Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs)
Abstract
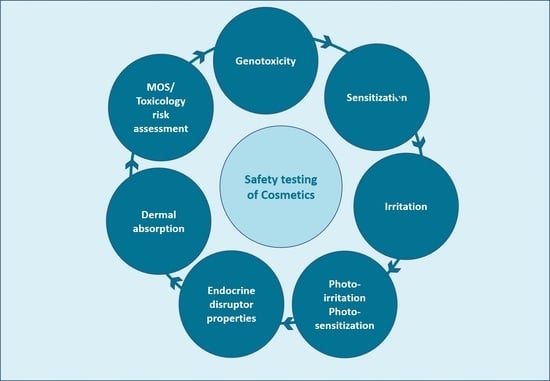
1. Introduction
2. Regulatory Requirements for Cosmetics Safety Assessments
Overall Context
- Substances restricted by an Annex
- Substances not restricted by an Annex
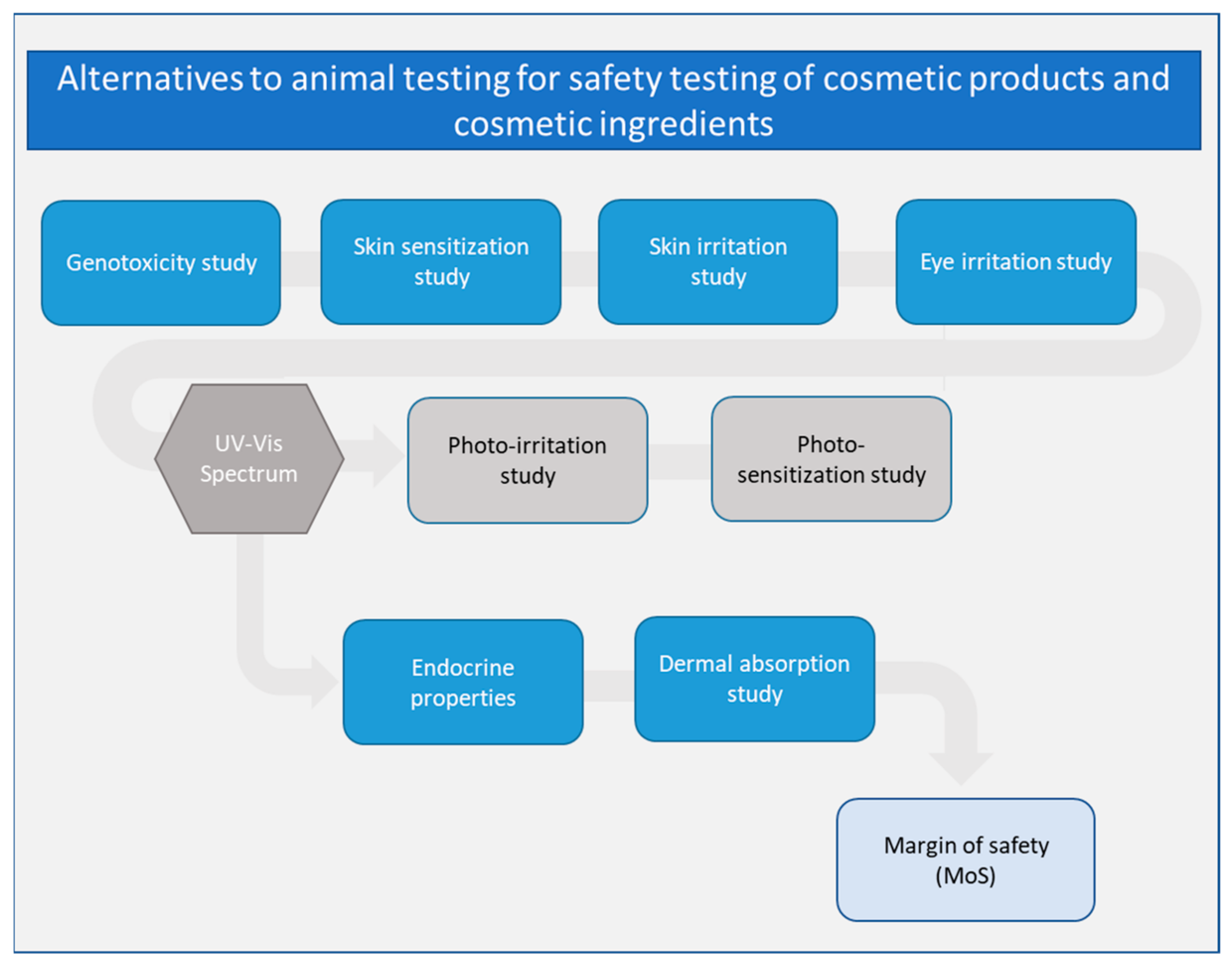
3. Genotoxicity Assessment of Cosmetic Products
4. Skin Sensitization Assessment of Cosmetic Products
5. Endocrine Properties Assessment of Cosmetic Products
6. Assessment of Dermal Absorption of Cosmetic Products
- Safety issues: the presence of systemic test item may lead to systemic adverse effects, the quantities absorbed is taken into consideration in toxicological risk assessment to extrapolate human exposure and calculate the margin of safety (MoS); and
- Therapeutic aspects: the quantities penetrated can be taken into consideration to predict the therapeutic concentration at the target sites in skin tissue.
