Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Aspects
2.2. Study Participants
2.3. Study Schedule
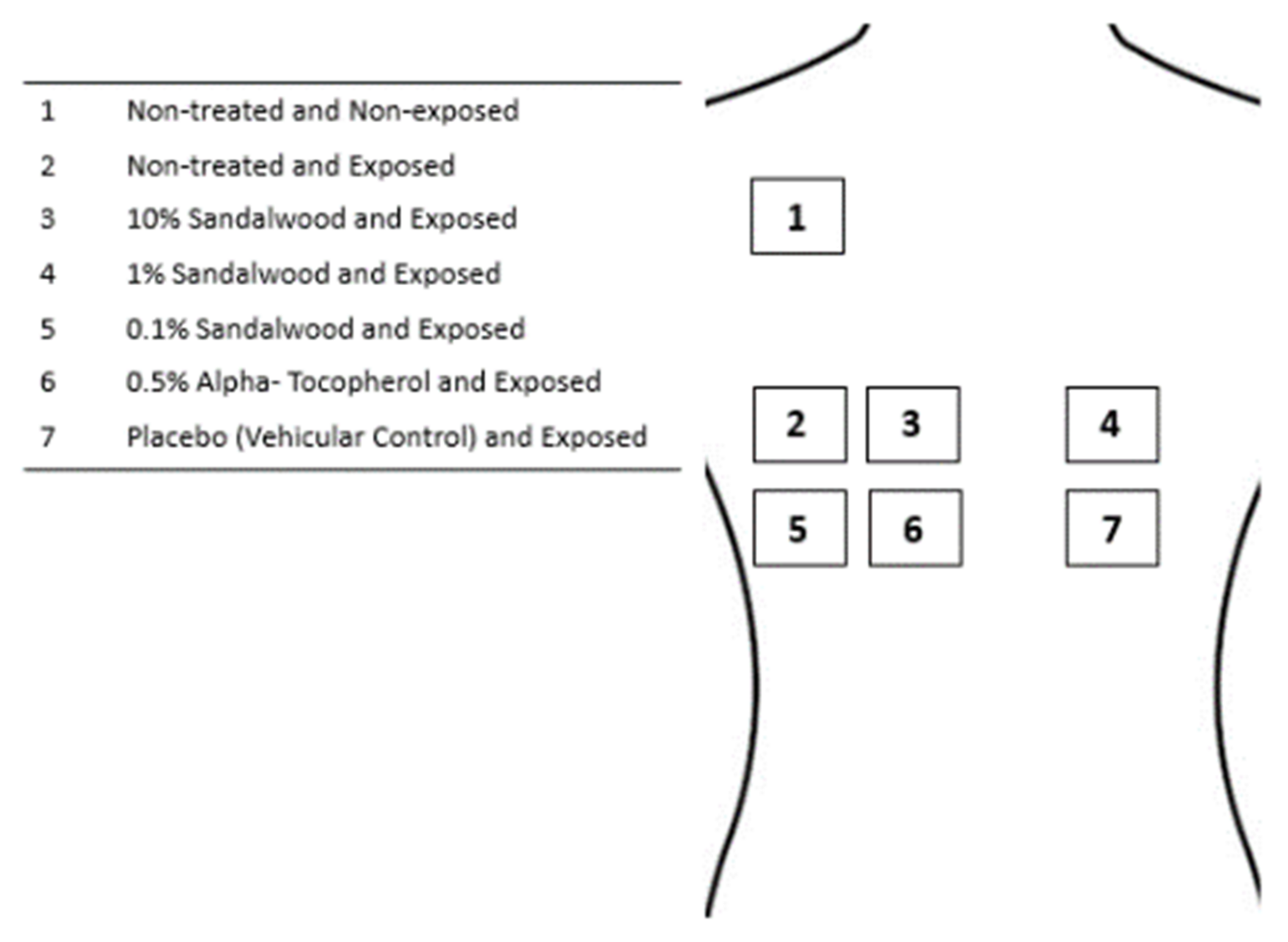
2.4. Investigational Products
2.5. Medical Examination
2.6. Ambient Dust Exposure
2.7. Blue Light Exposure
2.8. Swabbing and Sampling
2.9. Statistical Analysis
3. Results
3.1. Panel Description
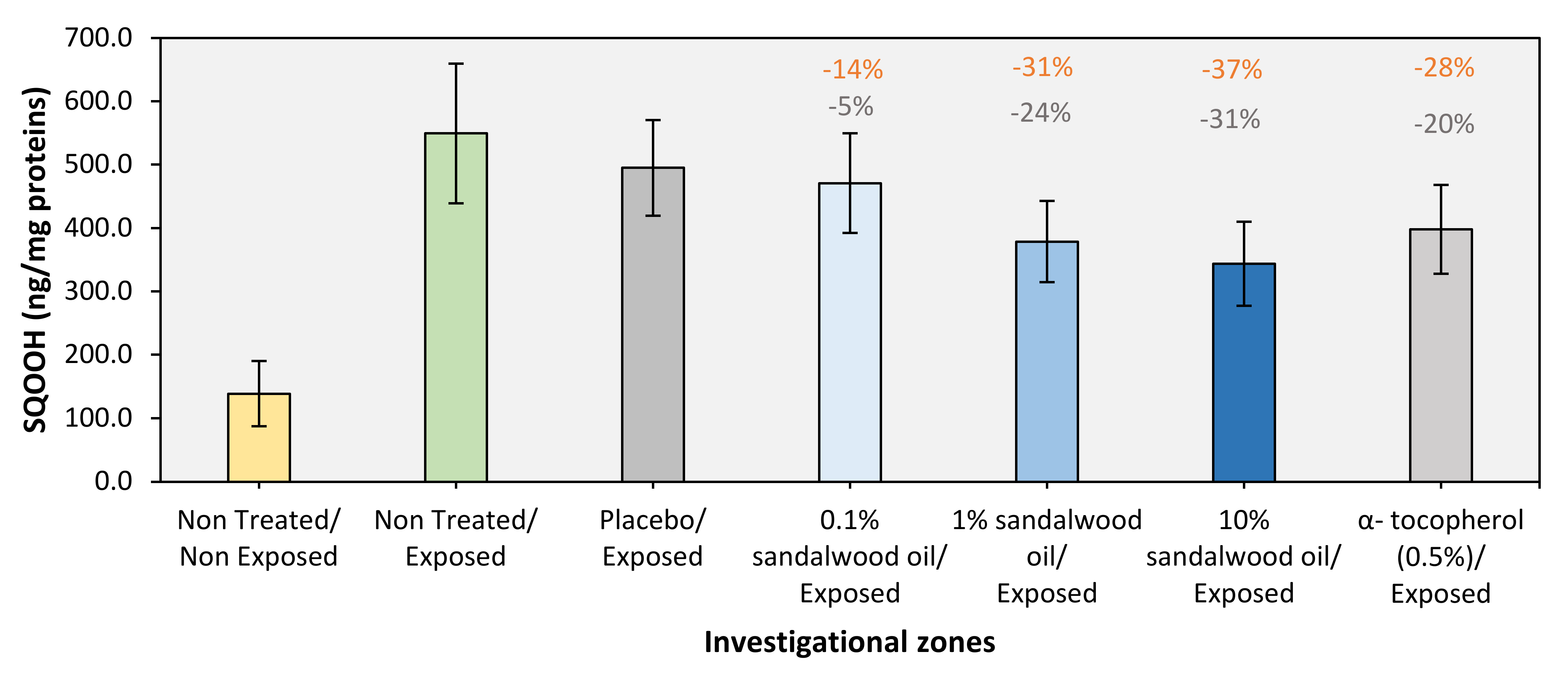
3.2. Protective Effect of Sandalwood Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wortzman, M.; Nelson, D.B. A comprehensive topical antioxidant inhibits oxidative stress induced by blue light exposure and cigarette smoke in human skin tissue. J. Cosmet. Dermatol. 2021, 20, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L. Pollution and sun exposure: A deleterious synergy. Mechanisms and opportunities for skin protection. Curr. Med. Chem. 2017, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Sœur, J.; Belaïdi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef]
- Coats, J.G.; Maktabi, B.; Abou-Dahech, M.S.; Baki, G. Blue light protection, part II—Ingredients and performance testing methods. J. Cosmet. Dermatol. 2021, 20, 718–723. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Sauce, R.; Oliveira, C.A.D.; Pinto, C.A.; Martinez, R.M.; Baah, S.; Almeida, T.S.; Rosado, C.; Baby, A.R. Active ingredients, mechanisms of action and efficacy tests of antipollution cosmetic and personal care products. Braz. J. Pharm. Sci. 2018, 54, e01003. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Kumar, A.A.; Joshi, G.; Ram, H.M. Sandalwood: History, uses, present status and the future. Curr. Sci. 2012, 1408–1416. [Google Scholar]
