Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation
Abstract
1. Introduction
Skin and Brain: A Psychobiological Concept
2. A Brief Overview: From Cosmetology and Cosmetic Functions to Neurocosmetics
- Anti-aging action: this refers to all ingredients that, via different pathways, combat the skin aging processes, preventing and fighting the signs of aging, revitalizing senescent skin, promoting the elimination of toxins, improving microcirculation, and reducing the number and the depth of wrinkles;
- Antioxidant action: this includes ingredients that can capture and inactivate free radicals at the skin level, reducing the “oxidative stress”;
- Anti-wrinkle action: this concerns ingredients that counteract the onset of wrinkles;
- Depigmenting action: this includes ingredients that lighten the skin color;
- Eudermic action: this considers ingredients that invoke a feeling of wellbeing when applied on the skin;
- Eutrophic action: this refers to substances that nourish the skin and improve its appearance;
- Soothing, anti-redness action: exhibited by ingredients able to soothe pain and reduce skin redness in cases of mild inflammation, counteracting irritation, and bringing relief to stressed skin;
- Regenerating action: this refers to ingredients with eutrophic properties, promoting cell regeneration, and providing the necessary elements for skin development and maintenance over time;
- Toning, “lifting” action: this concerns ingredients that restore skin tone and firmness by acting at both the epidermis and dermis levels, encouraging the typical turgor of young and healthy skin [1].
3. The Neurocosmetics
3.1. Towards Neurocosmetics and How to Understand It: A More in-Depth Insight about Skin Innervation and Neurotransmitters
3.2. Neurocosmetics, the Cosmetic of Neurotransmitters: The Brain and Skincare
- Directly on the cutaneous nervous fiber endings [9], as modulators of the neurotransmitter release [14]. Examples are the botulinum-like peptides that encourage facial musculature relaxation to obtain wrinkle-smoothing, and peptides that inhibit neurons, mainly used to make hypersensitive skin less reactive towards environmental stimuli (temperature changes, air humidity, smog, etc.) [9];
3.2.1. Feelings of Pleasantness and Wellbeing vs. Neurocosmetics
Psychocosmetics
Neurosciences
- Cellular Neurosciences study the behavior of nervous cells in vitro; these related experiments are usually performed by manufacturing companies on neurocosmetic functional ingredients;
- Behavioral Neurosciences study the cognitive (thoughts, memories, and other mental processes) and affective (emotions, feelings, and mood) behavior of individuals [11].
Neuroscience Applications in the Cosmetic Field
- Vital parameters such as heart activity recorded with ECG [64], respiratory activity/frequency, salivary samples for monitoring cortisol release [65,66] (i.e., the decrease in cortisol secretion in saliva after the use of a soothing cream should be observed) [56,67], thermography related to cutaneous blood flow (i.e., when blushing, etc.), the evolution of facial skin temperature under stress [64], and the electrical current perception threshold (CPT); the hyperexcitability of nerve endings (Aβ) increases with age, in which a threshold increase indicates a reduction of unpleasant excitability, (i.e., an improved state of wellness…) [64];
4. Neurocosmetic Ingredients
4.1. Neurocosmetic Ingredients for Combating Skin Stress
4.1.1. Skin Stress and the Stress Pathway: The Role of Cortisol
- Increased blood sugar levels, which promote “glycation” in the skin, damaging collagen and elastin. As a result, long-term aging and loss of the skin’s ability to fully bounce back are accelerated;
- Due to the strong catabolic effect of cortisol, the degradation of dermal proteins occurs;
- Skin dryness due to the reduction in the production of hyaluronic acid—a natural moisturizer for our skin, associated with an increase in transepidermal water loss (TEWL);
Neurocosmetic Ingredients for Rebalancing Cortisol Levels in the Skin
4.1.2. “Neuro-Relaxing” Anti-Aging Ingredients
The β-Endorphins: A Strategy for Skin Wellness
Discovering Neurocosmetic Ingredients from Plant Extracts
4.1.3. Neurocosmetics as a Strategy to Combat Inflammatory Responses Related to Skin Stress
4.1.4. Intriguing Hints about Sleep-Related Beauty: The Melatonin Receptor MT1
4.2. Neurocosmetic Ingredients for Sensitive Skin
4.2.1. Neuro-Sensitization of the Skin
The Molecular Basis of Sensitive Skin: TRPV-1-Mediated Neuroinflammation
4.2.2. Providing Solutions for Sensitive Skin: Neurocosmetic Ingredients
4.2.3. Providing Solutions for Sensitive Skin: “Coolant” Neurocosmetic Ingredients
An Insight about the Skin Sensation: Warm and Cold Receptors
4.2.4. Soothing Neurocosmetic Products on the Market
4.3. Neurocosmetic Ingredients for Skin Aging
- Intrinsic (chronological) aging or chrono-aging.
- Extrinsic aging
4.3.1. Physiological and Morphological Characteristics of Aged Skin
4.3.2. Skin Neuro-Aging and Neurocosmetic Ingredients
- The protection of nerve cells in the epidermis by providing good support for other skin cells;
- The maintenance of good barrier function in order to improve skin hydration (in the epidermis, nervous cells could stimulate the activity of keratinocytes, enhancing the barrier function that, in turn, causes hydrated and protected skin);
- The firming of skin by preserving the lipolytic activity in adipocytes. In the hypodermis, nervous cells stimulated the adipocytes’ lipolytic activity, which decreases with aging. By protecting nerve cells, good skin firmness is ensured [290].
Rethinking Skin Aging by Modulating Senescence Marker Proteins: The Case of Progerin
Cell Communication Supports by Stimulating POMC-Related Receptor Expression
A Strategy to Combat Dark Spots and Skin Stress
The Youth Proteins: KLOTHO and FOXO
4.4. Biomimetic Peptides
4.4.1. Topical Peptides and Their Skin Permeability
- The physicochemical properties of the substance (pKa, molecular size, stability, binding affinity, solubility, and partition coefficient);
- The time necessary for the permeation;
- The integrity, thickness, and components of the skin, and the cutaneous metabolism;
- The site, area, duration of application, and local depot at the site of application.
- Molecular weight of less than 500 Da;
- Moderate log of partition coefficient octanol/water between 1 and 3;
- Melting point less than 200 °C;
- Reasonable aqueous solubility (>1 mg mL−1);
- Zero or few polar centers.
4.4.2. Delivery Systems for Peptides
4.4.3. Neurotransmitter-Affecting Peptides
- Pentapeptide-3 (Vialox) is a synthetic peptide derived from snake venom, which acts at the postsynaptic membrane level by following a tubocurarine-like mechanism of action [301,335,344,348]. It is a competitive antagonist of the nicotinic acetylcholine membrane receptor [21,403]. Indeed, it prevents the release of sodium ions (Na+) required for the depolarization and contraction of muscle fibers, leading to muscle relaxation [21,335,336]. Less frequent contractions of muscles result in thinner lines. Softened wrinkles and reduced skin roughness were observed during in vivo and in vitro studies performed for testing this product; the results showed that, after 28 days of twice-daily use, wrinkle depth was reduced by about 49% [21,335];
- Vanistryl®, commercialized by Galena and Lipotec [404], is a complex of the bioactive peptides acetyl tripeptide-30 citrulline and pentapeptide-18 [110,404]. If acetyl tripeptide-30 citrulline is a signal peptide [301,335,336], conversely, pentapeptide-18 is a neurotransmitter inhibitor peptide [335,336,348,405]. These peptides are used in formulations for wrinkle smoothing, and act synergistically, when applied to the skin, to modulate muscular tension and inhibit matrix metalloproteinases (MMPs) [110,406]. Vanistryl® protects the connective tissue from degradation, and rebuilds ECM and dermal components, conferring to skin the integrity and elasticity needed. Furthermore, it exhibits wound-healing and smoothness effects, and reduces skin tension. In vivo skin surface studies (tightness and drying), color studies, and skin elasticity analysis were performed to demonstrate a visible attenuation of stretch marks when Vanistryl® is used [110,404,406].
4.4.4. Botulinum Neurotoxin: The First Neurotransmitter Inhibitor Anti-Wrinkle Ingredient
4.4.5. Topical Peptidomimetic Ingredients as Alternatives to Botox
- Acetyl hexapeptide-3 and -8—synthetic peptides that, by mimicking the portion of SNAP-25, compete with it for the SNARE complex—were the first Botox-like ingredients brought to market by LIPOTEC, with the trade name Argireline® [425,426]. When this analog peptide replaces SNAP-25, the SNARE complex is destabilized, and the release of acetylcholine is inhibited, resulting in muscle contraction being significantly reduced [334,337,353,354]. It was scientifically demonstrated that Argireline® is a safe, effective anti-wrinkle ingredient [337,352,427], particularly suitable for eye care product formulation [345,354]. The results derived from clinical tests showed a wrinkle depth reduction of up to 16.9% within 15 days, and up to 27% within 30 days [395,396]. For performing these tests, a cream containing 10% Argireline® was applied twice daily around the eyes by women aged 44. Moreover, when a cream containing 2% Argireline® was applied in the periorbital area by women aged 35–45, a reduction in wrinkle volume (up to 20.6%) and length (up to 15.9%) was observed within 7 days. An improvement of the skin tone and the presence of fewer wrinkles are the final visible benefits [303,334,335,402];
- Inyline® peptide (Acetyl Hexapeptide-30) by LIPOTEC uses a novel cosmetic approach to reduce muscle contraction and expression wrinkles. It targets the agrin/MuSK post-synaptic pathway, behaving as a competitive antagonist of MuSK (muscle-specific kinase) at the agrin-binding site [428], inactivating the formation of the agrin/MuSK complex and preventing acetylcholine receptor (AChR) clustering—a requirement for triggering muscle contraction [330,429,430,431]. Agrin is a proteoglycan involved in the organization of the basement membrane architecture [432,433]. A specific form of agrin, released by motor neurons, was mainly studied for developing skeletal muscle fibers; it represents a signal for maintaining the acetylcholine receptors’ aggregation, ensuring the neuromuscular junction (NMJ)’s assembly [434,435]. In detail, the agrin, produced by the growing ends of motor neuron axons, binds to MuSK—a tyrosine kinase receptor required for the formation of the neuromuscular junction [429,430,435,436]. In vitro and in vivo experiments were performed to assess the ability of Inyline® to attenuate expression lines. During in vivo tests, 20 female volunteers aged 41–50 applied a cream containing 5% Inyline® peptide solution to the crow’s feet area twice daily for 28 days. The obtained results showed that the treatment decreased wrinkle depth by 14.9% [437];
- BONT-L Peptide Solution (palmitoyl hexapeptide-19) is a synthetic Botox-like peptide [438] by INFINITEC [334], able to inactivate SNAP-25 by inhibiting the formation of the SNARE protein complex [439,440]. As a result, the release of acetylcholine into the synaptic cleft is prevented [440]. Moreover, an in vivo study on 15 volunteers who used a 5% cream applied twice daily showed a reduction in periorbital area micro-reliefs of 38% in 28 days [334];
- Acetyl octapeptide-3, marketed as Snap 8 by LIPOTEC [441], is an elongated sequence of Argireline peptide [303,442]. This analogy allows Snap 8 to mimic SNAP-25 in order to compete effectively in the SNARE complex, obtaining a significant inhibitory effect. It is promoted as the “next-generation” Botox alternative. When used in a 10% cream applied twice a day for 28 days, a reduction in wrinkle depth around the periorbital area of up to 63% was observed [334,336,441,442,443].
- Pentapeptide-18 (Leuphasyl) by LIPOTEC mimics the physiological mechanism of enkephalins and, by blocking calcium channels in the neuron, inhibits catecholamine and acetylcholine release [21,301,405,445]. As a consequence, the inhibitory G-protein-coupled receptor activation occurs with a decrease in the neuronal cell’s excitability, preventing the neurotransmitter release [336]. Its Botox-like effects and its safe efficacy were demonstrated, observing the reduction in the depth of fine lines and wrinkles. Moreover, other interesting properties include its hydration ability and improvement of skin firmness and tone [301,445,446]. Recently, another aspect was also explored, widening pentapeptide-18 (Leuphasyl)’s cosmetic qualities. Park et al. (2020) modified the commercial anti-aging pentapeptide-18 by substituting the N-terminal L-tyrosine with D-tyrosine, or adding L/D-tyrosine at the C-terminus. The role in the melanogenesis process was clearly demonstrated, particularly in the case of Leuphasyl peptide analogs containing C-terminal D-tyrosine, adding whitening properties to a remarkable anti-aging cosmeceutical [446]. It was also well proven that pentapeptide-18, when in the presence of acetyl hexapeptide-3, shows a synergistic effect [110,301,334]. Many studies showed that a cream containing 5% Leuphasyl with 5% Argireline caused a reduction of wrinkles in the periorbital area from 25% to 47% within 28 days [334,402];
- Acetyl dipeptide-1 cetyl ester (Calmosensine™), by SEDERMA—a synthetic replica of the naturally occurring peptide in the body—provides an in vitro Botox-like activity for the reversible inhibition of muscle contractions [334,447]. More specifically, it stimulates the release of pro-endorphins by keratinocytes, leading to the stimulation of relaxation messengers in the skin [334]. As a whole, Calmosensine™ prevents the onset of wrinkles and expression lines by relieving muscular tensions [334,447]. Furthermore, Calmosensine™ plays an important role in modulating the cutaneous perception of unpleasant sensations—such as heat—improving skin comfort, as confirmed by in vivo studies [303,448]. Another important cosmetic property of the active acetyl dipeptide-1 cetyl ester is its ability to significantly upregulate epidermal barrier genes, as shown by the results in the work of Khmaladze et al. (2020). [449] Moreover, acetyl dipeptide 1 cetyl ester can significantly reactivate elastogenesis by upregulating some of the most important dermal genes associated with skin wrinkling, such as alpha-1 type I collagen, decorin, lysyl oxidase-like 1, and fibrillin-1. Additionally, it showed interesting anti-glycation activity and promoted proteasomal activity. Thus, acetyl dipeptide 1 cetyl ester is a promising active compound for skincare formulations, especially if an improved barrier function is essential [449].
4.4.6. Plant Extract Alternatives to Botox
4.4.7. In Silico Designed Botox-Like Peptides
5. Anti-Aging Neurocosmetic Formulations on the Market
- Suitable for mature skin types, Bo2Look Therapy MD estetic serum, elixir, and wrinkle relaxer are formulated with the patented neuropeptide XEP™-018 [478,479]. The latter is a non-invasive anti-aging ingredient alternative to Botox, indicated for smoothing mimic wrinkles by relaxing facial muscles [480]. The main component of this formulation is the Mu-conotoxin CnIIIC, a synthetic biomimetic peptide inspired by that of venomous marine cone snail (Conus consors) [442,480,481,482,483]. This peptide blocks both voltage-gated sodium channels (Nav 1.4) and nicotinic acetylcholine receptors (nAChR), performing its action as a neuromuscular transmission modulator [442,480,483,484,485,486]. It instantly relaxes expression wrinkles when used for formulating a cosmetic product, giving a smooth skin appearance. In fact, in vivo studies conducted on 33 volunteers using a product containing 3% XEP-018 showed a visible reduction in wrinkle depth and rugosity 2 h after a single application [480,483,487];
- NEURO GABA&NANA THERAPY MD serum, cream, peel, and neuro gaba lift mask consist of a combined lifting and peeling instant treatment that contains GABA (gamma-aminobutyric acid) and NANA (N-acetyl-5-neuraminic acid) neurotransmitters, and lactic acid [488]. The smoothing of furrows and improvement of skin tension (“freezing effect”), immediate filling of wrinkles (“plumping effect”), and skin moisturization (“hydro effect”) are the ensured visible effects. When low- and high-molecular-weight hyaluronic acid is added, it deeply and long-lastingly moisturizes the skin, fills wrinkles, and makes the skin firm and flexible [489].
- Biomimetic Therapy PRO line is an intense home care treatment for mature skin types that prevents and reduces the signs of aging. This product, thanks to the presence of Progeline™, Adipofill’in™, and hyaluronic acid, is proposed for the loss of defined face contour, skin laxity, and drooping eyelids. It improves skin elasticity and flexibility, firms, and lifts wrinkles [504].
6. Neurocosmetics: What about Regulation?
- Product type;
- Application site;
- Cosmetic purpose.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bovero, A. Dermocosmetologia Dall’inestetismo al Trattamento Cosmetico, 1st ed.; Tecniche Nuove: Milano, Italy, 2011; ISBN 978-88-481-2626-7. [Google Scholar]
- Niedziela, M. Designing (Neuro) cosmetics for healthy mind, healthy body. Househ. Pers. Care Today 2019, 14, 21–22. [Google Scholar]
- Valéry, P. L’Idée Fixe ou Deux Hommes à la Mer; Collection Blanche; Gallimard, 1933; p. 172, (French Edition). [Google Scholar]
- McGlone, F.; Reilly, D. Sensitive skin and the somatosensory system, 2nd Edition. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 38–46. ISBN 978-1-4051-8635-3. [Google Scholar]
- Chamberlin, C.M.; Peschard, O.; Mondon, P.; Lintner, K. Quantifying Skin Relaxation and Well-Being. Cosmet. Toilet. Mag. 2004, 119, 65–70. [Google Scholar]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal Control of Skin Function: The Skin as a Neuroimmunoendocrine Organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef]
- Pincelli, C.; Bonté, F. The ‘beauty’ of skin neurobiology. J. Cosmet. Dermatol. 2003, 2, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, M. From the Skin Ego to the Psychic Envelope: An Introduction to the Work of Didier Anzieu BT—Skin, Culture and Psychoanalysis; Cavanagh, S.L., Failler, A., Hurst, R.A.J., Eds.; Palgrave Macmillan: London, UK, 2013; pp. 16–44. ISBN 978-1-137-30004-1. [Google Scholar]
- Misery, L. Les nerfs à fleur de peau. Int. J. Cosmet. Sci. 2002, 24, 111–116. [Google Scholar] [CrossRef] [PubMed]
- França, K.; Lotti, T.M. Psycho-Neuro-Endocrine-Immunology: A Psychobiological Concept BT—Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 123–134. ISBN 978-3-319-56017-5. [Google Scholar]
- Lombardi, S.A.; Ratti, A. Neurocosmesi, psicocosmesi e neuroscienze: Cosa sono? Kosmet. Numer. Due 2019, 40–42. Available online: https://www.bregaglio.eu/2018/09/18/neurocosmesi-psicocosmesi-e-neuroscienze-cosa-sono/ (accessed on 14 July 2021).
- Boulais, N. The epidermis: A sensory tissue. Eur. J. Dermatol. 2008, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Manco, M.; Oresajo, C. Epidermal Barrier. In Cosmetic Dermatology: Products and Procedures, 2nd Edition; Draelos, Z.D., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 3–12. ISBN 978-1-118-65546-7. [Google Scholar]
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-aging cosmetics: Facts and controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex Access to European Union Law Consolidated Text: Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast) (Text with EEA Relevance) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009R1223-20201203 (accessed on 17 May 2021).
- Ahsan, H. The biomolecules of beauty: Biochemical pharmacology and immunotoxicology of cosmeceuticals. J. Immunoass. Immunochem. 2019, 40, 91–108. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap#Definecosmetic (accessed on 10 May 2021).
- Surber, C.; Kottner, J. Skin care products: What do they promise, what do they deliver. J. Tissue Viability 2017, 26, 29–36. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- EUR-Lex Access to European Union Law Report From the COMMISSION to the European Parliament and the Council on Product Claims Made Based on Common Criteria in the Field of Cosmetics COM/2016/0580 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2016:0580:FIN (accessed on 17 May 2021).