- Formulation Screening: for selection of lead candidate formulation;
- Bioequivalence: to determine if the new product has the same degree of dermal absorption as reference product. In vitro dermal absorption assay was recently used to demonstrate bioequivalence, and the results of the comparison were accepted by the FDA in connection with the marketing authorization for Lotrimin Ultra cream [92];
- Cosmetics and consumer products: Dermal absorption rate is part of the toxicological profile of any ingredient. Almost always provided for any submission to the SCCS, the in vitro dermal absorption studies can then be part of the safety assessment of a cosmetic product;
- Pharmaceutical products: in vitro dermal absorption studies are part of safety and efficacy assessment of topical products;
- Chemical/agrochemical: in vitro dermal absorption studies are part of safety assessment purposes. With respect to pesticides, the results of the in vitro dermal absorption studies alone are accepted for pesticides risk assessment purposes in the European Union and other countries.
7. Skin and Eye Irritation Assessment of Cosmetic Products
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009, L396, 1–1355. Available online: http://data.europa.eu/eli/reg/2009/1223/oj (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision, 24–25 October 2018, SCCS/1602/18. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 11th Revision, 30–31 March 2021, SCCS/1628/21. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_250.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Resorcinol (CAS No 108-46-3, EC No 203-585-2), Preliminary Version of 16 October 2020, Final Version of 30–31 March 2021, SCCS/1619/20. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_241.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Propylparaben (CAS No 94-13-3, EC No 202-307-7), Preliminary Version of 27–28 October 2020, Final Version of 30–31 March 2021, SCCS/1623/20. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_243.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Octocrylene (CAS No 6197-30-4, EC No 228-250-8), Preliminary Version of 15 January 2021, Final Version of 30–31 March 2021, SCCS/1627/21. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_249.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Titanium Dioxide (TiO2), Preliminary Version of 7 August 2020, Final Version of 6 October 2020, SCCS/1617/20. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_238.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Addendum to the Scientific Opinion SCCS/1613/19 on the Safety of Aluminium in Cosmetic Products (Lipstick); SCCS/1626/20. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_248.pdf (accessed on 7 June 2021).
- ANSM. Evaluation du Risque Lié à L’utilisation du Phénoxyéthanol Dans Les Produits Cosmétiques. Available online: http://dev4-afssaps-marche2017.integra.fr/var/ansm_site/storage/original/application/58033db1a0bd86f6df50cf80b03e1839.pdf (accessed on 7 December 2016).
- BVL (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit). Technically avoidable heavy metal contents in cosmetic products. J. Consum. Prot. Food Saf. 2017, 12, 51–53. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) 1272/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2008, L 353, 1–1355. [Google Scholar]
- ECHA. ANNEX XVII TO REACH–Conditions of Restriction. Entry 70. Octamethylcyclotetrasiloxane (D4) Mdi; ECHA: 2010. Available online: https://echa.europa.eu/documents/10162/50e79685-efaf-ac9a-4acb-d8be3f0e9ddc (accessed on 7 June 2021).
- European Commission. Commission Implementation Decision of 25 November 2013 on Guidelines on Annex I to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; OJEU 2013/674/UE.; European Commission: Brussels, Belgium, 2013; Available online: http://data.europa.eu/eli/dec_impl/2013/674/oj (accessed on 7 June 2021).
