Abstract
People with self-reported sensitive skin may reluctantly use performing anti-ageing skin care products as it could elicit skin discomfort. We thus aimed to design and test an anti-ageing skin care routine that is suitable for people reporting sensitive skin. Key principles for developing products for sensitive skin were applied and formulas were screened for their mildness in vitro using the Reconstructed Human Epidermis ET50 method. Anti-ageing efficacy and mildness was evaluated during a clinical study in China, with 33 female volunteers aged 40–65 years, with sensitive skin. The anti-ageing benefits were measured using Primos 3D, the cutometer and clinical evaluation. Hallmarks for sensitive skin such as skin hydration, skin barrier, skin redness and response to lactic acid were also measured. The ET50 method yielded values suggesting moderate to mild expected irritancy effect in vivo for most of them, and non-irritating effect for the serum. During the clinical study, no physical or functional signs of discomfort were reported with twice-daily usage of the routine. Instrumental evaluation of Wrinkle depth, skin elasticity/firmness, skin hydration, skin barrier and skin redness revealed improvement at 4 and 8 weeks. Clinical evaluation evidenced skin smoothness, skin suppleness and radiance improvements. The skin was less reactive to lactic acid stimuli, while the sensitive skin burden was lowered according to the dermatological quality of life index. Lastly, a separate investigation suggested the potential relief aspect of such routines to alleviate discomforts from mask wearing. With the right formulation design, the benefits of layering products from a routine can be made accessible to people with sensitive skin while simultaneously alleviating the burden of sensitive skin.
1. Introduction
Prevalence of sensitive skin worldwide is said to have steadily increased over time. A review of the prevalence of sensitive skin communicated figures of ≈60–70% among women and ≈50–60% among men [1]. A recent large-scale survey conducted on 10,743 volunteers from Brazil, China, France Russia, and the United States, with an even distribution of men and women, reported a rate of 48.2% of self-reported sensitive skin [2].
Skin barrier defect is a frequently cited attribute of sensitive skin [3], notably as a more permeable skin would be more prone to react to external stimuli. Levels of inflammatory biomarkers such as cytokines, proinflammatory PUFAs and prostaglandin E2 are hallmarks of sensitive skin [4,5]. There is nowadays a greater emphasis on the skin’s nociception capabilities, or transduction of noxious stimuli to nerve fibre free endings located in the epidermis, to explain the pathophysiology of sensitive skin. The superfamily of Transient Receptor Protein (TRP), expressed in both neuronal cells and keratinocytes, can be elicited by stimuli of chemical or physical nature, to signal pain and itching. Vanilloid and Ankyrin families of TRP have been the foci of various investigations, notably TRPV1 and TRPA1 [6,7]. Descriptions of perceived discomfort may vary across ethnic groups as shown previously, with Caucasians relying more on visual cues (e.g., redness, swelling) than pain descriptors (e.g., stinging, burning), compared to Asians or African Americans [1].
Skin discomfort in response to climatic and environmental factors have been previously highlighted in a Korean population [8]. Pollution was reported as a key factor in another study among 286 Mexican women, particularly in the cohort based in Mexico City [9]. Temperature shift, sun exposure and stress have been cited in multiple countries. Moreover, newer insights from a lifestyle perspective have been uncovered, with greater instances of sleep disorders, fatigue, sweating or dust and food or tobacco consumption in people with sensitive skin, over people that do not report sensitive skin. A hormonal component was also suggested from these results, with a higher incidence of sensitive skin among pregnant women, women with painful menstruation or those using contraceptive pills [2].
Thus, skin sensitivity has become a dynamic skin care segment with targeted solutions. Products aimed at alleviating this burden can be found on the market and in the literature, with, for instance, a facial gel containing niacinamide, Hamamelis virginiana (witch hazel) and Avena sativa (oat) kernel extract, albeit without anti-ageing endpoints in vivo [10]. A recent review has compiled a thorough list of technologies addressing sensitive skin, launched over the last few years. Mentioned among the recurring mechanisms were: blockage of TRPV1 receptors, targeting key inflammatory pathways (NF-κB), modulation of inflammatory biomarker levels such as IL-1α/IL-1-/IL-8/CGRP/PEG2, NGF reduction, substance P reduction, inhibition of mast cells degranulation and binding to µ-opioid receptors [11]. We previously disclosed data on the cumulative anti-ageing effect of using a comprehensive skin care regimen in comparison to a simpler daily routine (e.g., cleanser and day cream only) [12]. Because extensive skin care routines may be met with reticence by people with reactive skin, we aimed at developing a 4-step routine that combines anti-ageing performance, desirable tolerability and ability to improve the burden of sensitive skin. In the present article, we disclose the approach to designing anti-ageing skin care routines suitable for sensitive skin from a formulation, safety and clinical efficacy standpoint.
2. Materials and Methods
2.1. Formulation Approach and Actives Selection
2.1.1. Formulation Approach
According to Draelos Z.D. [13], cosmetic and skincare product formulation considerations for sensitive skin include products with a paucity of ingredients, absence of common sensitizers, minimum number of irritants, and absence of cutaneous sensory or vasodilatory stimulants. Therefore, the selection of ingredients was key, and the following aspects were considered when designing the skincare range disclosed in this article:
- (A)
- Usage of ingredients that strengthen the skin barrier. As sensitive skin has characteristics of barrier function impairment [14], lipid layer enhancers, natural butters and oils were used across the formulations to protect and strengthen the skin barrier. Naturally occurring ingredients such as squalene and glyceryl oleate were used as a strategy to maintain the integrity and healthy appearance of the skin. Skin moisturising ingredients alleviate itchiness, dryness, and scaling while anti-inflammatory ingredients further attenuate skin redness and discomfort, thus leading to an improved consumer tolerance.