- Husein el Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.-B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef]
- Draelos, Z.D. Cosmetics, categories, and the future. Dermatol. Ther. 2012, 25, 223–228. [Google Scholar] [CrossRef]
- Misery, L. Neuro-immuno-cutaneous system (NICS). Pathol. Biol. 1996, 44, 867–874. [Google Scholar]
- Theoharides, T.C.; Stewart, J.M.; Taracanova, A.; Conti, P.; Zouboulis, C.C. Neuroendocrinology of the skin. Rev. Endocr. Metab. Disord. 2016, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Misery, L. Skin, immunity and the nervous system. Br. J. Dermatol. 1997, 137, 843–850. [Google Scholar] [CrossRef]
- Fatemi, S.A.; Jafarian-Dehkordi, A.; Hajhashemi, V.; Asilian-Mahabadi, A. Biomimetic proopiomelanocortin suppresses capsaicin-induced sensory irritation in humans. Res. Pharm. Sci. 2016, 11, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Global Cosmetic Industry. The Beauty Innovator’s Resource Chemical Reaction: Neurocosmetics. Available online: https://www.gcimagazine.com/business/rd/technology/7333696.html (accessed on 17 May 2021).
- Lintner, K.; Mas-Chamberlin, C.; Mondon, P.; Peschard, O.; Lamy, L. Cosmeceuticals and active ingredients. Clin. Dermatol. 2009, 27, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Chen, H.-D.; Gao, X.-H.; Gazzaniga, G.; Morganti, L. Natural Ingredients for advanced neurocosmetics. Pers. Care 2013, 6, 19–24. [Google Scholar]
- Wanninger, A. Well-being with Neurocosmetics? Available online: https://www.cossma.com/ingredients/article/well-being-with-neurocosmetics-36213.html (accessed on 12 May 2021).
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal Action of Menthol on the Transient Receptor Potential Channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef]
- Deckner, G. Cool Off or Warm Up with Neurocosmetics. Available online: https://knowledge.ulprospector.com/10629/pcc-cool-off-or-warm-up-with-neurocosmetics/ (accessed on 12 May 2021).
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gazitaeva, Z.I.; Drobintseva, A.O.; Chung, Y.; Polyakova, V.O.; Kvetnoy, I.M. Cosmeceutical product consisting of biomimetic peptides: Antiaging effects in vivo and in vitro. Clin. Cosmet. Investig. Dermatol. 2017, 10, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zappelli, C.; Barbulova, A.; Apone, F.; Colucci, G. Effective Active Ingredients Obtained through Biotechnology. Cosmetics 2016, 3, 39. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, A.; Gonçalves, C.L.; Aschner, M. Neurotoxicity of fragrance compounds: A review. Environ. Res. 2017, 158, 342–349. [Google Scholar] [CrossRef]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant Fortification of the Diet for Anti-Ageing Effects: A Review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef]
- Niedziela, M.M. Thoughtful packaging: Using applied consumer neuroscience to see what the consumer sees. Househ. Pers. Care Today 2016, 11, 14–16. [Google Scholar]
- Niedziela, M.M.; Ambroze, K. Chapter 17—Neuroscience tools: Using the right tool for the right question. In; Meiselman, H.L.B.T.-E.M., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 559–592. ISBN 978-0-12-821125-0. [Google Scholar]
- Frick, R. Happy Cosmetics: The Trend That Reconnects Beauty to Life? Available online: https://www.premiumbeautynews.com/en/happy-cosmetics-the-trend-that,16680 (accessed on 10 May 2021).
- KENZO Lotus Blanc. Available online: https://www.kenzoparfums.com/gb/en/white-lotus/ (accessed on 12 May 2021).
- KENZO Serum in a Mask KENZOKI WHITE LOTUS. Available online: https://www.kenzoparfums.com/gb/en/skincare/skincare-femme/kenzoki-lotus-blanc/serum-in-a-mask/K30400016.html (accessed on 17 May 2021).
- Tungmunnithum, D.; Kongsawadworakul, P.; Hano, C. A Cosmetic Perspective on the Antioxidant Flavonoids from Nymphaea lotus L. Cosmetics 2021, 8, 12. [Google Scholar] [CrossRef]
- Furrer, S.M.; Slack, J.P.; McCluskey, S.T.; Ungureanu, I.M.; Daniher, A.T.; Blancher, G.; Bell, K.; Krawec, P.; Cole, L.; Gray, K. New Developments in the Chemistry of Cooling Compounds. Chemosens. Percept. 2008, 1, 119–126. [Google Scholar] [CrossRef]
- Oh, S.-H.; Yu, J.-J.; Kim, H.-J.; Oh, K.-W.; Eun, J.-S. Effects of white lotus extracts on sleeping, chloride influx, and oxidation. Food Sci. Biotechnol. 2011, 20, 949. [Google Scholar] [CrossRef]
- Cosmetics & Toiletries Euphoryl Omega-3. Available online: http://dir.cosmeticsandtoiletries.com/detail/tradeName.html?id=19492 (accessed on 17 May 2021).
- Saengsorn, K.; Jimtaisong, A. Determination of hydrophilic–lipophilic balance value and emulsion properties of sacha inchi oil. Asian Pac. J. Trop. Biomed. 2017, 7, 1092–1096. [Google Scholar] [CrossRef]
- Penagos-Calvete, D.; Duque, V.; Marimon, C.; Parra, D.M.; Restrepo-Arango, S.K.; Scherf-Clavel, O.; Holzgrabe, U.; Montoya, G.; Salamanca, C.H. Glycerolipid Composition and Advanced Physicochemical Considerations of Sacha Inchi Oil toward Cosmetic Products Formulation. Cosmetics 2019, 6, 70. [Google Scholar] [CrossRef]
- Soimee, W.; Nakyai, W.; Charoensit, P.; Grandmottet, F.; Worasakwutiphong, S.; Phimnuan, P.; Viyoch, J. Evaluation of moisturizing and irritation potential of sacha inchi oil. J. Cosmet. Dermatol. 2020, 19, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Corradi, I.; De Souza, E.; Sande, D.; Takahashi, J.A. Correlation Between Phenolic Compounds Contents, Anti- tyrosinase and Antioxidant Activities of Plant Extracts. Chem. Eng. Trans. 2018, 64, 109–114. [Google Scholar] [CrossRef]
- PresseBox Euphoryl(TM) Omega -3 LS 9846—Naturally Enhances Beauty and Well-Being. Available online: https://www.pressebox.com/inactive/basf/Euphoryl-TM-Omega-3-LS-9846-naturally-enhances-beauty-and-well-being/boxid/258069 (accessed on 17 May 2021).
- Lombardi, S.A.; Ratti, A. Emotional effects induced by lip balms containing different emollients: Neuroscientific approach to studying the tactual experience. Househ. Pers. Care Today 2017, 12, 42–47. [Google Scholar]
- De Tollenaere, M.; Meunier, M.; Scandolera, A.; Sandre, J.; Lambert, C.; Chapuis, E.; Auriol, D.; Reynaud, R. Well-aging: A new strategy for skin homeostasis under multi-stressed conditions. J. Cosmet. Dermatol. 2020, 19, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Denda, M. Epidermis as the “Third Brain”? Dermatol. Sin. 2015, 33, 70–73. [Google Scholar] [CrossRef]
- Personal Care Creating Skin Wellbeing by Dopamine Stimulation. Available online: https://www.personalcaremagazine.com/story/5507/creating-skin-wellbeing-by-dopamine-stimulation (accessed on 12 May 2021).
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N., Jr.; Cicchetti, F.; Calon, F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Carnevali, L.; Pattini, E.; Carandina, A.; Tanzi, G.; Del Canale, C.; Goi, P.; De Felici del Giudice, M.B.; De Carne, B.; Fornari, M.; et al. Psychobiological evidence of the stress resilience fostering properties of a cosmetic routine. Stress 2021, 24, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Global Cosmetic Industry. The Beauty Innovator’s Resource Happy Talk is Serious Science. Available online: https://www.gcimagazine.com/business/rd/ingredients/Happy-Talk-is-Serious-Science-574289811.html (accessed on 17 May 2021).
- Effegilab The Era of Psycho-Cosmetics. Available online: https://effegilab.com/en/lera-della-psico-cosmesi/ (accessed on 17 May 2021).
- Giannakakis, G.; Grigoriadis, D.; Giannakaki, K.; Simantiraki, O.; Roniotis, A.; Tsiknakis, M. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 2019, 1. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Pössel, P.; Ahrens, S.; Hautzinger, M. Influence of cosmetics on emotional, autonomous, endocrinological, and immune reactions. Int. J. Cosmet. Sci. 2005, 27, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cosmetics Business Active Ingredient Neurophroline Blocks Stress Hormone to Control Skin. Available online: https://cosmeticsbusiness.com/news/article_page/Active_ingredient_Neurophroline_blocks_stress_hormone_to_control_skin/120025 (accessed on 17 May 2021).
- Researchgate Study of the Application and Validation of the Ethological Coding System for Interviews (ECSI). Available online: https://www.researchgate.net/publication/287225071_Study_of_the_application_and_validation_of_the_Ethological_Coding_System_for_Interviews_ECSI (accessed on 15 May 2021).
- Paas Oliveros, L.K.; Villanueva Valle, J.; González Arredondo, S.I.; Fresán, A.; Arango de Montis, I.; Brüne, M.; Muñoz Delgado, J. Study of the Application and Validation of the Ethological Coding System for Interviews (ECSI). Available online: http://www.revistasaludmental.mx/index.php/salud_mental/article/view/SM.0185-3325.2015.005 (accessed on 15 May 2021).
- Gabriel, D.; Merat, E.; Jeudy, A.; Cambos, S.; Chabin, T.; Giustiniani, J.; Haffen, E. Emotional Effects Induced by the Application of a Cosmetic Product: A Real-Time Electrophysiological Evaluation. Appl. Sci. 2021, 11, 4766. [Google Scholar] [CrossRef]
- Dunn, J.H.; Koo, J. Psychological Stress and skin aging: A review of possible mechanisms and potential therapies. Dermatol. Online J. 2013, 19, 18. [Google Scholar] [CrossRef]
- Fink, G. Stress: Definition and History. In Stress Science: Neuroendocrinology; Fink, G., Ed.; Academic Press: Oxford, UK, 2009; pp. 3–9. ISBN 9780123785718. [Google Scholar]
- Chen, Y.; Lyga, J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2014, 13, 177–190. [Google Scholar] [CrossRef]
- Kimyai-Asadi, A.; Usman, A. The Role of Psychological Stress in Skin Disease. J. Cutan. Med. Surg. 2001, 5, 140–145. [Google Scholar] [CrossRef]
- Folkman, S. Stress: Appraisal and Coping BT—Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1913–1915. ISBN 978-1-4419-1005-9. [Google Scholar]
- Antonelli, M.; Donelli, D. Effects of balneotherapy and spa therapy on levels of cortisol as a stress biomarker: A systematic review. Int. J. Biometeorol. 2018, 62, 913–924. [Google Scholar] [CrossRef]
- Dixon, L.J.; Witcraft, S.M.; McCowan, N.K.; Brodell, R.T. Stress and skin disease quality of life: The moderating role of anxiety sensitivity social concerns. Br. J. Dermatol. 2018, 178, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Givaudan Neurophroline® Overall Skin Stress Control. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/products/neurophroline (accessed on 17 May 2021).
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Evers, A.W.M.; Verhoeven, E.W.M.; Kraaimaat, F.W.; De Jong, E.M.G.J.; De Brouwer, S.J.M.; Schalkwijk, J.; Sweep, F.C.G.J.; Van De Kerkhof, P.C.M. How stress gets under the skin: Cortisol and stress reactivity in psoriasis. Br. J. Dermatol. 2010, 163, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.J.; Kim, D.; Kim, E.J.; Ahn, J.-S.; Choi, E.-J.; Son, E.D.; Lee, T.R.; Choi, E.H. Psychological Stress Deteriorates Skin Barrier Function by Activating 11β-Hydroxysteroid Dehydrogenase 1 and the HPA Axis. Sci. Rep. 2018, 8, 6334. [Google Scholar] [CrossRef]
- Maarouf, M.; Maarouf, C.L.; Yosipovitch, G.; Shi, V.Y. The impact of stress on epidermal barrier function: An evidence-based review. Br. J. Dermatol. 2019, 181, 1129–1137. [Google Scholar] [CrossRef]
- Tia, N.; Singh, A.K.; Pandey, P.; Azad, C.S.; Chaudhary, P.; Gambhir, I.S. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene 2018, 648, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tsitsipatis, D.; Klotz, L.O.; Steinbrenner, H. Multifaceted functions of the forkhead box transcription factors FoxO1 and FoxO3 in skin. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Meyrignac, C.; Plaza, C.; Capallere, C.; Botto, J. 263 Effect of environmental stress combined with “daily life stress” on skin aging. J. Investig. Dermatol. 2019, 139, S259. [Google Scholar] [CrossRef]
- Botto, J.; Meyrignac, C.; Plaza, C.; Lequoy, V.; Oger, E.; Coquet-Morel, C.; Capallere, C. 636 Effect of daily life stress on skin aging—Development of 3D skin reconstructed models. J. Investig. Dermatol. 2018, 138, S108. [Google Scholar] [CrossRef]
- DSM BEL-EVEN® Future-Proof Your Skin from the Effects of Daily Stress. Available online: https://www.dsm.com/personal-care/en_US/products/skin-bioactives/bel-even.html (accessed on 17 May 2021).
- Imfeld, D.; Jackson, E.; Seroul, P. Inhibition of cutaneous cortisol activation: A novel approach to protect skin from stress induced damage and aging. 30th IFSCC Congr. 2018, S1–S501. Available online: https://www.researchgate.net/publication/327816215_Inhibition_of_cutaneous_cortisol_activation_A_novel_approach_to_protect_skin_from_stress_induced_damage_and_aging (accessed on 14 July 2021).
- Carli, B. Feeling good about neuro-cosmetics. Househ. Pers. Care Today 2016, 11, 9–11. [Google Scholar]
- Schmelz, M.; Paus, R. Opioids and the skin: “Itchy” perspectives beyond analgesia and abuse. J. Investig. Dermatol. 2007, 127, 1287–1289. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Tobin, D.J.; Gaveriaux-Ruff, C.; Bigliardi-Qi, M. Opioids and the skin—Where do we stand? Exp. Dermatol. 2009, 18, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Dancik, Y.; Neumann, C.; Bigliardi-Qi, M. Opioids and skin homeostasis, regeneration and ageing—What’s the evidence? Exp. Dermatol. 2016, 25, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi-Qi, M.; Bigliardi, P.L.; Eberle, A.N.; Büchner, S.; Rufli, T. β-Endorphin Stimulates Cytokeratin 16 Expression and Downregulates μ-Opiate Receptor Expression in Human Epidermis. J. Investig. Dermatol. 2000, 114, 527–532. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Büchner, S.; Rufli, T.; Bigliardi-Qi, M. Specific Stimulation of Migration of Human Keratinocytes by μ -Opiate Receptor Agonists. J. Recept. Signal Transduct. 2002, 22, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wintzen, M.; Gilchrest, B.A. Proopiomelanocortin, Its Derived Peptides, and the Skin. J. Investig. Dermatol. 1996, 106, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A. Neuromediators—a crucial component of the skin immune system. J. Dermatol. Sci. 2002, 30, 87–93. [Google Scholar] [CrossRef]
- Luger, T.A.; Scholzen, T.; Brzoska, T.; Becher, E.V.A.; Slominski, A.; Paus, R. Cutaneous Immunomodulation and Coordination of Skin Stress Responses by α-Melanocyte-Stimulating Hormonea. Ann. N. Y. Acad. Sci. 1998, 840, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J.E.; Corliss, D.; Slominski, A. Spatiotemporal Expression, Distribution, and Processing of POMC and POMC-derived Peptides in Murine Skin. J. Histochem. Cytochem. 2000, 48, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2020, 30, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.; Dezutter, C.; Reymermier, C.; Vogelgesang, B.; Delay, E.; André, V. Age-related changes in pro-opiomelanocortin (POMC) and related receptors in human epidermis. Int. J. Cosmet. Sci. 2010, 32, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Ahn, S.S.; Lee, Y.H.; Shin, S.Y. Regulation of pro-opiomelanocortin (POMC) gene transcription by interleukin-31 via early growth response 1 (EGR-1) in HaCaT keratinocytes. Mol. Biol. Rep. 2020, 47, 5953–5962. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Zmijewski, M.; Slominski, R.M.; Kauser, S.; Wortsman, J.; Tobin, D.J. Corticotropin releasing hormone and the skin. Front. Biosci. 2006, 11, 2230–2248. [Google Scholar] [CrossRef]
- Luger, T.A.; Paus, R.; Slominski, A.; Lipton, J. The Proopiomelanocortin System in Cutaneous Neuroimmunomodulation: An Introductory Overview. Ann. N. Y. Acad. Sci. 1999, 885, xi–xiv. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Botchkarev, V.; Choudhry, M.; Fazal, N.; Fechner, K.; Furkert, J.; Krause, E.; Roloff, B.; Sayeed, M.; Wei, E.; et al. Cutaneous Expression of CRH and CRH-R: Is There a “Skin Stress Response System?”. Ann. N. Y. Acad. Sci. 1999, 885, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Mibelle Group Biochemistry Happybelle-PE Phyto-endorphins for a Youthful Glow. Available online: https://mibellebiochemistry.com/happybelle-pe (accessed on 12 May 2021).
- Prospector Happybelle-PE. Available online: https://www.ulprospector.com/en/eu/PersonalCare/Detail/2249/66038/Happybelle-PE (accessed on 17 May 2021).
- Schmidt, D.; Zülli, F. Role of Beta-Endorphin in the Skin. SÖFW J. 2005, 131, 1–4. [Google Scholar]
- Lima, T.N.; Moraes, C.A.P. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Schmid, D.; Belser, E.; Zülli, F. Self-tanning Based on Stimulation of Melanin Biosynthesis. Cosmet. Mag. 2007, 122, 55–62. [Google Scholar]
- Carli, B. Calm Down! Available online: https://search.informit.org/doi/10.3316/informit.409457987624542 (accessed on 17 May 2021).
- Sunar, K.; Kumar, U.; Deshmukh, S.K. Chapter 12—Recent Applications of Enzymes in Personal Care Products; Dhillon, G.S., Kaur, S.B.T.-A.-I.W. as F. for E.P., Eds.; Academic Press: San Diego, CL, USA, 2016; pp. 279–298. ISBN 978-0-12-802392-1. [Google Scholar]
- Zahid, H.; Rizwani, G.H.; Ishaqe, S. Phytopharmacological Review on Vitex agnus-castus: A Potential Medicinal Plant. Chin. Herb. Med. 2016, 8, 24–29. [Google Scholar] [CrossRef]
- Webster, D.E.; Lu, J.; Chen, S.-N.; Farnsworth, N.R.; Wang, Z.J. Activation of the μ-opiate receptor by Vitex agnus-castus methanol extracts: Implication for its use in PMS. J. Ethnopharmacol. 2006, 106, 216–221. [Google Scholar] [CrossRef]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Joyderm La Neurocosmesi Polisensoriale. La Nuova Frontiera Della Cosmesi. Available online: https://www.joyderm.it/neurocosmesi/ (accessed on 9 May 2021).
- Charles Dorni, A.I.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An Herb with Anti-Stress, Anti-Aging, and Immunostimulating Properties for Cancer Chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D.; Rhodiola Rosea, L. A critical review on biology, medicinal properties and pharmacological manifestations. J. Nat. Prod. Resour. 2015, 1, 4–9. [Google Scholar]
- Dieamant, G.C.; Velazquez Pereda, M.D.C.; Eberlin, S.; Nogueira, C.; Werka, R.M.; De Queiroz, M.L.S. Neuroimmunomodulatory compound for sensitive skin care: In vitro and clinical assessment. J. Cosmet. Dermatol. 2008, 7, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Nakamura, Y.; Nishino, J.; Sawayama, T.; Komiya, T.; Deguchi, T.; Kita, A.; Nakamura, H.; Matsumoto, J. A novel class of enkephalinase inhibitors containing a C-terminal sulfo group. J. Med. Chem. 1992, 35, 602–608. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakamura, S.; Sugimoto, S.; Tsukioka, J.; Hinomaru, F.; Nakashima, S.; Matsumoto, T.; Ohta, T.; Fujimoto, K.; Yoshikawa, M.; et al. Constituents of flowers of Paeoniaceae plants, Paeonia suffruticosa and Paeonia lactiflora. Phytochem. Lett. 2015, 12, 98–104. [Google Scholar] [CrossRef]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Kim, K.-H.; Shim, J.S.; Kim, H.-J.; Son, E.D. Penta-O-galloyl-β-D-glucose from Paeonia lactiflora Pall. root extract enhances the expression of skin barrier genes via EGR3. J. Ethnopharmacol. 2020, 248, 112337. [Google Scholar] [CrossRef]
- Letsiou, S.; Bakea, A.; Holefors, A.; Rembiesa, J. In vitro protective effects of Paeonia mascula subsp. hellenica callus extract on human keratinocytes. Sci. Rep. 2020, 10, 19213. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- NUXE Paris First Wrinkles Skincare Nirvanesque®. Available online: https://uk.nuxe.com/nirvanesque (accessed on 1 May 2021).
