Abstract
Acidic pH of the skin surface has been recognized as a regulating factor for the maintenance of the stratum corneum homeostasis and barrier permeability. The most important functions of acidic pH seem to be related to the keratinocyte differentiation process, the formation and function of epidermal lipids and the corneocyte lipid envelope, the maintenance of the skin microbiome and, consequently, skin disturbances and diseases. As acknowledged extrinsic factors that affect skin pH, topically applied products could contribute to skin health maintenance via skin pH value control. The obtained knowledge on skins’ pH could be used in the formulation of more effective topical products, which would add to the development of the so-called products ‘for skin health maintenance’. There is a high level of agreement that topical products should be acidified and possess pH in the range of 4 to 6. However, formulators, dermatologists and consumers would benefit from some more precise guidance concerning favorable products pH values and the selection of cosmetic ingredients which could be responsible for acidification, together with a more extensive understanding of the mechanisms underlaying the process of skin acidification by topical products.
1. Introduction
Skin pH has been recognized as an important factor and has been investigated for almost a century, ever since the term acid mantle was used by Heinrich Schade and Alfred Marchionini in 1928. Nevertheless, skin pH continues to be researched by the scientific community. Recent decades were marked with comprehensive research of different skin components, mechanisms and processes, relating to either healthy or diseased skin. Collected data provide new and/or better insight into skin characteristics. Among others, acidic pH of the skin surface has been recognized as a regulating factor for the maintenance of the stratum corneum homeostasis and barrier permeability. This review will summarize existing data that add to the understanding of the importance, origin and development of the acidic skin pH. Additionally, information related to skin pH and topical product formulations from accessible literature will be presented with the contemplation on the use of the obtained knowledge in the formulation of more effective topical products that could contribute to the development of the so-called products ‘for skin health maintenance’.
2. Methodology
Due to the very extensive research interest described in this paper, with the final goal to practically apply what is known about the pH of the skin in the development of topical products, two review questions were defined. The PubMed date base was used as a first instance to identify published papers based on our selected key terms. The first question was ‘What do we know about skin pH?’, and terms used for this search were: ‘skin pH’, ‘human pH’, ‘acid mantle’ and ‘skin barrier’. Inclusion criteria were review articles. In order to inquire deeper into the origins, formation, maintenance and importance of the skin pH for skin barrier homeostasis, the reference lists of review papers were investigated with an additional search of published papers based on defined terms: skin lipids, skin microbiome, acidic microdomain, skin buffer/buffering system, skin development and skin ageing. Except for several historically important papers, inclusion criteria were publication year (5–10 years), original research papers and the relation of the papers content to the review question; only papers which addressed skin pH at any point of interest were included. The second question was ‘How can topically applied product influence the skin barrier in relation to skin pH?’ Used terms were: effects on skin barrier, effects on skin pH, skin cleansers, skin pH and topical products, cosmetics and pH and acidic skin care products. Although some papers based on animal studies, the results of which present a key point in the research of skin pH, were included in the previous search, for the second question, inclusion criteria were original papers of experimental human studies with adequate study design (randomized controlled trials of topical products) and studies that performed statistical analyses. Exclusion criteria for the second question were dermatological treatments and therapy of different dermatoses. All included papers were peer-reviewed and published in established scientific or professional journals written by the authorities in the specific field.
3. Skin Barrier
For a long time, acid skin pH was allied to the ‘acid mantle’ concept and believed to be important for certain aspects, mainly protection from pathogenic microorganisms [1]. Today, due to technological developments and investigations in the field of dermatology, biochemistry, bioanalysis, physiology, immunology and genetics, the view has broadened, and various other aspects have been discovered.
Skin is now considered to be an active organ with a barrier function, organized in four functional levels: the microbiome barrier, the chemical barrier, the physical barrier and the immune barrier [2]. On the skin surface, hundreds of species of commensal organisms, including bacteria, fungi, viruses and mites, known as the microbiota, form the microbiome barrier [3,4]. There are a multitude of communication pathways and interactions between keratinocytes, immune cells and microbes maintain skin barrier permeability and homeostasis in healthy conditions, as well as under different stresses, such as wounding or infection [5]. The skin microbiota produces antimicrobial substances against invading bacterial competitors and promote the expression of certain potent and very conserved pathways of host defense [6,7].
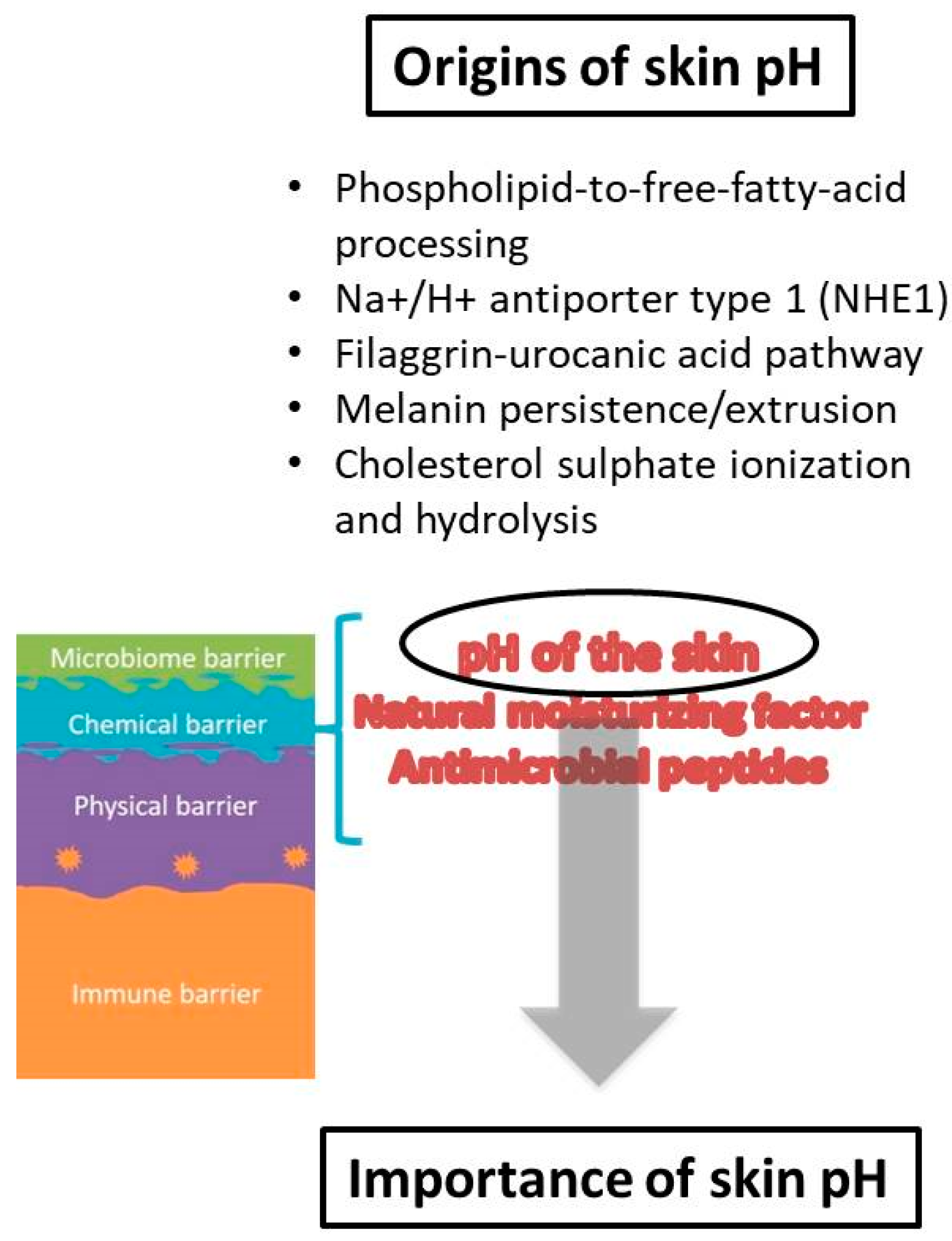
The chemical barrier has been recently recognized as a separate level of the skin barrier, albeit with a less sharp definition, but it undoubtedly includes factors that contribute to the acidic surface pH and compounds of the ‘natural moisturizing factor’ (NMF) [2]. Therefore, the ‘acid mantle’ of the skin is now considered as a part of the chemical barrier. The NMF are hygroscopic breakdown products from the proteolysis of the epidermal filaggrin, necessary for stratum corneum (SC) hydration maintenance, but also contributing to the overall skin pH [8].
Although the skin barrier function depends greatly upon its biochemical composition, without the appropriate tissue structure—a physical barrier—it would not exist. The physical barrier is the result of the skin’s characteristic feature to produce the stratum corneum [9]. The stratum corneum consists of keratinocytes that undergo terminal differentiation, being flattened and enucleated cornified keratinocytes (corneocytes) in the final stage, bounded by highly insoluble cornified envelopes together with equally cross-linked lipid envelopes [10,11]. Epidermal cells, keratinocytes, are closely connected by tight junctions in the stratum granulosum, and those tight junctions are another component of the physical barrier [12]. Keratinocytes are surrounded by cornified envelopes which stay linked in SC via corneodesmosomes, and their desquamation depends on the gradual degradation of these cell junctions [13,14]. Many proteins that are the structural components of these junctions are also part of the physical barrier [15]. Simultaneously with keratinocytes’ terminal differentiation, the cornified envelope and the accompanying external membrane monolayer, the corneocyte-bound lipid envelope (CLE), are being formed [16]. CLE is the structural ground for the extracellular lipid matrix, one more component of the physical barrier, which is highly ordered and has a specific lamellar organization providing the movement of water and SC general permeability [17,18].