- Braun, N.A.; Sim, S.; Kohlenberg, B.; Lawrence, B.M. Hawaiian sandalwood: Oil composition of Santalum paniculatum and comparison with other sandal species. Nat. Prod. Commun. 2014, 9, 1365–1368. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Ito, H.; Hayashi, K.; Hasegawa, T.; Machiguchi, T.; Yoshidad, T. Aromatic constituents from the heartwood of Santalum album L. Chem. Pharm. Bull. 2005, 53, 641–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francois-Newton, V.; Brown, A.; Andres, P.; Mandary, M.B.; Weyers, C.; Latouche-Veerapen, M.; Hettiarachchi, D. Antioxidant and Anti-Aging Potential of Indian Sandalwood Oil against Environmental Stressors In Vitro and Ex Vivo. Cosmetics 2021, 8, 53. [Google Scholar] [CrossRef]
- Mohankumar, A.; Kalaiselvi, D.; Levenson, C.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Antioxidant and stress modulatory efficacy of essential oil extracted from plantation grown Santalum album L. Ind. Crop. Prod. 2019, 140, 111–623. [Google Scholar]
- Moy, R.L.; Levenson, C. Sandalwood Album Oil as a Botanical Therapeutic in Dermatology. J. Clin. Aesthet. Dermatol. 2017, 10, 34–39. [Google Scholar] [PubMed]