- (B)
- Optimization of the number and concentration of ingredients. The choice of good multifunctional ingredients allows for more benefits with fewer ingredients. An example is how those formulas were preserved, with ingredients that not only provide antimicrobial properties but other functions such as moisturisation and soothing e.g., Hydroxyacetophenone [15]. Reduction of number and concentration of ingredients is also a strategy to deliver a minimalist approach. It is common knowledge that decreasing the number of ingredients in a formula will decrease the irritancy potential [16]. This concept won’t be applied for certain formula types, such as oil-based formulas, in which decreasing one raw material means increasing the main carrier oil. In such a case, the strategy would be the diversification of oils used.
- (C)
- The choice of the packaging also played an important role in the formula design. The usage of airless packs, helping to prevent microbial contamination [15], allowed the usage of the above mentioned multi-functional ingredients to preserve the formula. In addition, an auto-foaming pump was used for the cleanser, promoting the creation of dense and airy foam, while using a low concentration of mild surfactants.
- (D)
- Absence of ingredients known as irritants or that can affect the skin barrier were avoided to allow better performance of the final formulation [10,17]. It is of paramount importance that products designed for use on sensitive skin minimise exacerbation of the symptoms associated with this condition. Therefore, this range is free of alcohol, formaldehyde releasers, phenoxyethanol, SLES, fragrances and essential oils.
- (E)
- The natural-acidic pH of skin was targeted in all formulas [16].
2.1.2. Actives Selection
The desired efficacy of the range was twofold: (1) addressing the typical skin concerns associated with skin sensitivity, and (2) delivering anti-ageing performance, which ewrequired the selection of active ingredients that could independently target both concerns (i.e., skin sensitivity and ageing concerns). In this requirement, there was a very interesting dichotomy: it would require delivering both an efficacious anti-ageing regime that could, at the same time, not only respect the nature of sensitive skin, but also actively improve skin concerns associated with sensitive skin. The search included the review of well-known skin soothing functional ingredients such as Panthenol, Allantoin, Bisabolol, as well as lesser-known actives claiming benefits for sensitive skin such as Phaeodactylum tricornutum extract, and those seemingly gaining popularity in the industry such as Centella asiatica. From an anti-ageing active perspective, the focus was placed on reviewing actives that claimed retinol-like performance, such as Vigna aconitifolia, Cichorium intybus, and Nephelium lappaceum, and that also demonstrated good skin tolerability. Actives selection for both cases of sensitive skin and anti-ageing efficacies was based on criteria that included: (1) formulability; (2) in vitro/ex vivo data; (3) in vivo data, and (4) claims and concept fit.
The active Ophiopogon japonicus root extract was selected to target typical skin concerns associated with skin sensitivity. Ophiopogon japonicus root extract is a purified natural active ingredient (obtained from the roots of Ophiopogon japonicus) and displays the following activities [18]:
- Strengthening the skin barrier by activating epidermal differentiation and cohesion and by optimising the organisation and conformation of lipids.
- Restoring (Natural Moisturising Factors) NMFs and the water ratio in the skin, thereby improving skin hydration
- Re-equilibrating the cutaneous microbiota, by limiting the adhesion of bacteria (namely Staphylococcus aureus) on the skin.
- Inactivating inflammatory responses by reducing the synthesis of allergens
- Neuronal hyperactivity management by inhibiting the activation of TRPA1.
The active Nephelium lappaceum leaf extract was selected as the anti-ageing active in the range. Nephelium lappaceum leaf extract is a natural active ingredient obtained from a lychee-like fruit grown in clusters on the Rambutan tree in Vietnam. The active is rich in polyphenols (especially Corilagin) and has been identified through in vitro models and in vivo, to:
- Strengthen and preserve the elastic and collagen network of the skin
- Visibly reduce wrinkles and improve skin elasticity
- Rejuvenate the skin by strengthening crucial dermal fibres
Nephelium lappaceum leaf extract is an active that has been developed to replicate some of the mechanistic pathways observable for retinol (elastin and collagen stimulation, CCN1 inhibition), without some of the common drawbacks associated with retinol (stability, irritation). Although retinol’s irritation potential is more anecdotal, with little literature to conclusively state that it is irritating, it is known to be drying [19], which should be avoided in a range designed for sensitive skin. Nephelium lappaceum leaf extract plays in the same territory as other actives or compounds, for example Bakuchiol, which aim to show similar efficacy to retinol, but without its downsides [20]. A summary of formulation components and functions of key ingredients across the range can be found in Table S1.