The final part of the cutaneous barrier is the immune barrier, formed by cells (innate sentinels–resident antigen-presenting cells, innate lymphoid cells, innate-like cells, keratinocytes and adaptive tissue-resident memory cells) distributed throughout the skin [2]. Besides its major function in the initiation of an adequate immune response to a chemical or microbial hazard, this barrier contributes to barrier repair and homeostasis.
4. Skin pH
The pH represents the concentration of free hydrogen ions (H+) in a solution; specifically, pH is defined as the negative logarithm (ten base) of the H+ concentration. It is used as a measure of the acidity–alkalinity ratio with a scale ranging from 0 to 14 [19]. In the human organism, pH is regulated by acid-base homeostasis and varies from 1 to 8, depending on the organ and function.
From the beginning of its investigation, skin surface was considered to have an acidic nature [20]. Older publications, generally recognized, suggested that skin pH (forearm, adult male) ranges from 5.4 to 5.9 [21]. More recent studies have found that the skin pH is even more acidic, and today it is accepted that the pH of the skin ranges from 4.1 to 5.8, depending on the body part [22]. Exceptions are found in certain regions—in certain physiological gaps (axillae, groin, toe interdigits and anus), the pH values range from 6.1 to 7.4 [23]. All of the mentioned results were obtained by potentiometric methods, with the use of flat glass electrodes. Due to the measuring principle that only takes into account water-soluble components, some authors believe the actual skin pH could be closer to 6, with some more acidic microdomains [24].
Considering the importance of the pH in human physiology and pathology, it is equally relevant for each human organ to resist acid/alkaline aggression (maintain specific pH) to some extent, or in other words to possess a buffer system [25]. The skin’s buffering capacity has been investigated earlier and separately from the skin pH maintenance mechanisms, despite the fact that reliable data suggest certain co-dependence in those processes [1,26]. Existing data accredit free amino acids of the epidermis (originating from eccrine sweat, degradation of skin proteins and hair follicles) to be crucial SC components for the neutralization capacity of the skin. Other investigated components of the SC, such as sebum, keratin and CO2, although without a significant buffering role, still seem to have certain functions in the protection of skin from acids and bases [26].
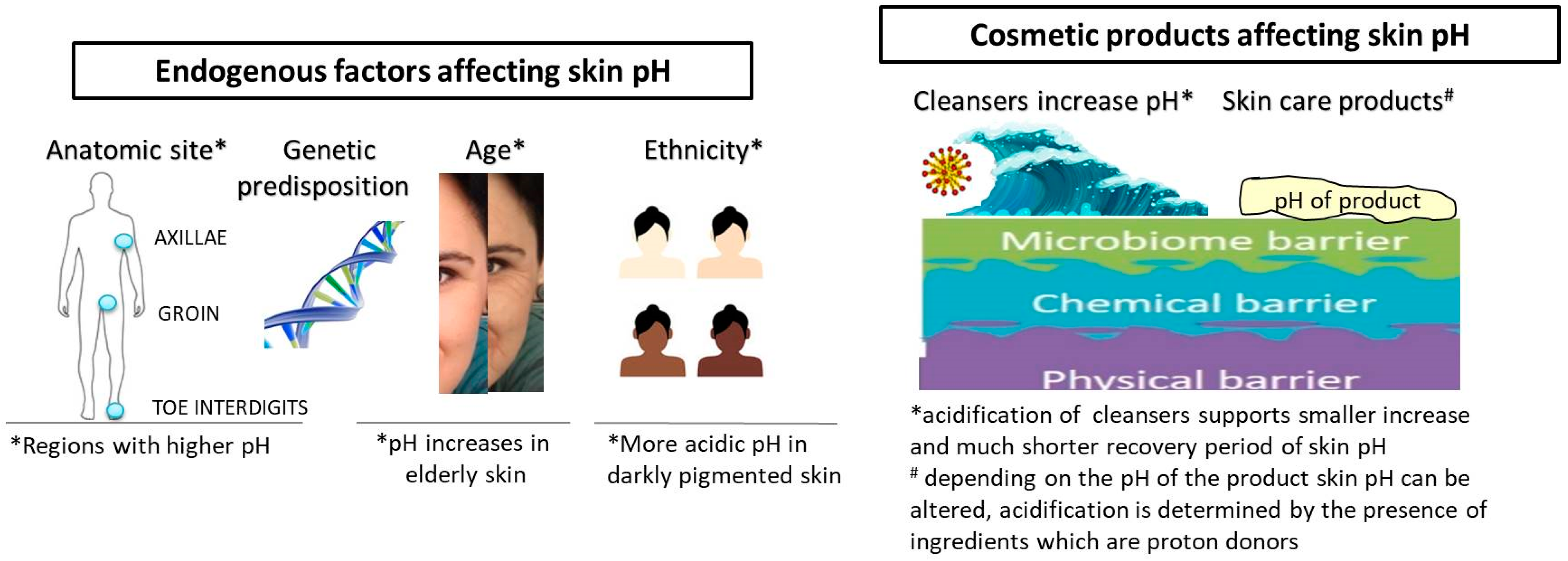
Obviously, different endogenous and exogenous factors affect skin pH (Figure 1). Besides the anatomic site and genetic predisposition, age and ethnicity are the most thoroughly investigated endogenous factors [1]. At birth, skin pH is higher, i.e., near neutral, and within 4 weeks, it reaches the physiological value, and usually remains as such until the end of the fifth decade of life [27,28]. Although not all published data are coherent regarding the age line when pH significantly alters, it is a fact that pH increases in elderly skin (after 70 years of age), which is coupled with a reduction in buffering capacity [26,29,30]. Gender-related differences, although frequently reported, are not generally considered significant [31]. Ehlers et al. reported that skin surface pH in women is somewhat lower, i.e., the average pH for men was 5.80, while that for women was 5.54 [32]. By contrast, another evaluation found pH values below 5.0. in men on the forehead, cheek, neck, forearm and hand, while in women the pH was always higher and below 5.0 only on the forehead [29].
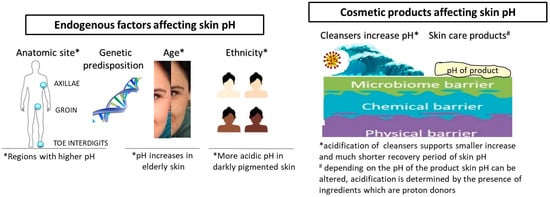
Figure 1.
Endogenous and exogenous factors affecting skin pH.
Ethnicity-related differences are also found for the skin pH in the context of barrier function. Namely, superior permeability barrier function is often displayed in humans with darker pigmented skin, in comparison with those with lighter pigmented skin [33]. Notable pigment type differences in barrier function and structure are coupled with differences in pH, with pH being more acidic in darker pigmented skin [34]. This is explained by the mechanisms involved in the maintenance of the acidic pH, namely increased epidermal lipid content and lamellar body density; based on more recent findings, this can be attributed to the pH-lowering effect of melanin and higher secretory phospholipase A2f expression [19,33].
The surface of the skin is acidic, but deeper in the skin, the pH of the SC increases, reaching neutral values in the viable epidermis [35]. Throughout the SC, acidic membrane compartments (so-called microdomains) have been identified, disclosing complexities in the pH gradient [36]. Fluorescence lifetime imaging was used as a sophisticated method to determine the presence of such acidic microdomains at the stratum granulosum–stratum corneum interface and in the lower SC interstices [35,36]. These gradients and acidic microdomains provide the acidification necessary for lipid processing and barrier homeostasis maintenance by the regulation of pH-dependent enzymes activity [37,38].
5. Origins, Formation and Maintenance of Skin pH
Despite the fact that neonatal skin development has not been completely elucidated, the majority of published data affirm that the skin surface pH is neutral at birth but afterwards shifts to acidic values independent of the newborn’s fetal age over the first few weeks of life [39,40,41]. In full-term neonates, all SC layers are developed, while vernix caseosa contributes to complete barrier formation over the range of protective and adaptive mechanisms [42]. Vernix is formed throughout the last trimester and, being a complex mixture of water, proteins and lipids, it could be considered as a naturally occurring protective cream with numerous effects [43]. Some findings even suggest that it supports acidic skin surface development [44]. Although the skin barrier at birth is generally considered as adequately functional, it is not yet fully mature, and complete development of the permeability barrier is also associated with SC acidification [41].
