Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Chromatographic Conditions
2.3. Preparation of Standard Solutions
2.4. Method Validation
2.5. Selection and Overview of the Analyzed Commercial Cosmetic Products
2.6. Analysis of the Commercial Cosmetic Products
2.6.1. Sample Preparation for the Analysis of Vitamin E and Coenzyme Q10
2.6.2. Sample Preparation for the Analysis of Vitamin A
2.6.3. Quantification of Vitamins A and E and Coenzyme Q10
3. Results
3.1. Validation of the HPLC–UV Methods
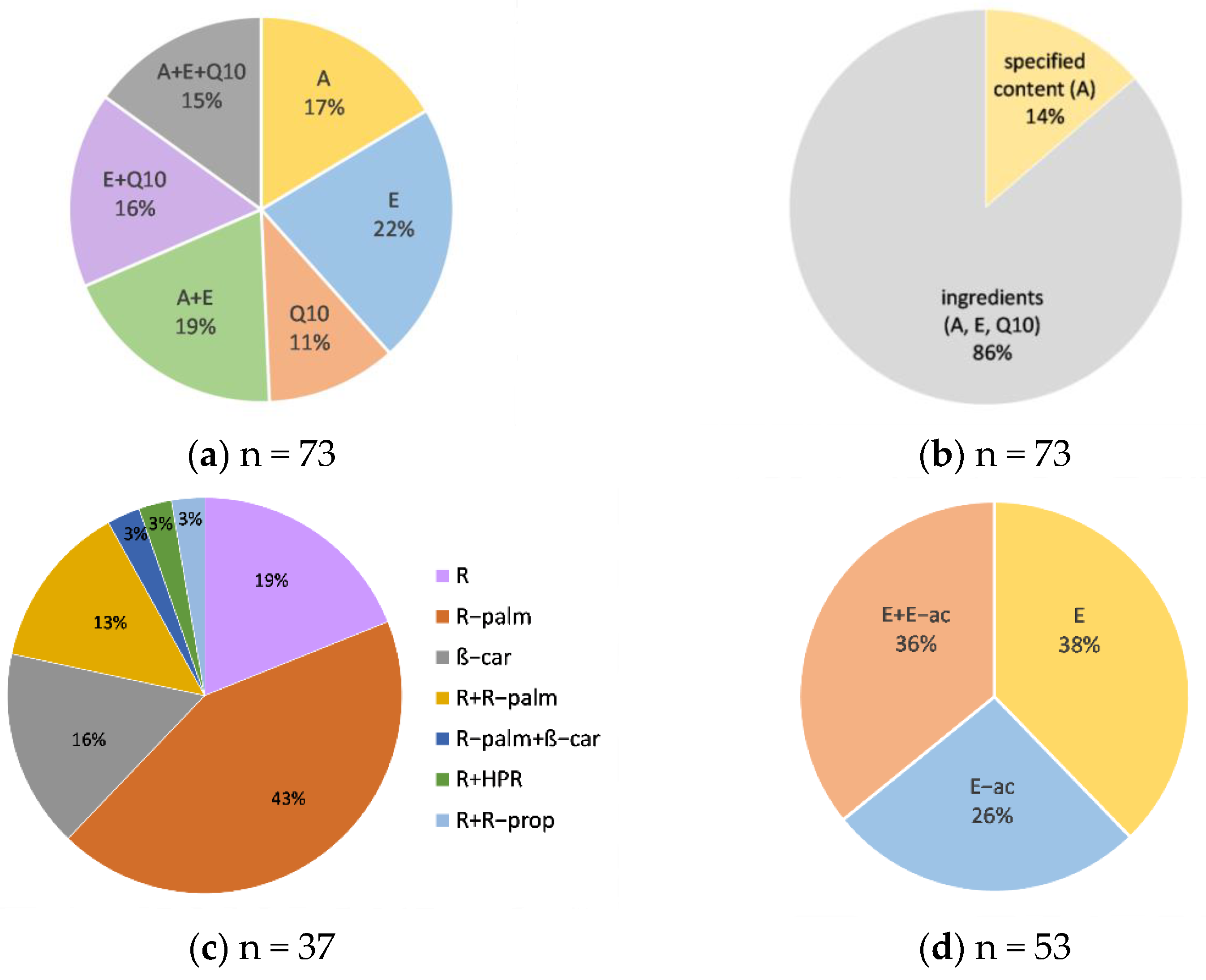
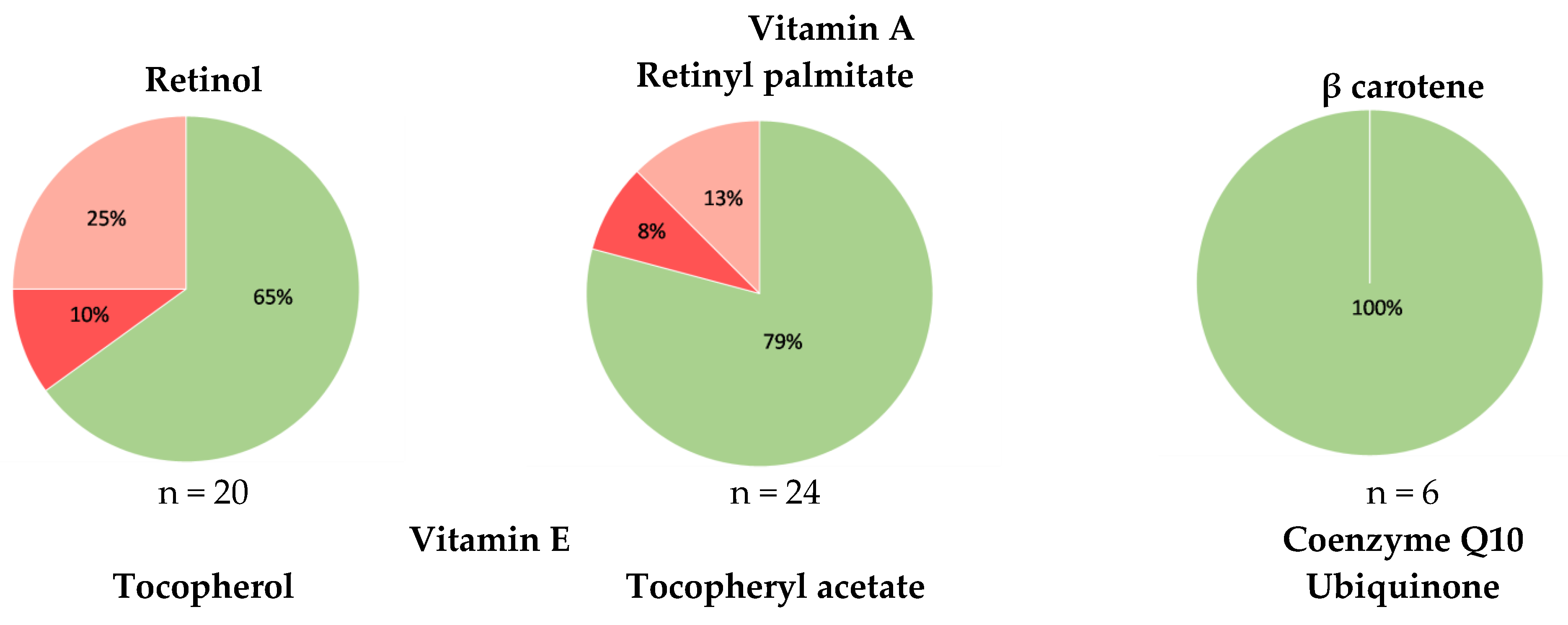
3.2. Overview of the Tested Cosmetics
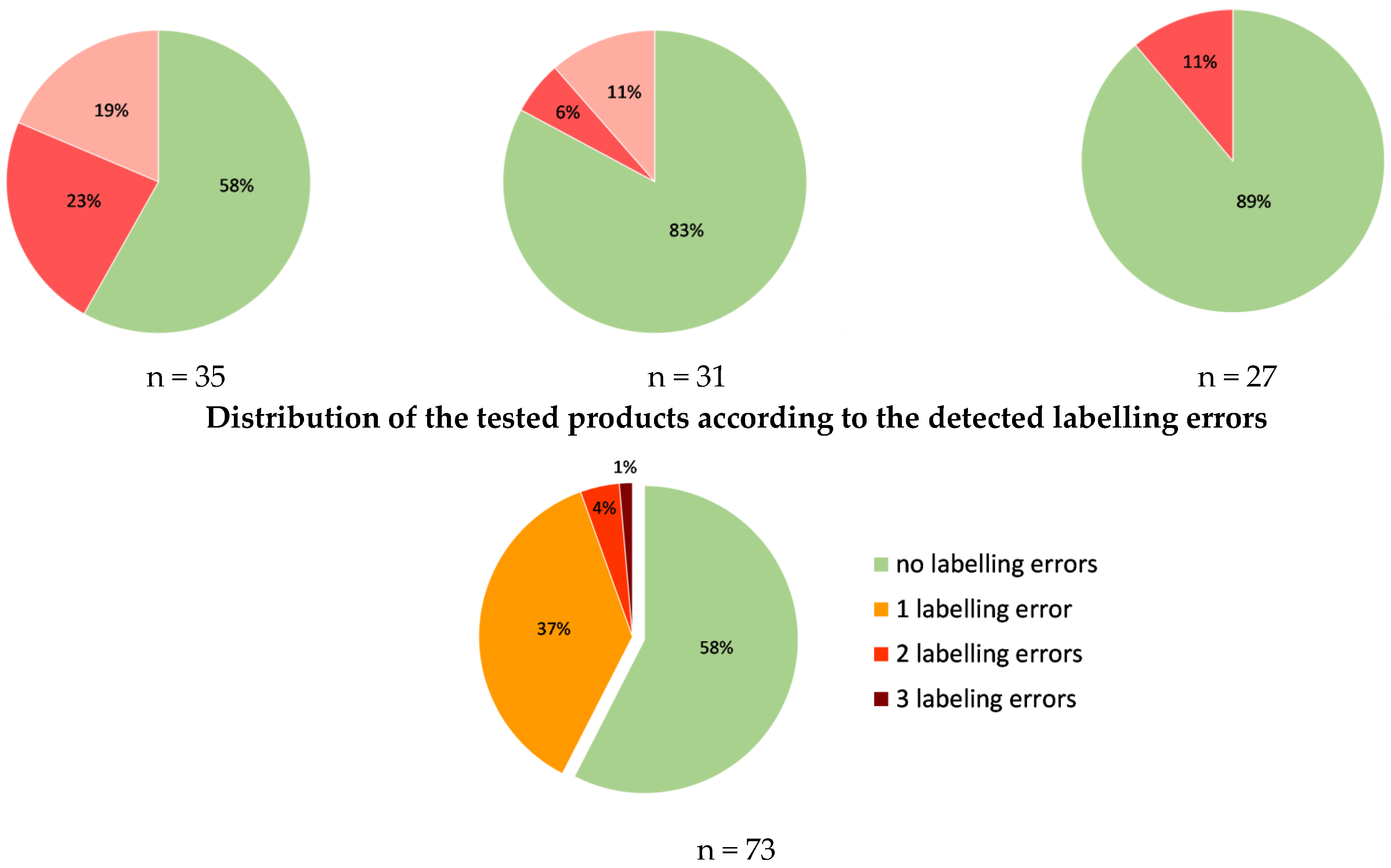
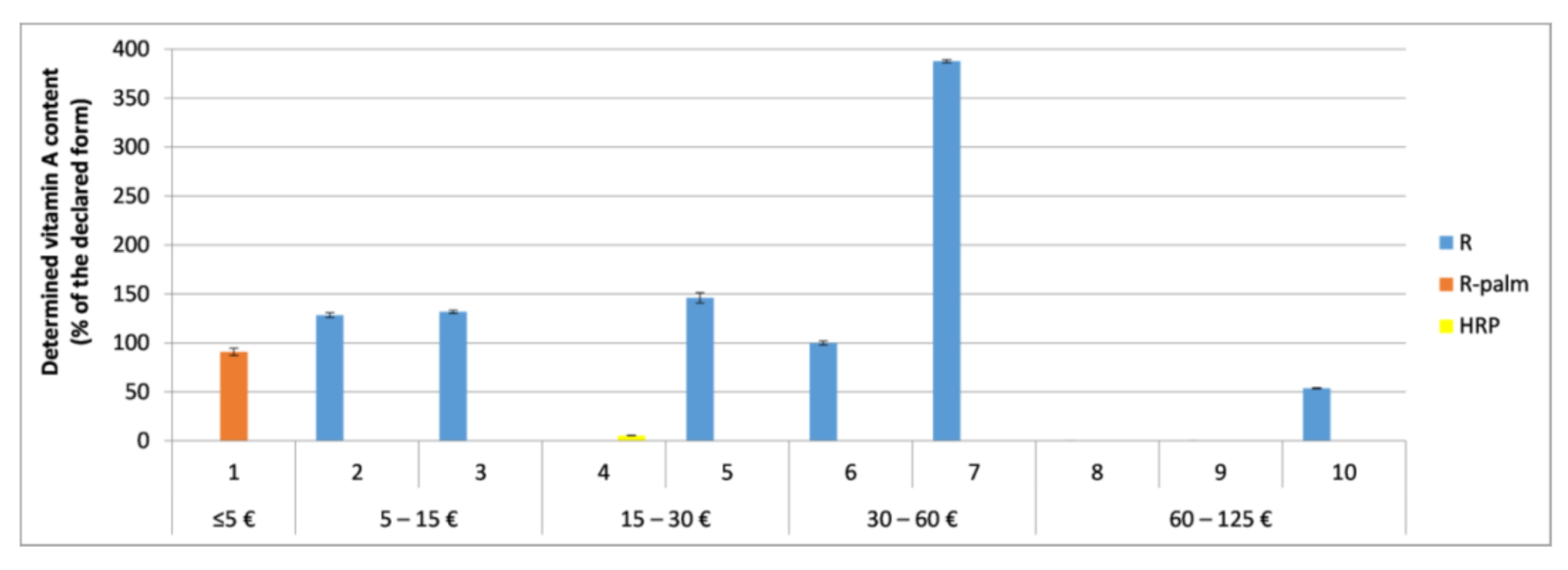
3.3. Accuracy of the Labeling of Vitamins A and E and Coenzyme Q10
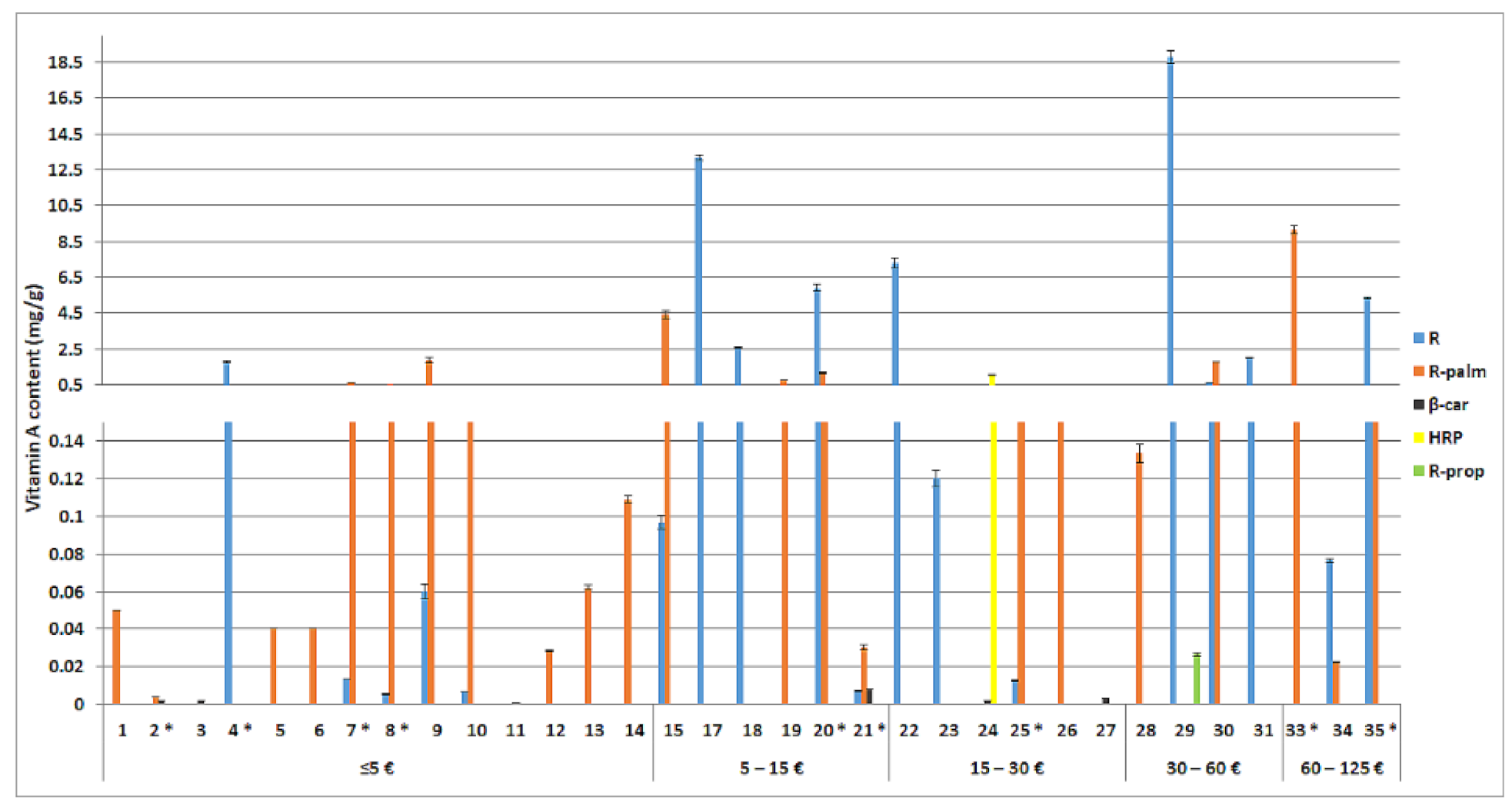
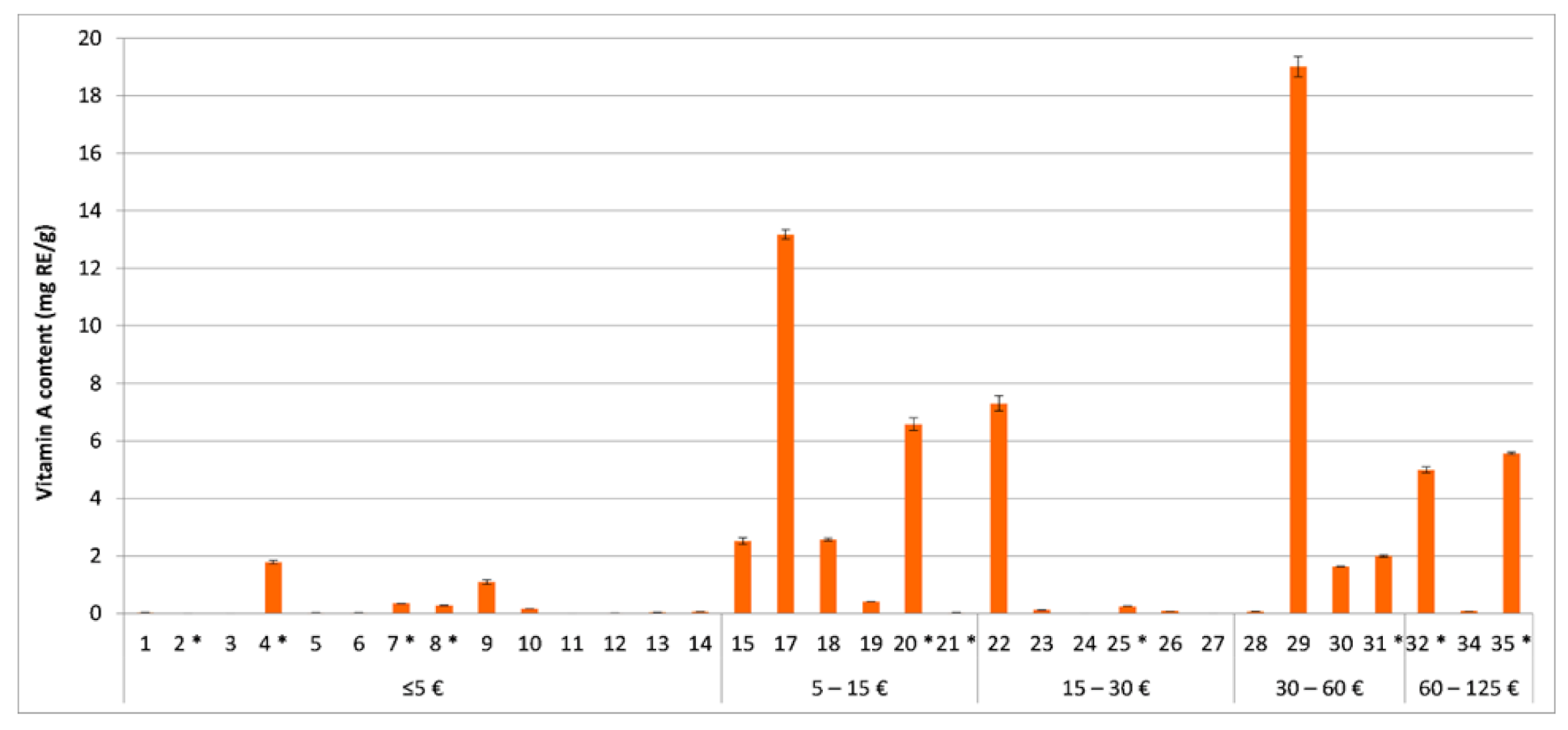
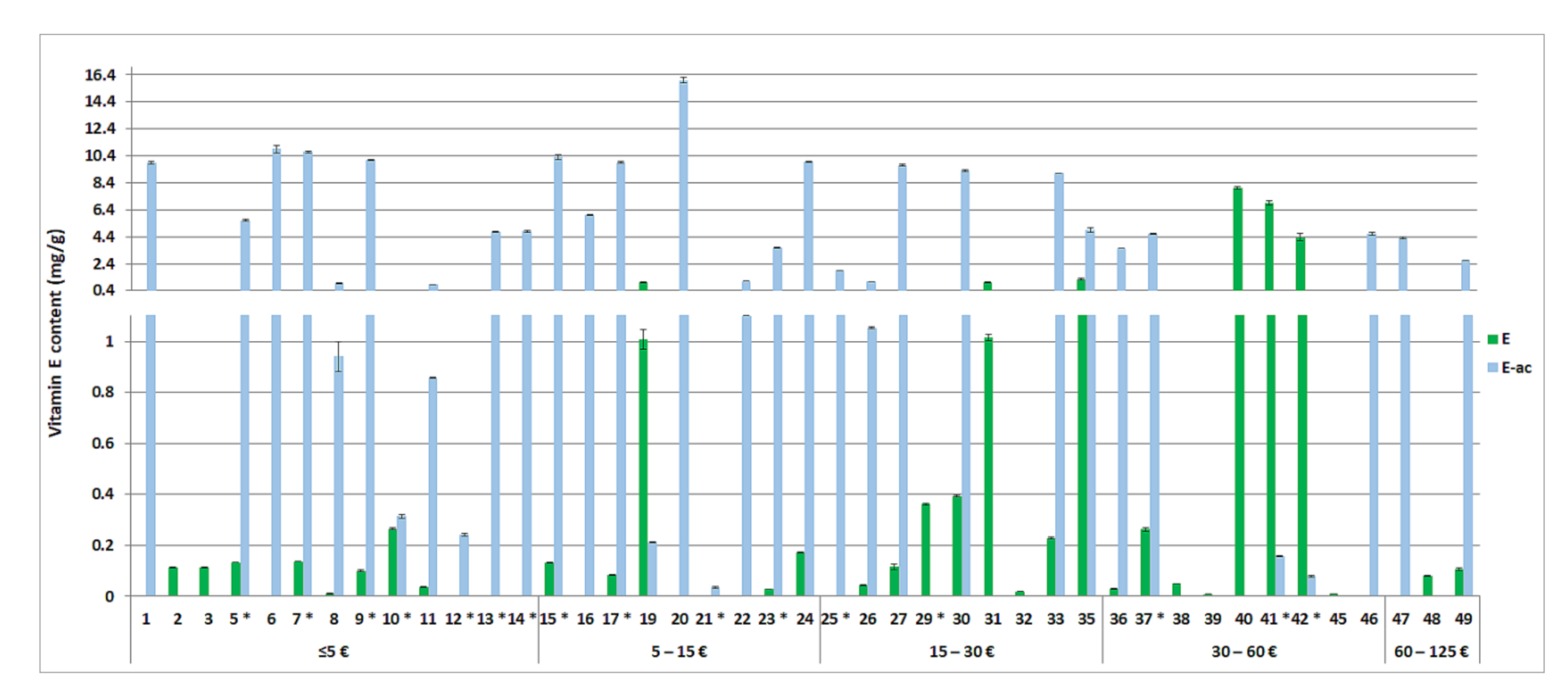
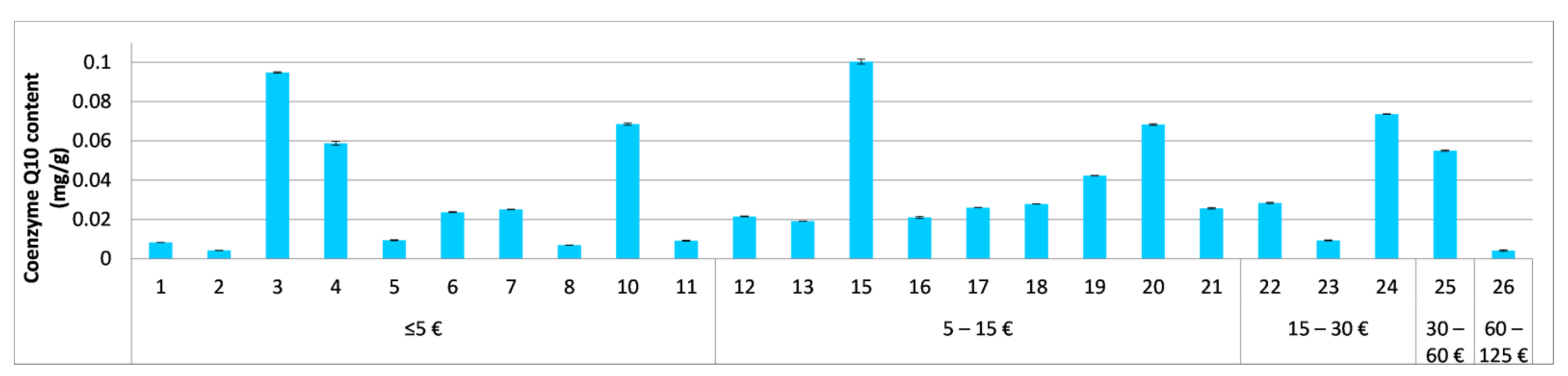
3.4. Quantitative Evaluation of Vitamins A and E and Coenzyme Q10 in the Tested Cosmetics
3.5. Content-Related Quality Control of Vitamin A in the Tested Cosmetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lupo, M.P. Antioxidants and Vitamins in Cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef]