2.2. In Vitro Study on Reconstructed Human Epidermis for Product Tolerance
The study was performed using the EpiDermTM reconstructed tissue model EPI-200 (MatTek Corporation, Ashland, OR, United States) and the method was adapted from the XCellR8 standard operating procedures based on the MatTek EpiDerm Skin Irritation Test. The in vitro Reconstructed Human Epidermis ET50 method is a screening method for skin irritation assessment. This method is based on the effective time at which material causes 50% reduction in tissue viability (ET50) compared to negative control. Using an adaptation of a regulatory skin irritation test, subtle differences between mild and ultra-mild formulations can be measured, allowing a series of products to be placed into rank order of expected irritation potential as outlined in Table 1. Expected in vivo irritancy according to ET50 values is based on a predictive model validated against human patch test. Figure 1 outlines the steps involved with this method.

Table 1.
Expected in vivo irritancy according to ET50 values.
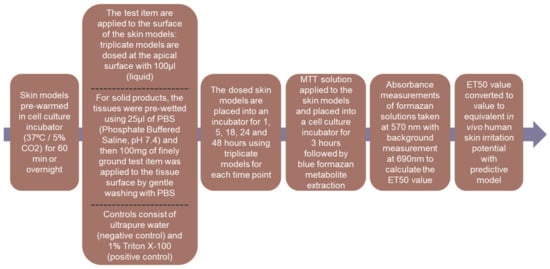
Figure 1.
Experimental flow of the Human Epidermis ET50 method.
The tested items were face products designed to be used in a skincare routine: cleanser, toner, serum, eye cream, day cream (without SPF) or day cream (with SPF) and a night cream. Three different cleansers were formulated during product development: Cleansers A, B and C (Table 2). The Cleanser B formulation contains the following anionic surfactants: Disodium Cocoamphodiacetate, Sodium Cocoamphodiacetate and Sodium Cocoyl Glycinate whereas Cleansers A and C contain Sodium Cocoyl Wheat Amino Acids. Furthermore, cleansers A and C included an amphoteric surfactant (Cocoamidopropyl Betaine) All cleansers contain the same level of non-ionic surfactant, Coco-Glucoside. The aim of the test was to (1) support the face cleanser selection between the three cleansers tested and (2) confirm the mildness of the rest of the test items (toner, serum, eye cream, day creams and night cream) based on the ET50 results and the correlation with the expected in vivo skin irritancy.

Table 2.
Reference codes and corresponding tested items.
2.3. Clinical Study
2.3.1. Study Panels
33 female volunteers aged 40–65 years old (mean age = 56.3, SD = 5.7) were recruited from the Beijing area in China. All procedures involved in the study were explained in detail and written informed consent was obtained from all volunteers. Enrolment was definite when volunteers met all inclusion and exclusion criteria from this open-label study. Main inclusion criteria notably included: self-reported sensitive skin, lactic acid respondent and showing slight/moderate crow’s feet wrinkles and uneven skin tone (skin redness). Prior to baseline measurements, all volunteers went through a wash out phase of two weeks, where usage of products with anti-ageing claimed benefits were not allowed. Following a period of 20 min acclimatisation under controlled temperature and humidity (21 °C ± 1 °C, relative humidity 50% ± 10%), volunteers went through a set of imaging and probe measurements at baseline, 28 days and 56 days later. The volunteers applied the skin care routine twice daily, with frequencies outlined in Table 3. The study was conducted under Good Clinical Practice (GCP) and in conformance with the most recent recommendations of the World Medical Association (Declaration of Helsinki 1964, amended in Fortaleza, Brazil, 2013) [21].

Table 3.
Products and usage frequency of each component from the routine.
2.3.2. Anti-Ageing Clinical Parameters
Topographic features of the face, such as wrinkle depth and skin roughness Ra, were collected on the crow’s feet area using the Primos 3D (Canfield Scientific GmbH, Berlin, Germany) a non-contact in vivo skin measurement device based on fringe projection. Skin extensibility (or “firmness”) and skin elasticity, respectively R0 and R2 parameters, were collected on the cheek by the mean of the Cutometer® Dual MPA 580 (CK Electronic GmbH, Cologne, Germany), a suction-based probe. Probe size was 2 mm, negative pressure was set at 450 mm, while both suction and release time were set at 2 s, for three cycles. Visia (Canfield Scientific, Fairfield, CT, USA) images were collected for illustration purposes. Clinical evaluation all over the face on photographs/live was performed by a clinical on the following parameters: wrinkles, skin smoothness, skin suppleness, complexion evenness and radiance on 4 to 7 point scales, according to the parameters evaluated.
2.3.3. Clinical Parameters Relevant to Sensitive Skin
Biophysical parameters such as superficial levels of hydration were collected using the Corneometer CM825 (CK Electronic GmbH, Cologne, Germany), while transepidermal water loss was collected with the Tewameter® TM 300 (CK Electronic GmbH, Cologne, Germany). Redness (a*) and erythema index (related to haemoglobin content) were measured using the spectrophotometer CM-700D (Konica Minolta, Tokyo, Japan) and the Mexameter® MX 18 (CK Electronic GmbH, Cologne, Germany), respectively. Product tolerance and soothing properties were assessed by the dermatologist (physical signs, e.g., erythema, desquamation) and the subject (functional signs, e.g., itching sensation, burning). Dermatology Quality of Life Index (DLQI) [22] was administered at baseline and only after 56 days, to minimise any recollection of the answers from the previous visit. The DLQI is calculated by summing the score of each question resulting in a maximum of 30 and a minimum of 0. The higher the score, the more quality of life is impaired. Lastly, itching and stinging severity tolerance to chemical stimuli was assessed with the lactic acid test: an aqueous solution of 10% [3,23,24] of lactic acid was applied to both nasolabial fold sides and discomfort was assessed at different time points after application (T0, Ti mm, T0.5 min, T1 min, …, T10 min) using a defined 4-point clinical scale (1. No reaction; 4. Severe reaction).
