Abstract
Sensitive skin is characterized by symptoms such as stinging and tingling in response to stimuli that usually do not cause unpleasant sensations. Epidemiological studies show that individuals with sensitive skin are more prone to developing skin allergies, although the link between both conditions is unknown. Aiming to evaluate the presence of allergens in facial-skin products for sensitive skin, a pool of 88 cosmetic products from international brands marketed in pharmacies and parapharmacies was analyzed. A list of allergens identified in product labels was compiled and grouped according to their function. Fragrances were the most common allergens, followed by skin-conditioning agents, surfactants, and preservatives. Fragrances presenting the highest use percentages were linalool, benzyl alcohol, geraniol, and citronellol. Overall, the majority of cosmetic formulations were absent of fragrance allergens, being present only in 7% of products. Other allergens were found in most products (95%). This finding should be interpreted with caution, since many of these compounds are rare sensitizers and studies demonstrating their risk for individuals with sensitive skin are lacking. With this study, useful information for health professionals is provided to support their advice and to help consumers choosing cosmetic products.
1. Introduction
Sensitive skin was first described by Maibach in 1987 under the name of Cosmetic Intolerance Syndrome, and it is clinically characterized by the occurrence of multiple sensations such as tightness, burning, tingling, pain, pruritus, and stinging [1,2,3]. Although the occurrence of visible dermatological reactions is rare, patients can also develop erythema, desquamation, and dryness [1,4]. These symptoms normally occur in response to a stimulus such as pollution, heat, cold, wind, and cosmetics, that usually lacks unpleasant sensations [5]. Psychological and physiological factors, namely stress, emotions, and the menstrual cycle, might also have a role in the appearance of symptoms identified in sensitive skin [6].
Currently, there are three hypotheses in the literature that try to explain its pathophysiology, namely the disruption of the epidermal-barrier function, enhanced immune responsiveness, and neurosensory dysfunction [1]. The disruption of the epidermal-barrier function can be related to a derangement of intracellular lipids due to a decrease in ceramide content and lower levels of pyrrolidone carboxylic acid, bleomycin hydrolase, and transglutaminase, as well as a higher number of smaller and immature corneocytes [7,8,9]. This can facilitate the penetration of irritants or allergens, provide inadequate protection of the nerve endings, and increase transepidermal water loss [1]. Notwithstanding, this hypothesis has been questioned. A recent study using confocal Raman microspectroscopy showed no significant differences in subjects with sensitive skin compared to those with nonsensitive skin regarding stratum-corneum thickness, transepidermal water loss, ceramide content, and natural moisturizing factors [10]. An individual with sensitive skin experiences a wide variety of symptoms, highlighting a possible relation with neurosensory dysfunction [11]. The skin possesses cutaneous nerve fibers, namely unmyelinated C fibers, with neurosensory receptors, such as endothelin and transient-receptor-potential channels, responsible for mediating pain, itches, and warmth. Individuals with sensitive skin demonstrated fewer unmyelinated C fibers, possibly caused by a degeneration after contact with environmental factors, which are thought to be involved in the appearance of sensitive skin. Consequently, this can overactivate the nerve-ending receptors and increase the release of neuropeptides, which may induce the release of proinflammatory cytokines and chemokines, resulting in an inflammatory response. Unpleasant sensations such as burning pain and pruritus may be perceived in a process where the transient-receptor-potential vanilloid type 1 (TRPV1) might be a key player [11,12,13,14,15]. It is thought that an overexpression of TRPV1 is involved in the pathophysiology of sensitive skin by increasing neuronal excitability. Moreover, Ehnis-Pérez et al., demonstrated that TRPV1 expression is upregulated in individuals with sensitive skin, and this correlates with the intensity of the symptoms [16].
A recent hypothesis correlating sensitive skin with microbiota changes is being studied, but contradictory results have been obtained. Sensitive skin demonstrated a higher phylogenetical diversity in skin microbiota, as well as a greater abundance of Lactobacillus and Mucor racemosus, and a smaller abundance of Malassezia restricta when compared with nonsensitive skin [17]. The abundance of Staphylococcus was also significantly decreased in sensitive skin [18]. However, in a study performed by Hillion et al. [19], no correlation between a genus, dominant bacterial species, or a phylum and sensitive skin was noted. Overall, the possible hypotheses behind the pathophysiological mechanisms of sensitive skin are complex, and they are not necessarily mutually exclusive, being possibly interlinked [20].
A study by Alani et al. [21] published in 2013 showed that men use up to 85 cosmetic ingredients and women up to 168 ingredients daily in their skincare routine, which can eventually lead to the occurrence of adverse reactions. Cosmetic-contact dermatitis is the cause of 2–4% of dermatological appointments, but this estimate must be higher, since most patients simply avoid the use of the suspected cosmetic. The importance of the identification of allergens responsible for these skin reactions increased when in 1997 it was made mandatory to include the list of the ingredients on the labels of cosmetics [22]. In 2004, cosmetic manufacturers were obligated to label the 26 fragrances present in the Annex III of EU Cosmetic Regulation 1223/2009 to alert consumers. This facilitated the identification of allergens by clinicians and their avoidance by consumers [21]. When products contain fragrance materials, they must be described on the label as “aroma”, or “parfum” [23]. Although there is no described mechanism by which skin allergens may play a role in the pathophysiology of sensitive skin, epidemiological studies support the association between sensitive skin and allergic predisposition [24]. In this regard, people with sensitive skin are estimated to be 1.8 to 5 times more prone to develop a skin allergy [25].
According to epidemiological studies, sensitive skin affects 71% of the adult global population. This condition is more frequent on the face, the corporal region in contact with the highest number of cosmetics, thinner skin barrier, and higher nerve density [6,14,26]. Several cosmetic products are available in the market targeting people with sensitive skin. The evaluation of the top 10 active ingredients present in cosmetic products from multinational brands for sensitive skin was already performed by our group [3]. However, to the best of our knowledge, the evaluation of the presence of allergens in those products remains unexplored. This study aims to unveil the presence of allergens in cosmetic products specially formulated for sensitive skin. For this study, ingredients were characterized as allergens if they are classified likewise by the FDA [27], if they are included in the European Standard Series for patch testing [28], if they are fragrances whose discrimination on product labels is mandatory according to Annex III of the EU Cosmetic Regulation 1223/2009 [23], or if skin sensitization was reported in recent studies [29,30].
2. Materials and Methods
2.1. Data Collection
To evaluate the presence of allergens in skincare products for sensitive skin, the composition of a series of cosmetic products from multinational manufacturers commercially available in the Portuguese market in 2019 was collected. All products marketed in pharmacies and parapharmacies which exhibited the following expressions in the label: “sensitive skin”, “reactive skin”, or “intolerant skin”, were included in the analysis. The information was collected from the products’ labels and on the manufacturers’ websites. A list of 117 cosmetic allergens was compiled based on the list of fragrance allergens presented on Annex III of EU Cosmetic Regulation 1223/2009 [23], the European Standard Series for patch testing [28], allergens classified by FDA [27], and the allergens which are used in cosmetic products documented by Goossens [29] and Uter et al. [30] and listed according to the International Nomenclature of Cosmetic Ingredients (INCI). The platforms of the Cosmetic Ingredient Review (CIR) [31] and the opinions of the Scientific Committee on Consumer Safety (SCCS) [32] were also consulted to verify the safety of the ingredients.
2.2. Data Analysis
Allergens were grouped according to their function, e.g., fragrance or others, which were then grouped as preservatives, ultraviolet (UV) filters, etc., based on CosIng ingredient database [33]. Data were analyzed regarding the relative number of products containing allergens, the occurrence of combinations of these ingredients, and the number of allergens for each function, in percentage. The percentage of cosmetic products containing parfum was also determined. Allergens were then ranked in descending order of occurrence.
3. Results
According with the methodology used, the list of ingredients from 88 products from 19 multinational brands was analyzed regarding the presence of fragrance allergens with regulatory restrictions in Europe [23], the European Standard Series for patch testing [28], allergens classified by FDA [27], and other allergens documented by Goossens [29] and Uter et al. [30].
