Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Data Collection
2.1.2. Participants
2.1.3. Source of Vibrational Stimuli
2.2. Statistical Analysis
2.3. Ethics
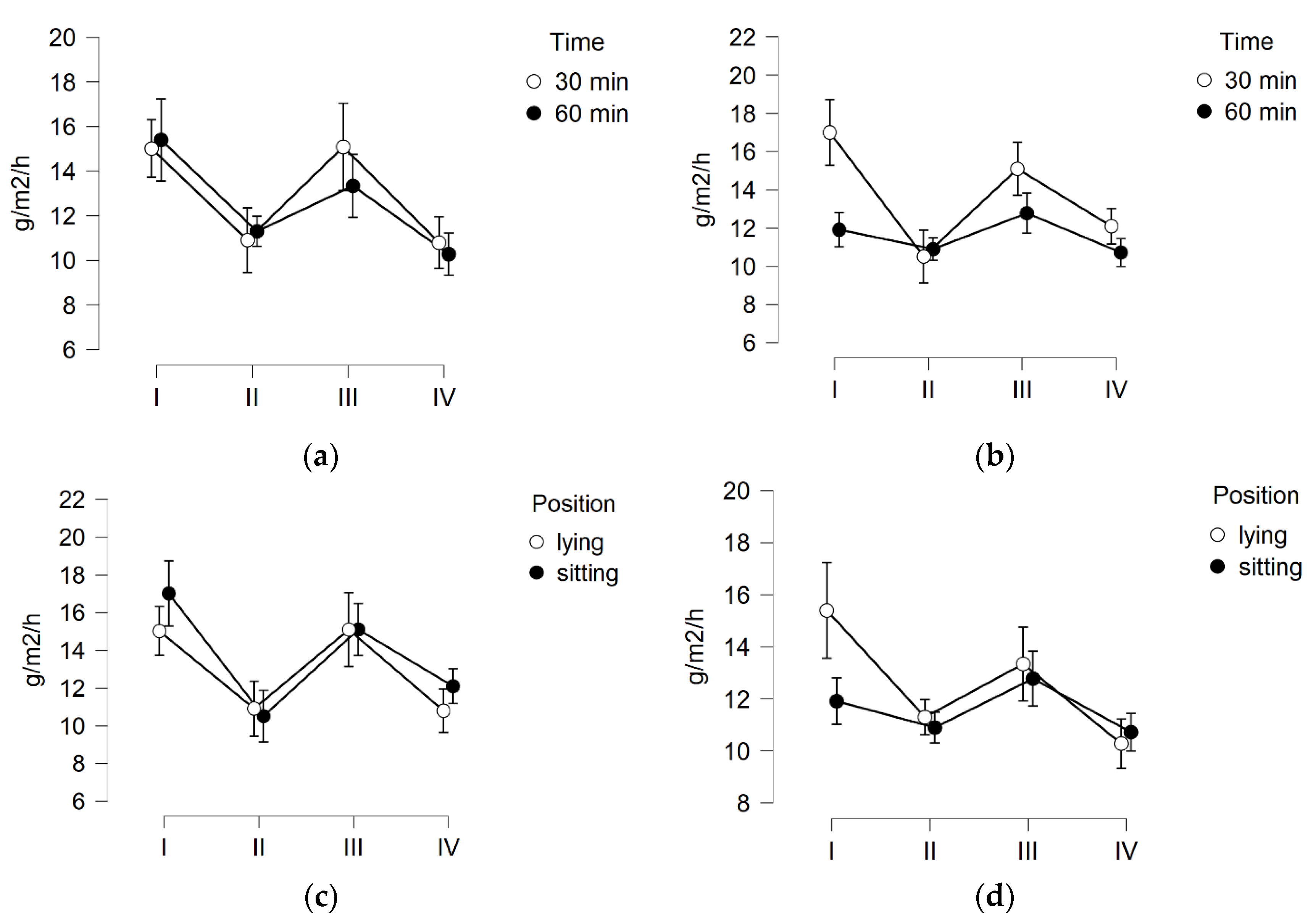
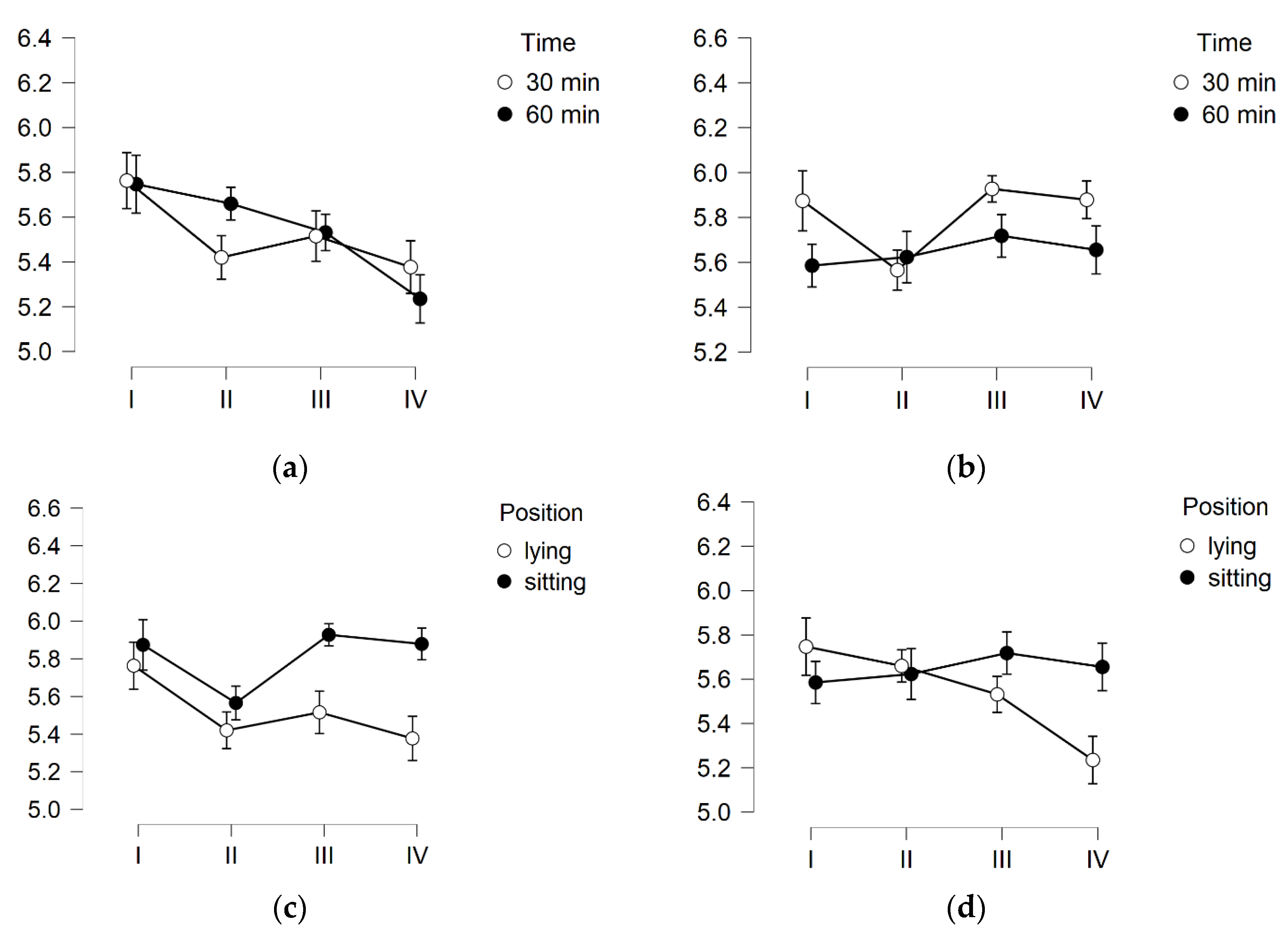
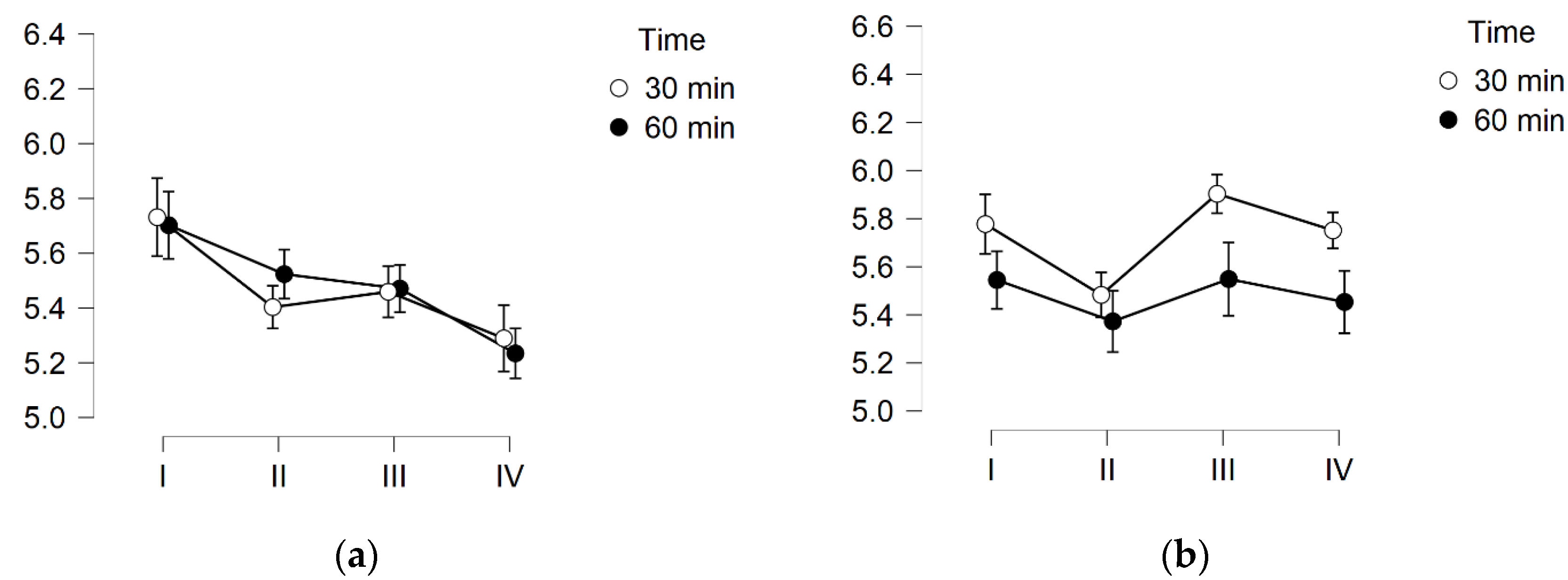
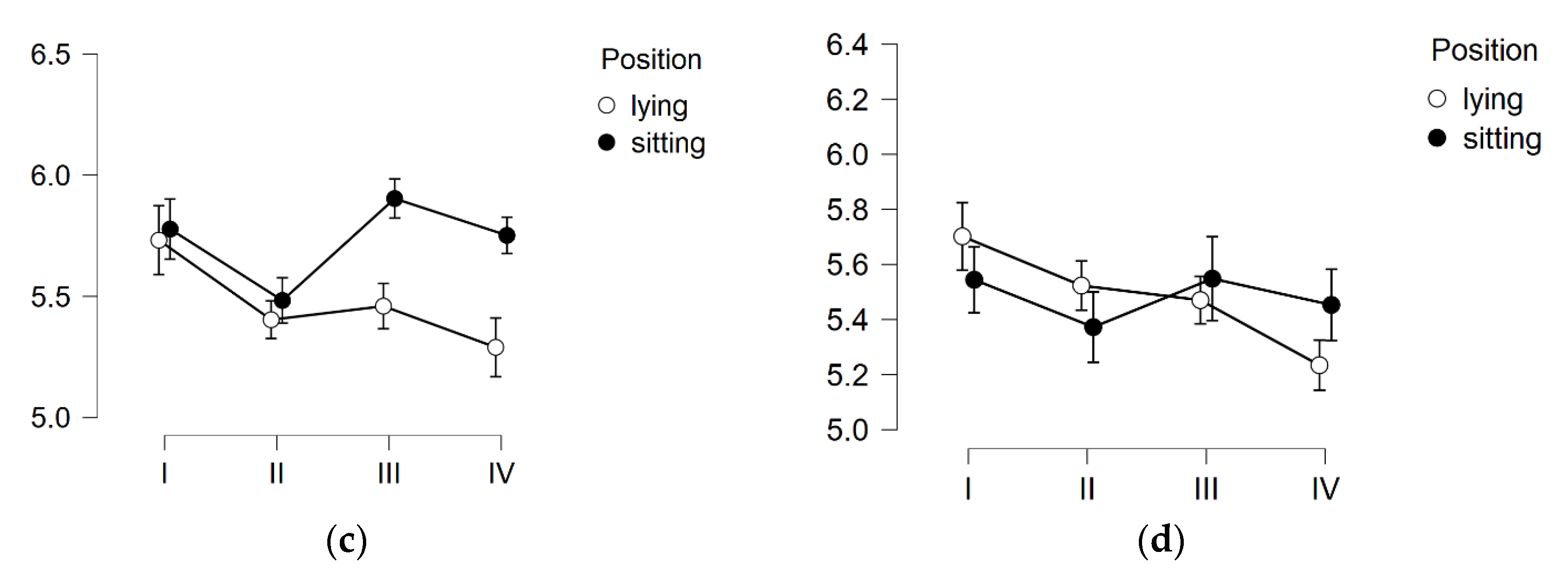
3. Results
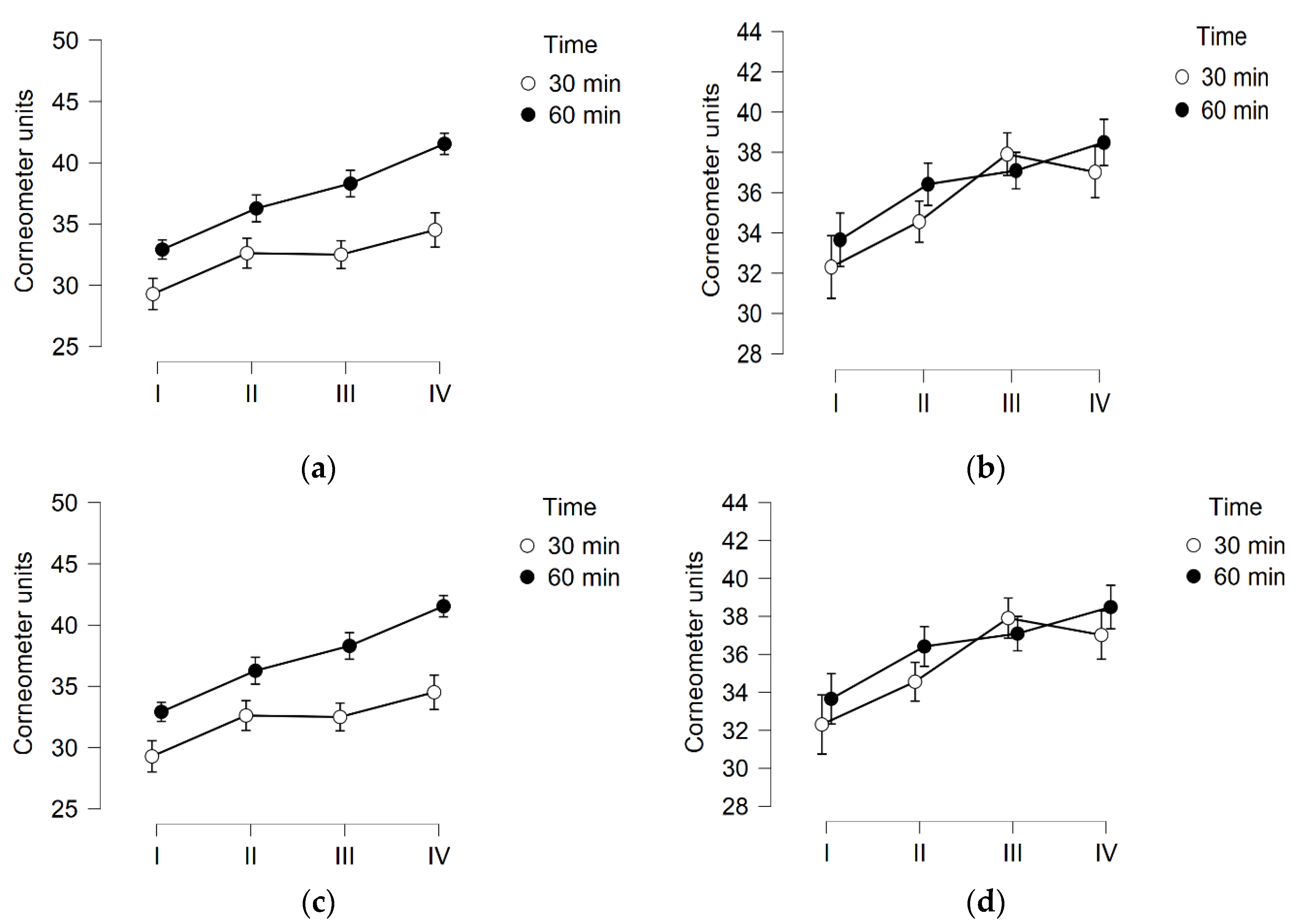
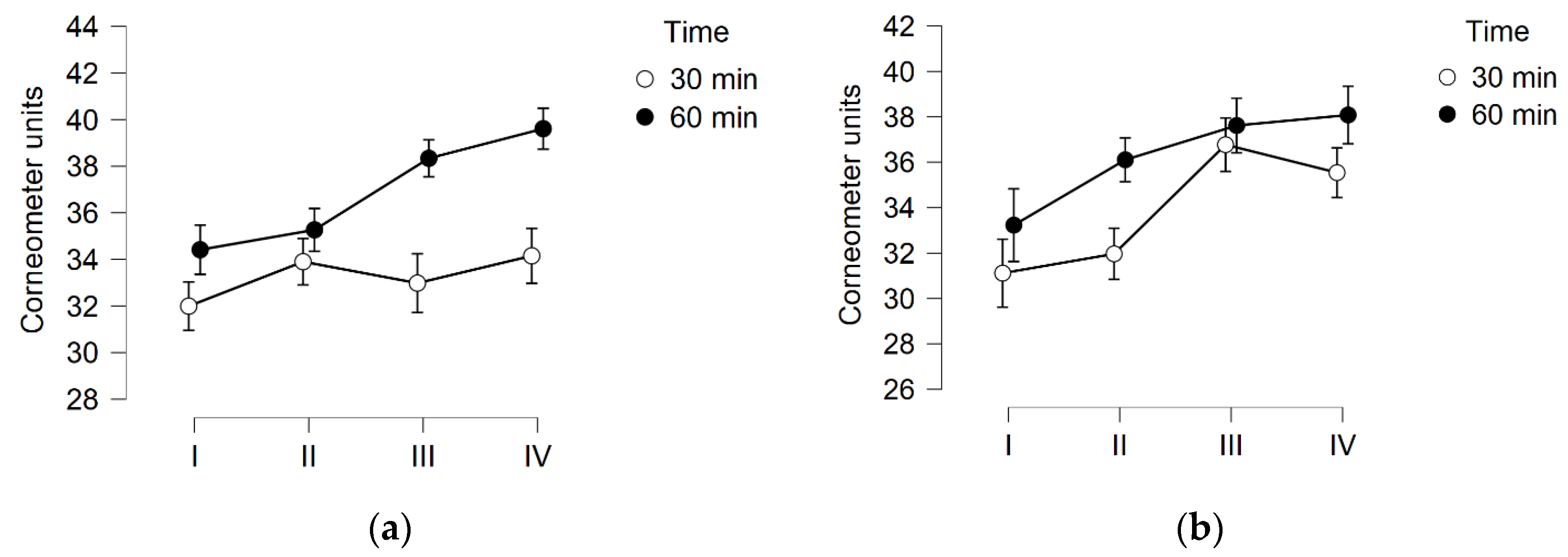
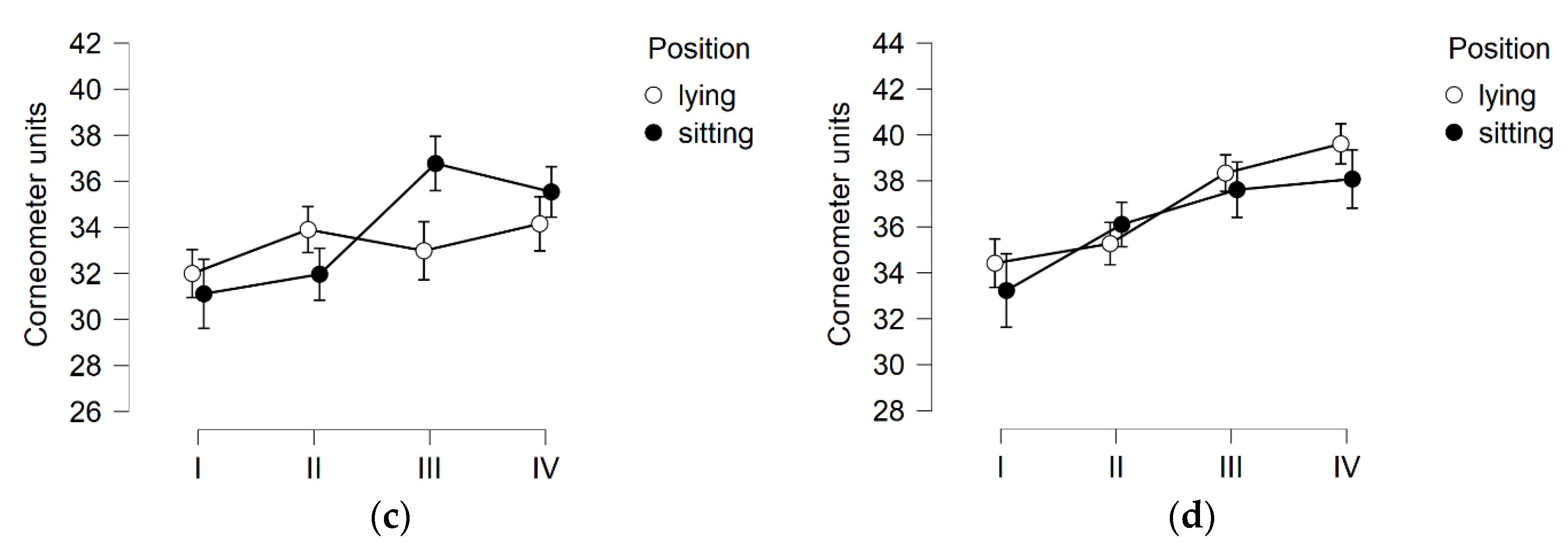
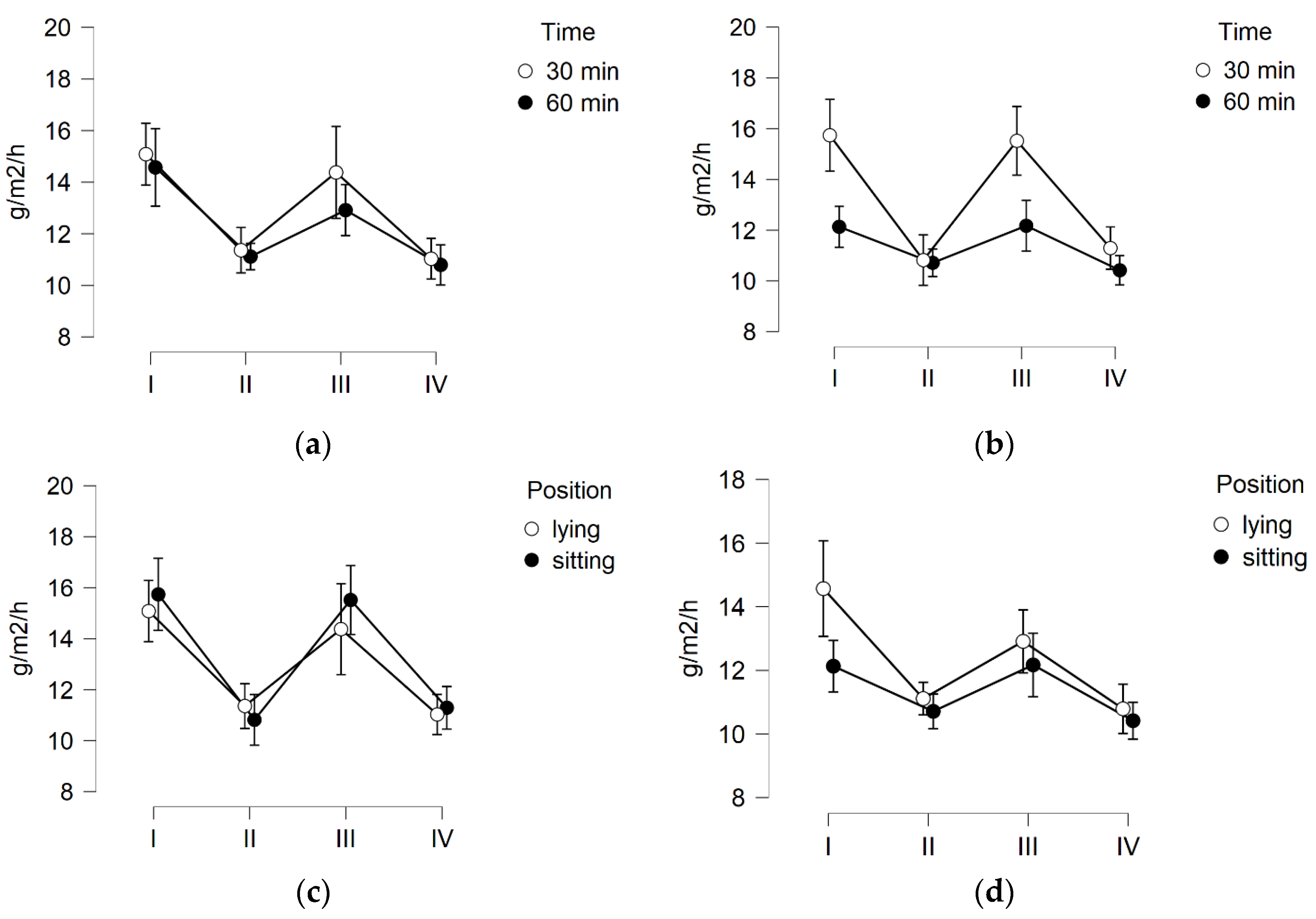
3.1. Skin Hydration
3.2. TEWL
3.3. Skin pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bass, L.S.; Kaminer, M.S. Insights Into the Pathophysiology of Cellulite: A Review. Dermatol. Surg. 2020, 46, S77–S85. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.P.; Vick, G.L.; Mishra, V. Cellulite: A review with a focus on subcision. Clin. Cosmet. Investig. Dermatol. 2017, 10, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.H.; Victor, F.; Rao, B.; Sadick, N.S. Treatment of cellulite. Part I. Pathophysiology. J. Am. Acad. Dermatol. 2010, 62, 361–370. [Google Scholar] [CrossRef]
- Migasiewicz, A.; Podbielska, H.; Bauer, J. Women’s quality of life depending on the cellulite stage. Acta Bio-Opt. Et Inform. 2014, 20, 217–226. [Google Scholar]
- Emanuele, E.; Bertona, M.; Geroldi, D. A multilocus candidate approach identifies ACE and HIF1A as susceptibility genes for cellulite. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, M.; Galęba, A.; Nurein, H. Cellulite as a medical and aesthetic problem—etiopathogenesis, symptoms, diagnosis and treatment. Hygeia Public Health 2014, 49, 425–430. [Google Scholar]