2.3.4. Statistical Analysis
Baseline comparisons were performed using paired t-test for Data derived from instruments (e.g., hydration, wrinkle depth, etc.), while Wilcoxon matched pairs test was used for parameters derived for lactic acid stinging test and DLQI. For clinical assessments, the proportion of volunteers who showed an improvement were compared versus a theoretical binomial distribution, using a Chi-square goodness of fit, within each timepoint. Threshold for significance was set at α = 0.05. Bar plots are shown as mean ± SEM. The statistical software used for statistical analysis was NCSS 10—PROFESSIONAL, vers. 10.0.7 released on 22 July 2015 running on Windows Server 2008 R2 Standard.
2.4. Mask-Wearing: Mask Relief from the Routine
A side investigation was conducted on the potential soothing effect of the skin care routine for people experiencing discomfort when wearing facial masks for an extended period. Respondents from Bray area in Ireland were asked to answer the following question “Does wearing mask negatively affect your skin?”. A total of 15 respondents, who answered, “My skin sometimes feels irritated” and “I often experience discomfort afterwards or a dryer skin”, were enrolled for a 4-weeks user trial with the same skin care routine outlined above (Table 3). Volunteers answered a questionnaire at the following occasions:
- After one application, at the end of a day when mask worn for a long period.
- After 4 weeks of use.
3. Results
3.1. In Vitro Study on Reconstructed Human Epidermis for Product Tolerance
Table 4 and Figure 1 summarise the nine items tested, their respective ET50 values and the expected in vivo irritancy correlated to the ET50 values. The expected in vivo irritancy was ‘Moderate to Mild’ for the Cleanser A, Day cream (without SPF), Cleanser C, Eye cream, Day cream (with SPF) and the Night cream; ‘Very Mild’ for the Toner and ‘Non-irritating’ for the Serum. Only the Cleanser B is expected to have ‘Moderate’ in vivo skin irritancy based on the calculated ET50 value.

Table 4.
ET50 values (in ascending order) and expected in vivo skin irritancy.
Figure 2 below summarises the calculated ET50 values (in ascending order) following normalisation to the negative control.
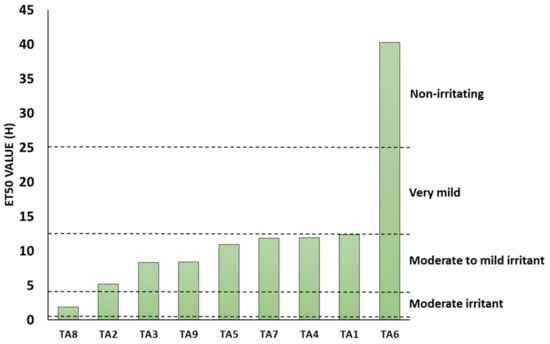
Figure 2.
ET50 value of the nine test items.
3.2. Anti-Ageing Clinical Results
Figure 3 depicts mean values of crow’s feet wrinkle depth, crow’s feet roughness, skin elasticity R2 and skin firmness R0, throughout the study and associated percentage improvements versus baseline, following usage of the skin care routine.
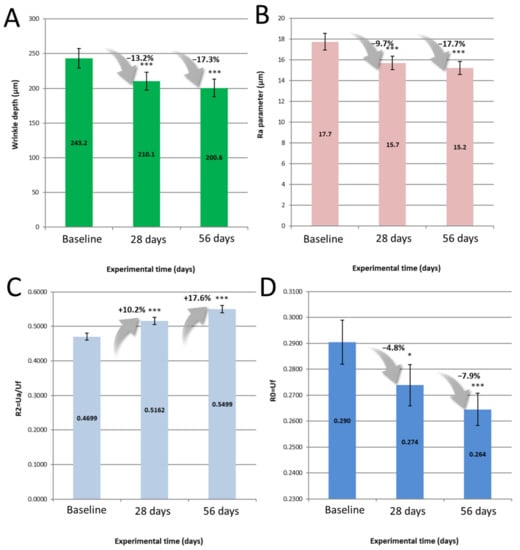
Figure 3.
Crow’s feet Wrinkles depth (A) Crow’s feet roughness (B), Skin elasticity R2 (C) and Skin firmness R0 (D) depth mean values at baseline, 28 and 56 days of use of the routine. Bar Plots describe mean ± SEM. Percentages describe parameter variation versus baseline. Asterisks signal a significant difference versus baseline as follows: * p < 0.05, *** p < 0.001 (n = 33, Paired t-test).
With regards to clinical evaluation, a significant proportion of volunteers showed improvement after 56 days of use for most parameters (Table 5), except for the homogeneity of complexion (Chi-square goodness of fit, p < 0.05).

Table 5.
Percentage of volunteers who showed an improvement during the clinical assessment, and respective p value (n = 33, Chi-square goodness of fit).