3.1. Fragrance Allergens
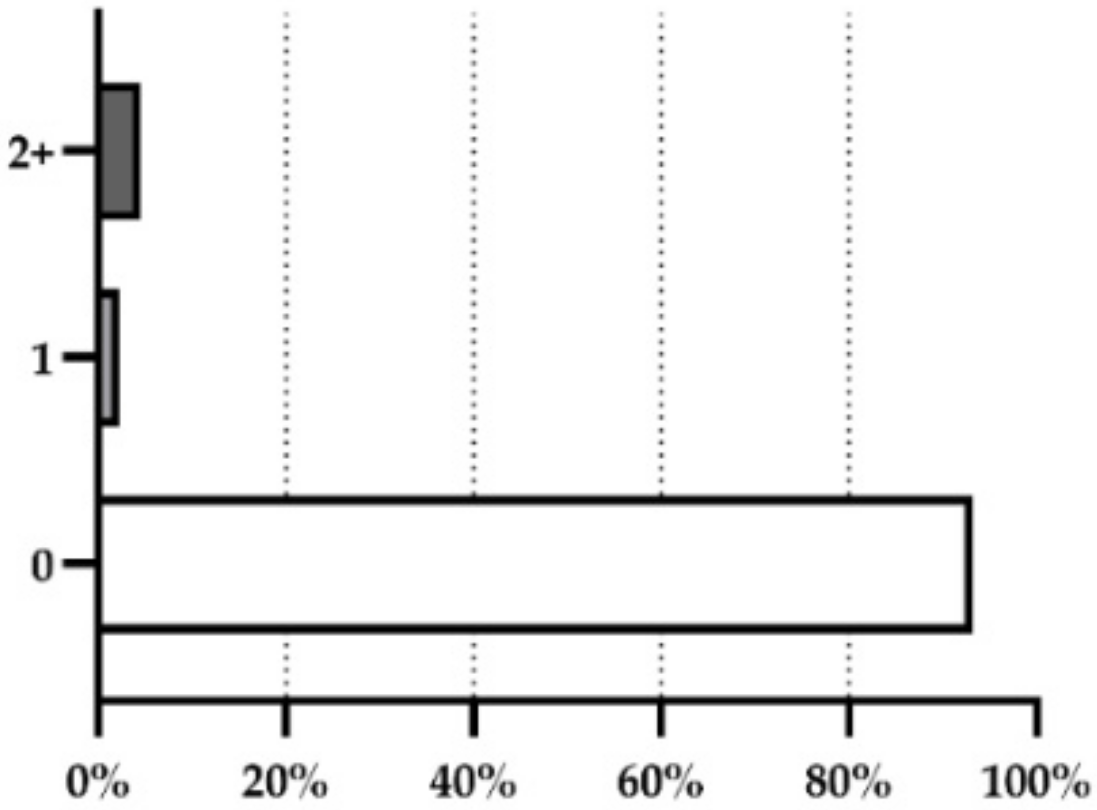
The occurrence of fragrance allergens in analyzed products is represented in Figure 1.
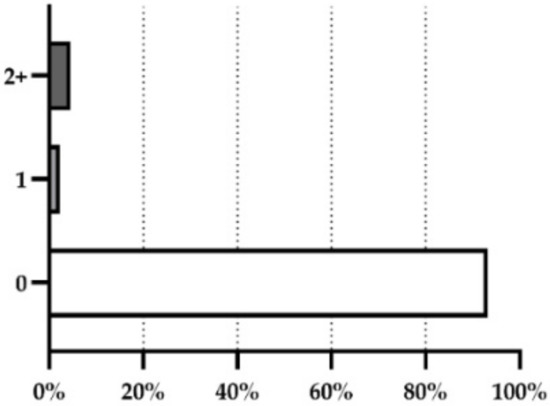
Figure 1.
Prevalence of products for sensitive skin that contained no fragrance allergens (0), one fragrance allergen (1), and combination of fragrance allergens (2+).
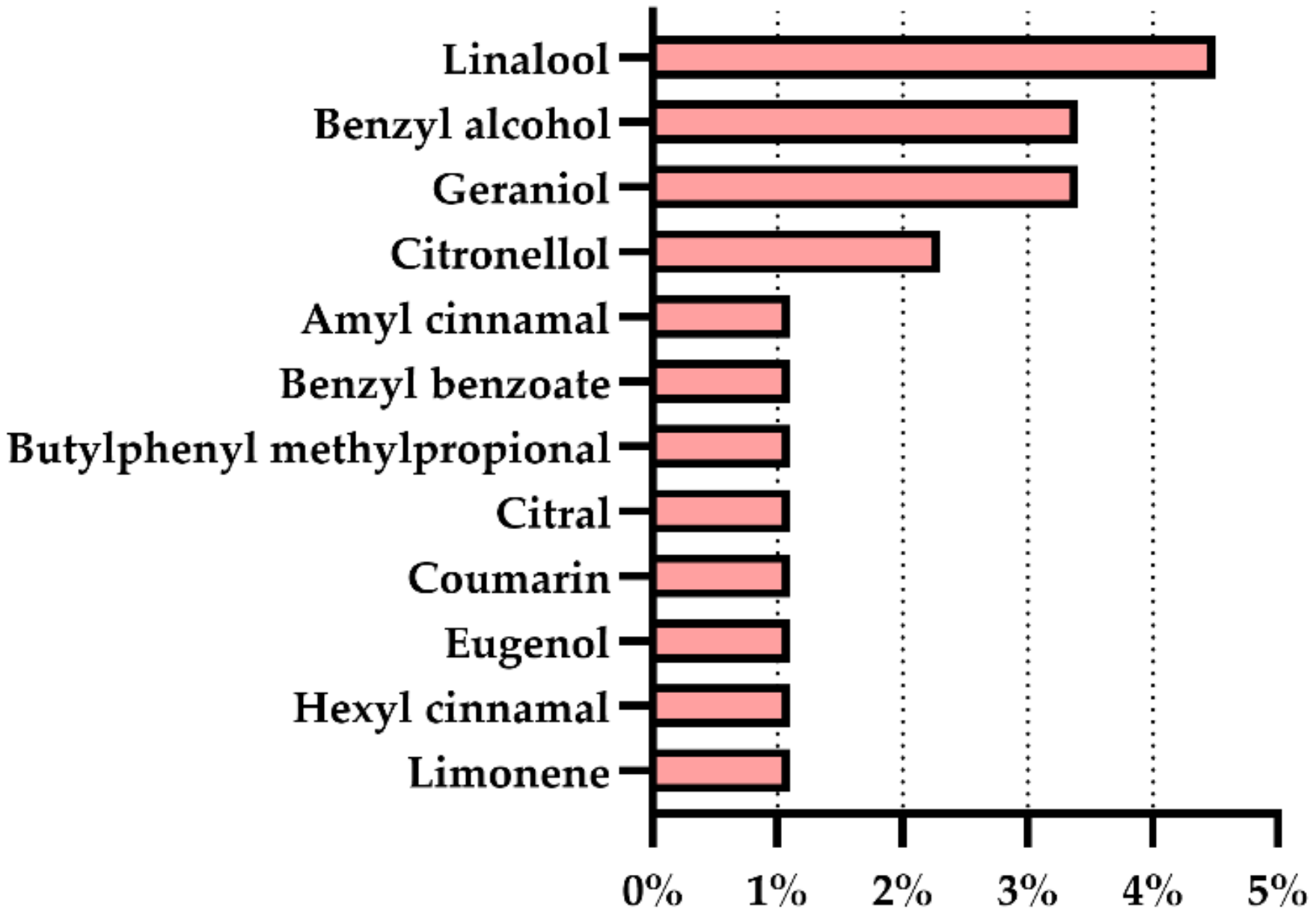
The presence of 12 fragrance allergens from the 26 fragrance allergens regulated in Europe was confirmed in six from the 88 cosmetic products for sensitive skin analyzed (7%). The percentages varied from 1.1% to 4.5%, with linalool being the allergen present in the most cosmetic products (4.5%), followed by benzyl alcohol and geraniol (3.4%), and citronellol (2.3%). The fragrances amyl cinnamal, benzyl benzoate, butylphenyl methylpropional, citral, coumarin, eugenol, hexyl cinnamal, and limonene were found in less than 2% of the analyzed products (Figure 2).