- Sadick, N. Treatment for cellulite. Int. J. Women’s Dermatol. 2019, 5, 68–72. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Halabchi, F.; Mazaheri, R.; Abolhasani, M.; Tabesh, M. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int. J. Endocrinol. Metab. 2016, 14, e36727. [Google Scholar] [CrossRef] [Green Version]
- Pilch, W.; Nastałek, M.; Piotrowska, A.; Czerwińska-Ledwig, O.; Zuziak, R.; Maciorowska, A.; Golec, J. The effects of a 4-week vibrotherapy programme on the reduction of adipose tissue in young women with cellulite—A pilot study. Rehabil. Med. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Pilch, W.; Czerwińska-Ledwig, O.; Chitryniewicz-Rostek, J.; Nastałek, M.; Krȩzałek, P.; Jȩdrychowska, D.; Totko-Borkusewicz, N.; Uher, I.; Kaško, D.; Tota, L.; et al. The Impact of Vibration Therapy Interventions on Skin Condition and Skin Temperature Changes in Young Women with Lipodystrophy: A Pilot Study. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Piotrowska, A.; Czerwińska-Ledwig, O. Effect of local vibrotherapy in sitting or lying position in two time protocols on the cellulite grade and change of body circumferences in women with cellulite. J. Cosmet. Dermatol. 2021, 00, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Savoia, A.; Landi, S.; Vannini, F.; Baldi, A. Low-level laser therapy and vibration therapy for the treatment of localized adiposity and fibrous cellulite. Dermatol. Ther. 2013, 3, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristovam, D.N.; Botelho, S.; Andrade, M.F.; Marques, J.; Sousa, L. Whole-body vibration in the reduction of the cellulite. J. Cosmet. Laser Ther. 2019, 21, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J.; Beller, G.; Armbrecht, G.; Mulder, E.; Buehring, B.; Gast, U.; Dimeo, F.; Schubert, H.; de Haan, A.; Stegeman, D.F.; et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone 2010, 46, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Wuestefeld, A.; Fuermaier, A.B.M.; Bernardo-Filho, M.; Da Cunha De Sá-Caputo, D.; Rittweger, J.; Schoenau, E.; Stark, C.; Marin, P.; Seixas, A.; Judex, S.; et al. Towards reporting guidelines of research using whole-body vibration as training or treatment regimen in human subjects—A Delphi consensus study. PLoS ONE 2020, 15, e0235905. [Google Scholar] [CrossRef] [PubMed]
- Van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Mar, P.J.; Taiar, R. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Otsuki, T.; Takanami, Y.; Aoi, W.; Kawai, Y.; Ichikawa, H.; Yoshikawa, T. Arterial stiffness acutely decreases after whole-body vibration in humans. Acta Physiol. 2008, 194, 189–194. [Google Scholar] [CrossRef]
- Uher, I. Vibration Therapy and Its Influence on Health. Biomed. J. Sci. Tech. Res. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Piotrowska, A.; Tota, Ł.; Czerwińska-Ledwig, O.; Bigosińska, M.; Potok, H.; Cisoń-Apanasewicz, U.; Zuziak, R.; Wrześniewski, K.; Tyka, A.; Żmuda-Pałka, M.; et al. Effect of Vibration Therapy on Fasting Glucose, Insulin Level and Homa2 Score in Women With Pre-Diabetes History. J. Kinesiol. Exerc. Sci. Antropomotoryka JKES 2018, 81, 11–19. [Google Scholar] [CrossRef]