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 1, 3. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). Memorandum on Use of Human Data in Risk Assessment of Skin Sensitisation, SCCS/1567/15, 15 December 2015. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_010.pdf (accessed on 7 June 2021).
- AFSSAPS. Test Clinique Final de Sécurité D’un Produit Cosmétique en Vue de Confirmer Son Absence de Potentiel Sensibilisant Cutané Retardé: Recommandations; AFSSAPS: 2008. Available online: https://ansm.sante.fr/documents/reference/recommandations-pour-les-produits-cosmetiques (accessed on 7 June 2021).
- Gilmour, N.; Kern, P.S.; Alépée, N.; Boislève, F.; Bury, D.; Clouet, E.; Hirota, M.; Hoffmann, S.; Kühnl, J.; Lalko, J.F.; et al. Development of a next generation risk assessment framework for the evaluation of skin sensitisation of cosmetic ingredients. Regul. Toxicol. Pharmacol. 2020, 116, 104721. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Gradin, R.; Forreryd, A.; Schmidt, J.; Id, A. Poster SOT: Quantitative Sensitizing Potency Assessment Using GARD Skin; 2021. Available online: https://senzagen.com/2021/04/15/poster-presented-at-sot-2021-quantitative-sensitizing-potency-assessment-using-gardskin-dose-response/ (accessed on 7 June 2021).
- Fioravanzo, E.; Bassan, A.; Pavan, M.; Mostrag-Szlichtyng, A.; Worth, A.P. Role of in silico genotoxicity tools in the regulatory assessment of pharmaceutical impurities. SAR QSAR Environ. Res. 2012, 23, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Rovida, C.; Barton-Maclaren, T.; Benfenati, E.; Caloni, F.; Chandrasekera, P.C.; Chesné, C.; Cronin, M.T.D.; De Knecht, J.; Dietrich, D.R.; Escher, S.E.; et al. Internationalization of read-across as a validated new approach method (NAM) for regulatory toxicology. ALTEX 2020, 37, 579–606. [Google Scholar] [CrossRef]
- Rogiers, V.; Benfenati, E.; Bernauer, U.; Bodin, L.; Carmichael, P.; Chaudhry, Q.; Coenraads, P.J.; Cronin, M.T.D.; Dent, M.; Dusinska, M.; et al. The way forward for assessing the human health safety of cosmetics in the EU-Workshop proceedings. Toxicology 2020, 436, 152421. [Google Scholar] [CrossRef] [PubMed]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative structure-skin permeability relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Shen, J.; Kromidas, L.; Schultz, T.; Bhatia, S. An in silico skin absorption model for fragrance materials. Food Chem. Toxicol. 2014, 74, 164–176. [Google Scholar] [CrossRef]
- Ates, G.; Steinmetz, F.P.; Doktorova, T.Y.; Madden, J.C.; Rogiers, V. Linking existing in vitro dermal absorption data to physicochemical properties: Contribution to the design of a weight-of-evidence approach for the safety evaluation of cosmetic ingredients with low dermal bioavailability. Regul. Toxicol. Pharmacol. 2016, 76, 74–78. [Google Scholar] [CrossRef]
- Kilbey, B.J.; Legator, M.; Nichols, W.; Ramel, C. (Eds.) Handbook of Mutagenesis Test Procedures 1973 and Novel Edition; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Ames, B.N.; Mccann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef]
- Marzin, D. La mutagénèse, principes, méthodes d’étude et législation. Parfums Cosmétiques Arôme N°32. In Proceedings of the Conférence Présentée Devant La SFC Paris, Paris, France, 25 October 1979. [Google Scholar]
- Shahin, M.M.; Chopy, C.; Mayet, M.J.; Lequesne, N. Mutagenicity of structurally related aromatic amines in the Salmonella/mammalian microsome test with various S-9 fractions. Food Chem. Toxicol. 1983, 21, 615–619. [Google Scholar] [CrossRef]
- OECD. (1997-corrected 26 June 2020). Test No. 471. Bacterial Reverse Mutation Test, OECD Guideline for the Testing of Chemicals. Available online: https://www.oecd-ilibrary.org/docserver/9789264071247-en.pdf?expires=1623341347&id=id&accname=guest&checksum=03521B29829FC1D20763B99E2FF5DA3B (accessed on 7 June 2021).
