trends and Challenges toward the Application of Pickering Emulsions in the Cosmetics Industry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Llamas, S.; Santini, E.; Liggieri, L.; Salerni, F.; Orsi, D.; Cristofolini, L.; Ravera, F. Adsorption of Sodium Dodecyl Sulfate at Water–Dodecane Interface in Relation to the Oil in Water Emulsion Properties. Langmuir 2018, 34, 5978–5989. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M.; Oh, H.M.; Lee, J.H. Controlling the emulsion stability of cosmetics through shear mixing process. Korea Aust. Rheol. J. 2020, 32, 243–249. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Hayase, M. Introduction to Cosmetic Materials. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–154. [Google Scholar]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Rossi, L.; Bouvier, M.; Ortega, F.; Rubio, R.G. Nanoemulsions for the Encapsulation of Hydrophobic Actives. Cosmetics 2021, 8, 45. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Mojahid, B.Z.E.; Guzmán, E.; Ortega, F.; Rubio, R.G. Performance of Oleic Acid and Soybean Oil in the Preparation of Oil-in-Water Microemulsions for Encapsulating a Highly Hydrophobic Molecule. Colloids Interfaces 2021, 5, 50. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Gutiérrez-Muro, S.; Guzmán, E.; Lucia, A.; Ortega, F.; Rubio, R.G. Oil-In-Water Microemulsions for Thymol Solubilization. Colloids Interfaces 2019, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Iqbal, H.M.N. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ramsdem, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.U. CXCVI—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- González-González, A.; Sánchez-Arribas, N.; Santini, E.; Rodríguez-Villafuerte, J.L.; Carbone, C.; Ravera, F.; Ortega, F.; Liggieri, L.; Rubio, R.G.; Guzmán, E. Effects of Oil Phase on the Inversion of Pickering Emulsions Stabilized by Palmitic Acid Decorated Silica Nanoparticles. Colloids Interfaces 2022, 6, 27. [Google Scholar] [CrossRef]
- Terescenco, D.; Hucher, N.; Picard, C.; Savary, G. Sensory perception of textural properties of cosmetic Pickering emulsions. Int. J. Cosmet. Sci. 2020, 42, 198–207. [Google Scholar] [CrossRef]
- Peito, S.; Peixoto, D.; Ferreira-Faria, I.; Martins, A.M.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nano- and microparticle-stabilized Pickering emulsions designed for topical therapeutics and cosmetic applications. Int. J. Pharm. 2022, 615, 121455. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Rel. 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Manga, M.S.; Cayre, O.J.; Williams, R.A.; Biggsa, S.; York, D.W. Production of solid-stabilised emulsions through rotational membrane emulsification: Influence of particle adsorption kinetics. Soft Matter 2012, 8, 1532–1538. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Z.; Ma, M.; Chao, Y.; Gao, Y.; Kong, T. Control of Particle Adsorption for Stability of Pickering Emulsions in Microfluidics. Small 2018, 14, 1802902. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Calabrese, V.; Courtenay, J.C.; Edler, K.J.; Scott, J.L. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr. Opin. Green Sustain. Chem. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Xia, T.; Xuea, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Guzmán, E.; Martínez-Pedrero, F.; Calero, C.; Maestro, A.; Ortega, F.; Rubio, R.G. A broad perspective to particle-laden fluid interfaces systems: From chemically homogeneous particles to active colloids. Adv. Colloid Interface Sci. 2022, 302, 102620. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Chen, Y.; Li, J.; Wang, L.; Li, C. A review of multiple Pickering emulsions: Solid stabilization, preparation, particle effect, and application. Chem. Eng. Sci. 2022, 248, 117085. [Google Scholar] [CrossRef]
- Binks, B.P. Colloidal Particles at a Range of Fluid–Fluid Interfaces. Langmuir 2017, 33, 6947–6963. [Google Scholar] [CrossRef]