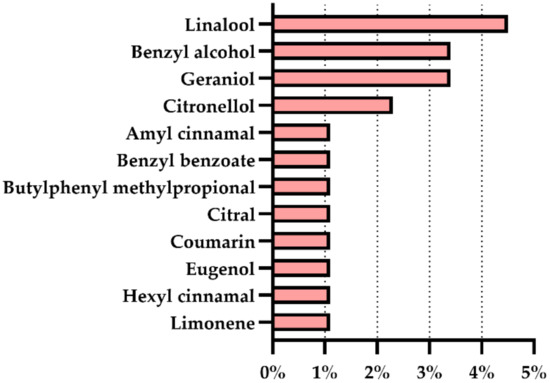
Figure 2.
Prevalence of the fragrance allergens regulated in Europe in products for sensitive skin.
3.2. Other Allergens
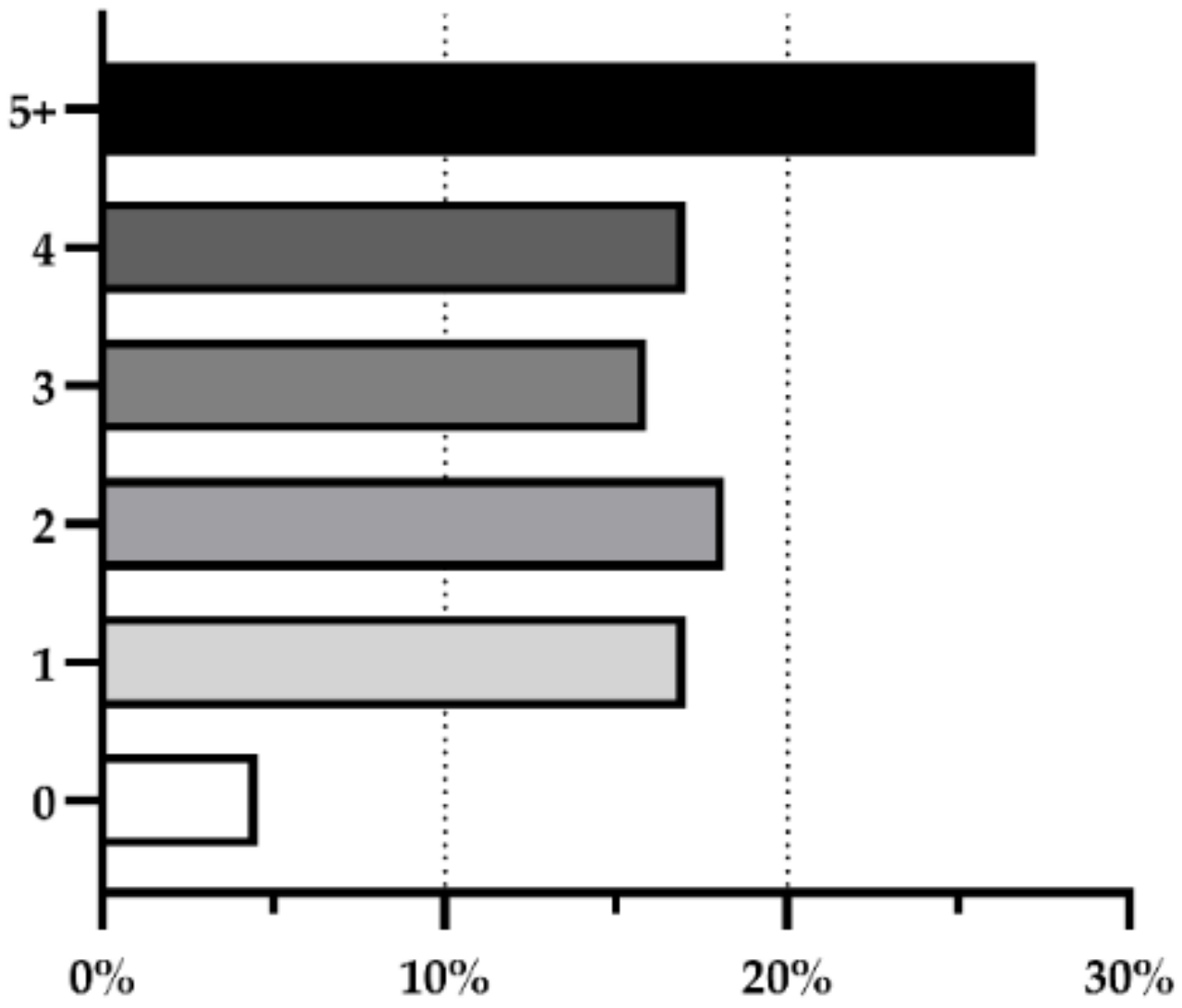
Regarding allergens other than fragrances, these ingredients were present in most products (95%). Of those, 78% possessed two or more allergens, with a combination of at least five being the most prevalent, with 27%. The following positions, with one, two, three, and four allergens were present between 16 and 18% of the cosmetic products. Only 5% of the products were absent of any allergen (Figure 3).
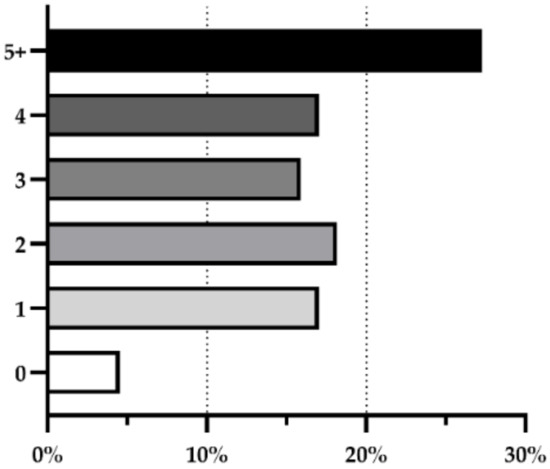
Figure 3.
Prevalence of products for sensitive skin that contain no allergens (0), one allergen (1), or combinations of allergens (2; 3; 4; 5+).