When it comes to the origin of skin pH, the first theories proposed that the acidic mantle was the result of molecules from a superficial film which were products of endogenous (sebum, sweat) and exogenous (microbes from the skin surface) sources [38]. Considerable research has been performed elucidating origins of protons on the skin surface, indicating a complex interplay of cellular and metabolic processes that are responsible for the skin surface pH acidification and maintenance [19]. Among them, several pathways appear to be the most important source of protons: (i) phospholipid-to-free-fatty-acid processing, (ii) Na+/H+ antiporter type 1 (NHE1), (iii) filaggrin-urocanic acid pathway, (iv) melanin persistence/extrusion and (v) cholesterol sulfate ionization and hydrolysis [34,36,37,45,46,47,48] (Figure 2). The free fatty acids (FFAs) that are products of phospholipids’ hydrolysis by secretory phospholipases (sPLA2) clearly contribute to the acidification of the SC [27,45]. FFAs produced from ceramides by ceramidases also make up part of the previous FFAs fraction [49]. The exchange of protons for sodium ions by the activity of the Na+/H+ antiporter 1 is an acknowledged mechanism that generates the acidic skin pH [36]. NHE1 expression in keratinocytes is a regulation mechanism activated by changes in external pH, which is significant for the formation of the aforementioned acidic microdomains within the SC [37]. Another pathway for proton formation is filaggrin degradation [50]. Metabolites of filaggrin are amino acids essential for NMF formation, while histidine alone has specific importance, as it is eventually metabolized to urocanic acid (UCA) and pyrrolidone-5-carboxylic acid (PCA), which add to the acidification of the SC [47,51,52]. Due to the role of filaggrin reduction in atopic dermatitis pathogenesis in some patients, this filaggrin-histidine-urocanic acid pathway was considered to have specific importance for acidification; nevertheless, it was not found to be essential [53,54]. Instead, it is more likely that all degradation components of filaggrin contribute to the maintenance of skin pH and chemical barrier [55]. The melanin granule persistence and extrusion contribute to the acidification of SC through the release of protons from the acidic milieu of phagolysosomes [33]. An additional mechanism that could contribute to skin pH acidification, which is suggested but not proven, is cholesterol sulfate ionization and/or hydrolysis [56]. Additionally, metabolites released from the skin’s microbiota augment the plethora of acidifying factors.
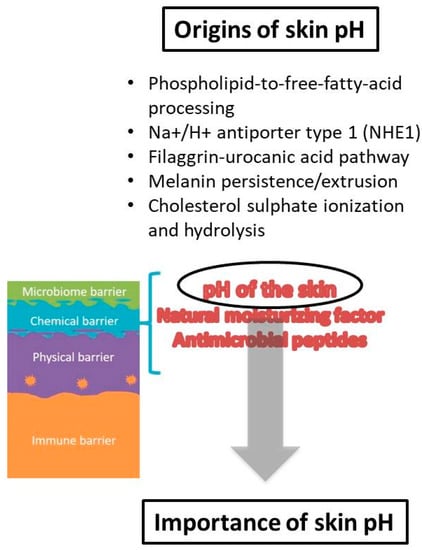
Figure 2.
Origins and importance of skin pH.
Due to the overall significance of pH in human organism, it is reasonable to expect that various mechanisms tend to form H+ ions, together with the maintenance of pH within tissue/organ specific limits. Different ion channels, receptors and transducers have attracted great attention for their part in pH maintenance: ionotropic pH sensors, acid-sensing ion channels, transient receptor potential, ionotropic purinoceptors, voltage-gated proton channels and metabotropic pH sensors are being intensively investigated [57].
6. Importance of Acidic Skin pH
The SC acid mantle’s importance has been well established through research of different skin aspects, but the most important functions of acidic pH seem to be related to the keratinocyte differentiation process, the formation and function of epidermal lipids and the corneocyte lipid envelope, the maintenance of the skin microbiome and, consequently, skin disturbances and diseases. In order to maintain its homeostasis, SC undergoes a number of well-organized and controlled events, which include epidermal proliferation, cell differentiation and exfoliation with simultaneous lipid lamellae formation [10].
In the epidermis, the highly organized lipid structures with a specific lipid ratio (over 50% being ceramides) enable the SC to act as a semi-permeable barrier [58]. The enzymes β-glucocerebrosidase and acid sphingomyelinase catalyze the last step of ceramide formation and their activity is pH-dependent [59,60]. Acidic skin pH regulates the activity of both enzymes, since the optimum action of β-glucocerebrosidase is reported at pH 5.5, while for acid sphingomyelinase, a pH of ∼5 is approximated [61,62]. Regarding the SC lipids, an acidic pH is necessary for promotion of specific bilayer structuring. It enables FFAs to persist in non-ionic form, minimizing their head-group repulsions, while supporting crystallinity and bilayer organization [63]. Consequently, strong membrane disruption and disturbance of lipid domains reported in skin models at pH 8 may be explained by ionization of free fatty acids and its influence on lipid–lipid interactions [64].
Another pH-dependent process is corneodesmosomes’ proteolytic degradation, which is a final step in keratinocyte differentiation, and its rate controls the desquamation process [65]. Degradation is regulated by kallikrein-regulated peptidases (KLKs), serine proteases and cathepsins [66]. pH is among the mechanisms regulating KLKs proteolytic activity and it is believed that proteases are being gradually activated by the pH gradient, and their activity at pH 5.6 has been reported [67,68].
One of the earliest supposed roles of the acidic mantle on skin surface was antimicrobial protection. Although acidic skin pH is a part of the skin’s ‘hostile’ environment for pathogenic microorganisms, the same acidic surrounding is necessary for the maintenance of normal skin microflora [69]. Resident microorganisms inhabiting the human body are impacting human health by influencing human adoptive and innate immune system [68,70]. This impact is the result of the restriction of growth of pathogenic microbes on one side and the influence skin microbiome has on human cells’ functioning on the other [71]. Antimicrobial compounds, also called bacterial antimicrobial peptides (AMPs) or bacteriocins are produced by skin commensals such as Staphylococcus epidermidis, a major constituent of the skin microbiome. Together with AMPs synthetized by epithelial cells, they are found to be key contributors of the innate immune system [70,72]. The commensal microbes produce signals which increase the expression of human AMPs and contribute to the maintenance of the epidermal barrier influencing cytokine production or increasing tight junction barrier [73,74]. A great diversity in skin microbes is the result of specific anatomical site conditions, such as pH, moisture, temperature, sebum content and exposure to external environments [75]. Factors also influencing skin microbiome diversity and balance are individual behavioral factors, with occlusion, cosmetic products, skin cleansers, detergents and topical antibiotics being the most important [5]. For more details on the topic of the skin microbiome and immune system, the reader is referred to Eyerich et al. 2018 and Sanford and Gallo 2013.
Everything that has been mentioned implies multiplicity and interconnection of cutaneous barrier parts and processes within it. Therefore, the fact that numerous skin diseases are related to disturbances in metabolism and/or functioning of skin lipids, skin proteins, skin microbiome, as well as skin acidic pH is not surprising. In inflammatory skin disorders, such as atopic dermatitis (AD), ichthyosis, irritant contact dermatitis, diaper and incontinence dermatitis, the pH of the skin is generally elevated [48,58,76,77,78]. Regarding the role of the skin pH in those disorders, a question that arises is whether increased pH is a cause or the consequence. The most common and largely investigated chronic inflammatory skin disorder is AD, characterized by elevated skin pH in both lesions and uninvolved skin sites [76] (Seidenari and Giusti, 1995). Molecular mechanisms underlining the AD complex pathophysiology which place skin pH in AD pathogenesis are the formation of FFAs from phospholipids by sPLA1, the transport of protons via NHE1 and the catabolism of amino acids—most importantly from filaggrin in AD [27,45]. Regardless, if the increased pH is a primary or a secondary phenomenon, cause or consequence, studies have shown that topical acidification interrupts the AD cycle and prevents the appearance of AD [79].
In addition to the topic of skin disorders, dysbiosis in human skin microbiome participates in various pathologies of the skin, and microbial infections are promoted in AD, psoriasis, mycosis, acne vulgaris, diaper dermatitis, etc. [80,81]. The role of the skin pH in diabetic wounds and wound healing in general is another area of research [82,83]. Although the role of pH in skin pathophysiology is not particularly investigated, evidence of its influence on skin homeostasis and cutaneous barrier are emerging.
7. pH of the Skin and Topical Formulations
The maintenance and the protection of the normal/physiological skin pH is a very important task. As acknowledged, extrinsic factors affect skin pH, and topically applied products have to be recognized as administrators of this task. Cosmetics, due to their extensive everyday usage could contribute to skin health maintenance, via skin pH value control. Additionally, in certain skin disorders, the use of topical products which could correct skin pH should be a part of the therapeutic regimen. Keeping this in mind, the pH of any topically applied product together with its buffering capacity must be considered carefully. In this part of the paper, information regarding the influence of different types of topical products on skin pH is collected and discussed.
7.1. Cleansers
Although the use of soap as a cleansing agent is a part of human history, foundations in contemporary skin cleansing were set at the beginning of the 20th century with the development of different synthetic substances. At the same time, with the expansion of cleansing products, dermatologists have become aware that the use of soaps could deteriorate the condition of existing dermatoses. In fact, pioneering studies and the first data on the influence of cosmetic products on the skin surface pH concerned the effect of skin cleansing products [84,85,86]. In order to accomplish their intended purpose—removal of contaminants from the skin—cleansers contain surfactants as key ingredients [87]. However, while exerting their cleansing function, these substances can negatively affect the structural and functional integrity of the skin. Their damaging effects are the result of an increase in the skin surface pH (alkalization), excessive removal of skin structural components (lipids and proteins), stratum corneum damage and even cytotoxicity [88,89,90]. Their skin-irritation activity is related to interactions with skin components and depends on the surfactant’s structure and physical properties in aqueous solutions [91]. A systematic review on the interactions between surfactants and the skin is given by Seweryn with a detailed discussion about surfactants’ structure and properties and how they relate to skin damage [91]. In order to exclude or reduce the risk of skin irritation, different formulation approaches in skin cleansing products development are being used, including the selection of adequate ingredients to obtain proper cleansing activity but a low skin irritation potency effect (mixture of surfactants), in addition to polymers and biopolymers or refatting substances in the formulation [92,93,94,95].