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering Emulsions for Sustainable Micro/Nanocellulose in Food and Cosmetic Applications. Polymers 2020, 12, 2385. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Maestro, A.; Guzman, E.; Ortega, F.; Rubio, R.G. Contact angle of micro- and nanoparticles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 355–367. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Development of high internal phase Pickering emulsions stabilised by ovotransferrin–gum Arabic particles as curcumin delivery vehicle. Int. J. Food Sci. Technol. 2019, 55, 1891–1899. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A. Soft Colloidal Particles at Fluid Interfaces. Polymers 2022, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Ridel, L.; Bolzinger, M.-A.; Gilon-Delepine, N.; Dugas, P.-Y.; Chevalier, Y. Pickering emulsions stabilized by charged nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A review of the rheological properties of dilute and concentrated food emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar] [CrossRef]
- Yuan, D.B.; Hu, Y.Q.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Development of stable Pickering emulsions/oil powders and Pickering HIPEs stabilized by gliadin/chitosan complex particles. Food Funct. 2017, 8, 2220–2230. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydrate Polym. 2020, 250, 116885. [Google Scholar] [CrossRef]
- Mason, T.G.; Bibette, J.; Weitz, D.A. Elasticity of Compressed Emulsions. Phys. Rev. Lett. 1995, 75, 2051. [Google Scholar] [CrossRef] [Green Version]
- Arditty, S.; Schmitt, V.; Giermanska-Kahn, J.; Leal-Calderon, F. Materials based on solid-stabilized emulsions. J. Colloid Interface Sci. 2004, 275, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Chan, H.K.; Mohraz, A. Characteristics of Pickering Emulsion Gels Formed by Droplet Bridging. Langmuir 2012, 28, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Nunes, A.; Martins, A.M.; Carvalheira, J.; Prazeres, P.; Gonçalves, L.; Marques, A.; Lucas, A.; Ribeiro, H.M. Pickering Emulsions Stabilized by Calcium Carbonate Particles: A New Topical Formulation. Cosmetics 2020, 7, 62. [Google Scholar] [CrossRef]
- Simon, S.; Theiler, S.; Knudsen, A.; Øye, G.; Sjöblom, J. Rheological Properties of Particle-Stabilized Emulsions. J. Dispers. Sci. Technol. 2010, 31, 632–640. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Santini, E.; Guzmán, E.; Ferrari, M.; Liggieri, L. Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water–hexane interface. Colloids Surf. A 2014, 460, 333–341. [Google Scholar] [CrossRef]
- Santini, E.; Guzmán, E.; Ravera, F.; Ferrari, M.; Liggieri, L. Properties and structure of interfacial layers formed by hydrophilic silica dispersions and palmitic acid. Phys. Chem. Chem. Phys. 2012, 14, 607–615. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E. Colloids at Fluid Interfaces. Processes 2019, 7, 942. [Google Scholar] [CrossRef] [Green Version]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Akanno, A.; Fernández-Peña, L.; Ortega, F.; Campbell, R.A.; Miller, R.; Rubio, R.G. Study of the Liquid/Vapor Interfacial Properties of Concentrated Polyelectrolyte–Surfactant Mixtures Using Surface Tensiometry and Neutron Reflectometry: Equilibrium, Adsorption Kinetics, and Dilational Rheology. J. Phys. Chem. C 2018, 122, 4419–4427. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Guzmán, E. Physico-chemical foundations of particle-laden fluid interfaces. Eur. Phs. J. E 2018, 41, 97. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Ascenso, A.; Simoes, S.; Almeida, A.J.; Ribeiro, H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert Opin. Drug Deliv. 2016, 13, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Koyama, J.; Ozawa, T. New Aspects of Cosmetics and Cosmetic Science. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–100. [Google Scholar]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly(vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Chewastowski, J.; Siudek, M.; Banach, M. Incorporation of Metallic Nanoparticles into Cosmetic Preparations and Assessment of Their Physicochemical and Utility Properties. J. Surfactants Deterg. 2018, 21, 575–591. [Google Scholar] [CrossRef]
- Jiménez-Pérez, Z.E.; Singh, P.; Kim, Y.-J.; Mathiyalagan, R.; Kim, D.-H.; Lee, M.H.; Yang, D.C. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng. Res. 2018, 42, 327–333. [Google Scholar] [CrossRef]