- Lancome Hydra Zen Anti-Stress Cream Hydra Zen Anti-Stress Cream. Available online: https://www.lancome.co.uk/skincare/by-product-category/moisturisers/hydra-zen-anti-stress-cream/085201-LAC.html (accessed on 12 May 2021).
- Rao, A.S.; Yadav, S.S.; Singh, P.; Nandal, A.; Singh, N.; Ganaie, S.A.; Yadav, N.; Kumar, R.; Bhandoria, M.S.; Bansal, P. A comprehensive review on ethnomedicine, phytochemistry, pharmacology, and toxicity of Tephrosia purpurea (L.) Pers. Phyther. Res. 2020, 34, 1902–1925. [Google Scholar] [CrossRef]
- Hubert, J.; Chollet, S.; Purson, S.; Reynaud, R.; Harakat, D.; Martinez, A.; Nuzillard, J.-M.; Renault, J.-H. Exploiting the Complementarity between Dereplication and Computer-Assisted Structure Elucidation for the Chemical Profiling of Natural Cosmetic Ingredients: Tephrosia purpurea as a Case Study. J. Nat. Prod. 2015, 78, 1609–1617. [Google Scholar] [CrossRef]
- Altemus, M.; Rao, B.; Dhabhar, F.S.; Ding, W.; Granstein, R.D. Stress-induced changes in skin barrier function in healthy women. J. Investig. Dermatol. 2001, 117, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bonte, F.; Dumas, M.; Lhermite, S.; Saunois, A. Use of Oligosaccharides to Stimulate Beta-endorphin Production. U.S. Patent Application No. 10/332,136, 21 August 2003. [Google Scholar]
- Ahn, K.S.; Aggarwal, B.B. Transcription Factor NF-κB: A Sensor for Smoke and Stress Signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.; Chrousos, G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016, 17, 295–304. [Google Scholar] [CrossRef]
- Cals-Grierson, M.-M.; Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef]
- Provital Do Care Agascalm. Available online: https://www.weareprovital.com/en/careactives/agascalm (accessed on 17 May 2021).
- Brooke Schleehauf Provital Group’s Agascalm. Available online: https://www.cosmeticsandtoiletries.com/formulating/category/skincare/Provital-Groups-Agascalm-477545493.html (accessed on 10 May 2021).
- Hakozaki, T.; Deyer, B.F.; Laughlin II, L.T. Skin Care Composition. 2019. Available online: https://patents.google.com/patent/US20200405614A1/en (accessed on 7 May 2021).
- Paufique, J. Active Ingredient Obtained From Nymphaea Alba Flowers. U.S. Patent Application No 16/912,958, 31 December 2020. [Google Scholar]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Chiang, B.-L. Sleep disorders and atopic dermatitis: A 2-way street? J. Allergy Clin. Immunol. 2018, 142, 1033–1040. [Google Scholar] [CrossRef]
- Oliveira, C.; Torres, T. More than skin deep: The systemic nature of atopic dermatitis. Eur. J. Dermatol. 2019, 29, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–147. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T.; Miki, M. Fluorescence emission from PAMAM and PPI dendrimers. J. Colloid Interface Sci. 2007, 306, 222–227. [Google Scholar] [CrossRef]
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue light disrupts the circadian rhythm and create damage in skin cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Ndiaye, M.A.; Nihal, M.; Wood, G.S.; Ahmad, N. Skin, Reactive Oxygen Species, and Circadian Clocks. Antioxid. Redox Signal. 2013, 20, 2982–2996. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Brown, A.; Aladren, S.; Narda, M. Night Cream Containing Melatonin, Carnosine and Helichrysum italicum Extract Helps Reduce Skin Reactivity and Signs of Photodamage: Ex Vivo and Clinical Studies. Dermatol. Ther. 2020, 10, 1315–1329. [Google Scholar] [CrossRef]
- Lan, A.L.; Lu, N.; Kang, D.; Ye, L.; Lintner, K.; Zappelli, C.; Apone, F.; Colucci, M.G.; Bimonte, M.; Bertelli, G.; et al. Neuro-Cosmetics Approach: TCM based formula with HACCE stem cell extract reduces stress symptoms by activating cutaneous melatonin receptor MT1. In Proceedings of the 25th IFSCC Conference CosmEthic Science and Conscience, Milan, Italy, 30 September 2019. [Google Scholar]
- Misery, L.; Ständer, S.; Szepietowski, J.; Reich, A.; Wallengren, J.; Evers, A.; Takamori, K.; Brenaut, E.; Le Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Wandrey, F.; Schmid, D.; Zülli, F. Peptide Inspired by Sea Anemone Venom Comforts Sensitive Skin. SOFW J. 2018, 19–23. Available online: https://www.sofw.com/de/hikashop-menu-for-categories-listing/product/221-peptide-inspired-by-sea-anemone-venom-comforts-sensitive-skin (accessed on 14 July 2021).
- Misery, L.; Loser, K.; Ständer, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, G.; Jiang, N. Study on the Repairing Effect of Cosmetics Containing Artemisia annua on Sensitive Skin. J. Cosmet. Dermatol. Sci. Appl. 2020, 10, 8–19. [Google Scholar]
- Prospector MarilianceTM. Available online: https://www.ulprospector.com/en/asia/PersonalCare/Detail/831/724171/Mariliance (accessed on 17 May 2021).
- Do, L.H.D.; Azizi, N.; Maibach, H. Sensitive Skin Syndrome: An Update. Am. J. Clin. Dermatol. 2020, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Morisset, S.; Seite, S.; Brenaut, E.; Ficheux, A.-S.; Fluhr, J.W.; Delvigne, V.; Taieb, C. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes : Results from a worldwide survey of 10,743 individuals. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1371–1376. [Google Scholar] [CrossRef]
- Kligman, A.M.; Sadiq, I.; Zhen, Y.; Crosby, M. Experimental studies on the nature of sensitive skin. Ski. Res. Technol. 2006, 12, 217–222. [Google Scholar] [CrossRef]
- Givaudan MarilianceTM Marine Neuro-Soother. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/products/mariliance (accessed on 17 May 2021).
- Talagas, M.; Lebonvallet, N.; Berthod, F.; Misery, L. Cutaneous nociception: Role of keratinocytes. Exp. Dermatol. 2019, 28, 1466–1469. [Google Scholar] [CrossRef]
- Misery, L. Sensitive Skins May Be Neuropathic Disorders: Lessons from Studies on Skin and Other Organs. Cosmetics 2021, 8, 14. [Google Scholar] [CrossRef]
- Pinolumin for Flawless Skin. Available online: https://www.personalcaremagazine.com/story/18396/pinolumin-for-flawless-skin (accessed on 17 May 2021).
- Wandrey, F.; Schmid, D.; Zülli, F. Flawless skin via Swiss stone pine extract. Pers. CareA Asia Pac. 2016, 27–30. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiJ39Xa3-LxAhVO4qQKHWZZApoQFnoECAUQAA&url=https%3A%2F%2Fesent.pl%2Fpl%2Fp%2Ffile%2F1d68c6828d2d78974480bee2712e0596%2FFlawless_Skin_Via_Swiss_Stone_Pine_Extract_Personal_Care_Magazine_November_2016-1.pdf&usg=AOvVaw1QJLnqDi0iNyb206vtRpQn (accessed on 14 July 2021).
- Talagas, M.; Misery, L. Role of Keratinocytes in Sensitive Skin. Front. Med. 2019, 6, 108. [Google Scholar] [CrossRef]
- Ehnis-Pérez, A.; Torres-Álvarez, B.; Cortés-García, D.; Hernández-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kang, S.M.; Chung, J.H. The role of TRPV1 channel in aged human skin. J. Dermatol. Sci. 2012, 65, 81–85. [Google Scholar] [CrossRef]
- Misery, L. Sensitive skin. Expert Rev. Dermatol. 2013, 8, 631–637. [Google Scholar] [CrossRef]
- Mandadi, S.; Roufogalis, B.D. ThermoTRP channels in nociceptors: Taking a lead from capsaicin receptor TRPV1. Curr. Neuropharmacol. 2008, 6, 21–38. [Google Scholar] [CrossRef]
- Cortright, D.N.; Szallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1. Eur. J. Biochem. 2004, 271, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Kueper, T.; Krohn, M.; Haustedt, L.O.; Hatt, H.; Schmaus, G.; Vielhaber, G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp. Dermatol. 2010, 19, 980–986. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, Y.K.; Chung, J.H. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp. Dermatol. 2009, 18, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Eberlin, S.; Polettini, A.J.; Da Costa Pereira, A.F.; Pereira, C.S.; Cortes Ferreira, N.M.; Dolis, E.; Oliveira Torloni, L.B. Neuromodulatory and Anti-Inflammatory Ingredient for Sensitive Skin: In Vitro Assessment. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2014, 13, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Garg, C.; Sharma, H.; Garg, M. Skin photo-protection with phytochemicals against photo-oxidative stress, photo-carcinogenesis, signal transduction pathways and extracellular matrix remodeling—An overview. Ageing Res. Rev. 2020, 62, 101127. [Google Scholar] [CrossRef]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef]
- Juráňová, J.; Franková, J.; Ulrichová, J. The role of keratinocytes in inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006, 83, 447S–455S. [Google Scholar] [CrossRef]
- Christopoulos, G.I.; Uy, M.A.; Yap, W.J. The Body and the Brain: Measuring Skin Conductance Responses to Understand the Emotional Experience. Org. Res. Methods 2019, 22, 394–420. [Google Scholar] [CrossRef]
- Matsumura, S.; Terao, M.; Murota, H.; Katayama, I. Th2 cytokines enhance TrkA expression, upregulate proliferation, and downregulate differentiation of keratinocytes. J. Dermatol. Sci. 2015, 78, 215–223. [Google Scholar] [CrossRef]
- Feliciani, C.; Gupta, A.K.; Saucier, D.N. Keratinocytes and Cytokine/Growth Factors. Crit. Rev. Oral Biol. Med. 1996, 7, 300–318. [Google Scholar] [CrossRef]
- Scandolera, A.; Hubert, J.; Humeau, A.; Lambert, C.; De Bizemont, A.; Winkel, C.; Kaouas, A.; Renault, J.-H.; Nuzillard, J.-M.; Reynaud, R. GABA and GABA-Alanine from the Red Microalgae Rhodosorus marinus Exhibit a Significant Neuro-Soothing Activity through Inhibition of Neuro-Inflammation Mediators and Positive Regulation of TRPV1-Related Skin Sensitization. Mar. Drugs 2018, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-W.; Seo, J.A.; Jeong, Y.S.; Bae, I.-H.; Jang, W.-H.; Lee, J.; Kim, S.-Y.; Shin, S.-S.; Woo, B.-Y.; Lee, K.-W.; et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. 2011, 62, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Voisin, T.; Chiu, I.M. Molecular link between itch and atopic dermatitis. Proc. Natl. Acad. Sci. USA 2018, 115, 12851–12853. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C. Atopic Dermatitis. N. Engl. J. Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef]
- Bonchak, J.G.; Swerlick, R.A. Emerging therapies for atopic dermatitis: TRPV1 antagonists. J. Am. Acad. Dermatol. 2018, 78, S63–S66. [Google Scholar] [CrossRef]
- Liu, T.; Ji, R.-R. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci. Bull. 2012, 28, 145–154. [Google Scholar] [CrossRef]
- Neuro-Soother for Comfort. Available online: https://www.personalcaremagazine.com/story/14348/formulations (accessed on 12 May 2021).
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Mibelle Group Biochemistry PinoluminTM Relax Your Skin—Enjoy a Flawless Complexion. Available online: https://mibellebiochemistry.com/pinolumintm (accessed on 15 May 2021).
- Human Research Stone Pine. Available online: http://humanresearch.at/newwebcontent/?page_id=96&lang=en (accessed on 16 May 2021).
- Ghadiriasli, R.; Mahmoud, M.A.A.; Wagenstaller, M.; Van de Kuilen, J.W.; Buettner, A. Molecular and sensory characterization of odorants in Cembran pine (Pinus cembra L.) from different geographic regions. Talanta 2020, 220, 121380. [Google Scholar] [CrossRef]
- Kotradyova, V.; Vavrinsky, E.; Kalinakova, B.; Petro, D.; Jansakova, K.; Boles, M.; Svobodova, H. Wood and Its Impact on Humans and Environment Quality in Health Care Facilities. Int. J. Environ. Res. Public Health 2019, 16, 3496. [Google Scholar] [CrossRef]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Nieminen, R.; Leppänen, T.; Eckerman, C.; Holmbom, B.; Moilanen, E. Pinosylvin and Monomethylpinosylvin, Constituents of an Extract from the Knot of Pinus sylvestris, Reduce Inflammatory Gene Expression and Inflammatory Responses in Vivo. J. Agric. Food Chem. 2015, 63, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B Irradiation and Matrix Metalloproteinases. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Bauerova, K.; Acquaviva, A.; Ponist, S.; Gardi, C.; Vecchio, D.; Drafi, F.; Arezzini, B.; Bezakova, L.; Kuncirova, V.; Mihalova, D.; et al. Markers of inflammation and oxidative stress studied in adjuvant-induced arthritis in the rat on systemic and local level affected by pinosylvin and methotrexate and their combination. Autoimmunity 2015, 48, 46–56. [Google Scholar] [CrossRef]
- Abbas, M.A. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem. Biol. Interact. 2020, 330, 109178. [Google Scholar] [CrossRef]
- BASF Skinasensyl® LS 9749. Available online: https://carecreations.basf.us/products/skinasensyl-ls-9749 (accessed on 17 May 2021).
- Schaefer, K. Tetrapeptide for Neurosensitive Skin. Available online: https://www.cosmeticsandtoiletries.com/formulating/function/antiirritant/35799934.html (accessed on 8 May 2021).
- BASF LS Skinasensyl—A Next-Generation Cosmeceutical for Sensitive Skin. Available online: https://www.pressebox.com/inactive/basf/LS-Skinasensyl-a-next-generation-cosmeceutical-for-sensitive-skin/boxid/215473 (accessed on 17 May 2021).
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-chemical characterization of formulations containing endomorphin-2 derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- BASF Skinasensyl. The Neurocosmeceutical Soother. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwixm9jK9NLwAhWGGewKHYZ0CfsQFjAAegQIBRAD&url=https%253A%252F%252Fbiakhim.com.ua%252Findex.php%253Foption%253Dcom_k2%2526Itemid%253D1173%2526id%253D617_5786cf0e9694d655383c8947159ba238%2526lang%253Dru%2526task%253 (accessed on 18 May 2021).
- Lintner, K. Chapter 36: Peptides and Proteins. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2016; pp. 308–317. ISBN 978-1-118-65558-0. [Google Scholar]
- Wandrey, F.; Schmid, D.; Zülli, F. Personal Care Europe. 2017, pp. 117–119.
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E. V Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [PubMed]
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in Pain Sensation. Front. Physiol. 2017, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Lewis, R.J. Natural Product Ligands of TRP Channels BT—Transient Receptor Potential Channels; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 41–85. ISBN 978-94-007-0265-3. [Google Scholar]
- Kozlov, S.A.; Andreev, Y.A.; Murashev, A.N.; Skobtsov, D.I.; D’yachenko, I.A.; Grishin, E.V. New polypeptide components from the Heteractis crispa sea anemone with analgesic activity. Russ. J. Bioorganic Chem. 2009, 35, 711. [Google Scholar] [CrossRef]
- Pauly, G.; Moussou, P.; Contet-Audonneau, J.-L.; Danoux, L.; Freis, O.; Sabadotto, M.; Benoit, I.; Misery, L.; Rathjens, A. New peptidic active ingredient to reduce discomfort and painful sensations in sensitive skin. Int. J. Cosmet. Sci. 2009, 31, 480. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 activation power can switch an action mode for its polypeptide ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef] [PubMed]
- Mibelle Group Biochemistry SensAmone P5: Immediate Comfort for Sensitive Skin. Available online: https://mibellebiochemistry.com/sensamone-p5 (accessed on 17 May 2021).
- SensAmone P5: Immediate Comfort for Sensitive Skin. Available online: https://www.cosmeticsbusiness.com/news/article_page/SensAmone_P5_immediate_comfort_for_sensitive_skin/128848 (accessed on 17 May 2021).
- Kimura, A.; Kanazawa, N.; Li, H.-J.; Yonei, N.; Yamamoto, Y.; Furukawa, F. Influence of chemical peeling on the skin stress response system. Exp. Dermatol. 2012, 21, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin Releasing Hormone and Proopiomelanocortin Involvement in the Cutaneous Response to Stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Coates, S.J.; Lee, E.H.; Granstein, R.D. Cutaneous Neuroimmunology BT—Clinical and Basic Immunodermatology; Gaspari, A.A., Tyring, S.K., Kaplan, D.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 179–199. ISBN 978-3-319-29785-9. [Google Scholar]
- Bonezzi, C.; Costantini, A.; Cruccu, G.; Fornasari, D.M.M.; Guardamagna, V.; Palmieri, V.; Polati, E.; Zini, P.; Dickenson, A.H. Capsaicin 8% dermal patch in clinical practice: An expert opinion. Expert Opin. Pharmacother. 2020, 21, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.E. Adrenocorticotropic Hormone. In Stress: Neuroendocrinology and Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 2, pp. 109–116. ISBN 9780128024232. [Google Scholar]
- Lintner, K.; Peschard, O. Biologically active peptides: From a laboratory bench curiosity to a functional skin care product. Int. J. Cosmet. Sci. 2000, 22, 207–218. [Google Scholar] [CrossRef]
- Perazzo, J.; Marb, C.; Santos, S.S. Pharmacological Potential of the Endogenous Dipeptide Kyotorphin and Selected Derivatives. Front. Pharmacol. 1979, 7, 530. [Google Scholar] [CrossRef]
- CRODA CalmosensineTM SP Sensual Healing, Embrace Yourself in Wellbeing. Available online: https://www.crodapersonalcare.com/en-gb/products-and-applications/product-finder/product/2960/Calmosensine_1_SP#tab-collapse-literature (accessed on 17 May 2021).
- RAHN DEFENSIL®-SOFT Help Your Skin Chill Out. Available online: https://www.rahn-group.com/en/cosmetics/product/29/ (accessed on 30 April 2021).
- Hettwer, S.; Bänziger, S.; Suter, B.; Obermayer, B. Grifolin derivatives from Albatrellus ovinus as TRPV1 receptor blockers for cosmetic applications. Int. J. Cosmet. Sci. 2017, 39, 379–385. [Google Scholar] [CrossRef]
- Meotti, F.C.; Lemos de Andrade, E.; Calixto, J.B. TRP Modulation by Natural Compounds BT—Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume II; Nilius, B., Flockerzi, V., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1177–1238. ISBN 978-3-319-05161-1. [Google Scholar]
- RAHN Rahn’s DEFENSIL -SOFT Offers Zen-Like Soothing and Anti-Ageing Effects. Available online: https://www.cosmeticsbusiness.com/news/article_page/Rahns_DEFENSIL_SOFT_offers_zen-like_soothing_and_anti-ageing_effects/132934 (accessed on 17 May 2021).
- LucasMeyer-Cosmetics Lipopeptide Derived from a Neuromediator. Available online: https://www.lucasmeyercosmetics.com/en/node/668 (accessed on 30 April 2021).
- LucasMeyer-Cosmetics NeutrazenTM The Soothing Neurocosmetic. Available online: https://www.lucasmeyercosmetics.com/en/taxonomy/term/123 (accessed on 30 April 2021).