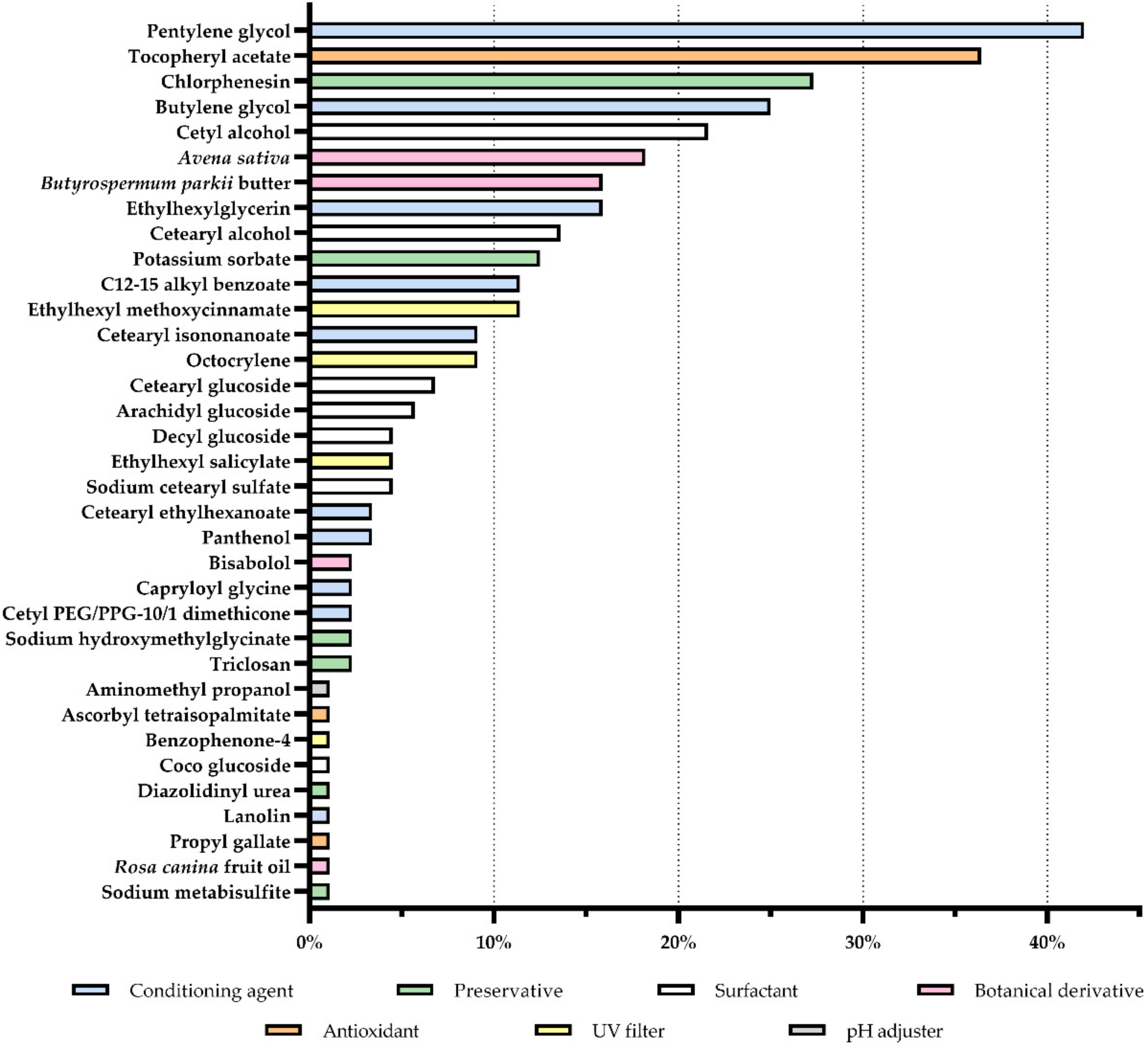
From the 35 allergens found summarized in Figure 4, pentylene glycol and tocopheryl acetate were clearly in evidence, being present in about 42% and 36% of the products, respectively. Other ingredients were also labeled in a major part of the cosmetic products analyzed. Chlorphenesin, butylene glycol, and cetyl alcohol were present in at least 20% of the formulations. Avena sativa derivatives, Butyrospermum parkii butter, ethylhexyglycerin, cetearyl alcohol, potassium sorbate, C12–15 alkyl benzoate, and ethylhexyl methoxycinnamate possessed a prevalence between 10 and 20%. The following positions were occupied by more 24 allergens which were found in less than 10% of the analyzed products.
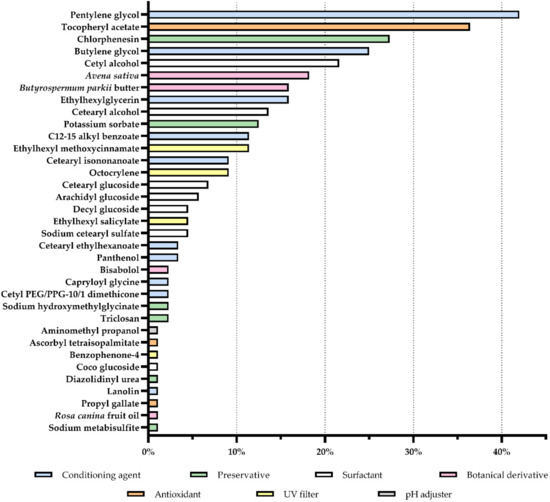
Figure 4.
Prevalence of allergens in products for sensitive skin. (UV = ultraviolet).
3.3. Allergens’ Function
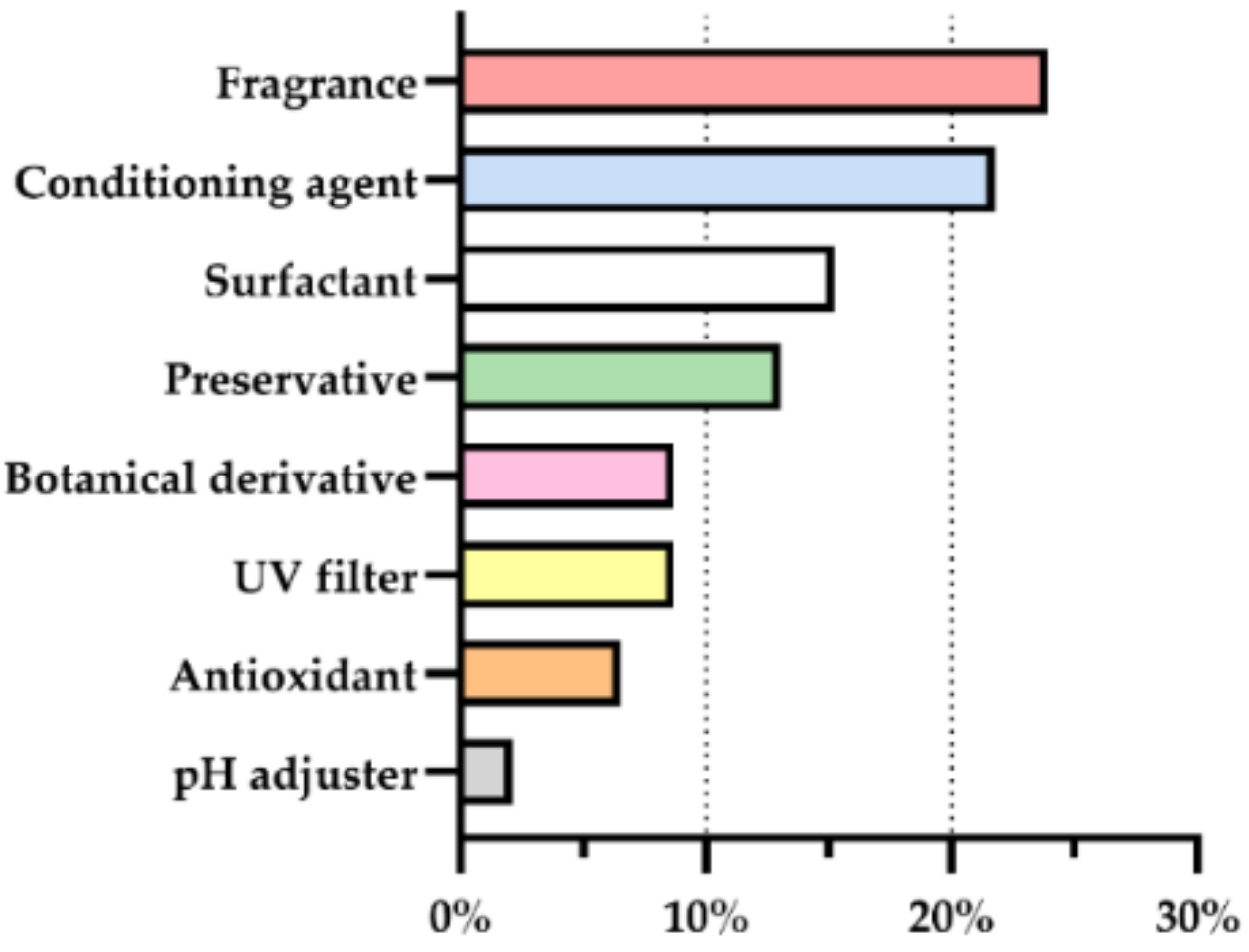
After the analysis of the constitution of the products for sensitive skin regarding the presence of allergens, these ingredients were categorized according to their function (Figure 5). Botanical derivatives were also grouped. From all the ingredients with potential to cause skin sensitization, fragrances and skin-conditioning agents were most common, with 24% and 22% usage frequency, respectively. They were followed by surfactants and preservatives with 15% and 13%, respectively. The remaining ingredients were botanical derivatives, UV filters, antioxidants, and pH adjusters with less than 10% usage.
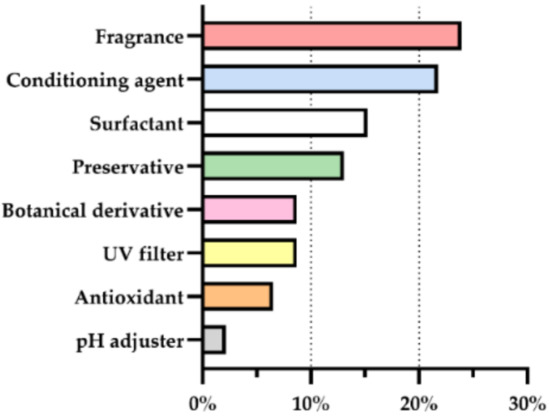
Figure 5.