- Piotrowska, A.M.; Bigosińska, M.; Potok, H.; Cisoń-Apanasewicz, U.; Czerwińska-Ledwig, O.; Tota, Ł.M.; Zuziak, R.; Pałka, T.; Pilch, W. Impact of oscillatory-cycloid vibration interventions on body composition, waist and hip circumference, and blood lipid profile in women aged over 65 years with hypercholesterolaemia. Prz. Menopauzalny 2018, 17, 161–167. [Google Scholar] [CrossRef]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nürnberger, F.; Müller, G. So-Called Cellulite: An Invented Disease. J. Dermatol. Surg. Oncol. 1978, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examinatory Survey (NHANES). Anthropometry Procedures Manual. 2007. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 3 December 2021).
- Çakar, H.I.; Doğan, S.; Kara, S.; Rittweger, J.; Rawer, R.; Zange, J. Vibration-related extrusion of capillary blood from the calf musculature depends upon directions of vibration of the leg and of the gravity vector. Eur. J. Appl. Physiol. 2017, 117, 1107–1117. [Google Scholar] [CrossRef]
- Ren, W.; Pu, F.; Luan, H.; Duan, Y.; Su, H.; Fan, Y. Effects of Local Vibration With Different Intermittent Durations on Skin Blood Flow Responses in Diabetic People. Front. Bioeng. Biotechnol. 2019, 7, 310. [Google Scholar] [CrossRef] [Green Version]