- Thody, A.J. α-MSH and the Regulation of Melanocyte Function. Ann. N. Y. Acad. Sci. 1999, 885, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Schulte, U.; Kalden, H.; Luger, T.A. Alpha-Melanocyte-Stimulating Hormone Modulates Activation of NF-κB and AP-1 and Secretion of Interleukin-8 in Human Dermal Fibroblasts. Ann. N. Y. Acad. Sci. 1999, 885, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R.; Wortsman, J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol. Cell. Endocrinol. 1993, 93, C1–C6. [Google Scholar] [CrossRef]
- Auriemma, M.; Brzoska, T.; Klenner, L.; Kupas, V.; Goerge, T.; Voskort, M.; Zhao, Z.; Sparwasser, T.; Luger, T.A.; Loser, K. α-MSH-Stimulated Tolerogenic Dendritic Cells Induce Functional Regulatory T Cells and Ameliorate Ongoing Skin Inflammation. J. Investig. Dermatol. 2012, 132, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, T.; Luger, T.A.; Maaser, C.; Abels, C.; Böhm, M. α-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects in Vitro and in Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases. Endocr. Rev. 2008, 29, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Scholzen, T.E.; Brzoska, T.; Böhm, M. New Insights into the Functions of α-MSH and Related Peptides in the Immune System. Ann. N. Y. Acad. Sci. 2003, 994, 133–140. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Scholzen, T.E.; Brzoska, T.; Kalden, D.-H.; O’Reilly, F.; Armstrong, C.A.; Luger, T.A.; Ansel, J.C. Effect of Ultraviolet Light on the Release of Neuropeptides and Neuroendocrine Hormones in the Skin: Mediators of Photodermatitis and Cutaneous Inflammation. J. Investig. Dermatol. Symp. Proc. 1999, 4, 55–60. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef]
- Loing, E. Reaching a Zen-like State in Skin: Biomimetic Peptide to Balance Sensitivity. Available online: https://www.cosmeticsandtoiletries.com/testing/sensory/Reaching-a-Zen-like-State-in-Skin-Biomimetic-Peptide-to-Balance-Sensitivity-420538914.html (accessed on 17 May 2021).
- Leffingwell, J.C. Cooling Ingredients and Their Mechanism of Action. In Handbook of Cosmetic Science and Technology; Barel, A.O., Paye, M., Maibach, H.I., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; pp. 661–676. ISBN 978-1-4200-6963-1. [Google Scholar]
- Salvona Encapsulation Technology HydroSal® SalCool. Available online: https://www.salvona.com/product/hydrosal-salcool/ (accessed on 13 May 2021).
- InfinityIngredients HydroSalTM SalCool. Available online: https://infinity-ingredients.co.uk/product/salvona-hydrosal-salcool (accessed on 14 May 2021).
- Givaudan Evercool® Skin Advanced Cooling Technology. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/products/evercool-skin (accessed on 17 May 2021).
- Leffingwell, J.C. Cool without Menthol & Cooler than Menthol and Cooling Compounds as Insect Repellents. Available online: http://www.leffingwell.com/cooler_than_menthol.htm#b (accessed on 18 May 2021).
- Rovner, S.L. Better Than Mint. Chem. Eng. News Arch. 2007, 85, 95. [Google Scholar] [CrossRef]
- Babor Doctor Babor—Neuro Sensitive Cellular Intensive Calming Cleanser. Available online: https://au.babor.com/products/doctor-babor/neuro-sensitive-cellular/59433-intensive-calming-cream.html#text (accessed on 17 May 2021).
- Muggli, R. Systemic evening primrose oil improves the biophysical skin parameters of healthy adults. Int. J. Cosmet. Sci. 2005, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Yasin, Z.A.M.; Ibrahim, F.; Rashid, N.N.; Razif, M.F.M.; Yusof, R. The Importance of Some Plant Extracts as Skin Anti-aging Resources: A Review. Curr. Pharm. Biotechnol. 2017, 18, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.; Sivamaruthi, B.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- LIPOTEC-Active Ingredients BODYFENSINE® Peptide. Available online: https://www.lipotec.com/en/products/bodyfensine-reg-peptide/ (accessed on 17 May 2021).
- Rull, M.; Davi, C.; Caufadas, E.; Cebriuen, J.; Delgado, R. Protect Skin, Reduce Discomfort. Available online: https://www.happi.com/issues/2013-10/view_features/protect-skin-reduce-discomfort (accessed on 15 May 2021).
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2012, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Molecular Mechanisms of Skin Aging. Ann. N. Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Wang, P.-W.; Huang, C.-H.; Chen, M.-H.; Wu, Y.-R.; Pan, T.-L. Skin aging caused by intrinsic or extrinsic processes characterized with functional proteomics. Proteomics 2016, 16, 2718–2731. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Functional and physiological characteristics of the aging skin. Aging Clin. Exp. Res. 2008, 20, 195–200. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Boland, B.; Yu, W.H.; Corti, O.; Mollereau, B.; Henriques, A.; Bezard, E.; Pastores, G.M.; Rubinsztein, D.C.; Nixon, R.A.; Duchen, M.R.; et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018, 17, 660–688. [Google Scholar] [CrossRef] [PubMed]
- Allan Butterfield, D. Amyloid β-peptide (1-42)-induced Oxidative Stress and Neurotoxicity: Implications for Neurodegeneration in Alzheimer’s Disease Brain. A Review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Ho, Y.-S.; So, K.-F.; Chang, R.C.-C. Anti-aging herbal medicine—How and why can they be used in aging-associated neurodegenerative diseases? Ageing Res. Rev. 2010, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kostomoiri, M.; Fragkouli, A.; Sagnou, M.; Skaltsounis, L.A.; Pelecanou, M.; Tsilibary, E.C.; Τzinia, A.K. Oleuropein, an Anti-oxidant Polyphenol Constituent of Olive Promotes α-Secretase Cleavage of the Amyloid Precursor Protein (AβPP). Cell. Mol. Neurobiol. 2013, 33, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Price, E.A.; Wu, G.; Crouthamel, M.-C.; Shi, X.-P.; Tugusheva, K.; Tyler, K.X.; Kahana, J.; Ellis, J.; Jin, L.; et al. In Vivo β-Secretase 1 Inhibition Leads to Brain Aβ Lowering and Increased α-Secretase Processing of Amyloid Precursor Protein without Effect on Neuregulin-1. J. Pharmacol. Exp. Ther. 2008, 324, 957–969. [Google Scholar] [CrossRef]
- Fukumoto, H.; Rosene, D.L.; Moss, M.B.; Raju, S.; Hyman, B.T.; Irizarry, M.C. β-Secretase Activity Increases with Aging in Human, Monkey, and Mouse Brain. Am. J. Pathol. 2004, 164, 719–725. [Google Scholar] [CrossRef]
- Del Crdenas-Aguayo, M.C.; Del Silva-Lucero, M.C.; Cortes-Ortiz, M.; Jimnez-Ramos, B.; Gmez-Virgilio, L.; Ramrez-Rodrguez, G.; Vera- Arroyo, E.; Fiorentino-Prez, R.; Garca, U.; Luna-Muoz, J.; et al. Physiological Role of Amyloid Beta in Neural Cells: The Cellular Trophic Activity. In Neurochemistry; IntechOpen: London, UK, 2014. [Google Scholar]
- Codif Technologie Naturelle; Codif STOECHIOL. Available online: https://cosmetics.specialchem.com/product/i-codif-stoechiol (accessed on 17 May 2021).
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science 1990, 250, 279–282. [Google Scholar] [CrossRef]
- CODIF R&N Neuroguard The New Science of Aging. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjfoYiB58bwAhVODOwKHZ1rC-UQFjAAegQIAxAD&url=http%3A%2F%2Fwww.codif-tn.com%2Fwp-content%2Fuploads%2F2016%2F02%2FNEUROGUARD-BROCHURE-GB.pdf&usg=AOvVaw0gbO4Tbd7Lg6SSJinuahoC (accessed on 17 May 2021).
- Codif Technologie Naturelle Neuroguard. Available online: http://www.codif-tn.com/en/principesactifs/neuroguard/ (accessed on 17 May 2021).
- Menéndez-González, M.; Pérez-Pinera, P.; Martínez-Rivera, M.; Calatayud, M.T.; Blázquez Menes, B. APP Processing and the APP-KPI Domain Involvement in the Amyloid Cascade. Neurodegener. Dis. 2005, 2, 277–283. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed]
- Codif Advanced Skin Care—Neurocosmetics—New Public 60+. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiHztyVrsbwAhVOhv0HHWqgBP8QFjABegQIBhAD&url=http%3A%2F%2Fwww.codif-tn.com%2Fwp-content%2Fuploads%2F2016%2F02%2FSTOECHIOL-TENDANCE-MARCHE.pdf&usg=AOvVaw1I4oHtceOwACmdIvUw9kZS (accessed on 17 May 2021).
- Tajima, S.; Inoue, H.; Kawada, A.; Ishibashi, A.; Takahara, H.; Hiura, N. Alginate oligosaccharides modulate cell morphology, cell proliferation and collagen expression in human skin fibroblasts in vitro. Arch. Dermatol. Res. 1999, 291, 432–436. [Google Scholar] [CrossRef]
- Park, R.-M.; Ahn, J.-Y.; Kim, S.Y.; Wee, J.-H.; Kim, Y.-H.; Min, J. Effect of Alginate Oligosaccharides on Collagen Expression in HS 27 Human Dermal Fibroblasts. Toxicol. Environ. Health Sci. 2019, 11, 327–334. [Google Scholar] [CrossRef]
- Alaca, S.; Gedik, G. Evaluation of the effect of anti-pollution anti-aging eye cream on the collagen contraction. J. Pharm. Technol. 2020, 1, 13–17. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Vieira, T.F.; Corrêa, R.C.G.; Peralta, R.A.; Peralta-Muniz-Moreira, R.F.; Bracht, A.; Peralta, R.M. An Overview of Structural Aspects and Health Beneficial Effects of Antioxidant Oligosaccharides. Curr. Pharm. Des. 2020, 26, 1759–1777. [Google Scholar] [CrossRef]
- Biosil Technologies Glistin. Available online: http://www.biosiltech.com/glistin/ (accessed on 17 May 2021).
- Kang, C.K.; Lim, H.J.; Kim, J.H.Y.; Cho, S.A.; Kim, J.H.Y.; Park, N.H.; Kim, Y.J.; Cho, J.C.; Han, S.H. Anti-Aging Cosmetic Composition Containing Glutamylamidoethyl Indole. 2010. Available online: https://patents.google.com/patent/KR101520333B1/en (accessed on 19 May 2021).
- Exsymol Neurocosmetics. Available online: https://www.exsymol.com/en/dossier/neuro-cosmetique/ (accessed on 17 May 2021).
- Exsymol Biosil Technologies Glutrapeptide. Available online: http://www.biosiltech.com/glutrapeptide/ (accessed on 17 May 2021).
- Prouheze, P.; Morand, B.; Nicolaÿ, J.-F.; Fréchet, M. Preservation of Sympathetic Neuron-Adipocyte Crosstalk May Limit Chronic Emotional Stress-Mediated Fat Accu-mulation. IFSCC Mag. 2014, 17, 17–21. [Google Scholar]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, A.; Cornick, S.; Chominski, V.; Ribeiro de Noronha, S.; Alves Corrêa de Noronha, S.; Ferreira, L. Review of Major Theories of Skin Aging. Adv. Aging Res. 2014, 3, 49375. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Budzisz, E.; Dana, A.; Rotsztejn, H. New look at the role of progerin in skin aging. Prz. Menopauzalny Menopause Rev. 2015, 14, 53–58. [Google Scholar] [CrossRef]
- Cao, K.; Blair, C.D.; Faddah, D.A.; Kieckhaefer, J.E.; Olive, M.; Erdos, M.R.; Nabel, E.G.; Collins, F.S. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Investig. 2011, 121, 2833–2844. [Google Scholar] [CrossRef]
- Takeuchi, H.; Rünger, T.M. Longwave UV Light Induces the Aging-Associated Progerin. J. Investig. Dermatol. 2013, 133, 1857–1862. [Google Scholar] [CrossRef]
- LucasMeyer-Cosmetics Biomimetic Peptide Derived from Elafin. Available online: https://www.lucasmeyercosmetics.com/en/node/677 (accessed on 17 May 2021).
- Schagen, S. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Loing, E.; Suere, T.; Lamarque, E. Trifluoroacetyl-Tripeptide-2 to Target Senescence for Anti-aging Benefits. Available online: https://www.cosmeticsandtoiletries.com/formulating/category/skincare/premium-Trifluoroacetyl-Tripeptide-2-to-Target-Senescence-for-Anti-aging-Benefits-227412651.html (accessed on 17 May 2021).
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Pain, S.; Nakajima, S.; Altobelli, C.; Boher, A.; Cittadini, L.; Favre-Mercuret, M.; Sohm, B.; Vogelgesang, B.; Andre-Frei, V. Achillea millefolium extract: An innovative anti-aging neuro-cosmetic ingredient. J. Dermatol. Sci. 2013, 69, e52. [Google Scholar] [CrossRef]
- Pain, S.; Altobelli, C.; Boher, A.; Cittadini, L.; Favre-Mercuret, M.; Gaillard, C.; Sohm, B.; Vogelgesang, B.; André-Frei, V. Surface rejuvenating effect of Achillea millefolium extract. Int. J. Cosmet. Sci. 2011, 33, 535–542. [Google Scholar] [CrossRef] [PubMed]
- BASF NEUROBIOX® BC10097 The Ultimate Biological Skin Imperfections Blurring Solution. Available online: https://www.carecreations.basf.com/product-formulations/product-highlights/product-highlights-detail/NEUROBIOXBC10097/30715899 (accessed on 17 May 2021).
- BASF Care Creations NeurobioxTM The Skin Biosurfacer. Available online: https://docplayer.net/63928594-Neurobiox-tm-by-beauty-creations-the-skin-biosurfacer.html (accessed on 17 May 2021).
- Lana, J.; Ascenso, A. New Trends in Anti-Aging Skin Care. In Carrier-Mediated Dermal Delivery: Applications in the Prevention and Treatment of Skin Disorders; Ascenso, A., Simões, S., Ribeiro, H., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017; pp. 3–41. ISBN 978-1-315-36447-6. [Google Scholar]
- Codif Technologie Naturelle Neurolight. Available online: http://www.codif-tn.com/en/principesactifs/neurolight/ (accessed on 17 May 2021).
- Legat, F.J.; Wolf, P. Photodamage to the cutaneous sensory nerves: Role in photoaging and carcinogenesis of the skin? Photochem. Photobiol. Sci. 2006, 5, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, E.C.; Sykes, S.M.; McMahon, S.B.; Murphy, M.E. The p53 family and programmed cell death. Oncogene 2008, 27, 6507–6521. [Google Scholar] [CrossRef]
- Gritsenko, D.A.; Orlova, O.A.; Linkova, N.S.; Khavinson, V.K. Transcription factor p53 and skin aging. Adv. Gerontol. 2017, 7, 114–119. [Google Scholar] [CrossRef]
- Brady, C.A.; Attardi, L.D. p53 at a glance. J. Cell Sci. 2010, 123, 2527–2532. [Google Scholar] [CrossRef]
- Rachmin, I.; Ostrowski, S.M.; Weng, Q.Y.; Fisher, D.E. Topical treatment strategies to manipulate human skin pigmentation. Adv. Drug Deliv. Rev. 2020, 153, 65–71. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Amano, Y.; Ohuchi, A.; Kitahara, T.; Takema, Y. The Essential Role of p53 in Hyperpigmentation of the Skin via Regulation of Paracrine Melanogenic Cytokine Receptor Signaling. J. Biol. Chem. 2009, 284, 4343–4353. [Google Scholar] [CrossRef] [PubMed]
- Elewa, R.; Makrantonaki, E.; Zouboulis, C.C. Neuropeptides and skin aging. Horm. Mol. Biol. Clin. Investig. 2013, 16, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, G.; Kaestner, K.H. SnapShot: Forkhead Transcription Factors I. Cell 2007, 130, 1160.e1–1160.e2. [Google Scholar] [CrossRef]
- Calissi, G.; Lam, E.W.-F.; Link, W. Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov. 2021, 20, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of Oxidative Stress by the Anti-aging Hormone Klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho and aging. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z. Current understanding of klotho. Ageing Res. Rev. 2009, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Jaiswal, K.K. The role of the Klotho protein in the function of aging and neurodegenerative disorders. Octa J. Biosci. 2019, 7, 113–118. [Google Scholar]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med 2019, 85, 1326–1350. [Google Scholar] [CrossRef] [PubMed]
- Codif Technologie Naturelle LAKESIS. Available online: http://www.codif-tn.com/en/principesactifs/lakesis/ (accessed on 17 May 2021).
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Tucker Zhou, T.B.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho is Mediated via Regulation of Members of the Redox System. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef]
- Yodoi, J.; Matsuo, Y.; Tian, H.; Masutani, H.; Inamoto, T. Anti-Inflammatory Thioredoxin Family Proteins for Medicare, Healthcare and Aging Care. Nutrients 2017, 9, 1081. [Google Scholar] [CrossRef]
- Bachem Cosmetic Peptides. Available online: https://www.bachem.com/knowledge-center/white-papers/ (accessed on 10 May 2021).
- Rodan, K.; Fields, K.; Falla, T. Bioactive Peptide. In Cosmeceuticals and Cosmetic Practice; Farris, P.K., Ed.; John Wiley & Sons, Ltd: Oxford, UK, 2014; pp. 142–152. ISBN 978-1-118-38483-1. [Google Scholar]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Carli, B. Stop the Clock: Botox Alternatives. HPC Today Househ. Pers. Care Today 2017, 12, 52–54. [Google Scholar]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Khalid, F.; Gorouhi, F.; Maibach, H.I. Anti-Aging Topical Peptides and Proteins. In Cosmeceuticals and Active Cosmetics; Sivamani, R.K., Jagdeo, J.R., Elsner, P., Maibach, H.I., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 127–161. ISBN 978-1-4822-1417-8. [Google Scholar]
- Łubkowska, B.; Grobelna, B.; Maćkiewicz, Z. The use of synthetic polypeptides in cosmetics. Copernic. Lett. 2010, 1, 75–82. [Google Scholar] [CrossRef][Green Version]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef]
- Hajfathalian, M.; Ghelichi, S.; García-Moreno, P.J.; Moltke Sørensen, A.-D.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef]
- Oshimura, E.; Sakamoto, K. Amino Acids, Peptides, and Proteins. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 285–304. ISBN 978-0-12-802005-0. [Google Scholar]
- Goldstein, M.; Lintner, K. Cosmetics & Toiletries® Magazine. 2007. Available online: https://www.cosmeticsandtoiletries.com/ (accessed on 10 June 2021).