3.3. Clinical Parameters Relevant to Sensitive Skin
Throughout the study, no functional signs (e.g., itching, stinging, burning etc.) were reported by the volunteers, nor physical signs during the dermatological evaluation (e.g., erythema, skin dryness etc.). With regards to instrumental data, Figure 4 depicts mean values of skin hydration and trans-epidermal water loss, and associated percentage improvements versus baseline, following usage of the skin care routine.
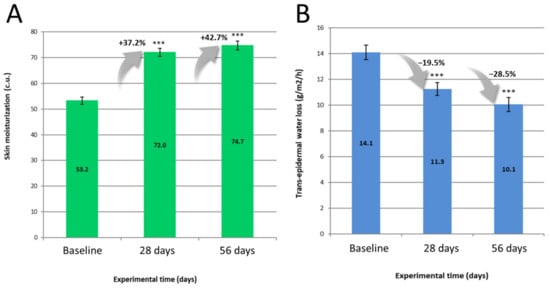
Figure 4.
Skin hydration (A) and Trans-epidermal water loss (B) mean values at baseline, 28 and 56 days of use of the routine. Bar Plots describe mean ± SEM. Percentages describe parameter variation versus baseline. Asterisks signal a significant difference versus baseline as follow: *** p < 0.001 (n = 33, Paired t-test).
Figure 5 outlines mean values of skin redness and erythema index, and associated percentage improvements versus baseline, following usage of the skin care routine.
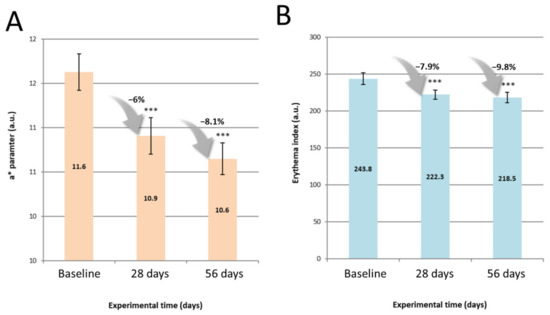
Figure 5.
Skin redness a* (A) and haemoglobin/erythema index (B) mean values at baseline, 28 and 56 days of use of the routine. Bar Plots describe mean ± SEM. Percentages describe parameter variation versus baseline. Asterisks signal a significant difference versus baseline as follow: *** p < 0.001 (n = 33, Paired t-test).
Figure 6 shows the mean stinging sensation at each visit, throughout the 10 min window following application. While the onset for stinging was immediate, the stinging score plateaued around 4 to 5 min post-application. Differences in stinging sensation between baseline and subsequent visit treatments were noticeably different as early as 30 s (T0.5), and observed until the end of the assessment at 10 min.
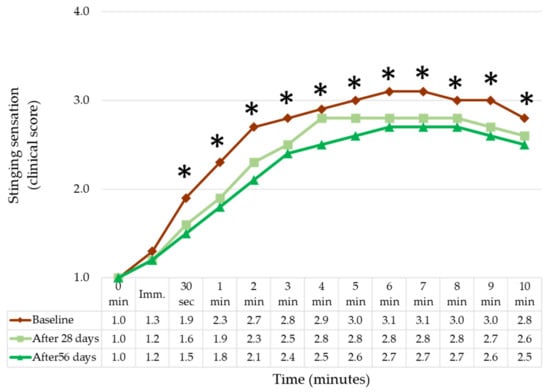
Figure 6.
Stinging sensation perceived following application of a 10% lactic acid on the nasolabial fold of the volunteers, at baseline, 28 and 56 days after using the routine. (n = 33, Paired t-test). Asterisks signal a significant difference after 56 days versus baseline as follow: * p < 0.05, (n = 33, Wilcoxon matched pairs test).
As outlined in Table 6, mean DLQI significantly dropped from 3.3 ± 0.9 at baseline to 1.0 ± 0.6, which corresponds to a shift from “Small effect” to “No effect” on patient life when it comes to interpreting the scores. Among the parameters included in this study, TEWL stood out as potential driver for improved DLQI (Figure 7). Figure 8 displays photographs of two volunteers throughout the study, under standard light or RBX modes from the Visia imaging system.

Table 6.
Mean DLQI score at baseline and after 56 days of treatment. (n = 33, Wilcoxon matched pairs test).
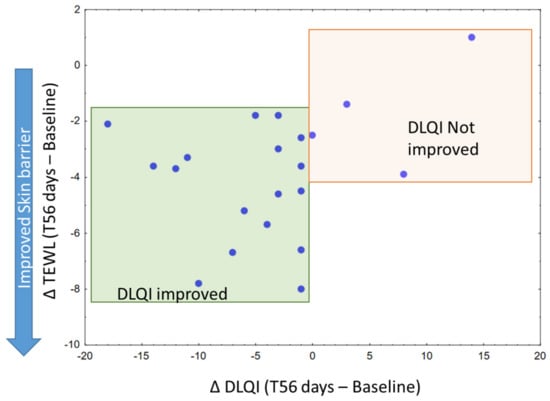
Figure 7.
Scatterplot of ΔTEWL (T56 days–Baseline) and ΔDLQI (T56 days–Baseline), showing volunteers whose dermatological quality of life index has improved (green) and those who did not (Orange) following 56 days of treatment.