Function of allergens other than fragrances present in the analyzed cosmetic products for sensitive skin, in percentage. (UV = ultraviolet).
The INCI names “parfum” or “aroma” were found in 64% of products’ labels.
4. Discussion
Cosmetic products, especially leave-on products, are in close contact with the skin for long periods of time, and they may trigger development of allergic reactions [34]. Individuals with sensitive skin have a reduced tolerance to the application of cosmetic products, being more prone to the appearance of multiple symptoms such as tightness, burning, tingling, pain, pruritus, and stinging [1], and to being diagnosed with allergic reactions [24,25]. Some ingredients, such as anti-inflammatory and antioxidant ingredients, are added to cosmetic formulations to reduce individuals’ symptoms and counter allergen potential.
In this study, the labels of cosmetic products for sensitive skin were analyzed regarding the presence of skin allergens. From the 88 products under study, only six were formulated with fragrance allergens present in the Annex III of EU Cosmetic Regulation 1223/2009, all being substances with a well-characterized allergenic potential. Linalool was the most common fragrance allergen, which is expected, since it is the most used fragrance substance, together with limonene [35]. On the contrary, limonene was one of the allergens with the lowest usage percentage. When they were present in products’ formulations, most of these ingredients were incorporated in the same formulation, creating combinations of allergens that can potentiate synergistic effects, increasing the risk of sensitization [36,37].
Fragrances are the most common cause of allergic-contact dermatitis, despite being widely used in cosmetic products [38]. From the 88 products analyzed, 32 contained “parfum”, affirming the presence of fragrances on their composition. According to the SCCS in its opinion on fragrance allergens in cosmetic products (SCCS/1459/11) [39], allergen fragrances go beyond the 26 that have to be listed in labels, and consumers must be aware of them. Therefore, alterations to the previous regulation are under study to expand the list of 26 fragrances to 87, but this modification is yet to come into force. The implementation of these alterations may increase the number of allergens that ought to be listed on cosmetic products’ labels [40].
New allergens are always appearing, and their identification is usually based on case notifications under the Cosmetovigillance system and reports by dermatologists/imunoalergologists [41]. Most of the analyzed products (95%) contained at least one allergen, and 78% contained allergens combinations that may further increase the occurrence of skin allergies. They were distributed into seven functions, where skin-conditioning agents, surfactants, and preservatives proved to be the most prevalent.
Seven allergens with conditioning-agent functions were found, with pentylene glycol being the most frequent. This 1,2-glycol, mostly used as a skin-conditioning agent and as a preservative [42], is considered a rare allergen, based on Local Lymph Node Assay results, with few cases of sensitization described in the literature [43,44]. Contact allergy to butylene glycol, frequently used due to its moisturizing and antibacterial properties, is not very common, but some cases were reported [45,46,47]. Regarding ethylhexylglycerin, with humectant, surfactant, emollient, and preservative properties, several allergic reactions were described [44,48,49], with it considered a moderate allergen based on Local Lymph Node Assay results [50]. C12-15 alkyl benzoate has emollient, antimicrobial, and skin-conditioning properties and can be an occasional sensitizer, with at least one case of contact allergy reported [51]. Scientific evidence supporting the allergenic character of the remaining conditioning agents, cetearyl isononanoate, cetearyl ethylhexanoate, panthenol, capryloyl glycine, and cetyl PEG/PPG-10/1 dimethicone, was also found [52,53,54,55,56,57,58]. Despite being used in some skin conditions for restoring skin-barrier function, lanolin has been associated with some cases of sensitization [59,60].
Surfactants were the third allergens function with the highest percentage, with cetyl alcohol and cetearyl alcohol being the two most frequently noted. Despite their wide use in cosmetics as emulsion stabilizers, surfactants, viscosity controllers, and opacifying agents [61], they are associated with cases of skin sensitization [62,63,64,65]. Decyl glucoside, arachidyl glucoside, cetearyl glucoside, and coco glucoside are alkyl glucosides, a family of surfactants elected “Allergen of the Year” in 2017 by the American Contact Dermatitis Society [66]. They are not potent sensitizers based on Local Lymph Node Assay results, but individuals with atopic skin or multiple skin allergies might have an increased risk of sensitization [66,67]. Sodium cetearyl sulfate, mostly used as a cleansing agent [68], is considered an extremely rare sensitizer based on Local Lymph Node Assay results, but at least one case of allergic-contact dermatitis was already reported [69].
The incidence of surfactants was followed by preservatives, one of the most frequent causes of allergic-contact dermatitis due of cosmetics [70]. In studies involving animals and humans, chlorphenesin, a chlorophenol derivative with antimicrobial properties [71], was not a sensitizer, nor a photosensitizer. However, some cases of skin irritation and eczema were described [72,73,74,75]. A positive reaction to potassium sorbate, a rare skin sensitizer with fungistatic activity, might reflect a sensitization to sorbic acid, meaning that consumers with sorbic-acid sensitivity must also avoid products containing potassium sorbate [76]. Sodium hydroxymethylglycinate and diazolidinyl urea are both formaldehyde-releasing preservatives. In Europe, the probability of sensitization to diazolidinyl urea occurring ranges between 0.5 to 1.4%, and about 24 to 75% of these reactions are relevant [22,77]. In vivo studies involving sodium hydroxymethylglycinate demonstrated its potential to cause skin sensitization [78]. In 2021, SCCS recommended the concentration reduction of these products to 0.001% to protect consumers sensitive to formaldehyde [79]. Triclosan, along with other 27 substances also suspected to be endocrine disruptors, is being analyzed by the SCCS regarding its safety for human health. Three other allergens present in this analysis, ethylhexyl methoxycinnamate, octocrylene, and benzophenone-4, are being evaluated for the same reasons [80,81]. The presence of sodium metabisulfite in cosmetics for sensitive skin is a concern, since the prevalence of allergic reactions to this preservative increased from 2.24% to 3.42% between 2011 and 2019 [82].
With awareness of the sun’s damaging effects, the incorporation of UV filters into everyday cosmetics besides sunscreens is increasing [83]. Four UV filters were noticed, with ethylhexyl methoxycinnamate being the most frequent. As triclosan, this ingredient has been associated to modifications on the endocrine system [84]. Octocrylene is considered a moderate sensitizer based on Local Lymph Node Assay results, with the occurrence of sensitization in those with sunscreen intolerance between 0.7 and 5% [85,86]. It is commonly associated with photoallergic-contact dermatitis, being responsible for approximately 80% of all reactions, which are probably secondary to previous photosensitization to ketoprofen [87]. In March 2021, SCCS considered octocrylene safe to be used in cosmetics regarding its contact sensitization and potential endocrine-disrupting properties [88]. Ethylhexyl salicylate is a UVB absorber and an uncommon allergen despite the existence of some case reports in the literature. Moreover, it can also cross-react with a common fragrance component, benzyl salicylate [89]. Benzophenones were considered the Allergen of the Year in 2004 by the American Contact Dermatitis Society, with benzophenone-3 being the leading allergen. Benzophenone-4 has been used to substitute benzophenone-3, but cases of skin sensitization may be increasing [85]. In a study, benzophenone-4 produced a higher number of positive patch-test results compared with other chemical UV filters tested, and was the third most-frequent positive test result overall [83].
Some consumers believe cosmetic products with natural ingredients, principally botanical derivatives, are safer and more beneficial for health and environment, increasing their preference [90]. However, these complex mixtures of chemicals may cause skin sensitization [91]. Avena sativa (oat) extracts can improve barrier function due to their anti-inflammatory, antioxidant, and antipruritic properties [90]. Despite all the benefits, cases of skin sensitization to oat were already reported [92,93], and the risk of being sensitized increased with an impaired skin barrier [90]. Butyrospermum parkii provides moisturizing and barrier-protective properties [94,95], but crude extracts of shea nuts might have allergenic properties [94], and a positive patch test in a patient suffering from cosmetic dermatitis was described [29]. Bisabolol, used in numerous cosmetics due to its anti-inflammatory, antibacterial, and skin-soothing properties [96], is being considered an allergen due to the report of allergic-contact dermatitis cases [96,97]. A case report of an allergic-contact dermatitis caused by Rosa canina, an ingredient with antioxidant and regenerative properties, has already been documented [98].