- Bissett, D.L. Common Cosmeceuticals. Clin. Dermatol. 2009, 27, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical Review of Its Current Use in Cosmetic and Clinical Dermatology. Dermatol. Surg. 2005, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in Human Skin: Organ-Specific Physiology and Considerations for Its Use in Dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of Avobenzone: Stabilization Effect of Antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of Efficacy and Tolerance of a Nighttime Topical Antioxidant Containing Resveratrol, Baicalin, and Vitamin e for Treatment of Mild to Moderately Photodamaged Skin. J. Drugs Dermatol. 2014, 13, 1467–1472. [Google Scholar]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Burke, K.E. Interaction of Vitamins C and E as Better Cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef]
- Alberts, D.S.; Goldman, R.; Xu, M.J.; Dorr, R.T.; Quinn, J.; Welch, K.; Guillen-Rodriguez, J.; Aickin, M.; Peng, Y.M.; Loescher, L.; et al. Disposition and Metabolism of Topically Administered Alpha-Tocopherol Acetate: A Common Ingredient of Commercially Available Sunscreens and Cosmetics. Nutr. Cancer 1996, 26, 193–201. [Google Scholar] [CrossRef]
- Baschong, W.; Artmann, C.; Hueglin, D.; Roeding, J. Direct Evidence for Bioconversion of Vitamin E Acetate into Vitamin E: An Ex Vivo Study in Viable Human Skin. J. Cosmet. Sci. 2001, 52, 155–161. [Google Scholar]
- Rangarajan, M.; Zatz, J.L. Kinetics of Permeation and Metabolism of Alpha-Tocopherol and Alpha-Tocopheryl Acetate in Micro-Yucatan Pig Sin. J. Cosmet. Sci. 2001, 52, 35–50. [Google Scholar]