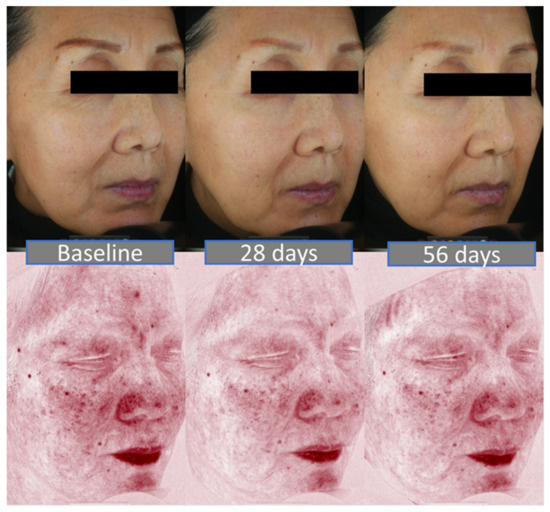
Figure 8.
Two examples of volunteers at each timepoints. Top: with Visia standard light mode, to appreciate topographic feature improvement. Bottom: with RBX red Visia mode, to appreciate redness improvement.
3.4. Mask-Wearing: Mask Relief from the Routine
Following a single application of the routine during a day where the mask was worn for an extended period of time (Median wear length = 5 h, Figure S1), volunteers provided positive feedback especially when it comes to the perceived dryness, itchiness or simply the relief brought by the application of the products (Figure 9A).
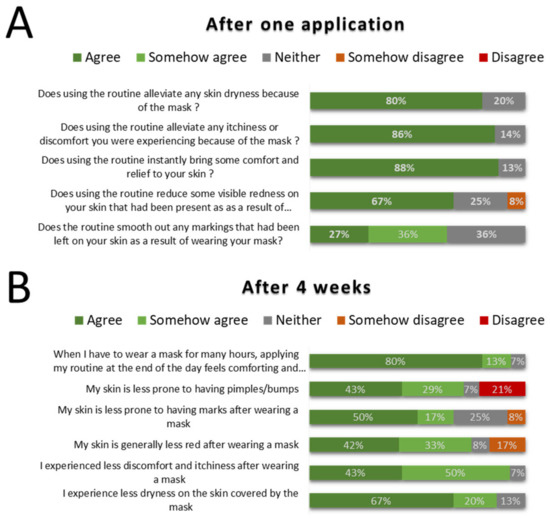
Figure 9.
Volunteers feedback on routine benefits in the context of prolonged facial mask wearing, following a single application (A) and four weeks of twice daily use (B) (n = 15).
Following four weeks of use, volunteers reported that using the routine alleviated some skin issues from mask wearing: dryness, redness, discomfort and marks and brought a sense of relief when using it. Unsurprisingly, the routine was not perceived to improve occurrence of pimples and bumps, as the routine was not designed to prevent microbiome shifts that may occur because of the heat/humidity from mask wearing (Figure 9B). Some volunteers expressed positive feedback, outlined below:
“Skin feels great…really no sign of marks or redness after 4 h of wearing disposable mask in a very warm environment (ICU ward in hospital). Disposable masks are much more comfortable to wear over a long period.”
“The routine is very comforting at the end of a day if I had to wear the mask for long periods. My skin around the mask area feels much more hydrated than before.”
4. Discussion
The in vitro assessment of the skin irritation potential following the XtraMild skin mildness test was performed, using reconstructed human epidermis method (ET50). This method provided a pre-screen, prior to human volunteer studies, driving key decisions in formulation development. The XtraMild testing was performed on nine items to define their ET50 values and correlate the results to the expected in vivo skin irritancy from applying the relevant product on the skin. Cleansers A and C are very similar in terms of formulation and their respective ET50 values were both higher than Cleanser B with an expected ‘Moderate to Mild’ in vivo skin irritancy. Ultimately, Cleanser C was chosen as Cleanser for the sensitive skin care range. Irrespective of the product tested, all scored at least ‘Moderate to Mild’ and were chosen to continue with clinical testing and product development. The serum exhibited—by far—the best mildness of all products screened in vitro. With regards to its composition, this formula stands out with much lower levels of tensioactive ingredients compared to the other products and may explain this excellent tolerability. Overall, there was a moderate correlation between the level of tensioactive ingredients, irrespective of function (e.g., surfactants, solubilizer, emulsifier) and the mildness score of the products (Figure S2), and this further highlights the need to optimize the levels of this category of ingredient.
Following the pre-screening in vitro, the benefits of a 4-step routine at improving signs of ageing and skin discomfort were assessed by means of instrumental and clinical evaluation. Topographic features such as wrinkles and roughness were improved as early as 28 days of treatment and sustained until 56 days. Similarly, biomechanical properties of the skin were enhanced at all timepoints, with a greater capability to withstand stretch and to return to its initial state following deformation. Both topographic features and skin biomechanical properties inevitably decline with age, have been extensively discussed in the literature among various ethnic groups [25,26] and, thus, were deemed ideal parameters to showcase anti-ageing benefits. In addition, clinical evaluation evidenced further benefits in terms of smoothness, suppleness and radiance after 56 days. Altogether, we can speculate that the choice of actives featured in the serum, conjugated with the layering effect of the routine yielded these positive outcomes. The full skincare range was well-tolerated by a carefully selected panel of sensitive skin volunteers, as evidenced with a lack of functional (e.g., stinging) and physical (e.g., erythema) signs throughout the study, thus confirming the relevance of the XtraMild ET50 in vitro approach used for mildness prediction prior to the clinical stage. Performance and gentleness to the skin should not be antinomic, and the present study features elements that invalidate a misconception popularized by widespread retinol use: skin reactions happen because the product is working on the skin [27]. Beyond being suitable for sensitive skin in terms of tolerability, the range was found to improve skin tolerance towards a chemical stimulus such as lactic acid, thus alleviating discomfort mediated by keratinocyte–nerve fibre interactions. Thus, following the guidelines described in the method for formulating a range of products enables us to seamlessly combine performance and tolerability. The decision to conduct this study with an Asian panel was mostly because Asian skin is prone to greater reactivity to cosmetic products, lactic acid, capsaicin and SLS, compared to Caucasian skin [28].