Natural (saponified) soaps have pH values around 9.5–10.5 by their very nature, and a single washing with a bar of soap tends to increase the pH from the normal range of 5–5.5 to 7.5. Over several hours after washing, due to the skin buffering capacity, the skin surface pH gradually decreases towards the normal range [45,96,97]. Studies investigating the use of tap water for washing also showed that water exposure increases skin pH and has a disrupting effect on skin barrier function [98,99,100]. The majority of published data imply that the use of acidic cleansers alters the skin’s pH to a lesser extent and with much shorter recovery period [101,102]. Some studies report a skin-irritancy effect of the cleansing products with a pH higher than 6 [95,100,103]. Although those results must take into account all mechanisms of surfactant–skin interactions (surfactant structure, adsorption of surfactant to the skin, etc.), the pH of the product may have a significant role in surfactant penetration through the skin. The increase in pH of a surfactant solution from 4 to 7 increased the barrier damage caused by the surfactant [104].
An additional relevant relation is the one between skin microflora and skin cleansers. It was reported by Korting et al. that the use of natural soap in healthy volunteers is followed by an increase in skin surface pH and a simultaneous increase in the number of propionibacteria compared to the use of synthetic cleansers [105,106,107]. A similar investigation with the group of patients in an intensive care unit by Duncan et al. confirmed alterations in the skin surface pH by two different cleansing regimens (natural soap and skin cleanser with pH = 5.5) but without significant differences between bacterial count [102]. A more recent study of Takagi et al. suggested that continuous use of a soap-based cleanser does not affect the maintenance of skin pH of volunteers with normal and healthy skin [108].
More detailed inquiry into available studies demonstrates differences between study protocols (frequency of washing and study duration), selection of volunteers (different skin types and conditions) and the measured parameters. It is a generally accepted fact that an extensive washing with alkaline soaps will lead to skin barrier impairment with a concomitant pH increase in any skin condition. Although the surfactants are deemed responsible for the skin irritation effect of the pertaining cleansers, it is shown that poor rinsability in combination with high pH may also increase the irritation potential of a product [95]. The use of a skin cleanser or even tap water will induce a short-term skin pH increase, but its duration depends on skin condition, frequency of washing and the composition of the cleansing product (surfactant/s, additives and the product’s pH). Therefore, every skin cleanser is a potential skin surface modifier and the pH of this product must be adjusted to a physiological pH during its development. Acidic skin cleansers should be used for frequent washing, by consumers with sensitive skin and different skin conditions and disorders related to a skin pH increase [109]. In order to accomplish an acidic pH of the product, skin cleansers are formulated with syndets, which have an acidic pH close to the skin’s pH. If the formulation needs a pH adjustment and/or stabilization, frequently used ingredients are lactic and citric acid, sodium acetate, sodium lactate, sodium citrate, diammonium citrate, etc. Acidic skin cleansers for normal, oily and acne-prone skin often contain acidic active ingredients, with hydroxy acids (salicylic acid, glycolic acid, lactic acid, mandelic acid, etc.), ascorbic acid, ferulic acid and linoleic acid being the most common.
It is interesting to mention the study of Baranda et al., who showed that products claimed to be suitable for sensitive skin in fact exerted a considerable irritating effect [103]. Although all products were formulated in accordance with contemporary design strategies for mild cleansing products, their pH values were 7 and higher (as high as 9.85–12.35) for all except two products, one with pH of 3.6 and one with pH 5.5, which were associated with the lowest irritation index. Another, more recent study investigated a pH of 67 children’s soaps and showed that the pH of the assessed soaps ranged from 4.4 to 11.5, with 11% of soaps with a pH < 5.9 and only two products with information about pH labeled on the product [110]. An additional survey made by Tarun et al. showed that soaps and shampoos commonly used by the Indian population have a pH of 9–10 (soaps) and 6–7 (shampoos), and thus, they can be considered as not ideal, especially for patients with sensitive or acne-prone skin [111].
An internet survey of available skin cleansers (gel cleanser type) shows that the aforementioned formulation strategies related to cleansers composition (i.e., use of surfactant mixtures, different polymers and refatting agents) are generally accepted, depending on the intended skin type. As for the cleansers’ pH, consumers are rarely informed about this characteristic by claims and other marketing information. In Table 1, some of the available claims and/or information on the selected products’ websites related to pH of the products or their effect on skin surface pH are listed.

Table 1.
Claims/information related to a product’s pH or its effect on the skin pH.
7.2. Skin Care Products
Skin care products aim at keeping or improving good condition/appearance of the skin. This is accomplished with the use of vehicles/carriers, which are mostly emulsions—creams, lotions or serums—intended to deliver product compounds to the skin. Scientific interest for emulsions is tremendous and researchers from various fields are engaged in their investigation. Nevertheless, research on skin care products’ pH in relation to skin surface pH is relatively new. Interrelation between product and skin surface pH is a complex issue with ambiguous interpretation of the obtained results. Further in the text, a summary of published papers addressing topical formulations’ pH and skin surface pH is given.
The work of Mauro et al. at the end of the 20th century showed that barrier recovery of the acetone-impaired hairless mouse skin was delayed by topical application of a neutral pH buffer (pH = 7.4) and accelerated when an acidic (pH = 5.5) solution was used [112]. Further studies from several groups confirmed and widened those findings, while elucidating mechanisms underlying them. It was shown that the neutralization of murine SC initiates SC functional abnormalities, barrier disruption and decline in SC integrity/cohesion [113]. In similar studies already mentioned in the text, the linkage between sPLA2, serine-proteases, lipid-processing enzymes and acidic skin pH was established [36,37,45,61].
Parallel to those investigations, SC lipids and their contribution to skin barrier homeostasis were explored. Some of the earliest studies described the negative effect of topically applied individual SC lipids or their incomplete mixtures on barrier recovery, while only mixtures with equimolar physiological ratio of lipids allowed normal recovery [114,115]. In contrast with the aforementioned are studies describing the positive effect of certain physiological lipids alone, some emollients (oils) alone or emulsions with physiological lipids on barrier recovery [116,117,118,119,120]. Differences in the experimental approaches, types of investigated topical formulations, study designs and protocols, measured parameters as well as investigated skin models (mouse/human, in vitro/in vivo) could explain the discrepancies in the obtained results. Nevertheless, this brought awareness of the possible effects of topical formulations to the higher level.
In order to investigate previous findings on the recovery of the skin barrier after neutral and acidic solution application, Buraczewska and Loden performed a similar study on human volunteers in surfactant-damaged skin. Based on visual scoring, transepidermal water loss (TEWL), blood flow and skin capacitance measurements, their study failed to show differences regarding skin barrier recovery between creams with pH 4 and 7.5 [121]. In contrast to these findings, it was shown that the aqueous cream BP, used as a barrier (protective) emollient, has a negative effect on the skin barrier. This was explained by the presence of sodium lauryl sulfate (SLS) as one of the stabilizers and the alkaline pH of this cream [122]. Although the efficacy of similar barrier creams has been well documented in many experiments, their pH was not taken into consideration in the study design nor the discussion of the obtained results [123].
There are far more studies comparing the efficacy of different vehicles and active substances rather than products with different pH. Additionally, there are studies that have investigated a sample’s influence on the skin pH, but they lack information on the investigated formulations’ pH. For example, in a study assessing different panthenol-containing creams, the obtained results confirmed that cream application increased skin hydration, decreased TEWL and shifted skin pH towards neutral values [124]. It was concluded that the results were influenced by the vehicle type and panthenol concentration, but no information regarding the formulation’s pH was revealed. In a recent study, the use of emollients in atopic dermatitis prevention in high-risk infants was investigated. A long-term (24 weeks) study determined that emollient use may contribute to the prevention of symptoms due to skin pH decrease and the increase in proportion of Streptococcus salivarius [125]. Nevertheless, the pH of the investigated formulation was once again not mentioned in the paper.
There is great interest in more acidic topical formulations, especially for the elderly population and AD patients [29,126]. One study, conducted on 20 subjects (mean age: 63.4 ± 6.8 years), investigated the effect of two water in oil (W/O) emulsions pH 4 and 5.8 and showed that the skin pH was significantly decreased after 4 weeks of treatment with the pH 4 emulsion, while results of skin hydration and TEWL were similar for both emulsions [127]. Furthermore, both emulsions significantly increased the overall skin lipid content, with the pH 4 emulsion being more effective. After this 4-week treatment, the skin was challenged with SLS, and although the results were similar for both emulsions, the skin treated with pH 4 emulsion had the lowest pH and its lipid structure was more resistant to SLS damage.
In the study performed by Blaak et al., oil in water (O/W) emulsion containing plant oils, adjusted to a pH of 4 by lactic acid, was investigated in the group of 23 subjects with dry skin in a 3-week study (mean age: 73.5 ± 3.4 years). The obtained results were compared to untreated control and showed that TEWL decreased and skin hydration increased, while skin pH remained unchanged after 3 weeks of application of the investigated product [128]. Nevertheless, the authors observed a lower variability of skin surface pH values after treatment, which they explained as a possible skin surface pH stabilization effect by the applied acidic emulsion. Finally, a significant increase in lipid lamellae in the SC after product application was obtained, along with an increase in ceramides levels and normalization of the lipid ratio in dry skin. This is especially interesting due to the fact that the investigated cosmetic product did not contain ceramides.