- Nabi, Z.; Tavakkol, A.; Dobke, M.; Polefka, T.G. Bioconversion of Vitamin E Acetate in Human Skin. Curr. Probl. Dermatol. 2001, 29, 175–186. [Google Scholar]
- Zondlo Fiume, M. Final Report on the Safety Assessment of Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, Potassium Ascorbyl Tocopheryl Phosphate, and Tocophersolan. Int. J. Toxicol. 2002, 21, 51–116. [Google Scholar]
- Rousselle, C. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Final Version of the Opinion on Vitamin A (Retinol, Retinyl Acetate and Retinyl Palmitate) in Cosmetic Products. Regul. Toxicol. Pharmacol. 2017, 84, 102–104. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Regulatution (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009, 52, L 342/83–L 342/127. [Google Scholar]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postepy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Srečnik, E.; Roškar, R. Novel HPLC-UV Method for Simultaneous Determination of Fat-Soluble Vitamins and Coenzyme Q10 in Medicines and Supplements. Acta Chim. Slov. 2017, 64, 523–529. [Google Scholar] [CrossRef][Green Version]
- Vinson, J.; Anamandla, S. Comparative Topical Absorption and Antioxidant Effectiveness of Two Forms of Coenzyme Q10 after a Single Dose and after Long-Term Supplementation in the Skin of Young and Middle-Aged Subjects. Int J. Cosmet. Sci. 2006, 28, 148. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical Treatment with Coenzyme Q10-Containing Formulas Improves Skin’s Q10 Level and Provides Antioxidative Effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Hoppe, U.; Bergemann, J.; Diembeck, W.; Ennen, J.; Gohla, S.; Harris, I.; Jacob, J.; Kielholz, J.; Mei, W.; Pollet, D.; et al. Coenzyme Q10, a Cutaneous Antioxidant and Energizer. Biofactors 1999, 9, 371–378. [Google Scholar] [CrossRef]
- Kaci, M.; Belhaffef, A.; Meziane, S.; Dostert, G.; Menu, P.; Velot, É.; Desobry, S.; Arab-Tehrany, E. Nanoemulsions and Topical Creams for the Safe and Effective Delivery of Lipophilic Antioxidant Coenzyme Q10. Colloids Surf. B Biointerfaces 2018, 167, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel for Cosmetic Ingredient Safety Members. Safety Assessment of Ubiquinone Ingredients as Used in Cosmetics. Cosmetic Ingredient Review. Available online: https://www.cir-safety.org/supplementaldoc/safety-assessment-ubiquinone-ingredients-used-cosmetics (accessed on 10 April 2021).
- Temova Rakuša, Ž.; Kristl, A.; Roškar, R. Stability of Reduced and Oxidized Coenzyme Q10 in Finished Products. Antioxidants 2021, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Rakuša, Ž.T.; Škufca, P.; Kristl, A.; Roškar, R. Retinoid Stability and Degradation Kinetics in Commercial Cosmetic Products. J. Cosmet. Dermatol. 