Ultimately, volunteers reported an improvement in their dermatological quality of life. Although significant, improvement in DLQI appeared to be of modest amplitude. It must be borne in mind that the burden of sensitive skin is nonetheless lower than known skin dermatoses, such as acne or psoriasis [29], thus with a reduced margin for improvement. From our findings, improvement in TEWL seemed to be the most apparent parameter to explain DLQI improvements, a sensible outcome since a less permeable skin would prevent most external stimuli (e.g., chemical, physical) to elicit a neurosensory response.
There are nonetheless opportunities to further collect evidence in the context of skincare solutions to manage sensitive skin burden. Assessing the mildness of similar product types for the rest of the product in the skincare range would be beneficial, as was done for the cleansers in this study. From a microbiome perspective, it appears that a shift in abundance of specific Proteobacteria types (Neisseriaceae and Neisseria), concomitantly with a lower diversity of species, are a footprint of sensitive skin, as reported in a recent investigation in women from Guangzhou [30]. Assessing the rebalancing effect of skincare routines is worth considering, bearing in mind the overlap of several preservative systems within a routine. More recently, cytokines and polyunsaturated fatty acid (PUFA) sampled in vivo, have been shown to be relevant inflammatory markers of sensitive skin in a paediatric cohort [4]. The lactic acid test used in this study remains a relevant trigger, as seen with its larger effect upon brain activity for people with sensitive skin [31], and provides evidence of a stronger resilience of the skin towards chemical stimuli. The additional investigation with regards to mask wearing relief hints at potential novel application of sensitive skin care and would deserve further focus as it was conducted with a very modest panel. On that note, it would be of interest to design protocols including other known skin discomfort triggers reported in the literature, such as temperature shift or pollution [2,8,9,32]. Inclusion of a Neurometer or Quantitative Sensory Testing is also relevant in in vivo instrumental approaches to directly assess the threshold needed to elicit itching/stinging in unmyelinated C-fibres and thickly myelinated Aδ-fibres [32,33].
A relevant skincare routine can improve both signs of ageing and skin resilience to external stimuli, thus reducing the likelihood of sensitive skin occurrence. Beyond TRPA1 Receptor potential channels (TRP), other targets can be considered for sensitive skin management, namely: G protein-coupled receptors (GPCRs), release of pruritogenic molecules involved in itching (e.g., Substance P, neutrophins) or TRP indirect activation mediated by GPCRs [34].
5. Conclusions
Given a suitable formulation and testing principles, potent anti-ageing benefits conveyed by the layering effect of a skin care regimen can be made accessible to people exhibiting sensitive facial skin. Furthermore, skin resilience to external stimuli can be improved along these anti-ageing benefits, thus reducing the likelihood of sensitive skin occurrence. With an increasing prevalence of sensitive skin worldwide, it is expected that formulation of skincare products suitable for sensitive skin, which goes beyond mere application of moisturizers, will become a new norm in the cosmetic industry.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cosmetics9020034/s1, Figure S1: Descriptive statistics for the number of hours of mask wearing (n = 15); Figure S2: Scatterplot and correlation of Mildness (ET50 score) versus percentages of tensioactives in all tested products; Table S1: Summary of formulation components and functions of key ingredients. Blue cells indicate inclusion of the ingredient in the product(s) within the range.
Author Contributions
C.M. designed the clinical study. I.D.P. conducted the clinical study. I.D.P. and C.M. analysed the results of the clinical study. J.D. coordinated the safety study. G.Z. designed and formulated the products. D.J. ideated the concept of the skin care range and actives selection. C.M., J.D., D.J. and G.Z. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted under Good Clinical Practice (GCP) and in conformance with the most recent recommendations of the World Medical Association (Declaration of Helsinki 1964, amended in Fortaleza, Brazil, 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
We would like to thank XCellR8 Ltd. for conducting the product tolerance screening and Complife Beijing for performing the clinical study. A special thanks to Rachel Mallon, Nahid Amini and Christina Osterlund for their support in reviewing the article.
Conflicts of Interest
Oriflame manufactures and sells skincare products individually and as part of routines.