The study performed by Angelova-Fischer et al. investigated the effects of two W/O formulations with pH 4 and 5.8 on skin barrier recovery [129]. In the first part of the study, 10 subjects (60–72 years) were submitted to acetone-induced barrier impairment; 10 min afterwards, the investigated emulsions were applied and the effects on TEWL and skin pH were measured. In the second study, the skin barrier integrity was investigated by tape stripping after 4 weeks of application of emulsions in 28 subjects (mean age: 67.1 years). The obtained results showed that the emulsion with pH 4 significantly reduced skin pH 3 h and 6 h after acetone induced barrier impairment, while the emulsion with pH 5.8 failed to induce any changes. Additionally, the effect on TEWL was significant only after pH 4 emulsion application, since the obtained values were decreased 6 h and 24 h after its application. The results of the long-term study showed a superior effect of pH 4 emulsions due to a significant decrease in skin pH, enhanced barrier integrity and reduced roughness and scaliness of the skin surface after 4 weeks of treatment.
The study by Tasic-Kostov et al. investigated the efficacy of lactobionic acid compared to glycolic acid from two types of vehicles, gels and O/W emulsions, and was conducted on 26 healthy young subjects (mean age: 25.1 ± 2.1). It showed that four tested formulations, each having the pH adjusted to 4, after being applied for two weeks did not alter baseline pH values. Initially, after 3 days of application, skin pH was decreased, but it returned to basal values after 14 days of application, due to the skin buffering capacity [130].
Although general conclusions of some publications tend to emphasize the efficacy of certain formulations just due to their acidic pH, from the formulators’ point of view, this should be reconsidered. For example, the adjustment of pH 4 of W/O emulsions, which was more effective than pH 5.8 emulsion, in research done by Kilic et al. and Angelova-Fischer et al. was accomplished by the mixture of glycolic acid and ammonia buffer. Glycolic acid is a well-established cosmetic and dermatologically active substance used for topical treatment of various skin conditions and disorders with a moisturizing, exfoliating and even anti-aging effect, depending on the used concentration [131]. Without any intention to disprove the obtained results, the possible contribution of the entrapped fraction of glycolic acid which could be slowly released from the inner phase of the applied pH 4 W/O emulsion should also be considered. In the work of Sahlin et al., acidification of formulations with different acids (hydrochloric acid vs. lactic acid) induced changes in the obtained results, indicating the relevance of the chosen acidifying agent [132]. In the study of Blaak et al., plant oil-based emulsion contained, among other ingredients, the following (INCI): prunus amygdalus dulcis oil, butyrospermum parkii, panthenol, tocopheryl acetate, cocos nucifera oil, persea gratissima oil, oenothera biennis oil, betaine, rhus verniciflua peel cera, phytosterols, allantoin, carthamus tinctorius seed oil, sambucus nigra seed oil, bellis perennis flower extract, olea europaea fruit oil, citrus aurantium dulcis peel oil, citrus limon peel oil, lactic acid and tocopherol. Although all of these ingredients may be considered as cosmetic actives, the positive effect after 3 weeks of application was only discussed in the light of the emulsion’s acidic pH. Additionally, the buffering capacity of a formulation was not mentioned in any of the aforementioned studies addressing the formulation’s pH.
For additional data on formulations’ pH and skin barrier function, two more studies will be mentioned. Enquiry into moisturizers’ influence on the skin barrier showed different effects of different creams—each having pH 5. The effects of some pH 5 moisturizers on barrier function and characteristics were positive, while some had a negative effect on TEWL, skin capacitance and skin irritation, which depended on the precise formulation composition [133]. The investigation of dermatotoxicity of salicylic acid pointed out that the formulation pH minimally influenced its efficacy, but the local dermal toxicity was significantly increased when the acidic pH of the solution was maintained (pH 3.3 vs. 6.95), resulting in skin barrier disruption [134].
It seems that different and sometimes contradictory results on products’ effects are obtained in the presented studies. For overall conclusions on the linkage between efficacy and pH of the product, more information is needed. It is necessary to establish more precise study protocols that involve human volunteers, as well as their measuring principles, in order to investigate the specific influence of the formulations’ pH per se in relation to topical products efficacy and beneficial effects.
With emulsions and skin pH measurements, several issues should be carefully considered. One of these issues is the very measurement of the skin pH after product application. The interesting work of Farage et al. highlighted the possibility that the measured pH after a product’s application may not indicate the real skin surface pH. The evidence that they provided shows that some topical products can support natural skin pH balance, rather than affect it [135]. Another important issue concerns the initial information on the emulsion type, its pH and its buffering capacity. Being a colloid dispersion and a mixture of water and oil phase, an emulsion is characterized by the pH value, which is chiefly determined by the pH of its water phase. If the emulsion is of an O/W type, the pH is measured upon direct immersion of a pH meter electrode. Usually, the pH of a W/O emulsion is determined after the breaking of an emulsion (emulsion separation) occurred, and is, thus, performed in the separated water phase of the emulsion. The buffering capacity may be determined via a titration method, i.e., by adding NaOH or HCl to the aqueous phase. As mentioned previously, the information regarding the emulsions buffering capacity is rarely provided in published papers, while the presented pH values of emulsions are not supported with description of methods used.
8. Skin Health Maintenance
During our entire life, the skin is exposed to hostile influence of external and internal factors and their interactions with each other, finally resulting in skin aging [136]. Therefore, the imperative in skin care became the prevention and reduction of signs of aging. Gathered information about the skin structure and functions together with the knowledge of the skin exosomes enabled the development of new topical product strategies. Nowadays, terminology used in cosmetic marketing campaigns avoids terms related to skin aging. Terms such as ‘aged skin’, ‘wrinkles’, ‘old’, etc., have been replaced with terms related to strength, youthfulness and skin wellbeing. Similar to this, ‘anti-age’ cosmetics has shifted to a category of cosmetics used for skin health maintenance.
However, the concept of skin health maintenance is bigger than anti-aging, not restricted only to the prevention of ageing and/or the skin care of aged skin but it takes into concern different skin types, conditions and needs of skin with the aim to keep it healthy. The basic elements of any strategy for skin health are cleansing, moisturization and protection from UV light [137]. Finally, based on all the presented data, additional compulsory element for any of these products should be a proper pH value.
Nevertheless, during the development of topical products potentially contributing to the skin health maintenance by affecting mechanisms related to skin acidification, the attention should be focused not only on the products’ pH but the presence of certain ingredients. Acids, such as alpha-, beta- and polyhydroxy acids, are active ingredients that have been intensively used for the last 30 years in both dermatology and cosmetology. Their established efficacy depends on the type, pH and strength of the used acid and, although not fully comprehended, their mechanism of action is related to the H+ ions concentration and dissociation of free acids, which should occur in the epidermis [138,139]. In a recent study, Lukic et al. have shown that topical products could induce skin acidification and that the effect is not in direct reciprocity to a products’ pH, although this depends on the presence of ingredients which are proton donors and on the activity of a skin buffering system [140]. Due to numerous difficulties related to the formulation of topical products with such actives, investigations in this field are still drawing great attention in relation to the development of stabile and safe products with rather low pH [87,140,141]. Undoubtedly, research in this area should be deepened by the interlinking efficacy of these products with skin pH formation, maintenance and acid microdomains.
Important ingredients which could be related to skin acidic pH are lipids—precursors of FFAs that are necessary for pH formation—as well as ingredients affecting the skin microbiome. During the development of products with these ingredients, their efficacy has to be investigated in the context of skin pH, among others. Additionally, substances affecting any pathway important for proton formation could be considered as candidates for actives in skin health maintenance products.
9. Concluding Remarks
Further research is obviously necessary and preferably delivered in the form of high-quality studies aiming to elucidate the possible interactions between products’ ingredients and acidic pH, studies on products’ buffering capacity, studies on the linkage between products’ pH and efficacy, etc. Even so, there is enough assessable evidence that confirms the importance of acidic skin surface, as well as the influence of topical products’ pH on skin surface pH. Consequently, there is a high level of agreement that topical products should be acidified and possess pH values in the range of 4 to 6. However, formulators, dermatologists and consumers would benefit from some more precise guidance concerning favorable product pH values. Further narrowing of the pH range that could be considered optimal for skin health maintenance may rely on the discussed studies of a number of skin enzymes (e.g., β-glucocerebrosidase, acid sphingomyelinase and proteases) that point towards the pH necessary for their optimal activity.