- Najafi, H.; Jafari, M.; Abolmaali, S. Recent Approaches in the Treatment of Skin Ageing by Synthetic Bioactive Peptides. Sadra Med. J. 2019, 7, 317–334. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Lupo, M.P.; Cole, A.L. Cosmeceutical Peptides. Dermatol. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef]
- Errante, F.; Ledwoń, P.; Latajka, R.; Rovero, P.; Papini, A.M. Cosmeceutical Peptides in the Framework of Sustainable Wellness Economy. Front. Chem. 2020, 8, 572923. [Google Scholar] [CrossRef]
- Ledwoń, P.; Errante, F.; Papini, A.M.; Rovero, P.; Latajka, R. Peptides as Active Ingredients: A Challenge for Cosmeceutical Industry. Chem. Biodivers. 2021, 18, e2000833. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Lupo, M.P. Cosmeceutical Peptides. Dermatol. Surg. 2005, 31, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Tadini, K.A.; Mercurio, D.G.; Campos, P.M.B.G.M. Acetyl hexapeptide-3 in a cosmetic formulation acts on skin mechanical properties—Clinical study. Braz. J. Pharm. Sci. 2015, 51, 901–909. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Zhou, W.; Wang, P.; Ogunsola, O.A. In vitro skin penetration of acetyl hexapeptide-8 from a cosmetic formulation. Cutan. Ocul. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.D.; Ellis, D.L.; Lupo, M.P. Facial Rejuvenation: Combining Cosmeceuticals With Cosmetic Procedures. Cutis 2014, 94, 122–126. [Google Scholar] [PubMed]
- Benson, H.A.E.; Namjoshi, S. Proteins and peptides: Strategies for delivery to and across the skin. J. Pharm. Sci. 2008, 97, 3591–3610. [Google Scholar] [CrossRef]
- Abu Samah, N.H.; Heard, C.M. Topically applied KTTKS: A review. Int. J. Cosmet. Sci. 2011, 33, 483–490. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Nanotechnology in Cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 337–369. ISBN 978-0-12-802005-0. [Google Scholar]
- Long, L.; Zhang, J.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Transdermal delivery of peptide and protein drugs: Strategies, advantages and disadvantages. J. Drug Deliv. Sci. Technol. 2020, 60, 102007. [Google Scholar] [CrossRef]
- Pillai, S.; Singh, S.; Oresajo, C. Percutaneous Delivery of Cosmetic Actives to the Skin. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; John Wiley & Sons, Ltd: Oxford, UK, 2016; pp. 65–74. ISBN 978-1-118-65558-0. [Google Scholar]
- Sugibayashi, K. Skin. In Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds; Sugibayashi, K., Ed.; Springer Japan KK: Tokyo, Japan, 2017; pp. 3–11. ISBN 978-4-431-56526-0. [Google Scholar]
- Förster, M.; Bolzinger, M.-A.; Fessi, H. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur. J. Dermatol. 2009, 19, 309–323. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Benson, H.A.E. Skin Structure, Function, and Permeation. In Transdermal and Topical Drug Delivery: Principles and Practice; Benson, H.A.E., Adam, C.W., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2012; pp. 3–22. ISBN 978-0-470-45029-1. [Google Scholar]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K. Related Topic: Chemical Permeation Through Impaired Skin. In Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds; Sugibayashi, K., Ed.; Springer Japan KK: Tokyo, Japan, 2017; pp. 87–91. ISBN 978-4-431-56526-0. [Google Scholar]
- Ham, S.W.; Kang, M.J.; Park, Y.-M.; Oh, I.-Y.; Kim, B.K.; Im, T.-J.; Kim, S.-H.; Choi, Y.W.; Lee, J. Transdermal Penetration of Synthetic Peptides and Their Penetration Enhancement Caused by Some Terpene Compounds. Bull. Korean Chem. Soc. 2007, 28, 1535–1538. [Google Scholar] [CrossRef][Green Version]
- Tadwee, I.K.; Gore, S.; Giradkar, P. Advances in Topical Drug Delivery System: A Review. Int. J. Pharm. Res. Allied Sci. 2012, 1, 14–23. [Google Scholar]
- Thong, H.-Y.; Zhai, H.; Maibach, H.I. Percutaneous Penetration Enhancers: An Overview. Skin Pharmacol. Physiol. 2007, 20, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Ashtiani, H.R.; Bishe, P.; Lashgari, N.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in Cosmetics. J. Ski. Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Eskens, O.; Amin, S. Challenges and effective routes for formulating and delivery of epidermal growth factors in skin care. Int. J. Cosmet. Sci. 2020, 43, 123–130. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Gad, S.; Desoqi, M.; El-Sawy, H.; Khafagy, E.; Ghourab, M. Drug Delivery Systems for Topical treatment of Inflammatory Skin Diseases. Rec. Pharm. Biomed. Sci. 2021, 5, 59–64. [Google Scholar] [CrossRef]
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal Delivery Systems for Biomolecules. J. Pharm. Innov. 2021, 1–14. [Google Scholar] [CrossRef]
- Castelletto, V.; Hamley, I.W.; Whitehouse, C.; Matts, P.J.; Osborne, R.; Baker, E.S. Self-Assembly of Palmitoyl Lipopeptides Used in Skin Care Products. Langmuir 2013, 29, 9149–9155. [Google Scholar] [CrossRef]
- Reissmann, S. Cell penetration: Scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. 2014, 20, 760–784. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-K.; Kuo, P.-H.; Lu, Y.-C.; Lin, H.-N.; Wang, L.H.-C.; Lin, Y.-C.; Kao, Y.-C.; Lai, H.-M.; Chang, M.D.-T. Cell Penetrating Peptide as a High Safety Anti-Inflammation Ingredient for Cosmetic Applications. Biomolecules 2020, 10, 101. [Google Scholar] [CrossRef]
- Ookubo, N.; Michiue, H.; Kitamatsu, M.; Kamamura, M.; Nishiki, T.; Ohmori, I.; Matsui, H. The transdermal inhibition of melanogenesis by a cell-membrane-permeable peptide delivery system based on poly-arginine. Biomaterials 2014, 35, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Zakrewsky, M.; Chen, M.; Menegatti, S.; Muraski, J.A.; Mitragotri, S. Peptides as skin penetration enhancers: Mechanisms of action. J. Control. Release 2015, 199, 168–178. [Google Scholar] [CrossRef]
- Chen, M.; Gupta, V.; Anselmo, A.C.; Muraski, J.A.; Mitragotri, S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J. Control. Release 2014, 173, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Yu, Y.; Chen, D.; Jiao, G.; Liu, X. Enhanced penetration strategies for transdermal delivery. Front. Chem. Sci. Eng. 2020, 14, 378–388. [Google Scholar] [CrossRef]
- Yang, J.-A.; Kim, E.-S.; Kwon, J.H.; Kim, H.; Shin, J.H.; Yun, S.H.; Choi, K.Y.; Hahn, S.K. Transdermal delivery of hyaluronic acid—Human growth hormone conjugate. Biomaterials 2012, 33, 5947–5954. [Google Scholar] [CrossRef]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef]
- Bakshi, P.; Vora, D.; Hemmady, K.; Banga, A.K. Iontophoretic skin delivery systems: Success and failures. Int. J. Pharm. 2020, 586, 119584. [Google Scholar] [CrossRef]
- Karimipour, D.J.; Karimipour, G.; Orringer, J.S. Microdermabrasion: An Evidence-Based Review. Plast. Reconstr. Surg. 2010, 125, 372–377. [Google Scholar] [CrossRef]
- Zhou, Y.; Banga, A.K. Enhanced delivery of cosmeceuticals by microdermabrasion. J. Cosmet. Dermatol. 2011, 10, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. Immunopharmacology and immunopathology of peptides and proteins in personal products. J. Immunoass. Immunochem. 2019, 40, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Slaga, T.J.; Snyder, P.W. Safety Assessment of Acetyl Hexapeptide-8 and Acetyl Hexapeptide-8 Amide as Used in Cosmetics. 2020. Available online: https://www.cir-safety.org/sites/default/files/acetyl122020revTR.pdf (accessed on 17 April 2021).
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Kang, N.J.; Jin, H.-S.; Lee, S.-E.; Kim, H.J.; Koh, H.; Lee, D.-W. New approaches towards the discovery and evaluation of bioactive peptides from natural resources. Crit. Rev. Environ. Sci. Technol. 2020, 50, 72–103. [Google Scholar] [CrossRef]
- Rona, C.; Vailati, F.; Berardesca, E. The cosmetic treatment of wrinkles. J. Cosmet. Dermatol. 2004, 3, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Campiche, R.; Pascucci, F.; Jiang, L.; Vergne, T.; Cherel, M.; Gougeon, S.; Préstat-Marquis, E.; François, G.; Laurent, G.; Gempeler, M. Facial Expression Wrinkles and Their Relaxation by a Synthetic Peptide. Int. J. Pept. Res. Ther. 2020. [Google Scholar] [CrossRef]
- Limbert, G.; Kuhl, E. On skin microrelief and the emergence of expression micro-wrinkles. Soft Matter 2018, 14, 1292–1300. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xiao, X.S.; Huo, J.; Zhang, W.D. The anti-wrinkle efficacy of Argireline. J. Cosmet. Laser Ther. 2013, 15, 237–241. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xiao, S.; Pan, P.; Li, P.; Huo, J. The Anti-Wrinkle Efficacy of Argireline, a Synthetic Hexapeptide, in Chinese Subjects. Am. J. Clin. Dermatol. 2013, 14, 147–153. [Google Scholar] [CrossRef]
- Sanders, L. Anti-ageing actives and technologies behind them. Pers. Care Eur. 2019, 85–89. [Google Scholar]
- Wongrattanakamon, P.; Nimmanpipug, P.; Sirithunyalug, B.; Jiranusornkul, S. Molecular modeling elucidates the cellular mechanism of synaptotagmin-SNARE inhibition: A novel plausible route to anti-wrinkle activity of botox-like cosmetic active molecules. Mol. Cell. Biochem. 2018, 442, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kavalali, E.T. Synaptic Vesicle-Recycling Machinery Components as Potential Therapeutic Targets. Pharmacol. Rev. 2017, 69, 141–160. [Google Scholar] [CrossRef]
- Pohanka, M. Alpha7 Nicotinic Acetylcholine Receptor Is a Target in Pharmacology and Toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Dixon, T.K.; Bhattacharyya, T.K. Effects of Topicals on the Aging Skin Process. Facial Plast. Surg. Clin. N. Am. 2013, 21, 55–60. [Google Scholar] [CrossRef]
- Mitchell, S. Best Peptide Face Creams. Available online: https://lumeskin.com/best-peptide-cream/ (accessed on 5 May 2021).
- Prokopowicz, M.; Różycki, K.M. Innovation in cosmetics. World Sci. News 2017, 72, 448–456. [Google Scholar]
- Lipotec VANISTRYL® Functional Ingredient. Available online: https://cosmetics.specialchem.com/product/i-lipotec-vanistryl-functional-ingredient (accessed on 5 May 2021).
- Diehl, C. Peptides in cosmeceuticals. Ukr. J. Dermatol. Venereol. Cosmetol. 2019, 28–35. [Google Scholar] [CrossRef]
- RobelynLabs ElastinMD Stretch Mark Repair. Available online: https://www.robelynlabs.com/shop/product_detail/elastinmd-stretch-mark-repair-therapy# (accessed on 5 May 2021).
- Cheng, C.M. Cosmetic use of botulinum toxin type A in the elderly. Clin. Interv. Aging 2007, 2, 81–83. [Google Scholar] [CrossRef]
- Jensen, J.D.; Freeman, S.R.; Cohen, J.L. Botulinum Toxins. In Cosmetic Dermatology Products and Procedures; Draelos, Z.D., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2016; pp. 364–374. ISBN 978-1-118-65558-0. [Google Scholar]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Popoff, M.R. Engineering Botulinum Neurotoxins for Enhanced Therapeutic Applications and Vaccine Development. Toxins 2021, 13, 1. [Google Scholar] [CrossRef]
- Carruthers, A.; Carruthers, J. Botulinum Toxin Products Overview. Available online: https://www.skintherapyletter.com/aging-skin/botulinum-toxin-overview/?amp=1 (accessed on 17 May 2021).
- França, K.; Kumar, A.; Fioranelli, M.; Lotti, T.; Tirant, M.; Roccia, M.G. The history of Botulinum toxin: From poison to beauty. Wien. Med. Wochenschr. 2017, 167, 46–48. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-aging and Sunscreens: Paradigm Shift in Cosmetics. Adv. Pharm. Bull. 2019, 9, 348–359. [Google Scholar] [CrossRef]
- Coleman, W.P., III. Handbook of Cosmetic Science and Technology, 3rd ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; ISBN 978-1-4200-6963-1. [Google Scholar]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef]
- Gallagher, C.J.; Ackerman, A. Botulinum Toxin: From Molecule to Medicine. In BotulinumToxins Cosmetic and Clinical Applications; Cohen, J.L., Ozog, D.M., Eds.; JohnWiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 37–51. ISBN 9781118661864. [Google Scholar]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef]
- Aoki, K.R. Review of a Proposed Mechanism for the Antinociceptive Action of Botulinum Toxin Type A. Neurotoxicology 2005, 26, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Grando, S.A.; Zachary, C.B. The non-neuronal and nonmuscular effects of botulinum toxin: An opportunity for a deadly molecule to treat disease in the skin and beyond. Br. J. Dermatol. 2018, 178, 1011–1019. [Google Scholar] [CrossRef]
- Sethi, N.; Singh, S.; DeBoulle, K.; Rahman, E. A Review of Complications Due to the Use of Botulinum Toxin A for Cosmetic Indications. Aesthetic Plast. Surg. 2020, 45, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Small, R. Botulinum Toxin Injection for Facial Wrinkles. Am. Fam. Physician 2014, 90, 168–175. [Google Scholar]
- Lim, S.H.; Sun, Y.; Thiruvallur Madanagopal, T.; Rosa, V.; Kang, L. Enhanced Skin Permeation of Anti-wrinkle Peptides via Molecular Modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Biopolymeric agents for skin wrinkle treatment. J. Cosmet. Laser Ther. 2016, 18, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lipotec ARGIRELINE® Peptide. Available online: https://www.lipotec.com/en/products/argireline-reg-peptide/# (accessed on 17 May 2021).
- Lipotec Argireline® Amplified Peptide. Available online: https://www.lipotec.com/en/products/argireline-reg-amplified-peptide/# (accessed on 17 May 2021).
- Grosicki, M.; Latacz, G.; Szopa, A.; Cukier, A.; Kieć-Kononowicz, K. The study of cellular cytotoxicity of argireline®—An anti-aging peptide. Acta Biochim. Pol. 2014, 61, 29–32. [Google Scholar] [CrossRef]
- Lipotec INYLINE® Peptide. Available online: https://www.lipotec.com/en/products/inyline-reg-peptide/# (accessed on 17 May 2021).
- Glass, D.J.; Bowen, D.C.; Stitt, T.N.; Radziejewski, C.; Bruno, J.; Ryan, T.E.; Gies, D.R.; Shah, S.; Mattsson, K.; Burden, S.J.; et al. Agrin Acts via a MuSK Receptor Complex. Cell 1996, 85, 513–523. [Google Scholar] [CrossRef]
- Zong, Y.; Jin, R. Structural mechanisms of the agrin–LRP4–MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 2013, 70, 3077–3088. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, B.; Gu, S.; Lee, K.; Zhou, J.; Yao, G.; Figueiredo, D.; Perry, K.; Mei, L.; Jin, R. Structural basis of agrin–LRP4–MuSK signaling. Genes Dev. 2012, 26, 247–258. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.J. Chapter Eight—The Basement Membrane Proteoglycans Perlecan and Agrin: Something Old, Something New. In Basement Membranes; Miner, J.H.B.T.-C.T. in M., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 76, pp. 255–303. ISBN 1063-5823. [Google Scholar]
- Bezakova, G.; Ruegg, M.A. New insights into the roles of agrin. Nat. Rev. Mol. Cell Biol. 2003, 4, 295–309. [Google Scholar] [CrossRef]
- Miner, J.H.; Abrahamson, D.R. Molecular and Cellular Mechanisms of Glomerular Capillary Development. In Seldin and Giebisch’s The Kidney Physiology and Pathophysiology; Alpern, R.J., Hebert, S.C.B.T.-S. and G.T.K. Fourth E., Eds.; Academic Press: San Diego, CL, USA, 2008; pp. 691–707. ISBN 978-0-12-088488-9. [Google Scholar]
- Bezakova, G.; Helm, J.P.; Francolini, M.; Lømo, T. Effects of Purified Recombinant Neural and Muscle Agrin on Skeletal Muscle Fibers in Vivo. J. Cell Biol. 2001, 153, 1441–1452. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef]
- Lipotec Inyline Peptide. A New Strategy to Escape from Expression Lines. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiertvooMfwAhXihv0HHf8vCpMQFjABegQIAxAD&url=https%3A%2F%2Fwww.quickbox.com%2Fwp-content%2Fuploads%2F2017%2F04%2FInyline.pdf&usg=AOvVaw3-6gl45MNLAmepiZqL6v9i (accessed on 17 May 2021).
- Prospector BONT-L Peptide Solution (PF). Available online: https://www.ulprospector.com/en/eu/PersonalCare/Detail/19323/601512/BONT-L-Peptide-Solution-PF (accessed on 5 May 2021).
- TAOS Inc. BoNT-L Peptide. Available online: https://www.technicalartofscience.com/product/bont-l-peptide/ (accessed on 10 May 2021).
- Shahi, S.; Athawale, R.B. Development and evaluation of Cosmeceutical Nanolipogel. Res. J. Top. Cosmet. Sci. 2010, 1, 18–24. [Google Scholar]
- Lipotec SNAP-8TM Peptide. Available online: https://www.lipotec.com/en/products/snap-8-trade-peptide/ (accessed on 17 May 2021).
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- LIPOTEC. A GMP Peptide for Cosmetic Applications; LIPOTEC: Barcelona, Spain, 2005. [Google Scholar]
- Infinitec X50® Myocept Lines of Expression: The Best Performance at Lowest Dose. Available online: https://infinitec.es/technology/x50-myocept/ (accessed on 17 May 2021).
- Dragomirescu, A.O.; Andoni, M.; Ionescu, D.; Andrei, F. The Efficiency and Safety of Leuphasyl—A Botox-Like Peptide. Cosmetics 2014, 1, 75–81. [Google Scholar] [CrossRef]
- Park, J.; Jung, H.; Jang, B.; Song, H.-K.; Han, I.-O.; Oh, E.-S. D-tyrosine adds an anti-melanogenic effect to cosmetic peptides. Sci. Rep. 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Sederma Prospector CalmosensineTM SP. Available online: https://www.ulprospector.com/en/na/PersonalCare/Detail/1240/44014/Calmosensine-SP (accessed on 17 May 2021).
- Sederma Calmosensine. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiGgL6misnwAhUEKuwKHfJQBuAQFjABegQIAhAD&url=https%3A%2F%2Fwww.bio-therapeutic.com%2Fwp-content%2Fuploads%2F2017%2F08%2FCHROMATIC_SERUM_WHITE_PAPERS.pdf&usg=AOvVaw0eY9TgKPNJeXcR9JdpYhM (accessed on 29 April 2021).
- Khmaladze, I.; Österlund, C.; Smiljanic, S.; Hrapovic, N.; Lafon-Kolb, V.; Amini, N.; Xi, L.; Fabre, S. A novel multifunctional skin care formulation with a unique blend of antipollution, brightening and antiaging active complexes. J. Cosmet. Dermatol. 2020, 19, 1415–1425. [Google Scholar] [CrossRef]
- BASF Care Creations; BASF Myoxinol® LS 9736. Available online: https://carecreations.basf.us/products/myoxinol-ls-9736 (accessed on 17 May 2021).
- Renzi, A.; Brillantino, A.; Di Sarno, G.; D’Aniello, F.; Ziccardi, S.; Paladino, F.; Iacobellis, F. Myoxinol (Hydrolyzed Hibiscus esculentus Extract) in the Cure of Chronic Anal Fissure: Early Clinical and Functional Outcomes. Gastroenterol. Res. Pract. 2015, 2015, 567920. [Google Scholar] [CrossRef]
- Benoit, I.; Danoux, L.; Gillon, V.; Moussou, P.; Pauly, G. Oligopeptides from Hibiscus esculentus seeds to smooth expression lines. SOFW J. 2004, 130, 64–71. [Google Scholar]
- Prospector MyoxinolTM LS 9736. Available online: https://www.ulprospector.com/en/na/PersonalCare/Detail/75/109267/Myoxinol-LS-9736 (accessed on 17 May 2021).
- Alqasoumi, S.I. ‘Okra’ Hibiscus esculentus L.: A study of its hepatoprotective activity. Saudi Pharm. J. 2012, 20, 135–141. [Google Scholar] [CrossRef] [PubMed]
- BASF Anti-wrinkle Eye Care Serum. Available online: https://www.carecreations.basf.com/product-formulations/formulations/formulations-detail/Anti-wrinkleEyecareSerum/sc-de-12-108-9 (accessed on 17 May 2021).
- A Natural Alternative to Botulinum Toxin. Available online: https://www.cosmeticsdesign.com/Article/2004/03/10/A-natural-alternative-to-botulinum-toxin (accessed on 17 May 2021).
- BASF Care Creations Myoxinol The Vegetal Answer to Smooth Expression Lines. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiO5ryehJ_wAhUNM-wKHbbACWEQFnoECAIQAA&url=https%253A%252F%252Fbiakhim.com.ua%252Findex.php%253Foption%253Dcom_k2%2526Itemid%253D1173%2526id%253D668_30edae9b008c91727b8d (accessed on 17 May 2021).