- Nakagami, G.; Sanada, H.; Matsui, N.; Kitagawa, A.; Yokogawa, H.; Sekiya, N.; Ichioka, S.; Sugama, J.; Shibata, M. Effect of vibration on skin blood flow in an in vivo microcirculatory model. BioSci. Trends 2007, 1, 161–166. [Google Scholar] [PubMed]
- Stewart, J.M.; Karman, C.; Montgomery, L.D.; McLeod, K.J. Plantar vibration improves leg fluid flow in perimenopausal women. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2005, 288, 623–629. [Google Scholar] [CrossRef]
- Farran, A.J.E.; Teller, S.S.; Jia, F.; Clifton, R.J.; Duncan, R.L.; Jia, X. Design and characterization of a dynamic vibrational culture system. J. Tissue Eng. Regen. Med. 2013, 7, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Jungbauer, S.; Gao, H.; Spatz, J.P.; Kemkemer, R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 2008, 95, 3470–3478. [Google Scholar] [CrossRef] [Green Version]
- Kutty, J.K.; Webb, K. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J. Tissue Eng. Regen. Med. 2010, 4, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Caberlotto, E.; Bernal, M.; Miller, Z.; Poole, A.; Ruiz, L.; Tanter, M.; Gennisson, J.L.; Querleux, B. Controlled mechanical vibration and impacts on skin biology. Ski. Res. Technol. 2019, 25, 881–889. [Google Scholar] [CrossRef]
- Tzen, Y.; Weinheimer-haus, E.M.; Corbiere, T.F.; Koh, T.J. Increased skin blood flow during low intensity vibration in human participants: Analysis of control mechanisms using short-time Fourier transform. PLoS ONE 2018, 13, e0200247. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.K.; Feland, J.B.; Johnson, A.W.; Mack, G.W.; Mitchell, U.H. Effect of Whole Body Vibration on Skin Blood Flow and Nitric Oxide Production. J. Diabetes Sci. Technol. 