2021, 20, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Benevenuto, C.G.; Guerra, L.O.; Gaspar, L.R. Combination of Retinyl Palmitate and UV-Filters: Phototoxic Risk Assessment Based on Photostability and in Vitro and in Vivo Phototoxicity Assays. Eur. J. Pharm. Sci. 2015, 68, 127–136. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Rossatto, V.; Gallarate, M.; Trotta, M.; Debernardi, F. Vitamin A Palmitate Photostability and Stability over Time. J. Cosmet. Sci. 2004, 55, 233–252. [Google Scholar] [CrossRef]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M.B.G.M. Stability of Cosmetic Formulations Containing Esters of Vitamins E and A: Chemical and Physical Aspects. Int. J. Pharm. 2006, 327, 12–16. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Gaspar, L.R.; Bueno de Camargo Júnior, F.; Campos, P.M.B.G.M. Benefits of Combinations of Vitamin A, C and E Derivatives in the Stability of Cosmetic Formulations. Molecules 2012, 17, 2219–2230. [Google Scholar] [CrossRef]
- Moyano, M.A.; Segall, A. Vitamin a Palmitate and α-Lipoic Acid Stability in O/W Emulsions for Cosmetic Application. J. Cosmet. Sci. 2011, 62, 405–415. [Google Scholar]
- Akhavan, A.; Levitt, J. Assessing Retinol Stability in a Hydroquinone 4%/Retinol 0.3% Cream in the Presence of Antioxidants and Sunscreen under Simulated-Use Conditions: A Pilot Study. Clin. Ther. 2008, 30, 543–547. [Google Scholar] [CrossRef]
- Ceugniet, C.; Lepetit, L.; Viguerie, N.L.D.; Jammes, H.; Peyrot, N.; Rivière, M. Single-Run Analysis of Retinal Isomers, Retinol and Photooxidation Products by High-Performance Liquid Chromatography. J. Chromatogr. A 1998, 810, 237–240. [Google Scholar] [CrossRef]