- Sharma, M.; Levenson, C.; Clements, I.; Castella, P.; Gebauer, K.; Cox, M.E. East Indian Sandalwood Oil (EISO) Alleviates Inflammatory and Proliferative Pathologies of Psoriasis. Front. Pharmacol. 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongratanaworakit, T.; Heuberger, E.; Buchbauer, G. Evaluation of the effects of East Indian sandalwood oil and α-santalol on humans after transdermal absorption. Planta Med. 2004, 70, 3–7. [Google Scholar]
- Curpen, S.; Francois-Newton, V.; Moga, A.; Hosenally, M.; Petkar, G.; Soobramaney, V.; Ruchaia, B.; Lutchmanen Kolanthan, V.; Roheemun, N.; Sokeechand, B.N. A novel method for evaluating the effect of pollution on the human skin under controlled conditions. Skin Res. Technol. 2020, 26, 50–60. [Google Scholar] [CrossRef]
- Tagle, D.A. The NIH microphysiological systems program: Developing in vitro tools for safety and efficacy in drug development. Curr. Opin. Pharmacol. 2019, 48, 146–154. [Google Scholar] [CrossRef]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a step toward sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef]
- Pham, D.M.; Boussouira, B.; Moyal, D.; Nguyen, Q.L. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Huang, C.-T.; Lee, C.-W.; Fang, J.-Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef]
- De Oliveira Pinto, C.A.S.; Martins, T.E.A.; Martinez, R.M.; Freire, B.T.; Velasco, M.V.R.; Baby, A.R. Vitamin E in Human Skin: Functionality and Topical Products. In Vitamin E in Health and Disease—Interactions, Diseases and Health Aspects; IntechOpen: London, UK, 2021. [Google Scholar]
- ISO 3518:2002; Oil of Sandalwood (Santalum album L.). International Standards Organization: Geneva, Switzerland, 2002.