- Barbosa, A.F.; De Carvalhoa, M.G.; Smith, R.E.; Sabaa-Srur, A.U.O. Spilanthol: Occurrence, extraction, chemistry and biological activities. Rev. Bras. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Silveira, N.; Sandjo, L.P.; Biavatti, M.W. Spilanthol-containing products: A patent review (1996–2016). Trends Food Sci. Technol. 2018, 74, 107–111. [Google Scholar] [CrossRef]
- Sabitha Rani, A.; Sana, H.; Sulakshana, G.; Keerti, E.; Shravya Puri, M. Spilanthes acmella- an important medicinal plant. Int. J. Minor Fruits Med. Aromat. Plants 2019, 5, 15–26. [Google Scholar]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Demarne, F.; Passaro, G. Use of an Acmella oleracea extract for the botulinum toxin-like effect thereof in an anti-wrinkle cosmetic composition. U.S. Patent No 7,531,193, 12 May 2009. [Google Scholar]
- Yang, D.; Li, W.; Fang, L.; Liu, C. Investigation of Controlled Release Molecular Mechanism of Oil Phase in Spilanthol Emulsion: Development and In Vitro, In Vivo Characterization. AAPS PharmSciTech 2019, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Baert, B.; Roche, N.; Burvenich, C.; De Spiegeleer, B. Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella (Compositae) extracts. J. Ethnopharmacol. 2010, 127, 77–84. [Google Scholar] [CrossRef]
- Surianarayanan, R.; Bhaskar, J.P. Herbal Cosmeceuticals. In Plant Metabolites: Methods, Applications and Prospects; Sukumaran, S.T., Sugathan, S., Abdulhameed, S., Eds.; Springer Nature: Singapore, 2020; pp. 217–238. ISBN 978-981-15-5136-9. [Google Scholar]
- Santana, M.; Oliveira, G.; Yoshida, V.; Sabha, M.; Oshima-Franco, Y. Naturally occurring ingredients as potential antiaging cosmetics. Lat. Am. J. Pharm. 2011, 30, 1531–1535. [Google Scholar]
- Muszyńska, B.; Fijałkowska, A.; Sułkowska-Ziaja, K.; Włodarczyk, A.; Kaczmarczyk, P.; Nogaj, E.; Piętka, J. Fomitopsis officinalis: A Species of Arboreal Mushroom with Promising Biological and Medicinal Properties. Chem. Biodivers. 2020, 17, e2000213. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Fauziah, N.; Herdiman, H.; Afni, M.; Afifah, E.; Sari, H.W.K.; Nufus, H.; Arumwardana, S.; Rihibiha, D.D. Antioxidant and Anti Aging Assays of Oryza Sativa Extracts, Vanillin and Coumaric Acid. J. Nat. Remedies 2016, 16, 88–99. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Ctpa Cosmetic Product Claims Regulatory Framework. Available online: https://www.ctpa.org.uk/resources-claims (accessed on 17 May 2021).
- Ctpa NEW—CTPA Guide to Advertising Claims. Available online: https://www.ctpa.org.uk/news/new-ctpa-guide-to-advertising-claims-4080 (accessed on 17 May 2021).
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- Dent, M.; Amaral, R.T.; Da Silva, P.A.; Ansell, J.; Boisleve, F.; Hatao, M.; Hirose, A.; Kasai, Y.; Kern, P.; Kreiling, R.; et al. Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput. Toxicol. 2018, 7, 20–26. [Google Scholar] [CrossRef]
- Juncan, A.M. Packaging Evaluation and Safety Assessment of a Cosmetic Product. Mater. Plast. 2018, 55, 644. [Google Scholar] [CrossRef]
- Juncan, A.M.; Rus, L.L. Influence of Packaging and Stability Test Assessment of an Anti-aging Cosmetic Cream. Mater. Plast. 2018, 55, 426. [Google Scholar] [CrossRef]
- Lionetti, N.; Rigano, L. Labeling of Cosmetic Products. Cosmetics 2018, 5, 22. [Google Scholar] [CrossRef]
- ARKANA. ARKANA Advanced Beauty Care. Available online: https://arkanacosmetics.com/ (accessed on 17 May 2021).
- ARKANA Bo2Look Elixir 20 ml. Available online: https://www.shop-arkana.com/b2c_en/bo2look-elixir-20-ml.html (accessed on 17 May 2021).
- Turner, A.; Kaas, Q.; Craik, D.J. Hormone-like conopeptides—New tools for pharmaceutical design. RSC Med. Chem. 2020, 11, 1235–1251. [Google Scholar] [CrossRef]
- BCR Biocomponent Research Activen XEPTM-018. Available online: https://www.bcringredients.com/activen-xep-018/ (accessed on 17 May 2021).
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef]
- Schroeder, C.I.; Craik, D.J. Therapeutic potential of conopeptides. Future Med. Chem. 2012, 4, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Activen Discover Cone Snail Peptidomimetic, the Revolutionary Anti-Wrinkles. Instant Line Relaxer. Available online: http://www.activen.ch/?page=xep (accessed on 17 May 2021).
- Favreau, P.; Benoit, E.; Hocking, H.G.; Carlier, L.; D’hoedt, D.; Leipold, E.; Markgraf, R.; Schlumberger, S.; Córdova, M.A.; Gaertner, H.; et al. A novel µ-conopeptide, CnIIIC, exerts potent and preferential inhibition of NaV1.2/1.4 channels and blocks neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2012, 166, 1654–1668. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Ramiro, I.B.L.; Yandell, M.; McIntosh, J.M.; Olivera, B.M.; Ellgaard, L.; Safavi-Hemami, H. Curses or Cures: A Review of the Numerous Benefits Versus the Biosecurity Concerns of Conotoxin Research. Biomedicines 2020, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Del Río-Sancho, S.; Cros, C.; Coutaz, B.; Cuendet, M.; Kalia, Y.N. Cutaneous iontophoresis of μ-conotoxin CnIIIC—A potent NaV1.4 antagonist with analgesic, anaesthetic and myorelaxant properties. Int. J. Pharm. 2017, 518, 59–65. [Google Scholar] [CrossRef]
- Elixseri Cone Snail Peptide/XEPTM- 018. Available online: https://www.elixseri.com/pta-cone-snail-peptide (accessed on 17 May 2021).
- ARKANA Neuro GABA & NANA Therapy. Available online: https://arkanacosmetics.com/md/neuro-gabanana-therapy/ (accessed on 17 May 2021).
- ARKANA Neuro GABA Therapy. Available online: https://www.shop-arkana.com/b2b_en/linie-arkana/neuro-gaba-nana-therapy.html (accessed on 17 May 2021).
- Ferrillo, M.; Vastarella, M.; Cantelli, M.; Mazzella, C.; Fabbrocini, G. Instrumental, clinical and subjective evaluation of the efficacy of a cosmetic treatment for home use. J. Cosmet. Laser Ther. 2019, 21, 190–195. [Google Scholar] [CrossRef]
- Mittapally, S.; Afnan, A.A. A review on nanotechnology in cosmetics. Pharma Innov. J. 2019, 8, 668–671. [Google Scholar]
- Chan, G.; Wong, Z.; Lam, K.; Cheng, L.; Zhang, L.; Lin, H.; Dong, T.; Tsim, K. Edible Bird’s Nest, an Asian Health Food Supplement, Possesses Skin Lightening Activities: Identification of N-Acetylneuraminic Acid as Active Ingredient. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 262–274. [Google Scholar] [CrossRef]
- Lodén, M. Moisturizers: Treatment of Dry Skin Syndrome and Barrier Defects. In Cosmeceuticals and Active Cosmetics; Sivamani, R.K., Jagdeo, J.R., Elsner, P., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 235–259. ISBN 9780429192388. [Google Scholar]
- Green, B.A.; Van Scott, E.J.; Yu, R.J. Clinical Uses of Hydroxyacids. In Cosmetic Dermatology Products and Procedures; Draelos, Z.D., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 346–356. ISBN 978-1-118-65558-0. [Google Scholar]
- ARKANA Professional CARBO V RECONSTRUCTOR. Available online: https://arkanaukprof.com/product/carbo-v-reconstructor/ (accessed on 17 May 2021).
- Sparavigna, A.; Tenconi, B.; De Ponti, I.; Guglielmini, G. Evaluation of the Activity and Tolerability of a Cosmetic Treatment for the Periocular Area on the Aging Face: Controlled Clinical and Instrumental Evaluation vs. Placebo. Cosmetics 2014, 1, 105–116. [Google Scholar] [CrossRef]
- Prospector Ximilene®. Available online: https://www.ulprospector.com/en/eu/PersonalCare/Detail/2736/90649/Ximilene (accessed on 17 May 2021).
- Satoto, G.; Fernandes, A.S.; Saraiva, N.; Santos, F.; Neng, N.; Nogueira, J.M.; Santos de Almeida, T.; Araujo, M.E. An Overview on the Properties of Ximenia Oil Used as Cosmetic in Angola. Biomolecules 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Dugall, M.; Maramaldi, G.; Togni, S.; Giacomelli, L. Evaluation of the efficacy of a new escin-based, multi-component formulation in reducing eyelid edema. Minerva Oftalmol. 2016, 58, 65–69. [Google Scholar]
- Vermaak, I.; Kamatou, G.P.P.; Komane-Mofokeng, B.; Viljoen, A.M.; Beckett, K. African seed oils of commercial importance—Cosmetic applications. S. Afr. J. Bot. 2011, 77, 920–933. [Google Scholar] [CrossRef]
- Shivatare, R.S.; Musale, R.; Lohakare, P.; Patil, D.; Choudhary, D.; Ganu, G.; Nagore, D.H.; Kewatkar, S.M. Isolation, Identification and Characterization of Ximenynic Acid with Anti-Aging Activity from Santalum Album. Int. J. Res. Pharm. Sci. 2020, 11, 1394–1399. [Google Scholar] [CrossRef]
- The Derm Review Amino Acids In Skincare: Arginine. Available online: https://thedermreview.com/arginine/ (accessed on 17 May 2021).
- BE BEAUTIFUL. What Is the Role of Arginine in Skincare. Available online: https://www.bebeautiful.in/all-things-skin/everyday/arginine-in-skincare (accessed on 17 May 2021).
- ARKANA Biomimetic Therapy—The Way to Regain Youth. Available online: https://arkanacosmetics.com/news/biomimetic-therapy-the-way-to-regain-youth/ (accessed on 17 May 2021).
- Prospector Adipofill’inTM. Available online: https://www.ulprospector.com/en/eu/PersonalCare/Detail/4499/215014/Adipofillin (accessed on 17 May 2021).
- LucasMeyer-Cosmetics L-Ornithine Vectorized in a IonosomeTM. Available online: https://www.lucasmeyercosmetics.com/en/node/578 (accessed on 10 May 2021).
- DeJohn, A. Volumizing Anti-aging Skin Care Ingredient. Available online: https://www.cosmeticsdesign.com/Article/2012/06/13/Volumizing-anti-aging-skin-care-ingredient (accessed on 10 May 2021).
- LucasMeyer-Cosmetics. Adipofill Bio-controlled Lipofilling. LucasMeyer-Cosmetics. Available online: www.lucasmeyercosmetics.com (accessed on 6 May 2021).
- Harada, D.; Nagamachi, S.; Aso, K.; Ikeda, K.; Takahashi, Y.; Furuse, M. Oral administration of l-ornithine increases the content of both collagen constituting amino acids and polyamines in mouse skin. Biochem. Biophys. Res. Commun. 2019, 512, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Loing, E.; Belhaj, N.; Ollier, V.; Bezivin, C. New generation of resistant delivery system for a better skin bioavailability and anti-aging targeted action. Available online: https://www.scconline.org/wp-content/uploads/2014/10/Loing.pdf (accessed on 10 June 2021).
- Stanek, J.; Wochner, M.; Gupta, S. Current and Future ‘Body-sculpting’Cosmetics. CoValence Lab. Res. CT 2015, 130, 20–31. [Google Scholar]
- Rattan, S.I.S. Hormetic Mechanisms of Anti-Aging and Rejuvenating Effects of Repeated Mild Heat Stress on Human Fibroblasts in Vitro. Rejuvenation Res. 2004, 7, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Kryzch, V.; Schnebert, S.; Perrier, E.; Nizard, C. Hormesis-Based Anti-Aging Products: A Case Study of a Novel Cosmetic. Dose Response 2012, 11. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Hormetins as Novel Components of Cosmeceuticals and Aging Interventions. Cosmetics 2015, 2, 11–20. [Google Scholar] [CrossRef]
- Does the Beauty Industry Need to Become More Transparent with Consumers? Available online: https://uk.fashionnetwork.com/news/Does-the-beauty-industry-need-to-become-more-transparent-with-consumers-,992826.html (accessed on 17 May 2021).
- The Provenance Team Transparency in Beauty & Wellness: Why 2020 Will be a Breakthrough Year. Available online: https://www.provenance.org/news/movement/transparency-in-beauty-wellness-why-2020-will-be-a-breakthrough-year (accessed on 10 May 2021).
- Rachel Brown Consumers Are Demanding Greater Transparency From Beauty Brands. What Does That Really Mean? Available online: https://www.beautyindependent.com/consumers-are-demanding-greater-transparency-from-beauty-brands-what-does-that-really-mean/ (accessed on 10 May 2021).
- Optel Transparency and Quality Guarantee for the Cosmetics Industry. Consumer Expectations: More Than Skin Deep. Available online: https://www.optelgroup.com/cosmetic-industry/ (accessed on 10 May 2021).
- Manteghi, M. European Cosmetics Industry: Main Aspects and Regulation. SSRN 2017, 21. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration Summary of Cosmetics Labeling Requirements. Available online: https://www.fda.gov/cosmetics/cosmetics-labeling-regulations/summary-cosmetics-labeling-requirements (accessed on 17 May 2021).
- Cosmetics Europe Understanding the Label. Available online: https://cosmeticseurope.eu/cosmetic-products/understanding-label/ (accessed on 17 May 2021).
- Canada, G. of Industry Guide for the Labelling of Cosmetics. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/labelling-cosmetics.html (accessed on 10 May 2021).
- European Commission Cosmetic Products—Specific Topics. Available online: https://ec.europa.eu/growth/sectors/cosmetics/products_en (accessed on 17 May 2021).
- Dorato, S. Chapter 1—General Concepts: Current Legislation on Cosmetics in Various Countries; Salvador, A., Chisvert, A.B.T.-A., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 3–37. ISBN 978-0-444-63508-2. [Google Scholar]
- EUR-Lex Access to European Union Law Commission Regulation (EU) No 655/2013 of 10 July 2013 Laying Down Common Criteria for the Justification of Claims Used in Relation to Cosmetic Products Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32013R0655 (accessed on 17 May 2021).
- European Commission Technical Document on Cosmetic Claims. Available online: https://ec.europa.eu/docsroom/documents/24847 (accessed on 17 May 2021).
- Ctpa Cosmetic Product Definition. Available online: https://www.ctpa.org.uk/definition-ofa-cosmetic (accessed on 17 May 2021).
- European Commission Cosmetics. Available online: https://ec.europa.eu/growth/sectors/cosmetics_en (accessed on 17 May 2021).
- European Commission Legislation. Available online: https://ec.europa.eu/growth/sectors/cosmetics/legislation_en (accessed on 17 May 2021).
- European Commission Manual of the Working Group on Cosmetic Products (Sub-Group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No 1223/2009 (ART. 2(1)(A)) Version 3.1. Available online: https://ec.europa.eu/docsroom/documents/29002 (accessed on 17 May 2021).
- Parliament, E. Question Reference: E-000056/2016. Available online: https://www.europarl.europa.eu/doceo/document/E-8-2016-000056-ASW_EN.html?redirect (accessed on 12 May 2021).
- EUR-Lex Access to European Union Law Consolidated text: Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/eli/dir/2001/83/2019-07-26 (accessed on 17 May 2021).
- EUR-Lex Access to European Union Law Consolidated text: Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products (Text with EEA relevance) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02012R0528-20210329 (accessed on 17 May 2021).
- EUR-Lex Access to European Union Law Consolidated text: Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/3. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0745-20200424 (accessed on 17 May 2021).
- European Commission Borderline Products. Available online: https://ec.europa.eu/growth/sectors/cosmetics/products/borderline-products_en (accessed on 17 May 2021).