- Wang, L.-H.; Huang, S.-H. Determination of Vitamins A, D, E, and K in Human and Bovine Serum, and B-Carotene and Vitamin A Palmitate in Cosmetic and Pharmaceutical Products, by Isocratic HPLC. Chromatographia 2002, 55, 289–296. [Google Scholar] [CrossRef]
- Gatti, R.; Gioia, M.G.; Cavrini, V. Analysis and Stability Study of Retinoids in Pharmaceuticals by LC with Fluorescence Detection. J. Pharm. Biomed. Anal. 2000, 23, 147–159. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Škufca, P.; Kristl, A.; Roškar, R. Quality Control of Retinoids in Commercial Cosmetic Products. J. Cosmet. Dermatol. 2021, 20, 1166–1175. [Google Scholar] [CrossRef]
- Hubinger, J.C. Determination of Retinol, Retinyl Palmitate, and Retinoic Acid in Consumer Cosmetic Products. J. Cosmet. Sci. 2009, 60, 485–500. [Google Scholar] [CrossRef]
- Grace, A.C.; Prabha, T.; Jagadeeswaran, M.; Srinivasan, K.; Sivakumar, T. Analytical Method Development for Simultaneous Determination of Ubidecarenone and Vitamin E Acetate in Capsule Dosage Form by HPLC. Int. J. Pharm. Pharm. Sci. 2019, 79–84. [Google Scholar] [CrossRef]
- ICH Q2(R1). Validation of Analytical Procedures: Text and Methodology. ICH Harmonised Tripartite Guideline Harmonised Tripartite Guideline. 2005. Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html (accessed on 11 June 2018).