References
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Morisset, S.; Séité, S.; Brenaut, E.; Ficheux, A.S.; Fluhr, J.W.; Delvigne, V.; Taieb, C. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes: Results from a worldwide survey of 10 743 individuals. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Farage, M.A.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.; De Belilovsky, C.; Brédif, S.; Baudouin, C.; Misery, L.; Bellemère, G. Clinical and instrumental exploration of sensitive skin in a pediatric population. Cosmetics 2021, 8, 43. [Google Scholar] [CrossRef]
- Reilly, D.M.; Parslew, R.; Sharpe, G.R.; Powell, S.; Green, M.R. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm. Venereol. 2000, 80, 171–174. [Google Scholar] [CrossRef]
- Talagas, M.; Misery, L. Role of Keratinocytes in Sensitive Skin. Front. Med. 2019, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, M.; Denda, S.; Ikeyama, K.; Goto, M.; Denda, M. Exposure to low temperature induces elevation of intracellular calcium in cultured human keratinocytes. J. Investig. Dermatol. 2010, 130, 1945–1948. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.R.; Cheon, H.I.; Misery, L.; Taieb, C.; Lee, Y.W. Sensitive skin in Korean population: An epidemiological approach. Skin Res. Technol. 2017, 24, 229–234. [Google Scholar] [CrossRef]
- Messaraa, C.; Richard, T.; McNamee, D.; Hurley, S.; Doyle, L. Exploratory investigation on the characteristics of Mexican women skin. In Proceedings of the IFSCC Conference—Poster Presentation, Cancun, Mexico, 18–28 October 2021. [Google Scholar]
- Heinicke, I.R.; Adams, D.H.; Barnes, T.M.; Greive, K.A. Evaluation of a topical treatment for the relief of sensitive skin. Clin. Cosmet. Investig. Dermatol. 2015, 8, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Messaraa, C.; Robertson, N.; Walsh, M.; Hurley, S.; Doyle, L.; Mansfield, A.; Daly, L.; Tansey, C.; Mavon, A. Clinical evidences of benefits from an advanced skin care routine in comparison with a simple routine. J. Cosmet. Dermatol. 2019, 19, 1993–1999. [Google Scholar] [CrossRef]
- Draelos, Z.D. Sensitive skin: Perceptions, evaluation, and treatment. Am. J. Contact Dermat. 1997, 8, 67–78. [Google Scholar] [CrossRef]
- Fan, L.; He, C.; Jiang, L.; Bi, Y.; Dong, Y.; Jia, Y. Brief analysis of causes of sensitive skin and advances in evaluation of anti-allergic activity of cosmetic products. Int. J. Cosmet. Sci. 2016, 38, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [Green Version]
- Duarte, I.; Silveira, J.; Hafner, M.; Toyota, R.; Pedroso, D. Sensitive skin: Review of an ascending concept. An. Bras. Dermatol. 2017, 92, 521–525. [Google Scholar] [CrossRef]
- Trapp, M. Is there room for improvement in the emollients for adjuvant therapy? J. Eur. Acad. Dermatol. Venereol. 2007, 21 (Suppl. S2), 14–18. [Google Scholar] [CrossRef]
- Silab. AD-RESYL® Supplier’s Technical Brochure; Silab: Saint-Viance, France.
- Gruber, J.V.; Stojkoska, V.; Riemer, J. Retinol Has a Skin Dehydrating Effect That Can Be Improved by a Mixture of Water-Soluble Polysaccharides. Cosmetics 2020, 7, 80. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Gao, Y.; Chen, S.; Liu, X.; Wang, H.; Dong, Y. CPT, the main test method of skin neuronal sensitivity. Cutan. Ocul. Toxicol. 2015, 34, 208–211. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Zhang, Y.; Wang, T.; Li, X.; Ma, Y. The evaluation of neural and vascular hyper-reactivity for sensitive skin. Skin Res. Technol. 2016, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Quantification of age-related facial wrinkles in men and women using a three-dimensional fringe projection method and validated assessment scales. Dermatol. Surg. 2014, 40, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, Y.S.; Kang, K.S. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, S.; Lee, J.; Cho, S.A.; Shin, K. Ethnic differences in objective and subjective skin irritation response: An international study. Skin Res. Technol. 2014, 20, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Sakurai, S.; Hoshii, N.; Nakagawa, H. Certolizumab Pegol for the Treatment of Moderate to Severe Plaque Psoriasis: 16-Week Results from a Phase 2/3 Japanese Study. Dermatol. Ther. 2021, 11, 513–528. [Google Scholar] [CrossRef]
- Qiao, Z.; Huang, S.; Leng, F.; Bei, Y.; Chen, Y.; Chen, M.; Hu, Y.; Huang, Y.; Xiang, Q. Analysis of the bacterial flora of sensitive facial skin among women in guangzhou. Clin. Cosmet. Investig. Dermatol. 2021, 14, 655–664. [Google Scholar] [CrossRef]
- Querleux, B.; Dauchot, K.; Jourdain, R.; Bastien, P.; Bittoun, J.; Anton, J.L.; Burnod, Y.; De Lacharrière, O. Neural basis of sensitive skin: An fMRI study. Skin. Res. Technol. 2008, 14, 454–461. [Google Scholar] [CrossRef]
- Huet, F.; Dion, A.; Batardière, A.; Nedelec, A.S.; Le Caër, F.; Bourgeois, P.; Brenaut, E.; Misery, L. Sensitive skin can be small fibre neuropathy: Results from a case–control quantitative sensory testing study. Br. J. Dermatol. 2018, 179, 1157–1162. [Google Scholar] [CrossRef]
- An, S.M.; Lee, E.J.; Lee, E.; Kim, H.O.; Koh, J.S. Itching sensation and neuronal sensitivity of the skin. Skin Res. Technol. 2016, 22, 104–107. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).