2014, 8, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arashi, M.; Sugama, J.; Sanada, H.; Konya, C.; Okuwa, M.; Nakagami, G.; Inoue, A.; Tabata, K. Vibration therapy accelerates healing of Stage I pressure ulcers in older adult patients. Adv. Ski. Wound Care 2010, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Okuwa, M.; Nakagami, G.; Sugama, J.; Sanada, H.; Konya, C. Acute effect of leg vibration on cutaneous microcirculation and macrocirculation in patients during hemodialysis. Jpn. J. Nurs. Art Sci. 2009, 8, 56–62. [Google Scholar]
- Ichioka, S.; Yokogawa, H.; Nakagami, G.; Sekiya, N.; Sanada, H. In vivo analysis of skin microcirculation and the role of nitric oxide during vibration. Ostomy Wound Manag. 2011, 57, 40–47. [Google Scholar]
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef]
- Leveque, J.L. Measurement of transepidermal water loss. Cutan. Investig. Health Dis. Non-Invasive Methods Instrum. 1989, 135–151. [Google Scholar]
- van Jansen Rensburg, S.; Franken, A.; Du Plessis, J.L. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Skin Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Skin Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2019, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm.-Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippke, F.; Berardesca, E.; Weber, T.M. PH and Microbial Infections. Curr. Probl. Dermatol. 2018, 54, 87–94. [Google Scholar] [PubMed]