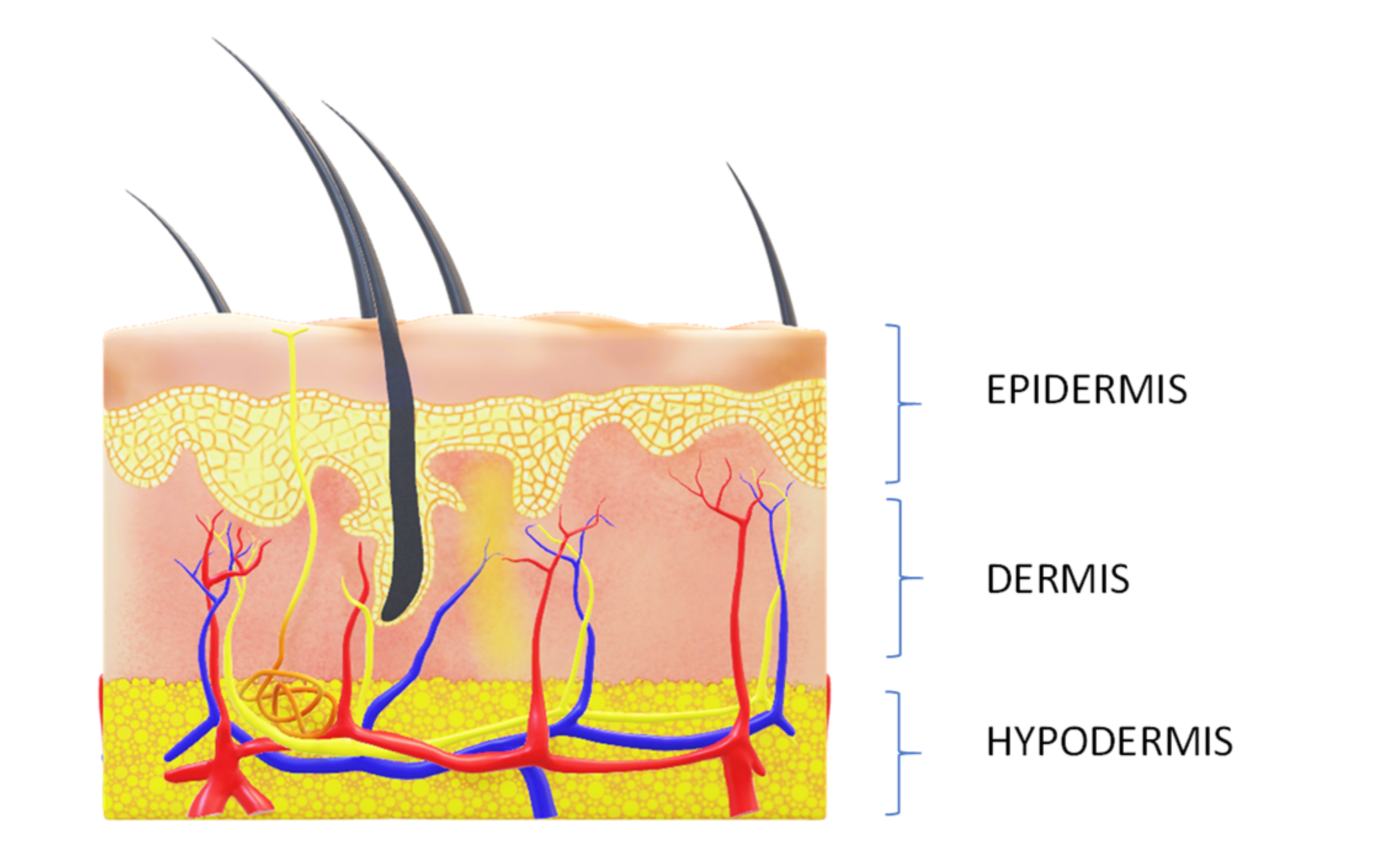
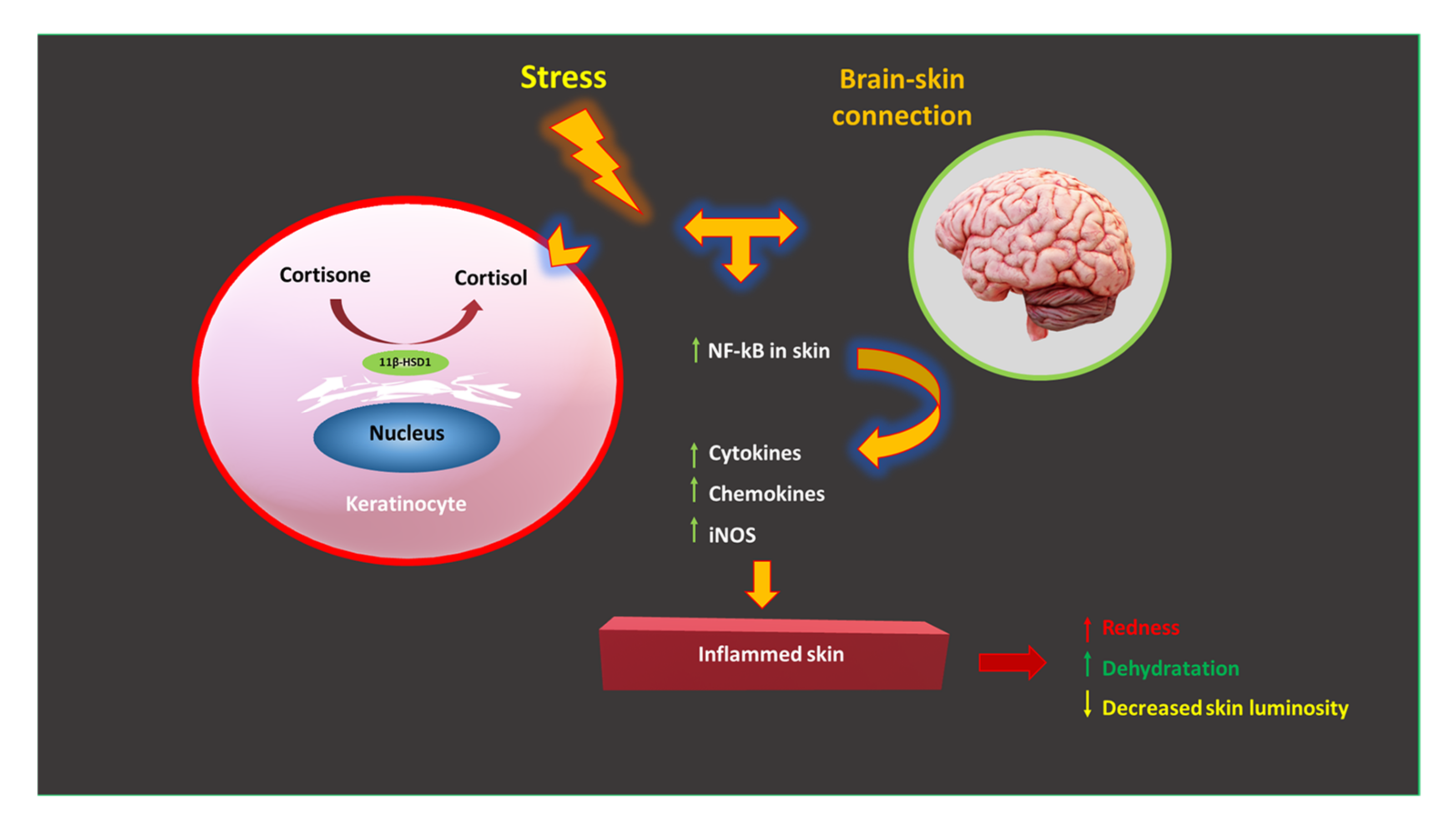
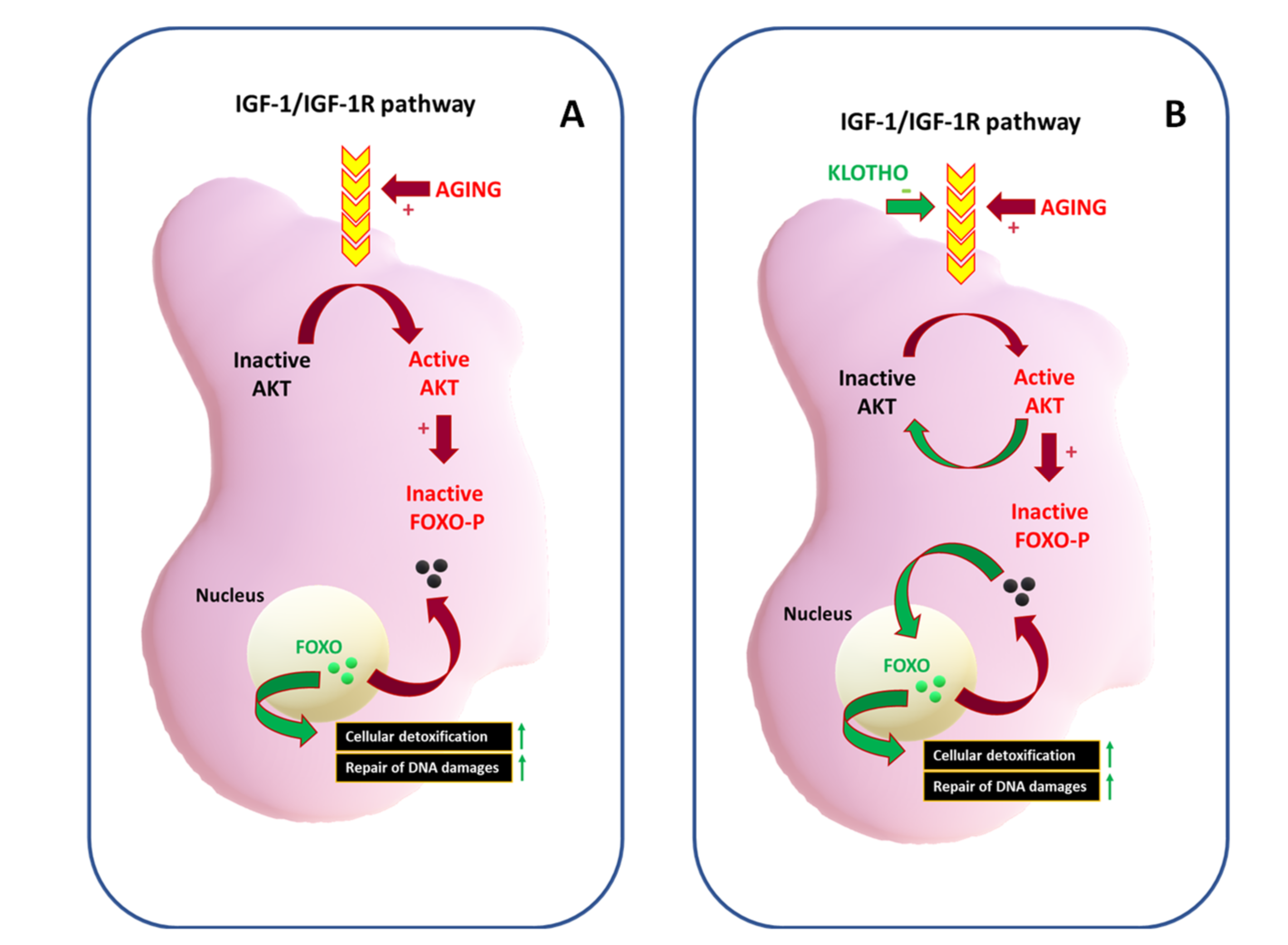
| Company/ Supplier | Product | Functionality Category | INCI | In Vitro/Ex vivo | In Vivo | Preservatives | Use Level | Formulation/ Processing | Features |
|---|---|---|---|---|---|---|---|---|---|
| Neurocosmetic ingredients for combating skin stress | |||||||||
| Provital Group | Agascalm | Skin protection. | Propanediol (and) glycerin (and) Agastache mexicana flower/leaf/stem extract. | Inhibition and release of chemokines (up to 104% in vitro). Reduction of the LKB protein degradation preventing the release of NF-κB, responsible for the transcription of proinflammatory agents; migration of the transcription factor NF-κB to the nucleus (up to 70% in vitro). | Skin redness reduction (−10%); vasodilation decrease (−30%); skin hydration improvement (+6%), TEWL (−13%). | None | 1–3% | Liquid; water-soluble. | Vascular tonicity improvement; restoration of the barrier function for moisturized skin; skin radiance enhancement and skin tone improvement. Suitable for sensitive skin. |
| Givaudan | Betaphroline | Body sculpting. | Aqua/water (and) butylene glycol (and) Tephrosia purpurea seed extract (and) phenoxyethanol. | Stimulation of β-endorphin release; adenylate cyclase activation; G3PDH inhibition; glycerol release. | Pain relief and improvement in the wellbeing of stomatodynia patients (oral application) (10%, D30; panelists’ evaluation. | Phenoxyethanol | 2–8% | Liquid; water-soluble. | Neurocosmetic and body shaping. |
| Mibelle Biochemistry | Happybelle-PE | Skin conditioning; anti-aging; antioxidant. | Happybelle-PE (standard version): lecithin (and) Vitex agnus-castus extract (and) glycerin (and) ascorbyl tetraisopalmitate (and) tocopherol (and) olus oil/vegetable oil (and) cyclodextrin (and) alcohol (and) aqua/ water. Happybelle O (oil-soluble version): Vitex agnus-castus extract (and) Helianthus annuus (sunflower) seed oil. | Stimulation of cell activity; increase in type I and III collagen (in vitro). | Increase in skin hydration and firmness; decrease in wrinkle depth. | None | 1–2% | Liquid; water-soluble. | Reduction of fine wrinkles and deeper lines; stimulation of collagen production; improvement in skin radiance; plumping and lifting effects. |
| Provital Group | Happy Skin | Anti-aging; antioxidant. | Glycerin (and) aqua/water, (and) Rhodiola rosea root extract (and) phenoxyethanol (and) potassium sorbate. Liposoluble version: Helianthus annuus (sunflower) seed oil (and) Rhodiola rosea root extract (and) Rosmarinus officinalis (rosemary) leaf extract. | Protection from free-radical-induced damage. | Increase in endorphin levels; enhancement of skin appearance. | Phenoxyethanol (and) potassium sorbate. No preservatives in the liposoluble version | 0.2–2.0% | Liquid; water-soluble. | Recommended to formulate facial, body, and hair care products. |
| Givaudan | Neurophroline | Skin protection. | Aqua/water (and) propanediol (and) Tephrosia purpurea seed extract. | Inhibition of stress hormone cortisol release; stimulation of β-endorphin production; global activation of antistress genes (transcriptomic on keratinocytes and fibroblasts); activation of antistress proteins HMOX1 and NQO1 (proteomic on keratinocytes and fibroblasts); increase in HMOX1 and NQO1 expression in the epidermis (ex vivo). | Efficacy on stressed (polluted air) skin: skin luminosity improvement; skin redness reduction; improvement of global skin color (when used at 2%); dark eye circle reduction. | None | 0.1–2% | Liquid; water-soluble. | Overall skin stress control. |
| Seppic | Sepicalm S WP | Protective agent; soothing agent; skin conditioning agent; skin moisturizing agent; sensorial modifier. | Sodium cocoyl amino acids (and) sarcosine (and) potassium aspartate (and) magnesium aspartate. | +48% production of ß-endorphins; Targets the key inflammation pathway (NF-κB): −32% of IL-6/-16% of IL-8; −78% of free radical induction (superoxide anion); −80% elastase inhibition; −100% hyaluronidase inhibition; −80% lipoxygenase inhibition. | Benefits on volunteers: skin water content is increased for up to 5 h after application (+15%); −7.4% redness after mechanical abrasion; −25% redness after solar erythema; −46% stinging sensation in 10 s. | None | 3% | Liquid; water-soluble. | Increased skin comfort for sensitive skin; suitable for sensitive skin. |
| Seppic | Sepicalm VG WP | Skin lightening agent; soothing care agent. | Sodium palmitoyl proline (and) Nymphaea alba flower extract. | Double skin lightening action: inhibition of inflammatory pigmentation induced by cutaneous stress, and modulation of gene expression in the basal pigmentation (MIC1, MITF, tyrosinase) after 16 h. | Benefits on volunteers: 90% lighter skin; 91% more radiant; 94% lighter spots; 73% smaller spots. | None | 3% | Liquid; amphiphilic. | Suitable for sensitive skin. |
| Neurocosmetic ingredients for sensitive skin | |||||||||
| Rahn | Defensil SOFT | Anti-aging; anti-inflammation; anti-redness; antistress; couperose; soothing. | Propanediol (and) Albatrellus ovinus extract (and) citric acid. | Affinity to TRPV1 (in vitro); Functionally blocks the TRPV1 receptor (in vitro); Neuron-driven inflammation (in vitro) counteraction. | Reduction of skin discomfort and irritation; protection from heat stress and soothing of hyperalgesic skin; prevention of IR-induced barrier damage (in vivo). | None | 1–3% | Liquid; water-soluble. Processing: incorporation at temperatures < 50 °C. | Increase in the overall tolerance of the skin to irritants; prevention of the sensitization of TRPV1 receptors by blocking serotonin receptors; prevention of IR-aging by protecting from IR-induced heat stress. |
| Givaudan | Mariliance | Skin protection. | Aqua/water (and) propanediol (and) Rhodosorus marinus extract. | Reduction of TRPV1 expression; inhibition of the expression of IL-1α, NRG, and NGF R, and reduction of NGF production; reduction of IL-1α synthesis; penetration of Mariliance in emulsion and validation of the results observed in vitro on IL-1α production (ex vivo). | Reduction of skin sensitivity (3%). | None | 1–3% | Liquid; water-soluble. Processing: incorporation at the end of the formulation under stirring or homogenization; can be heated for a short time < 80 °C. pH usage range: 2.0–10.0. | Neuro-soother; calming, anti-tightening. |
| Lucas Meyer Cosmetics | Neutrazen | Soothing agent. | Water (and) butylene glycol (and) dextran (and) palmitoyl tripeptide-8. | Biomimetic of neuromediator (POMC) :reduction of the consequences of substance P release; inhibition of the release of IL-1-induced IL-8 production (in vitro). | Reduction of sensitive skin symptoms related to cutaneous neurogenic inflammation. | None | 0.3–2.5% | Liquid; water-soluble; colorless; pH usage range: 5.2–7.2. | Designed to prevent and reverse signs of neurogenic inflammation (redness and swelling); calms and soothes irritated skin caused by UV, immune reactions, and mechanical stress; helps to maintain and restore a normal skin sensitivity threshold; suitable for sensitive and intolerant skin. |
| Givaudan | Ocaline PF | Skin protection. | Maris aqua (and) aqua/water (and) Cucurbita pepo seed extract (and) potassium sorbate (and) phenoxyethanol (and) citric Acid. | Inhibition of the mast cells degranulation (ex vivo) and histamine release induced by substance P. | Preventive effect on sensitive skin/stinging test (5%); soothing of irritated skin (10%, 60 min, 180 min, 24 h). | Potassium sorbate (and) phenoxyethanol | 3–10% | Liquid; water-soluble. | Prevention of sensitive skin by a neurocosmetic approach. |
| Givaudan | Ocaline XP | Skin protection. | Maris aqua (and) aqua/water (and) Cucurbita pepo seed extract (and) citric acid (and) benzyl alcohol (and) potassium sorbate. | Inhibition of the mast cells’ degranulation (ex vivo). | Preventive effect on sensitive skin/stinging test (5%); soothing of irritated skin (10%, 60 min, 180 min, 24 h). | Benzyl alcohol (and) potassium sorbate | 3–10% | Liquid; water-soluble. | Prevention of sensitive skin by a neurocosmetic approach; soothes away neurogenic inflammation. |
| Mibelle Biochemistry | Pinolumin | Antioxidant; energizing; radiance; anti-redness; whitening. | Standard version: Pinus cembra wood extract (and) alcohol (and) pentylene glycol (and) aqua/ water; Alcohol-free powder version, twofold concentrated: Pinus cembra wood extract (and) maltodextrin (and) aqua/water. | Inhibition of neuroinflammation (in vitro); inhibition of the function of TRPV1 (in vitro); anti-inflammatory activity: inhibition of the release of proinflammatory mediators (CGRP, IL-8, and PGE2) (in vitro); protection of collagen by inhibiting the UVA-induced MMP-1 production (in vitro). | Visible anti-redness effect; reduction of color irregularities; overall improvement of skin tone evenness. | None | 2% | Liquid; water-soluble. Incorporation: For cold processes, dissolve Pinolumin into the aqueous phase. In hot/cold processes, add during the cooling phase below 40 °C. Thermostability: temperatures of up to 40 °C for a short time will not affect the stability of Pinolumin. | Calms sensitive and irritated skin; visibly reduces redness and age spots; protects from the effects of environmental stress; suitable for sensitive skin. |
| Mibelle Biochemistry | SensAmone P5 | Skin conditioning. | Pentapeptide-59 (and) hydrogenated lecithin (and) Butyrospermum parkii (shea) butter (and) phenethyl alcohol (and) ethylhexylglycerin (and) maltodextrin (and) aqua/water. | Inhibition of TRPV1 receptor activation (in vitro). | Instant reduction of skin reactivity following a single application; reduction of skin sensitivity. | None | 1–2% | Liquid; water-soluble. | Calms over-reactive skin; minimizes skin’s response to stress; reduces the itching sensation of sensitive skin; suitable for sensitive skin. |
| Givaudan | Sensityl | Skin protection. | Water (and) Phaeodactylum tricornutum extract (and) pentylene glycol. | Epigenetic control of inflammation through exosomal communication; genetic control of skin soothing and calming (downregulation of a complete set of genes involved in anti-inflammatory and soothing activities); control of the acute phase of inflammation (reduction of immune cell recruitment); inhibition of immune cell recruitment (significant reduction of dendritic cells) (ex vivo 3%); reduction of proinflammatory signal through IL-8 inhibition (ex vivo 3%); reduction of pain sensation through TRPV1 expression reduction (ex vivo 3%); control of microbial proliferation (ex vivo 3%). | Protection of skin microbiota against sensitive skin conditions (3% vs. placebo), restoration and protection of sensitive skin (3%); improvement of cutaneous reactivity (3%). | Sodium benzoate (and) benzoic acid | 1–3% | Liquid; water-soluble. | Soothing; calming; neuro-soothing; skin microflora rebalancing. |
| BASF | Skinasensyl LS 9749 | Soothing agent. | Liquid synthetic tetrapeptide: water (and) glycerin (and) coco-glucoside (and) acetyl tetrapeptide-15. | Binding on the µ-opioid receptor (in vitro); inhibition of the CGRP release by sensory neurons (in vitro). | Increase of the skin’s tolerance threshold. | Sorbic acid | 1–3% | Liquid; water-soluble. Processing: it is incorporated into the finishing process below 50 °C, or at room temperature for cold processing; colorless. | Inhibits the release of the CGRP neuromediator from sensory neurons; increases the skin tolerance threshold; specifically designed to soothe sensitive skin. |
| BASF | Skinasensyl LS 9852 | Soothing agent. | Powder synthetic tetrapeptide: mannitol (and) sodium citrate (and) acetyl tetrapeptide-15. | Binding on the µ-opioid receptor (in vitro); inhibition of CGRP release by sensory neurons (in vitro). | Increase of the skin’s tolerance threshold (capsaicin, heat) (in vivo). | None | 0.3–1.0% | Powder; water-soluble; colorless. | Inhibits the release of the neuromediator CGRP from sensory neurons; increases the skin tolerance threshold; specifically designed to soothe sensitive skin. |
| Seppic | Sepicalm S WP | Protective agent; soothing agent; skin conditioning agent; skin moisturizing agent; sensorial modifier. | Sodium cocoyl amino acids (and) sarcosine (and) potassium aspartate (and) magnesium aspartate. | +48% production of ß-endorphins; Targets the key inflammation pathway (NF-κB): −32% of IL-6/−16% of IL-8; −78% of free radical induction (superoxide anion); −80% elastase inhibition; −100% hyaluronidase inhibition; −80% lipoxygenase inhibition. | Benefits on volunteers: skin water content is increased for up to 5 h after application (+15%); −7.4% redness after mechanical abrasion; −25% redness after solar erythema; −46% stinging sensation in 10 s. | None | 3% | Liquid; water-soluble. | Increase skin comfort for sensitive skin; suitable for sensitive skin. |
| Seppic | Sepicalm VG WP | Skin lightening agent; soothing care agent. | Sodium palmitoyl proline (and) Nymphaea alba flower extract | Double skin lightening action: inhibition of inflammatory pigmentation induced by cutaneous stress, and modulation of gene expression in the basal pigmentation (MIC1, MITF, tyrosinase) after 16 h. | Benefits on volunteers: 90% lighter skin, 91% more radiant, 94% lighter spots, 73% smaller spots. | None | 3% | Liquid; Amphiphilic | Suitable for sensitive skin |
| “Coolant” neurocosmetic ingredients for sensitive skin | |||||||||
| Givaudan | Evercool Skin | Cooling. | Menthyl PCA (and) Lactamide MEA (and) menthane carboxamide ethylpyridine. | Activation of cooling sensation receptor TRMP8. | Long-lasting cooling effect (2%, 30 min, 60 min, 120 min). | None | 1–4% | Water-soluble; soluble in alcohol; odorless. | Patented combination of Givaudan cooling activities; improved, long-lasting freshness. |
| Salvona | HydroSal SalCool | Cooling; delivery systems/carriers. | Propylene glycol (and) hydroxypropyl Cellulose (and) methyl diisopropyl propionamide (and) menthyl lactate (and) ethyl menthane carboxamide (and) phenethyl alcohol (and) caprylyl glycol (and) ethylhexylglycerin. | The release kinetics of the ingredients are optimized and synchronized to result in continuous, long-lasting, enhanced refreshment. | In a consumer perception test, the intensity of the sensation from an aftershave balm containing HydroSal™ SalCool was compared to an aftershave balm containing free menthol at 1% loading: HydroSal™ SalCool lasted twice as long as the freementhol at the same strength. | None | 7.0–15.0% | Water-soluble; compatible with hydro-alcoholic formulations; odorless. Processing: incorporation to the water phase during production, or at the end of the process when the temperature is below 30 °C. | Suitable for sensitive skin; it can be incorporated in skin, lip, scalp, and hair care formulations. |
| Neurocosmetic ingredients for skin aging | |||||||||
| Lucas Meyer Cosmetics | Adipofill’in | Anti-aging; anti-wrinkle; skin conditioning; delivery systems/ carriers. | Aqua/water (and) propanediol (and) ornithine (and) phospholipids (and) glycolipids. | Activation of HIF-1α; increase in lipid storage in human adipocytes; decrease in fatty acids released from adipocytes. | Lipofilling-like effect; decrease in nasogenian fold depth and skin roughness. | None | 0.5–2% | Liquid; water-soluble; pH usage range: 4.5–6.0. Incorporation/processing: at the end of the formulation (< 40˚C). | Skin smoother, firmer, less tired, plumper and lifted; reduction of nasogenian folds. |
| Biosil Technologies | Glistin | Anti-aging; antioxidant | Glutamylamidoethyl indole (and) aqua/water | Neurotrophic effect (NGF-like); neuro-cutaneous messenger; cutaneous neuroprotection: anti-apoptotic effect. | «Antistress» (cutaneous sensitivity). | None | 1% | Liquid; water-soluble; miscible with alcohols and glycols; colorless; pH usage range: 7.0. | Prevention of neurodegeneration. |