- Lu, P.J.; Fang, S.W.; Cheng, W.L.; Huang, S.C.; Huang, M.C.; Cheng, H.F. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J. Food Drug Anal. 2018, 26, 1192–1200. [Google Scholar] [CrossRef]
- Nafisi, S.; Schafer-Korting, M.; Maibach, H.I. Perspectives on percutaneous penetration: Silica nanoparticles. Nanotoxicology 2015, 9, 643–657. [Google Scholar] [CrossRef]
- Guo, Q. Progress in the preparation, stability and functional applications of Pickering emulsion. IOP Conf. Ser. Earth Environ. Sci. 2021, 639, 012028. [Google Scholar] [CrossRef]
- Wu, F.; Deng, J.; Hu, L.; Zhang, Z.; Jiang, H.; Li, Y.; Yi, Z.; Ngai, T. Investigation of the stability in Pickering emulsions preparation with commercial cosmetic ingredients. Colloids Surf. A 2020, 602, 125082. [Google Scholar] [CrossRef]
- Marku, D.; Wahlgren, M.; Rayner, M.; Sjöö, M.; Timgren, A. Characterization of starch Pickering emulsions for potential applications in topical formulations. Int. J. Pharm. 2012, 428, 1–7. [Google Scholar] [CrossRef]
- Ali, A.; Yilmaz, E.; Sjöö, M. A Novel Technology for Personal Care Emulsions. SOFW J. 2015, 141, 11–15. [Google Scholar]
- Wei, Y.-S.; Niu, Z.-C.; Wang, F.-Q.; Feng, K.; Zong, M.-H.; Wu, H. A novel Pickering emulsion system as the carrier of tocopheryl acetate for its application in cosmetics. Mat. Sci. Eng. C 2020, 109, 110503. [Google Scholar] [CrossRef] [PubMed]
- Sharkawy, A.; .Casimiro, F.M.; Barreiro, M.F.; Rodrigues, A.E. Enhancing trans-resveratrol topical delivery and photostability through entrapment in chitosan/gum Arabic Pickering emulsions. Int. J. Biol. Macromol. 2020, 147, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Preparation of chitosan/gum Arabic nanoparticles and their use as novel stabilizers in oil/water Pickering emulsions. Carbohydr. Polym. 2019, 224, 115190. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, F.; Ugarte, C.; Günther, G.; Larraín, M.A.; Guarnizo-Herrero, V.; Nonell, S.; Morales, J. Carminic Acid Linked to Silica Nanoparticles as Pigment/Antioxidant Bifunctional Excipient for Pharmaceutical Emulsions. Pharmaceutics 2020, 12, 376. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. New Pickering emulsions stabilized with chitosan/collagen peptides nanoparticles: Synthesis, characterization and tracking of the nanoparticles after skin application. Colloids Surf. A 2021, 616, 126327. [Google Scholar] [CrossRef]
- Simovic, S.; Ghouchi-Eskandar, N.; Prestidge, C.A. Pickering emulsions for dermal delivery. J. Drug. Deliv. Sci. Tec. 2011, 21, 123–133. [Google Scholar] [CrossRef]
- Hougeir, F.G.; Kircik, L. A review of delivery systems in cosmetics. Dermatol. Ther. 2012, 25, 234–237. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, S.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. A novel cholesterol-free mayonnaise made from pickering emulsion stabilized by apple pomace particles. Food Chem. 2021, 353, 129418. [Google Scholar] [CrossRef] [PubMed]


| Type | Particles | Oil Phase | Application | References |
|---|---|---|---|---|
| O/W | quinoa starch | Miglyol paraffin sheanut oil | skin care | Marku et al. [63] |
| O/W | PLGA/PSS | medium chain triglyceride | skin care (tocopheryl acetate delivery) | Wei et al. [65] |
| O/W | chitosan/gum arabic | olive oil | skin care (trans-resveratrol delivery) | Sharkawy et al. [66,67] |
| O/W | core-mesoporous shell silica particles | oil mixture (mineral oil and castor oil) | skin care (vitamin E delivery) | Arriagada et al. [68] |
| O/W | chitosan/collagen | olive oil | skin care | Sharkawy et al. [69] |
| O/W | calcium carbonate | caprylic/capric acid triglyceride | skin care | Marto et al. [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics 2022, 9, 68. https://doi.org/10.3390/cosmetics9040068
Guzmán E, Ortega F, Rubio RG. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics. 2022; 9(4):68. https://doi.org/10.3390/cosmetics9040068
Chicago/Turabian StyleGuzmán, Eduardo, Francisco Ortega, and Ramón G. Rubio. 2022. "Pickering Emulsions: A Novel Tool for Cosmetic Formulators" Cosmetics 9, no. 4: 68. https://doi.org/10.3390/cosmetics9040068
APA StyleGuzmán, E., Ortega, F., & Rubio, R. G. (2022). Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics, 9(4), 68. https://doi.org/10.3390/cosmetics9040068