- Miyaji, A.; Sugimori, K.; Hayashi, N. Short- and long-term effects of using a facial massage roller on facial skin blood flow and vascular reactivity. Complement. Ther. Med. 2018, 41, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; AlEnezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of Olive and Sunflower Seed Oil on the Adult Skin Barrier: Implications for Neonatal Skin Care. Pediatr. Dermatol. 2013, 30, 42–50. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1525-1470.2012.01865.x (accessed on 3 December 2021). [CrossRef]
| Total | Sitting | Lying | p | |||
|---|---|---|---|---|---|---|
| 30′ | 60′ | 30′ | 60′ | |||
| (n) | 57 | 15 | 15 | 13 | 14 | |
| Age (years) | 22.84 ± 5.34 | 22.47 ± 4.76 | 21.67 ± 3.39 | 23.92 ± 7.18 | 23.50 ± 5.91 | 0.2512 |
| Hight (cm) | 166.42 ± 5.62 | 164.86 ± 4.07 | 156.87 ± 5.16 | 165.69 ± 5.99 | 169.36 ±5.58 | 0.1364 |
| Body mas (kg) | 62.28 ± 8.43 | 60.05 ± 6.96 | 60,73 ± 8.18 | 63.58 ± 9.67 | 65.1 ± 8.76 | 0.0748 |
| BMI | 22.45 ± 2.55 | 22.06 ± 2.17 | 22.00 ± 1.88 | 23.16 ± 3.34 | 22.69 ± 2.81 | 0.1942 |
| Fat (%) | 23.75 ± 4.08 | 24.92 ± 3.58 | 23.59 ± 4.16 | 24.63 ± 4.44 | 21.84 ± 3.83 | 0.3248 |
| Water (%) | 56.08 ± 3.23 | 56.14 ± 3.00 | 56.13 ± 2.92 | 55.3 ± 3.67 | 56.68 ± 3.56 | 0.8885 |
| Arm circumference (cm) | 27.71 ± 2.88 | 27.62 ± 3.03 | 28.01 ± 2.25 | 27.87 ± 2.85 | 27.34 ± 3.54 | 0.7789 |
| Waist circumference (cm) | 72.55 ± 6.39 | 71.16 ± 6.03 | 72.17 ± 5.40 | 74.55 ± 7.47 | 72.61 ± 6.87 | 0.2706 |
| Hip circumference (cm) | 97.61 ± 6.68 | 96.03 ± 7.19 | 97.83 ± 6.83 | 98.41 ± 6.82 | 98.31 ± 6.29 | 0.4239 |