| Biosil Technologies | Glutrapeptide | Slimming and firming agent; anti-aging; antioxidant. | Pyroglutamylamidoethyl indole (and) butylene glycol. | Enhancement of neurons’ ability to support lipolysis; synergy with caffeine for slimming effect; enhancement of fibroblast contractile ability together with NGF. | Skin appearance, firmness, density, and elasticity improvement after 28 days of treatment; amelioration of cellulite appearance after 28 days of treatment; overall slimming effect up to 4 cm for the arms, 6 cm for the waist, 4 cm for the abdomen, and 3.5 cm for the thighs. | None | 1–5% | Liquid; water-soluble; colorless; pH usage range: 6.0; miscible with alcohols and glycols. | Neuroprotection; neuroslimming; body firming; cell detoxification; prevention of neurodegeneration. |
| Codif Technologie Naturelle | Lakesis | Anti-aging; firming. | Caprylic/capric triglyceride (and) Pistacia lentiscus (mastic) gum. | Activation of youth proteins KLOTHO and FOXO; reactivation of cellular detoxification processes; improvement of cellular activity; improvement of type I collagen synthesis by 47%; reactivation of fibrillin synthesis. | Redensification of the dermis; improvement of facial contours. | None | 0.2% | Liquid; liposoluble. Processing: stable up to 80 °C. | Lifting action on the oval face. |
| BASF | Neurobiox | Anti-wrinkle; anti-aging; brightening agent. | Water (and) butylene glycol (and) pentylene glycol (and) Achillea millefolium extract (and) xanthan gum. | Improvement of the epidermal thickness (ex vivo); stimulation of epidermal differentiation (ex vivo); stimulation of synthesis: filaggrin, cytokeratin 10, MO-R1, and MC-2R receptors (ex vivo); dose-effect on the gene expression of MC-2R and MOR-1 receptors; effect on the protein synthesis of MC-2R and MOR-1; Stimulation of involucrin gene expression (in vitro). | Pore refining; skin brightening; skin softness; stimulation of epidermal renewal; wrinkle smoothening. | None | 0.5–2% | Liquid; water-soluble; colorless; low-pH tolerant; high-pH tolerant; cation compatible. | Support of cell communication by stimulating the expression of POMC-related receptors; improvement of epidermal differentiation; acceleration of skin renewal; improvement of skin softness, radiance, and visible reduction of the appearance of wrinkles and pores. |
| Codif Technologie Naturelle | Neuroguard | Anti-aging; anti-wrinkle. | Glycerin (and) aqua/water (and) hydrolyzed algin | Increase in the production of neuroprotector sAPPα by 87% (in vitro); protects the communication between nerves and fibroblasts from the toxicity of neuro-aging and preserves fibroblasts’ activity (in vitro); protection of the extracellular matrix from the toxicity of neuro-aging (in vitro); protection of the synthesis of collagen and elastin from neuro-aging toxicity (in vitro). | Reduce the volume and area of crow’s feet wrinkles. | Neuroguard P: phenoxyethanol; Neuroguard G: preservative-free. | Neuroguard P: 1.5%; Neuroguard G: 3% | Liquid; Water-soluble; Processing: incorporation at less than 50 °C. | Protection of neurons from neuro-aging; prevention from fibro-aging; decrease in wrinkle depth. |
| Codif Technologie Naturelle | Neurolight.61G | Skin lightening. | Glycerin (and) water (and) Pancratium maritimum extract. | Inhibition of POMC expression and intracellular melanin synthesis (in vitro); reduction of the melanocyte dendricity and the synthesis of receptors for substance P (in vitro); inhibition of the effects of substance P on the export of melanin (in vitro); inhibition of the synthesis and export of melanin (in vitro). | Reduction of the surface of the pigment spots; clarification of the pigmentation of dark spots without lightening the pigmentation of the skin. | None | 1.5% | Liquid; water-soluble. | Neurocosmetic treatment of dark spots. |
| Lucas Meyer Cosmetics | Progeline | Anti-aging. | Glycerin (and) water (and) dextran (and) trifluoroacetyl tripeptide-2. | Reduction of progerin synthesis; inhibition of MMPs and elastase; increase in syndecans. | Improvement of cutaneous elasticity up to 93%; improvement of cutaneous firmness up to 82%; improvement of cutaneous viscoelasticity up to 42%. | None | 0.5–2% | Liquid; Water-soluble; Odorless; Colorless; pH usage range: 4–6; Processing: incorporation at the end of the formulation at a temperature of < 40 °C. | Exhibition of a remodeling effect and reduction of signs of aging: sagging and wrinkles; lifting effect on jawlines. |
| Neurotransmitter-Affecting Peptides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Company/ Supplier | Product | Functionality Category | INCI Name | In vitro/Ex vivo | In vivo | Preservatives | Use Level | Formulation/ processing | Features |
| DSM | Vialox | Anti-aging; anti-wrinkle; myorelaxant agent. | Pentapeptide-3. | Competitive antagonist effect towards the acetylcholine receptors coupled with the enkephalin receptor and closure of the calcium channels, leading to muscle relaxation (in vitro); reduction of muscle contractions by 71% within 1 min after treatment, and 58% 2 h later (in vitro). | Decrease in wrinkle size (−49%) and skin roughness (−47%) after 28 days of application. | None | - | Powder; water-soluble. | A topical alternative to muscle relaxing injections; an intensive treatment for fighting expression lines. |
| Galena; Lipotec | Vanistryl | Firming agent; delivery systems/carriers. | Water/aqua (and) caprylyl/capryl glucoside (and) lecithin (and) glycerin (and) Pseudoalteromonas ferment extract (and) acetyl tripeptide-30 citrulline (and) pentapeptide-18 (and) xanthan gum (and) caprylyl glycol (and) potassium sorbate (and) phenoxyethanol. | Inhibition of extracellular matrix (ECM) degradation (in vitro); modulation of acetylcholine release from neuron cell cultures by preventing the entry of Ca2+ into the neuron: attenuation of muscle contraction and relaxation of the muscle tissue surrounding striae (in vitro); increases dermal protein synthesis: type I collagen raised to 128%, type IV to 81%, and elastin to 31%, in 15 days (in vitro); increases fibroblast adhesion (125% in 5 h) and keratinocyte growth (36% after 48 h) (in vitro); enhancement of wound healing effect on keratinocytes (in vitro). | Improvement of the skin surface, dryness, and firmness; decrease in erythema (−18%); attenuation of stretch marks; improvement of the color of stretch marks. | Phenoxyethanol (and) potassium sorbate | 5% | Liquid; Water-soluble. Incompatibilities: oxidants and electrophiles. Processing: Incorporate in the aqueous phase in the final step of the manufacturing process. In the case of emulsion preparation, it should be added once the emulsion is formed. Generally, add at a temperature below 40 °C; pH usage range: 4.0–8.0. | Maintenance and improvement of the dermal structure; regeneration of thethe epidermis, reconstruction of the connective tissue by protecting and boosting dermal proteins; increase in skin elasticity. |
| Topical peptidomimetic ingredients as alternatives to Botox | |||||||||
| Lipotec | Argireline | Anti-aging; anti-wrinkle; myorelaxant agent. | Aqua/water (and) acetyl hexapeptide-8 (and) caprylyl glycol. | Modulation of SNARE complex formation (in vitro); modulation of catecholamine release in chromaffin cells (in vitro). | Reduction of expression of wrinkles (volume by 20.6% and length by 15.9%) in just 1 week, with a solution of Argireline peptide (2%). | None | Max 10% | Liquid; water-soluble; colorless. Processing: incorporation at the final stage of the manufacturing process, at a temperature below 40 °C.pH usage range: 3.0–6.0. | Reduction of wrinkle depth caused by the contraction of muscles of facial expression, especially on the forehead and around the eyes, after 1 week. |
| Lipotec | Argirelox | Anti-aging; anti-wrinkle; myorelaxant agent. | Aqua/water (and) glycerin (and) acetyl hexapeptide-8 (and) pentapeptide-18 (and) citric acid (and) caprylyl glycol. | Modulation of glutamate release (in vitro). | Reduction of expression lines in frontal and periorbital regions, prolonging the effects of BoNT-A even after 6 months. | None | 10% | Liquid; water-soluble. Incompatibilities: oxidants and electrophiles. Processing: incorporation in the aqueous phase of emulsions and gels in the final step of the manufacturing process.When preparing the emulsion, it should be added once the emulsion is formed at a temperature below 40 °C;colorless; pH usage range: 3.0–8.0 | Muscle relaxation by modulating acetylcholine (ACh) release; smoothing effect on expression lines by modulating the SNARE complex and calcium channels; reduction of reappearance of expression lines in the crow’s feet area. |
| Infinitec | BONT-L Peptide Solution | Anti-aging; anti-wrinkle; myorelaxant agent. | Water/aqua (and) palmitoyl hexapeptide-19. | SNARE protein complex inhibition by 30%. | A cream containing 5% BONT-L Peptide was tested for 28 days on 15 volunteers; an average reduction of 38% of macro-relief of human skin. | Phenoxyethanol | 3–5% | Liquid; water-soluble. | Muscles’ relaxation by minimizing the release of acetylcholine, and consequent wrinkle reduction, in 4 weeks. |
| Infinitec | BONT-L Peptide Powder | Anti-aging; anti-wrinkle; myorelaxant agent. | Dextran (and) palmitoyl hexapeptide-19. | SNARE protein complex inhibition by 30%. | A cream containing 5% BONT-L Peptide was tested for 28 days on 15 volunteers; an average reduction of 38% of macro-relief of human skin. | None | 3–5% | Powder; water-soluble. | Muscles’ relaxation by minimizing the release of acetylcholine, and consequent wrinkle reduction, in 4 weeks. |
| Sederma | Calmosensine | Anti-Aging; anti-wrinkle; skin moisturizer; soothing agent; smoothing agent;cooling agent. | Butylene glycol (and) aqua/water (and) laureth-3 (and) hydroxyethyl cellulose (and) acetyl dipeptide-1 cetyl ester. | Stimulation of the release of pro-endorphins by keratinocyte; progressive reduction in muscle contraction, with levels as low as 1 ppm leading to total muscular inhibition within 2 h (in vitro). | Modulation of the cutaneous perception to lessen unpleasant sensations; reduction of the perception of heat. | None | 3% | Liquid; water-soluble. Processing: incorporation at room temperature. | Enhancement of skin comfort; relief of tension to help prevent the onset of wrinkles and expression lines. Suitable for sensitive skin |
| Lipotec | Inyline | Anti-aging; anti-wrinkle; myorelaxant agent. | Aqua/water (and) acetyl hexapeptide-30 (and) arginine (and) caprylyl glycol. | Reduction of AChR clustering, a key step in the post-synaptic functionality of the neuromuscular junction (NMJ) (in vitro). | Decrease in wrinkle depth of up to 14.9%. | None | 5% | Liquid; water-soluble. | Attenuation of expression wrinkles. |
| Lipotec | Leuphasyl | Anti-aging; anti-wrinkle; myorelaxant agent. | Aqua/water (and) glycerin (and) pentapeptide-18 (and) caprylyl glycol. | Modulation of glutamate release in a neuron cell culture(in vitro). | Decrease in depth of expression wrinkles (−11.64%). | None | 3–10% | Liquid; water-soluble; colorless; pH usage range: 2.0–5.0.Processing: incorporation at the final stage of the manufacturing process at a temperature below 40 °C. | Reduction of wrinkle depth caused by the contraction of muscles responsible for facial expression, especially in the forehead and around the eyes; has a synergistic effect with Argireline. |
| Lipotec | Snap 8 | Anti-aging; anti-wrinkle; myorelaxant agent. | Aqua/water (and) acetyl octapeptide-3 (and) caprylyl glycol. | Inhibition of SNARE complex formation (in vitro); modulation of catecholamine release in chromaffin cells (in vitro); modulation of glutamate release in a neuron cell culture (in vitro). | Wrinkle depth reduction (63%). | None | 3–10% | Liquid; water-soluble; colorless. | By reducing muscle contractions, depth of fine lines and expression wrinkles is reduced within one month of treatment. |
| DSM | Syn-ake | Anti-aging; anti-wrinkle; smoothing agent. | Glycerin (and) aqua/water (and) dipeptide diaminobutyroyl benzylamide diacetate. | Reversible antagonist of the muscular nicotinic acetylcholine receptor (mnAChR) (in vitro); inhibition of the Na+ uptake at the postsynaptic membrane to attenuate muscle cell contractions (in vitro); mimics/mimic waglerin-1 functionality. | Wrinkle reduction up to 52% after 28 days; measurable smoothing effect on 80% of the volunteers; measurable wrinkle reduction on 73% of the volunteers; reduction of wrinkle depth; smoothing of crow’s feet lines and forehead wrinkles. | None | 1–4% | Liquid; water-soluble. | Reduction of the appearance of mimic wrinkles. |
| Infinitec | X50 Myocept | Anti-aging; anti-wrinkle; myorelaxant agent; cosmetic drone. | Powder form: lactic acid/glycolic acid copolymer (and) palmitoyl hexapeptide-52 (and) polyvinyl alcohol (and) palmitoyl heptapeptide-18.Liquid suspension: water/aqua (and) xanthan gum (and) lactic acid/glycolic acid copolymer (and) palmitoyl hexapeptide-52 (and)polyvinyl alcohol (and) palmitoyl heptapeptide-18. Additives: phenoxyethanol (and) caprylyl glycol (and) glycerin (and) glyceryl caprylate (and) phenylpropanol. | Reduction of the entry of calcium into the neuron, and inhibition of the formation of the SNARE protein complex; reduction of the neuronal exocytosis. | −20% wrinkle depth and length reduction in just 4 weeks. | Phenoxyethanol | 1% | Powder/liquid suspension; water-soluble. | Reduction of the expression lines. |
| Plant extract alternatives to Botox | |||||||||
| Gattefossé | Gatuline Expression | Anti-aging; anti-wrinkle; myorelaxant agent; smoothing agent. | Alcohol (and) water (and) Acmella oleracea extract. | Inhibition of muscle contractions (in vitro). | Smoothing efficacy on crow’s feet wrinkles. | None | 2–5% | Liquid; water-soluble; insoluble in oils; color: yellow to amber; odor: characteristic; pH usage range: 5.0–7.0. Processing: incorporation at cold temperatures. | Improvement of the eye contour by reducing expression lines and wrinkles. |
| BASF | Myoxinol | Anti-aging; Botox-like agent. | Hydrolyzed Hibiscus esculentus extract (and) dextrin. | Inhibition of muscle cell contraction; free radical scavenging. | Wrinkle smoothening (1%). | None | 0.5–2% | Powder; water-soluble; insoluble in oils and fats; color: beige to pale yellow; odor: characteristic; pH usage range: 5.0–7.0. Processing: incorporate below 50 °C during the finishing process, or at room temperature for cold processing. | Anti-oxidant; smooths expression lines. |
| Codif Technologie Naturelle | Stoechiol | Anti-aging; anti-wrinkle; Botox-like agent. | Caprylic/capric triglyceride (and) Lavandula stoechas | Wrinkle relaxation; stimulation of the division of keratinocytes in the basal layer to restore the density of the epidermis; promotes the production of the lipid cement involved in the restructuring of the stratum corneum. By increasing the expression of the adhesion proteins, Stoechiol increases the formation of inter-corneocyte links, promoting the reorganization of the corneocytes and, therefore, the cohesion of the corneal layer. Immobilization of the wrinkle coupled with a re-densifying and smoothing effect of the epidermis leads to a rapid and lasting reduction in the depth of expression lines. | Immediate anti-age effect from 0.25%. Within just 1 h, the amplitude of the cutaneous relief is smoothed; after 24 h wrinkles are less visible; 7 days after the end of treatment, wrinkles are visibly attenuated. An immediate anti-wrinkle action coupled with a long-term anti-age effect that continues beyond the end of treatment. | None | 0.25% | Liquid; liposoluble. | Reversible and repetitive inhibition of muscle contractions; redensifying and restructuring action on the epidermis; immediate and long-term reduction in the main wrinkle; immediate and long-term smoothing action on the roughness of the skin; decrease in the expression lines (visible within 24 h). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. https://doi.org/10.3390/cosmetics8030066
Rizzi V, Gubitosa J, Fini P, Cosma P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics. 2021; 8(3):66. https://doi.org/10.3390/cosmetics8030066
Chicago/Turabian StyleRizzi, Vito, Jennifer Gubitosa, Paola Fini, and Pinalysa Cosma. 2021. "Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation" Cosmetics 8, no. 3: 66. https://doi.org/10.3390/cosmetics8030066
APA StyleRizzi, V., Gubitosa, J., Fini, P., & Cosma, P. (2021). Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics, 8(3), 66. https://doi.org/10.3390/cosmetics8030066