Author Contributions
Conceptualization: M.L., Investigation: M.L., Writing—Original Draft Preparation: M.L., Writing—Review & Editing: I.P., S.D.S., Supervision: S.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia (No. 451-03-9/2021-14/200161). The APC was funded by Sebapharma GmbH & Co. KG, Binger Straße 80, D-56154 Boppard/Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rippke, F.; Schreiner, V.; Schwanitz, H.V. The Acidic Milieu of the Horny Layer New Findings on the Physiology and Path-ophysiology of Skin pH. Am. J. Clin. Dermatol. 2002, 3, 261–272. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef]
- Wierzbicka, D.M.; Karczewski, J.; Dobrowolska-Zachwieja, A.; Adamski, Z. The microbiome and dermatological diseases. Postepy Hig. Med. Dosw. 2015, 7, 978–985. [Google Scholar]
- Kong, J.; Segre, H. The Molecular Revolution in Cutaneous Biology: Investigating the Skin Microbiome. J. Investig. Dermatol. 2017, 137, e119–e122. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schröder, J.-M.; Gläser, R. The skin surface as antimicrobial barrier: Present concepts and future outlooks. Exp. Dermatol. 2012, 22, 1–5. [Google Scholar] [CrossRef]
- Fry, L.; Baker, B.S.; Powles, A.V.; Fahlen, A.; Engstrand, L. Is chronic plaque psoriasis triggered by microbiota in the skin? Br. J. Dermatol. 2013, 169, 47–52. [Google Scholar] [CrossRef]
- Fowler, J. Understanding the Role of Natural Moisturizing Factor in Skin Hydration. Pract. Dermatol. 2012, 7, 36–40. [Google Scholar]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Haftek, M. Epidermal barrier disorders and corneodesmosome defects. Cell Tissue Res. 2015, 360, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Van Smeden, J.; Janssens, M.; Gooris, G.; Bouwstra, J. The important role of stratum corneum lipids for the cutaneous barrier function. BBA Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Igawa, S. Genetic skin diseases related to desmosomes and corneodesmosomes. J. Dermatol. Sci. 2014, 74, 99–105. [Google Scholar] [CrossRef]
- Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. 2017, 469, 3–14. [Google Scholar] [CrossRef]
- Elias, P.M.; Gruber, R.; Crumrine, D.; Menon, G.; Williams, M.L.; Wakefield, J.S.; Holleran, W.M.; Uchida, Y. Formation and functions of the corneocyte lipid envelope (CLE). BBA Mol. Cell Biol. Lipids 2014, 1841, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Bouwstra, J.A. Stratum corneum lipids: Their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [PubMed]
- Bhattacharya, N.; Sato, W.J.; Kelly, A.; Ganguli-Indra, G.; Indra, A.K. Epidermal Lipids: Key Mediators of Atopic Dermatitis Pathogenesis. Trends Mol. Med. 2019, 25, 551–562. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Schade, H.; Marchionini, A. Zur physikalischen Chemie der Hautoberfläche. Arch. Dermatol. Res. 1928, 154, 690–716. [Google Scholar] [CrossRef]
- Braun-Falco, O.; Korting, H.C. Normal pH value of the skin. Hautarzt 1986, 37, 126–129. [Google Scholar] [PubMed]
- Segger, D.; Aßmus, U.; Brock, M.; Erasmy, J.; Finkel, P.; Fitzner, A.; Heuss, H.; Kortemeier, U.; Munke, S.; Rheinländer, T.; et al. Multicenter study on measurement of the natural pH of the skin surface. Int. J. Cosmet. Sci. 2008, 30, 75. [Google Scholar] [CrossRef]
- Kleesz, P.; Darlenski, R.; Fluhr, J. Full-Body Skin Mapping for Six Biophysical Parameters: Baseline Values at 16 Anatomical Sites in 125 Human Subjects. Skin Pharmacol. Physiol. 2012, 25, 25–33. [Google Scholar] [CrossRef]
- Schmitt, T.; Neubert, R.H. State of the art in Stratum Corneum research: The biophysical properties of ceramides. Chem. Phys. Lipids 2018, 216, 91–103. [Google Scholar] [CrossRef]
- Levin, J.; Maibach, H. pH buffering considerations in mature skin. Cosm. Toil. 2011, 126, 422–428. [Google Scholar]
- Levin, J.; Maibach, H. Human skin buffering capacity: An overview. Skin Res. Technol. 2008, 14, 121–126. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Behne, M.J.; Brown, B.E.; Moskowitz, D.G.; Selden, A.; Mao-Qiang, M.; Mauro, T.M.; Elias, P.M.; Feingold, K.R. Stratum Corneum Acidification in Neonatal Skin: Secretory Phospholipase A2 and the Sodium/Hydrogen Antiporter-1 Acidify Neonatal Rat Stratum Corneum. J. Investig. Dermatol. 2004, 122, 320–329. [Google Scholar] [CrossRef]
- Fluhr, J.; Darlenski, R.; Lachmann, N.; Baudouin, C.; Msika, P.; De Belilovsky, C.; Hachem, J.-P. Infant epidermal skin physiology: Adaptation after birth. Br. J. Dermatol. 2012, 166, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Age-related changes in skin barrier function—Quantitative evaluation of 150 female subjects. Int. J. Cosmet. Sci. 2013, 35, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Man, M.; Xin, S.; Song, S.; Cho, S.; Zhang, X.; Tu, C.; Feingold, K.; Elias, P. Variation of Skin Surface pH, Sebum Content and Stratum Corneum Hydration with Age and Gender in a Large Chinese Population. Skin Pharmacol. Physiol. 2009, 22, 190–199. [Google Scholar] [CrossRef]
- Bailey, S.H.; Oni, G.; Brown, S.A.; Kashefi, N.; Cheriyan, S.; Maxted, M.; Stewart, C.; Jones, C.; Maluso, P.; Kenkel, A.M.; et al. The use of non-invasive instruments in characterizing human facial and abdominal skin. Lasers Surg. Med. 2012, 44, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.; Ivens, U.I.; Møller, M.L.; Senderovitz, T.; Serup, J. Females have lower skin surface pH than men. A study on the influence of gender, forearm site variation, right/left difference and time of the day on the skin surface pH. Skin Res. Technol. 2001, 7, 90–94. [Google Scholar] [CrossRef]
- Man, M.-Q.; Lin, T.-K.; Santiago, J.L.; Celli, A.; Zhong, L.; Huang, Z.-M.; Roelandt, T.; Hupe, M.; Sundberg, J.P.; Silva, K.A.; et al. Basis for Enhanced Barrier Function of Pigmented Skin. J. Investig. Dermatol. 2014, 134, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, R.; Schurer, N.Y.; Shoo, B.A.; Celli, A.; Hachem, J.P.; Crumrine, D.; Sirimanna, G.; Feingold, K.R.; Mauro, T.M.; Elias, P.M. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J. Investig. Dermatol. 2009, 129, 1719–1729. [Google Scholar] [CrossRef]
- Hanson, K.M.; Behne, M.J.; Barry, N.P.; Mauro, T.M.; Gratton, E.; Clegg, R.M. Two-Photon Fluorescence Lifetime Imaging of the Skin Stratum Corneum pH Gradient. Biophys. J. 2002, 83, 1682–1690. [Google Scholar] [CrossRef]
- Behne, M.J.; Meyer, J.W.; Hanson, K.M.; Barry, N.P.; Murata, S.; Crumrine, D.; Clegg, R.W.; Gratton, E.; Holleran, W.M.; Elias, P.M.; et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J. Biol. Chem. 2002, 277, 47399–47406. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Behne, M.; Aronchik, I.; Demerjian, M.; Feingold, K.; Elias, P.; Mauro, T. Extracellular pH Controls NHE1 Expression in Epidermis and Keratinocytes: Implications for Barrier Repair. J. Investig. Dermatol. 2005, 125, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Matousek, J.L.; Campbell, K.L. A comparative review of cutaneous pH. Vet. Dermatol. 2002, 13, 293–300. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Maayan-Metzger, A.; Merlob, P.; Sirota, L. Skin barrier properties in different body areas in neonates. Pediatrics 2000, 106, 105–108. [Google Scholar] [CrossRef]
- Fox, C.; Nelson, D.; Wareham, J. The timing of skin acidification in very low birth weight infants. J. Perinatol. 1998, 18, 272–275. [Google Scholar] [PubMed]
- Behne, M.J.; Barry, N.P.; Hanson, K.M.; Aronchik, I.; Clegg, R.W.; Gratton, E.; Feingold, K.; Holleran, W.M.; Elias, P.M.; Mauro, T.M. Neonatal Development of the Stratum Corneum pH Gradient: Localization and Mechanisms Leading to Emergence of Optimal Barrier Function. J. Investig. Dermatol. 2003, 120, 998–1006. [Google Scholar] [CrossRef][Green Version]
- Visscher, M.O.; Adam, R.; Brink, S.; Odio, M. Newborn infant skin: Physiology, development, and care. Clin. Dermatol. 2015, 33, 271–280. [Google Scholar] [CrossRef]
- Pickens, W.L.; Warner, R.R.; Boissy, Y.L.; Boissy, R.E.; Hoath, S.B. Characterization of Vernix Caseosa: Water Content, Morphology, and Elemental Analysis. J. Investig. Dermatol. 2000, 115, 875–881. [Google Scholar] [CrossRef]
- Visscher, M.O.; Narendran, V.; Pickens, W.L.; LaRuffa, A.A.; Meinzen-Derr, J.; Allen, K.; Hoath, S.B. Vernix caseosa in neo-natal adaptation. J. Perinatol. 2005, 25, 440–446. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Kao, J.; Ahn, S.K.; Feingold, K.R.; Elias, P.M.; Jain, M. Generation of Free Fatty Acids from Phospholipids Regulates Stratum Corneum Acidification and Integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef]
- Jang, H.; Matsuda, A.; Jung, K.; Karasawa, K.; Matsuda, K.; Oida, K.; Ishizaka, S.; Ahn, G.; Amagai, Y.; Moon, C.; et al. Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J. Investig. Dermatol. 2016, 136, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, K.; Henkes, D.; Strüver, K.; Sochorová, M.; Školová, B.; Witting, M.Y.; Friess, W.; Schreml, S.; Meier, R.J.; Schäfer-Korting, M.; et al. Filaggrin Deficiency Leads to Impaired Lipid Profile and Altered Acidification Pathways in a 3D Skin Construct. J. Investig. Dermatol. 2014, 134, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Hman, H.; Vahlquist, A. The pH Gradient over the Stratum Corneum Differs in X-Linked Recessive and Autosomal Dominant Ichthyosis: A Clue to the Molecular Origin of the “Acid Skin Mantle”? J. Investig. Dermatol. 1998, 111, 674–677. [Google Scholar]