Analyzing antioxidants, tocopheryl acetate is considered a nonsensitizer [99], but it is one of the suspects for sensitization in a case report of contact dermatitis [100]. Ascorbyl tetraisopalmitate, considered nonirritant and nonsensitizer, and propyl gallate, with high sensitizing potential, also demonstrated their capability to cause contact dermatitis [101,102,103].
Despite not being considered an allergen, a reported case of allergic-contact dermatitis was hypothesized to be linked to the pH adjuster aminomethyl propanol [104].
Overall, although these ingredients are safe, several case reports regarding skin sensitization are found in the literature. These findings unveil a high number of allergens in cosmetic products for sensitive skin, which may pose concerns.
5. Conclusions
It is estimated that a major part of the global population suffers from sensitive skin, and the cosmetic industry formulates products especially designed for this condition which are intended to avoid and minimize its symptoms. The presence of allergens in cosmetic formulations is not related with the pathophysiology of sensitive skin, but a higher probability of developing skin allergies has been described.
Fragrance allergens were found in a minority of products (7%) included in this study, and of those, the majority were present in allergen combinations (5%), with the remaining products containing only one ingredient (2%). The fragrances that presented the highest percentages were linalool, benzyl alcohol, geraniol, and citronellol. Noteworthy, benzyl alcohol may also be used as a preservative ingredient. Other allergens were grouped in seven categories according to their function, and after fragrances, the most prevalent group, in descending order, were skin-conditioning agents, surfactants, preservatives, botanical derivatives, UV filters, antioxidants, and pH adjusters. In the pool of analyzed products, 35 allergens were found, and 12 were present in more than 10% of the products, those being pentylene glycol, tocopheryl acetate, chlorphenesin, butylene glycol, cetyl alcohol, Avena sativa derivatives, Butyrospermum parkii butter, ethylhexylglycerin, cetearyl alcohol, potassium sorbate, C12-15 alkyl benzoate, and ethylhexyl methoxycinnamate. Moreover, these allergens were present mainly in combinations (78%) and the remaining (17%) were present alone in the formulation.
Overall, it was noted that most cosmetic formulations were absent of fragrances recognized as allergens. Allergens other than fragrances were present in most products. Of those, the majority were considered rare skin sensitizers, with few cases of skin sensitization reported. It is also important to note that the usage concentrations in cosmetic products of these ingredients were not disclosed in the labels, and published reports of reactions at high concentrations may not be relevant when the ingredients are used at lower levels. Despite allergens being found in cosmetics for sensitive skin, these allergens do not cause sensitive skin, although they may induce sensitized/allergic skin in rare cases.
This work unveils the most-used allergens in cosmetic products for sensitive skin marketed in Europe, and the scientific evidence supporting their sensitization risk. This insight highlights the importance of reading labels prior to suggesting or using cosmetic products, contributing to the avoidance of skin allergens by susceptible consumers.
6. Limitations
This study was performed in a pool of cosmetic products of the Portuguese cosmetic market (pharmacies and parapharmacies), but all from multinational cosmetic brands. This may, however, result in discrepancies when compared with other markets. From the listed allergens, some compounds are widely recognized as such, through regulations and patch-testing series, while others were identified through isolated case reports. Moreover, the concentrations of these ingredients are undisclosed, and published reports of reactions at high concentrations may not be relevant at lower concentration levels. Therefore, their ability to elicit skin allergies or aggravate symptoms of sensitive skin is difficult to estimate.
Author Contributions
Conceptualization, I.F.A.; methodology, M.S.F.; investigation, M.S.M.; data curation, M.S.F.; writing—original draft preparation, M.S.M.; writing—review and editing, I.F.A., E.S. and M.S.F.; supervision, I.F.A. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is also supported by national funds through FCT within the scope of UIDB/04423/2020, UIDP/04423/2020 (Group of Natural Products and Medicinal Chemistry-CIIMAR), as well as the structured program of R&D&I ATLANTIDA—Platform for the monitoring of the North Atlantic Ocean and tools for the sustainable exploitation of the marine resources (reference NORTE-01-0145-FEDER-000040), supported by the North Portugal Regional Operational Programme (NORTE2020) through the European Regional Development Fund (ERDF). Marta Salvador Ferreira and Márcia Silva Martins acknowledge the Ph.D. research grants SFRH/BD/144864/2019 and 2021.05964.BD, respectively, fully supported by national funding by FCT, Foundation for Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is supported by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Misery, L.; Loser, K.; Ständer, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.; Silveira, J.E.P.S.; Hafner, M.d.F.S.; Toyota, R.; Pedroso, D.M.M. Sensitive skin: Review of an ascending concept. An. Bras. Dermatol. 2017, 92, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Sousa Lobo, J.M.; Almeida, I.F. Sensitive skin: Active ingredients on the spotlight. Int. J. Cosmet. Sci. 2021, 44, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis. Cosmetics 2021, 8, 81. [Google Scholar] [CrossRef]
- Cho, H.J.; Chung, B.Y.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J. Dermatol. 2012, 39, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Voegeli, R.; Rawlings, A.V.; Doppler, S.; Imfeld, D.; Munday, M.R.; Lane, M.E. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int. J. Cosmet. Sci. 2017, 39, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef]
- Richters, R.J.; Falcone, D.; Uzunbajakava, N.E.; Varghese, B.; Caspers, P.J.; Puppels, G.J.; van Erp, P.E.; van de Kerkhof, P.C. Sensitive Skin: Assessment of the Skin Barrier Using Confocal Raman Microspectroscopy. Skin Pharmacol. Physiol. 2017, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Schneider, S.W.; Weishaupt, C.; Luger, T.A.; Misery, L. Putative neuronal mechanisms of sensitive skin. Exp. Dermatol. 2009, 18, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Buhé, V.; Vié, K.; Guéré, C.; Natalizio, A.; Lhéritier, C.; Le Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm. Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Montiel, A.; Camprubí-Robles, M.; García-Sanz, N.; Sempere, A.; Valente, P.; Van Den Nest, W.; Carreño, C. The contribution of neurogenic inflammation to sensitive skin: Concepts, mechanisms and cosmeceutical intervention. Int. J. Cosmet. Sci. 2009, 31, 477. [Google Scholar] [CrossRef]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Weisshaar, E.; Brenaut, E.; Evers, A.W.M.; Huet, F.; Ständer, S.; Reich, A.; Berardesca, E.; Serra-Baldrich, E.; Wallengren, J.; et al. Pathophysiology and management of sensitive skin: Position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ehnis-Pérez, A.; Torres-Álvarez, B.; Cortés-García, D.; Hernández-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Keum, H.L.; Kim, H.; Kim, H.-J.; Park, T.; Kim, S.; An, S.; Sul, W.J. Structures of the Skin Microbiome and Mycobiome Depending on Skin Sensitivity. Microorganisms 2020, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liang, H.; Li, Z.; Tang, M.; Song, L. Skin microbiome in sensitive skin: The decrease of Staphylococcus epidermidis seems to be related to female lactic acid sting test sensitive skin. J. Dermatol. Sci. 2020, 97, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Hillion, M.; Mijouin, L.; Jaouen, T.; Barreau, M.; Meunier, P.; Lefeuvre, L.; Lati, E.; Chevalier, S.; Feuilloley, M.G. Comparative study of normal and sensitive skin aerobic bacterial populations. Microbiologyopen 2013, 2, 953–961. [Google Scholar] [CrossRef]
- Huet, F.; Misery, L. Sensitive skin is a neuropathic disorder. Exp. Dermatol. 2019, 28, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Alani, J.I.; Davis, M.D.; Yiannias, J.A. Allergy to cosmetics: A literature review. Dermatitis 2013, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, P.; Conde-Salazar, L.; Vañó-Galván, S. Allergic Contact Dermatitis Caused by Cosmetic Products. Actas Dermosifiliogr. 2014, 105, 822–832. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament; The Council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, L342, 59. [Google Scholar]
- Richters, R.; Falcone, D.; Uzunbajakava, N.; Verkruysse, W.; van Erp, P.; van de Kerkhof, P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol. Physiol. 2015, 28, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. Self-reported immunological and familial links in individuals who perceive they have sensitive skin. Br. J. Dermatol. 2008, 159, 237–238. [Google Scholar] [CrossRef]
- Chen, W.; Dai, R.; Li, L. The prevalence of self-declared sensitive skin: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- FDA. Allergens in Cosmetics. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/allergens-cosmetics (accessed on 15 February 2022).