- Ruth, N.; Mammone, T. Anti-Aging Effects of Retinoid Hydroxypinacolone Retinoate on Skin Models. J. Invest. Dermatol. 2018, 138, S223. [Google Scholar] [CrossRef]
- Bjerke, D.L.; Li, R.; Price, J.M.; Dobson, R.L.M.; Rodrigues, M.; Tey, C.; Vires, L.; Adams, R.L.; Sherrill, J.D.; Styczynski, P.B.; et al. The Vitamin A Ester Retinyl Propionate Has a Unique Metabolic Profile and Higher Retinoid-Related Bioactivity over Retinol and Retinyl Palmitate in Human Skin Models. Exp. Dermatol. 2021, 30, 226–236. [Google Scholar] [CrossRef]
- Kikuchi, K.; Suetake, T.; Kumasaka, N.; Tagami, H. Improvement of Photoaged Facial Skin in Middle-Aged Japanese Females by Topical Retinol (Vitamin A Alcohol): A Vehicle-Controlled, Double-Blind Study. J. Dermatol. Treat. 2009, 20, 276–281. [Google Scholar] [CrossRef]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A Comparative Study of the Effects of Retinol and Retinoic Acid on Histological, Molecular, and Clinical Properties of Human Skin. J. Cosm. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Randomized Parallel Control Trial Checking the Efficacy and Impact of Two Concentrations of Retinol in the Original Formula on the Aging Skin Condition: Pilot Study. J. Cosmet. Dermatol. 2020, 19, 437–443. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.S.R.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of Naturally Aged Skin with Vitamin A (Retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef]
- Gold, M.H.; Kircik, L.H.; Bucay, V.W.; Kiripolsky, M.G.; Biron, J.A. Treatment of Facial Photodamage Using a Novel Retinol Formulation. J. Drugs. Dermatol. 2013, 12, 533–540. [Google Scholar]
- Tolleson, W.; Cherng, S.-H.; Xia, Q.; Boudreau, M.; Yin, J.; Wamer, W.; Howard, P.; Yu, H.; Fu, P. Photodecomposition and Phototoxicity of Natural Retinoids. Int. J. Environ. Res. Public Health 2005, 2, 147–155. [Google Scholar] [CrossRef]
- Rutkowski, M.; Grzegorczyk, K. Adverse Effects of Antioxidative Vitamins. Int. J. Occup. Med. Environ. Health 2012, 25, 105–121. [Google Scholar] [CrossRef]
- Nada, A.; Krishnaiah, Y.S.R.; Zaghloul, A.-A.; Khattab, I. Analysis of Vitamin E in Commercial Cosmetic Preparations by HPLC. J. Cosmet. Sci. 2010, 61, 353–365. [Google Scholar]
- Nada, A.; Krishnaiah, Y.S.R.; Zaghloul, A.-A.; Khattab, I. In Vitro and in Vivo Permeation of Vitamin E and Vitamin E Acetate from Cosmetic Formulations. Med. Princ. Pract. 2011, 20, 509–513. [Google Scholar] [CrossRef]
- Trevithick, J.R.; Mitton, K.P. Topical Application and Uptake of Vitamin E Acetate by the Skin and Conversion to Free Vitamin E. Biochem. Mol. Biol. Int. 1993, 31, 869–878. [Google Scholar]
- Prahl, S.; Kueper, T.; Biernoth, T.; Wöhrmann, Y.; Münster, A.; Fürstenau, M.; Schmidt, M.; Schulze, C.; Wittern, K.-P.; Wenck, H.; et al. Aging Skin Is Functionally Anaerobic: Importance of Coenzyme Q10 for Anti Aging Skin Care. Biofactors 2008, 32, 245–255. [Google Scholar] [CrossRef]
| R | R-palm | R-ac | β-car | E | E-ac | Q10 | |
|---|---|---|---|---|---|---|---|
| Calibration standards | 0.25 | 0.38 | 0.25 | 0.25 | 8.00 | 8.00 | 2.50 |
| 1.00 | 1.50 | 1.00 | 1.00 | 40.0 | 40.0 | 12.5 | |
| 10.0 | 15.0 | 10.0 | 10.0 | 80.0 | 80.0 | 25.0 | |
| 25.0 | 37.5 | 25.0 | 25.0 | 320 | 320 | 100 | |
| 75.0 | 113 | 75.0 | 75.0 | 480 | 480 | 150 | |
| 100 | 150 | 100 | 100 | 800 | 800 | 250 | |
| QC solutions | 5.00 | 7.50 | 5.00 | 5.00 | 16.0 | 16.0 | 5.00 |
| 15.0 | 22.5 | 15.0 | 15.0 | 160 | 160 | 50.0 | |
| 50.0 | 75.0 | 50.0 | 50.0 | 640 | 640 | 200 |
| No. | Form | Vitamin A | Vitamin E | Coenzyme Q10 | Price (€/50 mL) |
|---|---|---|---|---|---|
| 1 | C | 0.0055% R-palm | ≤5 | ||
| 2 | DC | β-car | E | ubiquinone | ≤5 |
| 3 | NC | β-car | E | ubiquinone | ≤5 |
| 4 | DC | R-palm | E | ubiquinone | ≤5 |
| 5 | C | R-palm | E | ubiquinone | ≤5 |
| 6 | C | R-palm | E | ubiquinone | ≤5 |
| 7 | C | R-palm | ≤5 | ||
| 8 | C | R-palm | E | ≤5 | |
| 9 | NC | R | E-ac | ≤5 | |