- Houben, E.; Hachem, J.; De Paepe, K.; Rogiers, V. Epidermal Ceramidase Activity Regulates Epidermal Desquamation via Stratum Corneum Acidification. Skin Pharmacol. Physiol. 2008, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Elias, P.M. Stratum corneum pH: Formation and function of the‘Acid Mantle’. Exogen. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Krien, P.M.; Kermici, M. Evidence for the Existence of a Self-Regulated Enzymatic Process within the Human Stratum Corneum –An Unexpected Role for Urocanic Acid. J. Investig. Dermatol. 2000, 115, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Elias, P.M.; Man, M.Q.; Hupe, M.; Selden, C.; Sundberg, J.P.; Tschachler, E.; Eckhart, L.; Mauro, T.M.; Feingold, T.R. Is the Filaggrin-Histidine-Urocanic Acid Pathway Essential for Stratum Corneum Acidification? J. Investig. Dermatol. 2010, 130, 2141–2144. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Kezic, S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Matts, P.J. Stratum Corneum Moisturization at the Molecular Level: An Update in Relation to the Dry Skin Cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. The how, why and clinical importance of stratum corneum acidification. Exp. Dermatol. 2017, 26, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Biro, T.; Olah, A.; Toth, B.I.; Szollosi, A.G. Endogenous Factors That Can Influence Skin pH. In pH of the Skin: Issues and Challenges; Surber, C., Abels, C., Maibach, H., Eds.; Karger: Basel, Switzerland, 2018; Volume 54, pp. 54–63. [Google Scholar]
- Eberting, C.L.; Blickenstaff, N.; Goldenberg, A. Pathophysiologic Treatment Approach to Irritant Contact Dermatitis. Curr. Treat. Options Allergy 2014, 1, 317–328. [Google Scholar] [CrossRef][Green Version]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Meijling, K.G.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J. Dermatol. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Mao-Qiang, M.; Brown, B.E.; Hachem, J.-P.; Moskowitz, D.G.; Demerjian, M.; Haftek, M.; Serre, G.; Crumrine, D.; Mauro, T.M.; et al. Functional Consequences of a Neutral pH in Neonatal Rat Stratum Corneum. J. Investig. Dermatol. 2004, 123, 140–151. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Lieckfeldt, R.; Villalaín, J.; Gómez-Fernández, J.C.; Lee, G. Apparent pK a of the fatty acids within ordered mixtures of model human stratum corneum lipids. Pharm. Res. 1995, 12, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, I.; Norlén, L.; Bagatolli, L.A. Direct Visualization of Lipid Domains in Human Skin Stratum Corneum’s Lipid Membranes: Effect of pH and Temperature. Biophys. J. 2007, 93, 3142–3155. [Google Scholar] [CrossRef] [PubMed]
- Haftek, M.; Simon, M.; Kanitakis, J.; Maréchal, S.; Claudy, A.; Serre, G.; Schmitt, D. Expression of corneodesmosin in the granular layer and stratum corneum of normal and diseased epidermis. Br. J. Dermatol. 1997, 137, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y. Implications of normal and disordered remodeling dynamics of corneodesmosomes in stratum corneum. Dermatol. Sin. 2015, 33, 58–63. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Roelandt, T.; Schürer, N.; Pu, X.; Fluhr, J.; Giddelo, C.; Man, M.-Q.; Crumrine, D.; Roseeuw, D.; Feingold, K.R.; et al. Acute Acidification of Stratum Corneum Membrane Domains Using Polyhydroxyl Acids Improves Lipid Processing and Inhibits Degradation of Corneodesmosomes. J. Investig. Dermatol. 2010, 130, 500–510. [Google Scholar] [CrossRef]
- Caubet, C.; Jonca, N.; Brattsand, M.; Guerrin, M.; Bernard, D.; Schmidt, R.; Egelrud, T.; Simon, M.; Serre, G. Degradation of Corneodesmosome Proteins by Two Serine Proteases of the Kallikrein Family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Investig. Dermatol. 2004, 122, 1235–1244. [Google Scholar] [CrossRef]
- Blum, H.E. The human microbioma. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Gallo, R.L.; Nakatsuji, T. Microbial Symbiosis with the Innate Immune Defense System of the Skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.-I.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Degrades Specific Proteins Associated with Staphylococcus aureus Biofilm Formation and Host-Pathogen Interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef]
- Yuki, T.; Yoshida, H.; Akazawa, Y.; Komiya, A.; Sugiyama, Y.; Inoue, S.; Mesin, L.; Di Niro, R.; Thompson, K.M.; Lundin, K.E.A.; et al. Activation of TLR2 Enhances Tight Junction Barrier in Epidermal Keratinocytes. J. Immunol. 2011, 187, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Seidenari, S.; Giusti, G. Objective assessment of the skin of children affected by atopic dermatitis: A study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm. Venereol. 1995, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Rojas, R.; Tirado-Sánchez, A.; Chávez-López, D.; Mena, C.; Calderón, L.; María, P.O. Superficial Mycoses Asso-ciated with Diaper Dermatitis. Mycopathologia 2016, 181, 671–679. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Incontinence in the aged: Contact dermatitis and other cutaneous consequences. Contact Dermat. 2007, 57, 211–217. [Google Scholar] [CrossRef]
- Danby, S.G.; Cork, M.J. pH in Atopic Dermatitis. In pH of the Skin: Issues and Challenges; Surber, C., Abels, C., Maibach, H., Eds.; Karger: Basel, Switzerland, 2018; Volume 54, pp. 95–107. [Google Scholar]
- Kong, H.H. Skin microbiome: Genomics-based insights into the diversity and role of skin microbes. Trends Mol. Med. 2011, 17, 320–328. [Google Scholar] [CrossRef]
- Oh, J.; Freeman, A.F.; Park, M.; Sokolic, R.; Candotti, F.; Holland, S.M.; Segre, J.A.; Kong, H.; Program, N.C.S. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 2013, 23, 2103–2114. [Google Scholar] [CrossRef]
- Percival, S.L.; Mccarty, S.; Hunt, J.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Dissemond, J.; Witthoff, M.; Brauns, T.C.; Haberer, D.; Goos, M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt 2003, 54, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, R.J.; Ross, L. Effects of surfactants and solvents on the permeability of the epidermis. J. Soc. Cosmet. Chem. 1970, 21, 853–873. [Google Scholar]
- Madison, K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Watkinson, A.; Rogers, J.; Mayo, A.; Hope, J.; Scott, I.A. Abnormalities in stratum corneum structure, lipid composition, and desmosome degradation in soap-induced winter xerosis. J. Soc. Cosmet. Chem. 1994, 45, 203–220. [Google Scholar]
- Lukic, M.; Pantelic, I.; Savic, S. An Overview of Novel Surfactants for Formulation of Cosmetics with Certain Emphasis on Acidic Active Substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrierand the technology of mild cleansing. Dermatol. Ther. 2004, 7, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Rhein, L.D. Review of properties of surfactants that determine their interactions with stratum corneum. J. Soc. Cosmet. Chem. 1997, 48, 253–274. [Google Scholar]
- Corazza, M.; Lauriola, M.M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, skin cleansing protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin–Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Jackson, C.T.; Paye, M.; Maibach, H.I. Mechanism of skin irritation by surfactants and anti-irritants for surfactant-based products. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Draelos, Z.; Hornby, S.; Walters, R.; Appa, Y. Hydrophobically modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J. Cosmet. Dermatol. 2013, 12, 314–321. [Google Scholar] [CrossRef]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Abbas, S.; Goldberg, J.W.; Massaro, M. Personal cleanser technology and clinical performance. Dermatol. Ther. 2004, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mücke, H.; Mohr, K.T.; Rümmler, A.; Wutzler, T. Untersuchungen über den Haut-pH-Wert der Hand nach Anwendung von Seife, Reinigungs- und Händedesinfektionsmitteln. Pharmazie 1993, 48, 468–469. [Google Scholar]
- Trobaugh, C.M.; Wickett, R.R. Personal care products: Effects on skin surface pH. Cosm. Toil. 1990, 105, 41–46. [Google Scholar]
- Firooz, A.; Aghazadeh, N.; Estarabadi, A.R.; Hejazi, P. The effects of water exposure on biophysical properties of normal skin. Skin Res. Technol. 2015, 21, 131–136. [Google Scholar] [CrossRef]