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2016. [Google Scholar] [CrossRef]
- Kirkland, D.; Aardema, M.; Müller, L.; Makoto, H. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens II. Further analysis of mammalian cell results, relative predictivity and tumour profiles. Mutat. Res. 2006, 608, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, D.; Reeve, L.; Gatehouse, D.; Vanparys, P. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat. Res. 2011, 721, 27–73. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Brusick, D. Genotoxic effects in cultured mammalian cells produced by low pH treatment conditions and increased ion concentrations. Environ. Mutagenesis 1986, 8, 879–886. [Google Scholar] [CrossRef]
- Aardema, M.J.; Galloway, S.; Zeiger, E.; Cimino, M.C.; Hayashi, M. Guidance for understanding solubility as a limiting factor for selecting the upper test concentration in the OECD in vitro Micronucleus Assay Test Guideline No. 487. Mutat. Res. 2011, 722, 89–90. [Google Scholar] [CrossRef]
- Thompson, C.; Morley, P.; Kirkland, D.; Proudlock, R. Modified bacterial mutation test procedures for evaluation of peptides and amino acid-containing material. Mutagenesis 2005, 20, 345–350. [Google Scholar] [CrossRef]
- Hamel, A.; Roy, M.; Proudlock, R. The Bacterial Reverse Mutation Test. Genetic Toxicology Testing; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Schimmer, O.; Häfele, F.; Krüger, A. The mutagenic potencies of plant extracts containing quercetin in Salmonella typhimurium TA98 and TA100. Mutat. Res. 1988, 206, 201–208. [Google Scholar] [CrossRef]
- OECD. Principles for the Validation, for Regulatory Purposes, of (Quantitative) Structure-Activity Relationship Models. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/37849783.pdf (accessed on 21 May 2021).
- Fowler, P.; Smith, K.; Young, J.; Jeffrey, L.; Kirkland, D.; Pfuhler, S.; Carmichael, P. Reduction of misleading (“false”) positive results in mammalian cell genotoxicity assays. I. Choice of cell type. Mutat. Res. 2012, 742, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Bryce, S.M.; Bemis, J.C.; Avlasevich, S.L.; Dertinger, S.D. In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity. Mutat. Res. 2012, 630, 78–91. [Google Scholar] [CrossRef]
- Verma, J.R.; Rees, B.J.; Wilde, E.C.; Thornton, C.A.; Jenkins, G.J.S.; Doak, S.H.; Johnson, G.E. Evaluation of the automated MicroFlow® and Metafer™ platforms for high-throughput micronucleus scoring and dose response analysis in human lymphoblastoid TK6 cells. Arch. Toxicol. 2017, 91, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- MˈKacher, R.; Maalouf, E.E.; Ricoul, M.; Heidingsfelder, L.; Laplagne, E.; Cuceu, C.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; Sabatier, L. New tool for biological dosimetry: Reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat. Res. 2014, 770, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Finot, F.; Kaddour, A.; Morat, L.; Mouche, I.; Zaguia, N.; Cuceu, C.; Souverville, D.; Négrault, S.; Cariou, O.; Essahli, A.; et al. Genotoxic risk of ethyl-paraben could be related to telomere shortening. J. Appl. Toxicol. 2017, 37, 758–771. [Google Scholar] [CrossRef]
- Chetelat, A.A.; Albertini, S.; Gocke, E. The Photomutagenicity of fluoroquinolones in tests for gene mutation, chromosomal aberration, gene conversion and DNA breakage (Comet assay). Mutagenesis 1996, 11, 497–504. [Google Scholar] [CrossRef]
- Barcham, R.; Orsini, N.; Andres, E.; Hundt, A.; Luzy, A.P. Successful proof of concept of a micronucleus genotoxicity assay on reconstructed epidermis exhibiting intrinsic metabolic activity. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2018, 829–830, 75–86. [Google Scholar] [CrossRef]
- OECD. Test No.231. Guidance Document on the In Vitro Bhas 42 Cell Transformation Assay Series on Testing Assessment; ENV/JM/MONO(2016)1; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Mascolo, M.G.; Perdichizzi, S.; Vaccari, M.; Rotondo, F.; Zanzi, C.; Grilli, S.; Paparella, M.; Jacobs, M.N.; Colacci, A. The transformics assay: First steps for the development of an integrated approach to investigate the malignant cell transformation in vitro. Carcinogenesis 2018, 39, 955–967. [Google Scholar] [CrossRef]