- Acer, E.; Kaya Erdogan, H.; Batan, T.; Saracoglu, Z.N. European Standard Series Patch Test Results in Contact Dermatitis Patients in a Tertiary Care Hospital. Med. Bull. Sisli Etfal Hosp. 2020, 54, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Goossens, A. Cosmetic Contact Allergens. Cosmetics 2016, 3, 5. [Google Scholar] [CrossRef]
- Uter, W.; Werfel, T.; Lepoittevin, J.-P.; White, I.R. Contact Allergy-Emerging Allergens and Public Health Impact. Int. J. Environ. Res. Public Health 2020, 17, 2404. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review. Available online: https://www.cir-safety.org/ (accessed on 7 February 2022).
- Scientific Committee on Consumer Safety (SCCS)—Opinions. Available online: https://ec.europa.eu/health/scientific-committees/scientific-committee-consumer-safety-sccs/sccs-opinions_en (accessed on 7 February 2022).
- CosIng. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.simple (accessed on 15 February 2022).
- Laguna, C.; de la Cuadra, J.; Martín-González, B.; Zaragoza, V.; Martínez-Casimiro, L.; Alegre, V. Allergic Contact Dermatitis to Cosmetics. Actas Dermosifiliogr. 2009, 100, 53–60. [Google Scholar] [CrossRef]
- Bennike, N.H.; Oturai, N.B.; Müller, S.; Kirkeby, C.S.; Jørgensen, C.; Christensen, A.B.; Zachariae, C.; Johansen, J.D. Fragrance contact allergens in 5588 cosmetic products identified through a novel smartphone application. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 79–85. [Google Scholar] [CrossRef]
- Johansen, J.D.; Skov, L.; Volund, A.; Andersen, K.; Menné, T. Allergens in combination have a synergistic effect on the elicitation response: A study of fragrance-sensitized individuals. Br. J. Dermatol. 1998, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld, C.M.; Nielsen, M.M.; Rubin, I.M.; Vennegaard, M.T.; Dabelsteen, S.; Gimenéz-Arnau, E.; Lepoittevin, J.P.; Geisler, C.; Johansen, J.D. Enhanced sensitization and elicitation responses caused by mixtures of common fragrance allergens. Contact Derm. 2011, 65, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zug, K.A. Fragrance allergic contact dermatitis. Dermatitis 2014, 25, 232–245. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Fragrance Allergens in Cosmetic Products. 2012. SCCS/1459/11. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_102.pdf (accessed on 20 February 2022).
- Labelling Fragrance Allergens. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/2009-Labelling-fragrance-allergens_en (accessed on 27 January 2022).
- Davies, R.F.; Johnston, G.A. New and emerging cosmetic allergens. Clin. Dermatol. 2011, 29, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of 1,2-glycols as used in cosmetics. Int. J. Toxicol. 2012, 31, 147s–168s. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Schenetti, C.; Schettini, N.; Zedde, P.; Borghi, A. Pentylene glycol: An emerging cosmetic allergen? Contact Derm. 2022, 86, 44–46. [Google Scholar] [CrossRef]
- Mortz, C.G.; Otkjaer, A.; Andersen, K.E. Allergic contact dermatitis to ethylhexylglycerin and pentylene glycol. Contact Derm. 2009, 61, 180. [Google Scholar] [CrossRef]
- Aizawa, A.; Ito, A.; Masui, Y.; Ito, M. Case of allergic contact dermatitis due to 1,3-butylene glycol. J. Dermatol. 2014, 41, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.; Johansen, J.D. Allergic contact dermatitis due to butylene glycol in cosmetics. Contact Derm. 2020, 83, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Hayakawa, R. Contact dermatitis due to 1,3-butylene glycol. Contact Derm. 1997, 37, 90. [Google Scholar] [CrossRef]
- Aerts, O.; Verhulst, L.; Goossens, A. Ethylhexylglycerin: A low-risk, but highly relevant, sensitizer in ‘hypo-allergenic’ cosmetics. Contact Derm. 2016, 74, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, D.; Stanciu, M. Allergic contact dermatitis from ethylhexylglycerin in sunscreens. Dermatitis 2014, 25, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Canavez, A.; de Oliveira Prado Corrêa, G.; Isaac, V.L.B.; Schuck, D.C.; Lorencini, M. Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives. J. Appl. Toxicol. 2021, 41, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Werbrouck, J.; Lambrecht, C.; Goossens, A. C12–15 alkyl benzoate: A new cosmetic allergen? Contact Derm. 2015, 73, 249–250. [Google Scholar] [CrossRef]
- Álvarez-Chinchilla, P.; Poveda-Montoyo, I.; González, I.; Silvestre, J.F. Cetearyl isononanoate, an underestimated contact allergen? Contact Derm. 2018, 79, 243–244. [Google Scholar] [CrossRef]
- Ito, K.; Fujimura, N.; Uchida, T.; Ikezawa, Z.; Aihara, M. Contact dermatitis with systemic reactions caused by cetearyl isononanoate. Contact Derm. 2013, 69, 315–316. [Google Scholar] [CrossRef]
- Chin, M.F.; Hughes, T.M.; Stone, N.M. Allergic contact dermatitis caused by panthenol in a child. Contact Derm. 2013, 69, 321–322. [Google Scholar] [CrossRef]
- Fernandes, S.; Macias, V.; Cravo, M.; Amaro, C.; Santos, R.; Cardoso, J. Allergic contact dermatitis caused by dexpanthenol: Report of two cases. Contact Derm. 2012, 66, 160–161. [Google Scholar] [CrossRef]
- Mangodt, E.A.; Dendooven, E.; De Fré, C.; Lambert, J.; Aerts, O. Capryloyl glycine: A polyfunctional cosmetic ingredient and potential skin sensitizer. Contact Derm. 2019, 80, 400–402. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.-A.; Gatica-Ortega, M.-E.; Alcántara-Nicolás, F.-D.-A.; Pérez-Mesonero, R.; Gil-Redondo, R.; Martín-Alcalde, E.; De Eusebio, E. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Derm. 2018, 78, 236–239. [Google Scholar] [CrossRef]
- Roberts, H.; Williams, J.; Tate, B. Allergic contact dermatitis to panthenol and cocamidopropyl PG dimonium chloride phosphate in a facial hydrating lotion. Contact Derm. 2006, 55, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Overgaard, L.E.K.; Johansen, J.D.; Thyssen, J.P. Contact allergy to lanolin: Temporal changes in prevalence and association with atopic dermatitis. Contact Derm. 2018, 78, 70–75. [Google Scholar] [CrossRef]
- McFadden, J.; Puangpet, P.; Pongpairoj, K.; Thaiwat, S.; Lee, S.X. (Eds.) Lanolin. In Common Contact Allergens: A Practical Guide to Detecting Contact Dermatitis, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 311–315. [Google Scholar]
- McFadden, J.; Puangpet, P.; Pongpairoj, K.; Thaiwat, S.; Lee, S.X. (Eds.) Cetearyl Alcohol. In Common Contact Allergens: A Practical Guide to Detecting Contact Dermatitis, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 317–320. [Google Scholar]
- Aakhus, A.E.; Warshaw, E.M. Allergic Contact Dermatitis from Cetyl Alcohol. Dermatitis 2011, 22, 56–57. [Google Scholar] [CrossRef]
- Kiec-Swierczynska, M.; Krecisz, B.; Swierczynska-Machura, D. Photoallergic and allergic reaction to 2-hydroxy-4-methoxybenzophenone (sunscreen) and allergy to cetyl alcohol in cosmetic cream. Contact Derm. 2005, 53, 170–171. [Google Scholar] [CrossRef]
- Soga, F.; Katoh, N.; Kishimoto, S. Contact dermatitis due to lanoconazole, cetyl alcohol and diethyl sebacate in lanoconazole cream. Contact Derm. 2004, 50, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Oiso, N.; Fukai, K.; Ishii, M. Concomitant allergic reaction to cetyl alcohol and crotamiton. Contact Derm. 2003, 49, 261. [Google Scholar] [CrossRef]