| Constituent | Concentration (% w/w) |
|---|---|
| Z α-santalol | 48.2 |
| Z β-santalol | 20.5 |
| trans α-bergamotol | 5.9 |
| epi β-santalol | 4.0 |
| Required Age Range? | Yes | 22 | 100.0% |
| No | 0 | 0.0% | |
| Gender | Female | 15 | 68.0% |
| Male | 7 | 32.0% |
| Nonexposed | Exposed | ||||||
|---|---|---|---|---|---|---|---|
| Zone | Nontreated | Nontreated | Placebo | 0.1% Sandalwood Oil | 1% Sandalwood Oil | 10% Sandalwood Oil | α-Tocopherol (0.5%) |
| Mean | 138.75 | 549.46 | 495.13 | 470.80 | 378.75 | 343.66 | 398.23 |
| Median | 100.69 | 494.54 | 483.77 | 463.11 | 353.07 | 309.92 | 380.73 |
| Minimum | 44.71 | 213.36 | 189.84 | 102.36 | 139.94 | 68.78 | 126.62 |
| Maximum | 459.63 | 1078.65 | 823.29 | 794.50 | 740.94 | 577.97 | 843.50 |
| SD | 116.25 | 248.16 | 170.74 | 177.30 | 144.54 | 149.38 | 157.77 |
| Investigational Zone (I) | Zone of Comparison (J) | Mean (%) Change | Mean Difference (I-J) | p-Value |
|---|---|---|---|---|
| Nontreated/Exposed | Nontreated/Nonexposed | 296 | 410.706 | <0.001 |
| Placebo/Exposed | Nontreated/Exposed | −10 | −54.332 | 0.586 |
| α-Tocopherol (0.5%)/Exposed | −28 | −151.234 | <0.001 | |
| 0.1% Sandalwood oil/Exposed | −14 | −78.658 | 0.158 | |
| 1% Sandalwood oil/Exposed | −31 | −170.713 | <0.001 | |
| 10% Sandalwood oil/Exposed | −37 | −205.803 | <0.001 | |
| 0.1% Sandalwood oil/Exposed | Placebo/Exposed | −5 | −24.326 | 0.986 |
| 1% Sandalwood oil/Exposed | −24 | −116.381 | 0.005 | |
| 10% Sandalwood oil/Exposed | −31 | −151.470 | <0.001 | |
| 1% Sandalwood oil/Exposed | 0.1% Sandalwood oil/Exposed | −20 | −92.055 | 0.055 |
| 10% Sandalwood oil/Exposed | 1% Sandalwood oil/Exposed | −9 | −35.089 | 0.918 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutchmanen Kolanthan, V.; Brown, A.; Soobramaney, V.; Philibert, E.G.; Francois Newton, V.; Hosenally, M.; Sokeechand, B.N.; Petkar, G.; Moga, A.; Andres, P.; et al. Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome. Cosmetics 2022, 9, 35. https://doi.org/10.3390/cosmetics9020035
Lutchmanen Kolanthan V, Brown A, Soobramaney V, Philibert EG, Francois Newton V, Hosenally M, Sokeechand BN, Petkar G, Moga A, Andres P, et al. Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome. Cosmetics. 2022; 9(2):35. https://doi.org/10.3390/cosmetics9020035
Chicago/Turabian StyleLutchmanen Kolanthan, Vimi, Andrew Brown, Vitisha Soobramaney, Evans Georges Philibert, Veronique Francois Newton, Muzzammil Hosenally, Bibi Nusayha Sokeechand, Gitanjali Petkar, Alain Moga, Philippe Andres, and et al. 2022. "Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome" Cosmetics 9, no. 2: 35. https://doi.org/10.3390/cosmetics9020035
APA StyleLutchmanen Kolanthan, V., Brown, A., Soobramaney, V., Philibert, E. G., Francois Newton, V., Hosenally, M., Sokeechand, B. N., Petkar, G., Moga, A., Andres, P., Mandary, M. B., & Hettiarachchi, D. (2022). Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome. Cosmetics, 9(2), 35. https://doi.org/10.3390/cosmetics9020035