- Pfuhler, S.; van Benthem, J.; Curren, R.; Doak, S.H.; Dusinska, M.; Hayashi, M.; Heflich, R.H.; Kidd, D.; Kirkland, D.; Luan, Y.; et al. Use of in vitro 3D models in genotoxicity testing strategic fit, validation status and way forward. Report of working group of the 7th International workshop on genotoxicity testing (IWGT). Mutat. Res. 2020, 850–851, 503135. [Google Scholar] [CrossRef]
- Kimber, I.; Basketter, D.A.; Gerberick, G.F.; Ryan, C.A.; Dearman, R.J. Chemical allergy: Translating biology into hazard characterization. Toxicol. Sci. 2011, 120 (Suppl. 1), S238–S268. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.B.; Strickland, J.; Allen, D.; Casati, S.; Zuang, V.; Barroso, J.; Whelan, M.; Régimbald-Krnel, M.J.; Kojima, H.; Nishikawa, A.; et al. International regulatory requirements for skin sensitization testing. Regul. Toxicol. Pharmacol. 2018, 95, 52–65. [Google Scholar] [CrossRef]
- Commission Regulation (EU). 2017/706 of 19 April 2017 amending Annex VII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards skin sensitisation and repealing Commission Regulation (EU) 2016/1688 (Text with EEA relevance.) C/2017/2369. Off. J. Eur. Union 2017, 8–11. Available online: http://data.europa.eu/eli/reg/2017/706/oj (accessed on 7 June 2021).
- OECD. Test No. 406: Skin Sensitisation, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 1992. [Google Scholar] [CrossRef]
- OECD. Test No. 429: Skin Sensitisation: Local Lymph Node Assay, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2010. [Google Scholar] [CrossRef]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins, OECD Series on Testing and Assessment; n° 168; OECD: Paris, France, 2014. [Google Scholar] [CrossRef]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Hoffmann, S.; Alepee, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches (*). Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test Guidelines Programme Work Plan. Available online: https://www.oecd.org/chemicalsafety/testing/Test_Guidelines_Workplan_2020.pdf (accessed on 29 April 2021).
- Roberts, D.W. Is a combination of assays really needed for non-animal prediction of skin sensitization potential? Performance of the GARD (Genomic Allergen Rapid Detection) assay in comparison with OECD guideline assays alone and in combination. Regul. Toxicol. Pharmacol. 2018, 98, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mehling, A.; Adriaens, E.; Casati, S.; Hubesch, B.; Irizar, A.; Klaric, M.; Letasiova, S.; Manou, I.; Müller, B.P.; Roggen, E.; et al. In vitro RHE skin sensitisation assays: Applicability to challenging substances. Regul. Toxicol. Pharmacol. 2019, 108, 104473. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Lindstedt, M.; Albrekt, A.S.; Borrebaeck, C.A. A genomic biomarker signature can predict skin sensitizers using a cell-based in vitro alternative to animal tests. BMC Genom. 2011, 12, 399. [Google Scholar] [CrossRef]
- Johansson, H.; Gradin, R.; Johansson, A.; Adriaens, E.; Edwards, A.; Zuckerstätter, V.; Jerre, A.; Burleson, F.; Gehrke, H.; Roggen, E.L. Validation of the GARD™ skin assay for assessment of chemical skin sensitizers: Ring trial results of predictive performance and reproducibility. Toxicol. Sci. 2019, 170, 374–381. [Google Scholar] [CrossRef]
- Larne, O.; Mattson, U.; Gradin, R.; Hohansson, H. Extended applicability domain of the GARD platform by solvent-extraction protocols allows for accurate assessment of sensitizing mixtures and UVCBs. In Proceedings of the SOT Annual Meeting 2020, Anaheim, CA, USA, 15–19 March 2020. [Google Scholar]
- Johansson, A.; Larne, O.; Pedersen, E.; Berglin, M.; Petersen, H.; Jenvert, R.-M.; Johansson, H. Evaluation of the Applicability of GARDskin to Predict Skin Sensitizers in Leachables from Medical Device Materials. 2021. (Unpublished; Manuscript in Preparation). Available online: https://www.sartorius.com/en/services/validation-service/extractables-leachables-testing?gclid=EAIaIQobChMI64a9jbiP8QIVTNiWCh1ofgGTEAAYASAAEgKNW_D_BwE (accessed on 7 June 2021).