- Sasseville, D. Alkyl Glucosides: 2017 “Allergen of the Year”. Dermatitis 2017, 28, 296. [Google Scholar] [CrossRef]
- Loranger, C.; Alfalah, M.; Le Bouedec, M.C.F.; Sasseville, D. Alkyl Glucosides in Contact Dermatitis. Dermatitis 2017, 28, 5–13. [Google Scholar] [CrossRef]
- Fiume, M.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Alan Andersen, F. Final report on the safety assessment of sodium cetearyl sulfate and related alkyl sulfates as used in cosmetics. Int. J. Toxicol. 2010, 29, 115s–132s. [Google Scholar] [CrossRef]
- Oscoz-Jaime, S.; Hervella-Garcés, M.; de Espronceda-Ezquerro, I.M.; Yanguas-Bayona, J.I. Allergic contact dermatitis caused by sodium cetearyl sulfate. Contact Derm. 2018, 78, 426–427. [Google Scholar] [CrossRef]
- Lee, S.S.; Hong, D.K.; Jeong, N.J.; Lee, J.H.; Choi, Y.S.; Lee, A.Y.; Lee, C.H.; Kim, K.J.; Park, H.Y.; Yang, J.M.; et al. Multicenter study of preservative sensitivity in patients with suspected cosmetic contact dermatitis in Korea. J. Dermatol. 2012, 39, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Chlorphenesin as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 5s–15s. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.L.; Orton, D.I. Two cases of facial dermatitis due to chlorphenesin in cosmetics. Contact Derm. 2005, 52, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.A. Allergic contact sensitivity to chlorphenesin. Contact Derm. 1986, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.H.; White, I.R. Dermatitis from chlorphenesin in a facial cosmetic. Contact Derm. 1997, 37, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Dyring-Andersen, B.; Elberling, J.; Duus Johansen, J.; Zachariae, C. Contact allergy to chlorphenesin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1019. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, E.; Kerre, S.; Foubert, K.; Pieters, L.; Lambert, J.; Goossens, A.; Aerts, O. Allergic contact dermatitis from potassium sorbate and sorbic acid in topical pharmaceuticals and medical devices. Contact Derm. 2021, 85, 171–177. [Google Scholar] [CrossRef]
- De Groot, A.C.; White, I.R.; Flyvholm, M.-A.; Lensen, G.; Coenraads, P.-J. Formaldehyde-releasers in cosmetics: Relationship to formaldehyde contact allergy. Contact Derm. 2010, 62, 2–17. [Google Scholar] [CrossRef]
- Russell, K.; Jacob, S.E. Sodium hydroxymethylglycinate. Dermatitis 2010, 21, 109–110. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Scientific Advice on the Threshold for the Warning ‘Contains Formaldehyde’ in Annex V, Preamble point 2 for Formaldehyde-Releasing Substances. 2021. SCCS/1632/21. Available online: https://ec.europa.eu/health/system/files/2021-05/sccs_o_254_0.pdf (accessed on 20 February 2022).
- Call for Data on Ingredients with Potential Endocrine-Disrupting Properties Used in Cosmetic Products. Available online: https://ec.europa.eu/newsroom/growth/items/702447/en (accessed on 27 January 2022).
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; Guido, M.; Congedo, M. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Latheef, F.; Wilkinson, M. Trends in preservative allergy: Benzisothiazolinone emerges from the pack. Contact Derm. 2021, 85, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Stone, N.M. Benzophenone 4: An emerging allergen in cosmetics and toiletries? Contact Derm. 2007, 56, 153–156. [Google Scholar] [CrossRef]
- Manová, E.; von Goetz, N.; Hungerbuehler, K. Aggregate consumer exposure to UV filter ethylhexyl methoxycinnamate via personal care products. Environ. Int. 2015, 74, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Cheng, H. Ultraviolet filter, fragrance and preservative allergens in New Zealand sunscreens. Aust. J. Dermatol. 2021, 63, E21–E25. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Vanden Broecke, K.; Mårtensson, J.; Goossens, A.; Börje, A. Clinical and experimental studies of octocrylene’s allergenic potency. Contact Derm. 2011, 64, 343–352. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.; Roberts, D. Contact and photocontact allergy to octocrylene: A review. Contact Derm. 2014, 70, 193–204. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Octocrylene (CAS No 6197-30-4, EC No 228-250-8). 2021. SCCS/1627/21. Available online: https://ec.europa.eu/health/system/files/2021-04/sccs_o_249_0.pdf (accessed on 20 February 2022).
- Dens, A.-C.; Goossens, A.; Darcis, J.; Huygens, S.; Lambrecht, C.; Gilissen, L. Allergic contact dermatitis caused by ethylhexyl salicylate with possible cross-reactivity with benzyl salicylate. Contact Derm. 2019, 81, 317–318. [Google Scholar] [CrossRef]
- Bruusgaard-Mouritsen, M.A.; Johansen, J.D.; Zachariae, C.; Kirkeby, C.S.; Garvey, L.H. Natural ingredients in cosmetic products-A suggestion for a screening series for skin allergy. Contact Derm. 2020, 83, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Jorizzo, M.; Parente, G.; Tosti, A. Allergic contact dermatitis due to avena extract. Contact Derm. 2000, 42, 364. [Google Scholar]
- Vansina, S.; Debilde, D.; Morren, M.-A.; Goossens, A. Sensitizing oat extracts in cosmetic creams: Is there an alternative? Contact Derm. 2010, 63, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J., Jr.; Silverberg, N. Active naturals have a key role in atopic dermatitis. Semin. Cutan. Med. Surg. 2008, 27, 8–10. [Google Scholar] [CrossRef]
- Andersson, A.-C.; Alander, J. Shea butter extract for bioactive skin care. Cosmet. Toilet. 2015, 130, 18–25. [Google Scholar]
- Jacob, S.E.; Matiz, C.; Herro, E.M. Compositae-associated allergic contact dermatitis from bisabolol. Dermatitis 2011, 22, 102–105. [Google Scholar] [CrossRef]
- Jacob, S.E.; Hsu, J.W. Reactions to Aquaphor: Is bisabolol the culprit? Pediatr. Dermatol. 2010, 27, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Ibernón, G.; Schneller-Pavelescu, L.; Silvestre-Salvador, J.F. Allergic contact dermatitis caused by “Rosa mosqueta” oil. Contact Derm. 2018, 79, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Zondlo Fiume, M. Final Report on the Safety Assessment of Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, Potassium Ascorbyl Tocopheryl Phosphate, and Tocophersolan. Int. J. Toxicol. 2002, 21 (Suppl. S3), 51–116. [Google Scholar] [CrossRef] [PubMed]
- Ohko, K.; Ito, A.; Ito, M. Allergic Contact Dermatitis Syndrome Due to Tocopherol Acetate, in Addition to Glycyrrhetinic Acid. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 38–40. [Google Scholar] [CrossRef][Green Version]
- Perez, A.; Basketter, D.A.; White, I.R.; McFadden, J. Positive rates to propyl gallate on patch testing: A change in trend. Contact Derm. 2008, 58, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Assier, H.; Wolkenstein, P.; Grille, C.; Chosidow, O. Contact dermatitis caused by ascorbyl tetraisopalmitate in a cream used for the management of atopic dermatitis. Contact Derm. 2014, 71, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, I.; Goossens, A. Allergic contact dermatitis caused by ascorbyl tetraisopalmitate. Contact Derm. 2011, 64, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Geier, J.; Forkel, S.; Heetfeld, A.; Lessmann, H.; Buhl, T. Contact allergy to 2-amino-2-methyl-1-propanol in a metalworking fluid. Contact Derm. 2019, 80, 323–324. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).