| 10 | DC | R, R-palm | ≤5 | ||
| 11 | DC | E-ac | ubiquinone | ≤5 | |
| 12 | DC | E-ac | ubiquinone | ≤5 | |
| 13 | S | E-ac | ubiquinone | ≤5 | |
| 14 | NC | E, E-ac | ubiquinone | ≤5 | |
| 15 | DC | E-ac | ubiquinone | ≤5 | |
| 16 | S | E-ac | ubiquinone | ≤5 | |
| 17 | NC | β-car | E | ubiquinone | ≤5 |
| 18 | C | R-palm | E, E-ac | ubiquinone | ≤5 |
| 19 | C | R-palm | E, E-ac | ≤5 | |
| 20 | DC | E, E-ac | ubiquinone | ≤5 | |
| 21 | C | E, E-ac | ubiquinone | ≤5 | |
| 22 | T | R-palm | ≤5 | ||
| 23 | S | 1% R | 5–15 | ||
| 24 | S | 0.2% R | 5–15 | ||
| 25 | DC | R-palm | E-ac | 5–15 | |
| 26 | C | R-palm, β-car | E, E-ac | 5–15 | |
| 27 | EC | ubiquinone | 5–15 | ||
| 28 | AC | E-ac | ubiquinone | 5–15 | |
| 29 | DC | R-palm | E, E-ac | ubiquinone | 5–15 |
| 30 | S | R, R-palm | E, E-ac | 5–15 | |
| 31 | S | E-ac | ubiquinone | 5–15 | |
| 32 | C | R-palm | E | ubiquinone | 5–15 |
| 33 | DC | ubiquinone | 5–15 | ||
| 34 | NC | E-ac | ubiquinone | 5–15 | |
| 35 | C | ubiquinone | 5–15 | ||
| 36 | C | ubiquinone | 5–15 | ||
| 37 | C | E-ac | 5–15 | ||
| 38 | S | R-palm | E,E-ac | ubiquinone | 5–15 |
| 39 | S | 0.5%R | 15–30 | ||
| 40 | C | 2%HRP, R | 15–30 | ||
| 41 | C | E, E-ac | 15–30 | ||
| 42 | AC | R-palm | E, E-ac | 15–30 | |
| 43 | DC | β-car | E, E-ac | ubiquinone | 15–30 |
| 44 | NC | ubiquinone | 15–30 | ||
| 45 | C | E, E-ac | ubiquinone | 15–30 | |
| 46 | C | E | 15–30 | ||
| 47 | C | E | 15–30 | ||
| 48 | S | R-palm | E, E-ac | 15–30 | |
| 49 | DC | E | 15–30 | ||
| 50 | C | β-car | E, E-ac | 15–30 | |
| 51 | C | β-car | E, E-ac | 15–30 | |
| 52 | C | E, E-ac | 15–30 | ||
| 53 | C | 0.5% R, R-prop | E, E-ac | 30–60 | |
| 54 | C | 0.2% R | 30–60 | ||
| 55 | AC | ubiquinone | 30–60 | ||
| 56 | C | R-palm | E-ac | 30–60 | |
| 57 | C | E | 30–60 | ||
| 58 | S | E | 30–60 | ||
| 59 | EC | E | 30–60 | ||
| 60 | NC | E | 30–60 | ||
| 61 | S | E | 30–60 | ||
| 62 | AC | R, R-palm | E | 30–60 | |
| 63 | S | E | 30–60 | ||
| 64 | S | E | 30–60 | ||
| 65 | DC | E-ac | 30–60 | ||
| 66 | S | 0.03% R, R-palm | 60–125 | ||
| 67 | C | 1% R | 60–125 | ||
| 68 | S | 2.5% R | 60–125 | ||
| 69 | C | E-ac | 60–125 | ||
| 70 | C | R, R-palm | E | 60–125 | |
| 71 | EC | ubiquinone | 60–125 | ||
| 72 | C | ubiquinone | 60–125 | ||
| 73 | C | E, E-ac | 60–125 |
| R | R-palm | R-ac | β-car | E | E-ac | Q10 | |
|---|---|---|---|---|---|---|---|
| Range (mg/L) | 0.25–100 | 0.38–150 | 0.25–100 | 0.25–100 | 8.00–800 | 8.00–800 | 2.50–250 |
| R2 | 0.9996 | 1.0000 | 0.9998 | 0.9998 | 0.9999 | 0.9999 | 0.9999 |
| LOD (ng/g) | 1.88 | 2.12 | 1.52 | 55.0 | 97.6 | 85.8 | 12.3 |
| LOQ (ng/g) | 6.20 | 7.00 | 5.02 | 182 | 322 | 283 | 40.7 |
| Intra-day accuracy (%) | 101.5 ± 1.1 | 101.4 ± 0.7 | 101.4 ± 0.9 | 100.2 ± 1.7 | 100.6 ± 2.5 | 101.2 ± 2.0 | 100.3 ± 3.3 |
| Inter-day accuracy (%) | 99.8 ± 3.7 | 100.5 ± 4.3 | 102.2 ± 2.8 | 98.2 ± 3.2 | 96.9 ± 3.0 | 97.2 ± 2.4 | 107.0 ± 0.1 |
| Intra-day precision (%) | 0.88 ± 0.64 | 0.92 ± 0.70 | 0.90 ± 0.65 | 1.67 ± 0.72 | 1.48 ± 0.68 | 1.46 ± 0.75 | 1.31 ± 0.78 |
| Inter-day precision (%) | 1.46 ± 1.02 | 1.66 ± 0.56 | 3.40 ± 1.54 | 1.97 ± 1.08 | 1.39 ± 0.59 | 1.62 ± 0.82 | 1.24 ± 0.85 |
| Injection repeatability (%) | 0.20 | 0.08 | 0.12 | 0.11 | 0.09 | 0.15 | 0.35 |
| Stability (%) | 100.8 ± 0.4 | 100.5 ± 0.7 | 100.9 ± 0.4 | 101.0 ± 0.5 | 96.3 ± 2.5 | 101.8 ± 0.3 | 103.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. https://doi.org/10.3390/cosmetics8030061
Temova Rakuša Ž, Roškar R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics. 2021; 8(3):61. https://doi.org/10.3390/cosmetics8030061
Chicago/Turabian StyleTemova Rakuša, Žane, and Robert Roškar. 2021. "Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products" Cosmetics 8, no. 3: 61. https://doi.org/10.3390/cosmetics8030061
APA StyleTemova Rakuša, Ž., & Roškar, R. (2021). Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics, 8(3), 61. https://doi.org/10.3390/cosmetics8030061