- Voegeli, D. The Effect of Washing and Drying Practices on Skin Barrier Function. J. Wound Ostomy Cont. Nurs. 2008, 35, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gfatter, R.; Hackl, P.; Braun, F. Effects of Soap and Detergents on Skin Surface pH, Stratum corneum Hydration and Fat Content in Infants. Dermatology 1997, 195, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.; Megele, M.; Mehringer, L.; Vieluf, D.; Zienicke, H.; Hamm, G.; Braun-Falco, O. Influence of skin cleansing preparation acidity on skin surface properties. Int. J. Cosmet. Sci. 1991, 13, 91–102. [Google Scholar] [CrossRef]
- Duncan, C.N.; Riley, T.V.; Carson, K.C.; Budgeon, C.A.; Siffleet, J. The effect of an acidic cleanser versus soap on the skin pH and micro-flora of adult patients: A non-randomized two group crossover study in an intensive care unit. Intensive Crit. Care Nurs. 2013, 29, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Baranda, L.; González-Amaro, R.; Torres-Alvarez, B.; Alvarez, C.; Ramírez, V. Correlation between pH and irritant effect of cleansers marketed for dry skin. Int. J. Dermatol. 2002, 41, 494–499. [Google Scholar] [CrossRef]
- Lu, G.; Moore, D.J. Study of surfactant-skin interactions by skin impedance measurements. Int. J. Cosmet. Sci. 2012, 34, 74–80. [Google Scholar] [CrossRef]
- Korting, H.C.; Kober, M.; Mueller, M.; Braun-Falco, O. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. Results of a cross-over trial in health probationers. Acta Derm. Venereol. 1987, 67, 41–47. [Google Scholar]
- Korting, H.C.; Braun-Falco, O. The effect of detergents on skin pH and its consequences. Clin. Dermatol. 1996, 14, 23–27. [Google Scholar] [CrossRef]
- Barel, A.O.; Lambrecht, R.; Clarys, P.; Morrison, B.M., Jr.; Paye, M. A comparative study of the effects on the skin of a classical bar soap and a syndet cleansing bar in normal use conditions and in the soap chamber test. Skin Res. Technol. 2001, 7, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kaneda, K.; Miyaki, M.; Matsuo, K.; Kawada, H.; Hosokawa, H. The long-term use of soap does not affect the pH-maintenance mechanism of human skin. Skin Res. Technol. 2015, 21, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Bornkessel, A.; Flach, M.; Elsner, P.; Fluhr, J.W.; Arens-Corell, M. Functional assessment of a washing emulsion for sensitive skin: Mild impairment of stratum corneum hydration, pH, barrier function, lipid content, integrity and cohesion in a controlled washing test. Skin Res. Technol. 2005, 11, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.R.; Shimabukuro, D.M.; Uber, M.; Abagge, K.T. Critical assessment of the pH of children’s soap. J. Pediatr. 2016, 92, 290–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarun, J.; Susan, V.J.; Susan, J.; Suria, J.; Criton, S. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J. Dermatol. 2014, 59, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Mauro, T.; Grayson, S.; Gao, W.N.; Man, M.-Q.; Kriehuber, E.; Behne, M.; Feingold, K.R.; Elias, P.M. Barrier recovery is impeded at neutral pH, independent of ionic effects: Implications for extracellular lipid processing. Arch. Dermatol. Res. 1998, 290, 215–222. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. pH Directly Regulates Epidermal Permeability Barrier Homeostasis, and Stratum Corneum Integrity/Cohesion. J. Investig. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef]
- Man, M.Q.; Feingold, K.R.; Elias, P.M. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Arch. Dermatol. 1993, 129, 728–738. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Feingold, K.R.; Thornfeldt, C.R.; Elias, P.M. Optimization of Physiological Lipid Mixtures for Barrier Repair. J. Investig. Dermatol. 1996, 106, 1096–1101. [Google Scholar] [CrossRef]
- Zhang, Z.; Lukic, M.; Savic, S.; Lunter, D.J. Reinforcement of barrier function—Skin repair formulations to deliver physiological lipids into skin. Int. J. Cosmet. Sci. 2018, 40, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, K.; Hrabalek, A.; Mac-Mary, S.; Humbert, P.; Muret, P. Ceramide analogue 14S24 selectively recovers perturbed human skin barrier. Br. J. Dermatol. 2007, 157, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nakagawa, H.; Higuchi, K.; Imokawa, G. Characterization of Surfactant-Induced Skin Damage through Barrier Recovery Induced by Pseudoacylceramides. Dermatology 2005, 211, 128–134. [Google Scholar] [CrossRef]
- Fukunaga, K.; Yoshida, M.; Nakajima, F.; Uematsu, R.; Hara, M.; Inoue, S.; Kondo, H.; Nishimura, S. Design, synthesis, and evaluation of beta-galactosylceramide mimics promoting beta-glucocerebrosidase activity in keratinocytes. Bioorg. Med. Chem. Lett. 2003, 13, 813–885. [Google Scholar] [CrossRef]
- Darmstadt, G.L.; Man, M.Q.; Chi, E.; Saha, S.K.; Ziboh, V.A.; Black, R.E.; Santosham, M.; Elias, P.M. Impact of topical oils on the skin barrier: Possible implications for neonatal health in developing countries. Acta Paediatr. 2002, 91, 546–554. [Google Scholar] [CrossRef]
- Buraczewska, I.; Lodén, M. Treatment of Surfactant-Damaged Skin in Humans with Creams of Different pH Values. Pharmacology 2005, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.; Danby, S. Aqueous cream damages the skin barrier. Br. J. Dermatol. 2011, 164, 1179–1180. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H.I. Barrier creams—Skin protectants: Can you protect skin? J. Cosmet. Dermatol. 2002, 1, 20–23. [Google Scholar] [CrossRef]
- Pavlačková, J.; Egner, P.; Sedláček, T.; Mokrejš, P.; Sedlaříková, J.; Polášková, J. In vivo efficacy and properties of semisolid formulations containing panthenol. J. Cosmet. Dermatol. 2019, 18, 346–354. [Google Scholar] [CrossRef]
- Glatz, M.; Jo, J.-H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Age-Related Changes in Male Skin: Quantitative Evaluation of One Hundred and Fifty Male Subjects. Skin Pharmacol. Physiol. 2014, 27, 9–17. [Google Scholar] [CrossRef]
- Kilic, A.; Masur, C.; Reich, H.; Knie, U.; Dähnhardt, D.; Dähnhardt-Pfeiffer, S.; Abels, C. Skin acidification with a water-in-oil emulsion (pH 4) restores disrupted epidermal barrier and improves structure of lipid lamellae in the elderly. J. Dermatol. 2019, 46, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Dähnhardt, D.; Dähnhardt-Pfeiffer, S.; Bielfeldt, S.; Wilhelm, K.P.; Wohlfart, R.; Staib, P. A plant oil-containing pH 4 emulsion improves epidermal barrierstructure and enhances ceramide levels in aged skin. Int. J. Cosmet. Sci. 2017, 39, 284–291. [Google Scholar] [CrossRef]
- Angelova-Fischer, I.; Fischer, T.W.; Abels, C.; Zillikens, D. Accelerated barrier recovery and enhancement of the barrier in-tegrity and properties by topical application of a pH 4 vs. a pH 5.8 water-in-oil emulsion in aged skin. Br. J. Dermatol. 2018, 179, 471–477. [Google Scholar]
- Tasic-Kostov, M.; Savic, S.; Lukic, M.; Tamburic, S.; Pavlovic, M.; Vuleta, G. Lactobionic acid in a natural alkylpolygluco-side-based vehicle: Assessing safety and efficacy aspects in comparison to glycolic acid. J. Cosmet. Dermatol. 2010, 9, 3–10. [Google Scholar] [CrossRef]
- Yu, R.J.; Van Scott, E.J. Alpha-hydroxyacids and carboxylic acids. J. Cosmet. Dermatol. 2004, 3, 76–87. [Google Scholar] [CrossRef]
- Sahlin, A.; Edlund, F.; Lodén, M. A double-blind and controlled study on the influence of the vehicle on the skin susceptibility to stinging from lactic acid. Int. J. Cosmet. Sci. 2007, 29, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Buraczewska, I.; Berne, B.; Lindberg, M.; Törmä, H.; Lodén, M. Changes in skin barrier function following long-termtreatment with moisturizers, a randomized controlled trial. Br. J. Dermatol. 2007, 156, 492–498. [Google Scholar] [CrossRef]
- Bashir, S.; Dreher, F.; Chew, A.; Zhai, H.; Levin, C.; Stern, R.; Maibach, H. Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm. 2005, 292, 187–194. [Google Scholar] [CrossRef]
- Farage, M.A.; Hood, W.; Berardesca, E.; Maibach, H. Intrinsic and Extrinsic Factors Affecting Skin Surface pH. In pH of the Skin: Issues and Challenges; Surber, C., Abels, C., Maibach, H., Eds.; Karger: Basel, Switzerland, 2018; Volume 54, pp. 33–47. [Google Scholar]
- . Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Nash, J.F.; Matts, P.J.; Ertel, K.D. Maintenance of healthy skin: Cleansing, moisturization, and ultraviolet protection. J. Cosmet. Dermatol. 2007, 6, 7–11. [Google Scholar] [CrossRef]
- Jiang, M.; Qureshi, S. Assessment of in vitro percutaneous absorption of glycolic acid through human skin sections using a flow-through diffusion cell system. J. Dermatol. Sci. 1998, 18, 181–188. [Google Scholar] [CrossRef]
- Draelos, Z.D. α-Hydroxy acids, β-hydroxy acid, and other topical agents. Dermatol. Ther. 2000, 13, 154–158. [Google Scholar] [CrossRef]
- Lukic, M.; Filipovic, M.; Nevena, P.; Lunter, D.; Bozic, D.; Savic, S. Formulation of topical acidic products and acidification of the skin—Contribution of glycolic acid. Int. J. Cosmet. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tasić-Kostov, M.; Lukić, M.; Savić, S. A 10% Lactobionic acid-containing moisturizer reduces skinsurface pH without irrita-tion—An in vivo/in vitro. J. Cosmet. Dermatol. 2019, 18, 1705–1710. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).