- Api, A.M.; Basketter, D.A.; Cadby, P.A.; Cano, M.F.; Ellis, G.; Gerberick, G.F.; Griem, P.; McNamee, P.M.; Ryan, C.A.; Safford, R. Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul. Toxicol. Pharmacol. 2008, 52, 3–23. [Google Scholar] [CrossRef]
- Basketter, D.; Safford, B. Skin sensitization quantitative risk assessment: A review of underlying assumptions. Regul. Toxicol. Pharmacol. 2016, 74, 105–116. [Google Scholar] [CrossRef]
- Hirota, M.; Fukui, S.; Okamoto, K.; Kurotani, S.; Imai, N.; Fujishiro, M.; Kyotani, D.; Kato, Y.; Kasahara, T.; Fujita, M.; et al. Evaluation of combinations of in vitro sensitization test descriptors for the artificial neural network-based risk assessment model of skin sensitization. J. Appl. Toxicol. 2015, 35, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Gradin, R.; Forreryd, A.; Mattson, U.; Jerre, A.; Johansson, H. Quantitative assessment of sensitizing potency using a dose-response adaptation of GARDskin. 2021. (Unpublished; Manuscript in Preparation). [Google Scholar]
- Steiling, W. Safety evaluation of cosmetic ingredients regarding their skin sensitization potential. Cosmetics 2016, 3, 14. [Google Scholar] [CrossRef]
- Commission regulation (EU). 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties (Text with EEA relevance). Off. J. Eur. Union 2018, L101, 33–36. [Google Scholar]
- IPCS Global Assessments of EDCS. Chapter 1: Executive Summary. Available online: https://www.who.int/ipcs/publications/en/ch1.pdf (accessed on 21 May 2021).
- OECD. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption, OECD Series on Testing and Assessment; n° 150; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- OECD. Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- OECD. Test No. 456: H295R Steroidogenesis Assay, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2011. [Google Scholar] [CrossRef]
- Loughlin, K.R. The clinical applications of five-alpha reductase inhibitors. Can. J. Urol. 2021, 28, 10584–10588. [Google Scholar]
- Rossier, N.M.; Chew, G.; Zhang, K.; Riva, F.; Fent, K. Activity of binary mixtures of drospirenone with progesterone and 17α-ethinylestradiol in vitro and in vivo. Aquat. Toxicol. 2016, 174, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Leusch, F.D.L.; Aneck-Hahn, N.H.; Cavanagh, J.E.; Du Pasquier, D.; Hamers, T.; Hebert, A.; Neale, P.A.; Scheurer, M.; Simmons, S.O.; Schriks, M. Comparison of in vitro and in vivo bioassays to measure thyroid hormone disrupting activity in water extracts. Chemosphere 2018, 191, 868–875. [Google Scholar] [CrossRef]
- OECD. Test No. 248: Xenopus Eleutheroembryonic Thyroid Assay (XETA), OECD Guidelines for the Testing of Chemicals; Section 2; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Belanger, S.E.; Balon, E.K.; Rawlings, J.M. Saltatory ontogeny of fishes and sensitive early life stages for ecotoxicology tests. Aquat. Toxicol. 2010, 97, 88–95. [Google Scholar] [CrossRef]
- Petersen, K.; Fetter, E.; Kah, O.; Brion, F.; Scholz, S.; Tollefsen, K.E. Transgenic (cyp19a1b-GFP) zebrafish embryos as a tool for assessing combined effects of oestrogenic chemicals. Aquat. Toxicol. 2013, 138–139, 88–97. [Google Scholar] [CrossRef]
- Spirhanzlova, P.; Leleu, M.; Sébillot, A.; Lemkine, G.F.; Iguchi, T.; Demeneix, B.A.; Tindall, A.J. Oestrogen reporter transgenic medaka for non-invasive evaluation of aromatase activity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 179, 64–71. [Google Scholar] [CrossRef]
- European Chemical Agency (ECHA); European Food Safety Authority (EFSA); Joint Research Centre (JRC); Andersson, N.; Arena, M.; Auteri, D.; Barmaz, S.; Grignard, E.; Kienzler, A.; Lepper, P.; et al. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018, 16, e05311. [Google Scholar] [CrossRef]