| Thigh circumference (cm) | 56.98 ± 4.27 | 56.13 ± 4.60 | 57.05 ± 3.99 | 57.24 ± 4.29 | 57.56 ± 4.51 | 0.4769 |
| Calf circumference (cm) | 36.39 ± 2.54 | 36.00 ± 2.63 | 36.25 ± 2.53 | 36.48 ± 1.88 | 36.89 ± 3.11 | 0.4088 |
| Triceps skinfold (mm) | 26.05 ± 6.73 | 27.00 ± 4.36 | 27.73 ± 6.17 | 27.12 ± 7.01 | 22.25 ± 8.2 | 0.1213 |
| Subscapular skinfold (mm) | 20.84 ± 7.00 | 21.03 ± 7.51 | 20.53 ± 5.71 | 22.46 ± 8.22 | 19.46 ± 6.84 | 0.9474 |
| Abdominal skinfold (mm) | 28.59 ± 8.60 | 29.87 ± 7.51 | 27.60 ± 9.70 | 31.38 ± 8.89 | 25.68 ± 8.00 | 0.8943 |
| Hip skinfold (mm) | 21.23 ± 7.85 | 21.90 ± 8.36 | 22.27 ± 7.47 | 22.54 ± 8.61 | 18.17 ± 6.96 | 0.3906 |
| Thigh skinfold (mm) | 40.29 ± 8.88 | 40.73 ± 7.43 | 42.13 ± 8.14 | 40.58 ± 10.70 | 37.57 ± 9.54 | 0.3096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, A.; Czerwińska-Ledwig, O. Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite. Cosmetics 2022, 9, 16. https://doi.org/10.3390/cosmetics9010016
Piotrowska A, Czerwińska-Ledwig O. Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite. Cosmetics. 2022; 9(1):16. https://doi.org/10.3390/cosmetics9010016
Chicago/Turabian StylePiotrowska, Anna, and Olga Czerwińska-Ledwig. 2022. "Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite" Cosmetics 9, no. 1: 16. https://doi.org/10.3390/cosmetics9010016
APA StylePiotrowska, A., & Czerwińska-Ledwig, O. (2022). Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite. Cosmetics, 9(1), 16. https://doi.org/10.3390/cosmetics9010016