- Sébillot, A.; Damdimopoulou, P.; Ogino, Y.; Spirhanzlova, P.; Miyagawa, S.; Du Pasquier, D.; Mouatassim, N.; Iguchi, T.; Lemkine, G.F.; Demeneix, B.A.; et al. Rapid fluorescent detection of (anti)androgens with spiggin-gfp medaka. Environ. Sci. Technol. 2014, 48, 10919–10928. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 428: Skin Absorption: In Vitro Method, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies, OECD Series on Testing and Assessment; n° 28; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 156, Guidance Notes for the Estimation of Dermal Absorption Values, OECD Series on Testing and Assessment; ENV/JM/MONO (2011)36; OECD: Paris, France, 2011. [Google Scholar]
- The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients. Updated October 2003; SCCNFP/0750/03. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out231_en.pdf (accessed on 7 June 2021).
- SCCS (Scientific Committee on Consumer Safety). Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients, 22 June 2010. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_002.pdf (accessed on 7 June 2021).
- Mitra, A.; Kim, N.; Spark, D.; Toner, F.; Craig, S.; Roper, C.; Meyer, T.A. Use of an in vitro human skin permeation assay to assess bioequivalence of two topical cream formulations containing butenafine hydrochloride (1%, w/w). Regul. Toxicol. Pharmacol. 2016, 82, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.M.; Frasch, H.F. Effect of Frozen human epidermis storage duration and cryoprotectant on barrier function using two model compounds. Skin Pharmacol. Physiol. 2016, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wester, R.C.; Christoffel, J.; Hartway, T.; Poblete, N.; Maibach, H.I.; Forsell, J. Human cadaver skin viability for in vitro percutaneous absorption: Storage and detrimental effects of heat-separation and freezing. Pharm. Res. 1998, 15, 82–84. [Google Scholar] [CrossRef]
- Osman-Ponchet, H.; Boulai, A.; Kouidhi, M.; Sevin, K.; Alriquet, M.; Gaborit, A.; Bertino, B.; Comby, P.; Ruty, B. Characterization of ABC transporters in human skin. Drug Metab. Pers. Ther. 2014, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Alriquet, M.; Sevin, K.; Gaborit, A.; Comby, P.; Ruty, B.; Osman-Ponchet, H. Characterization of SLC transporters in human skin. ADMET DMPK 2015, 3, 34–44. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, R.; Takenaka, S.; Hashimoto, M.; Narawa, T.; Itoh, T. Expression of human solute carrier family transporters in skin: Possible contributor to drug-induced skin disorders. Sci. Rep. 2014, 4, 5251. [Google Scholar] [CrossRef] [PubMed]
- Clerbaux, L.A.; Paini, A.; Lumen, A.; Osman-Ponchet, H.; Worth, A.P.; Fardel, O. Membrane transporter data to support kinetically-informed chemical risk assessment using non-animal methods: Scientific and regulatory perspectives. Environ. Int. 2019, 126, 659–671. [Google Scholar] [CrossRef]
- Rougier, A.; Lotte, C.; Maibach, H.I. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: Predictive assessment by the stripping method. J. Pharm. Sci. 1987, 76, 451–454. [Google Scholar] [CrossRef]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef]
- Marrakchi, S.; Maibach, H.I. Biophysical parameters of skin: Map of human face, regional, and age-related differences. Contact Dermat. 2007, 57, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shriner, D.L.; Maibach, H.I. Regional variation of nonimmunologic contact urticaria: Functional map of the human face. Skin Pharmacol. 1996, 9, 312–321. [Google Scholar] [CrossRef]
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar] [CrossRef]
- Endringer-Pinto, F.; Bagger, C.; Kunze, G.; Joly-Tonetti, N.; Thénot, J.P.; Osman-Ponchet, H.; Janfelt, C. Visualization of penetration of topical antifungal drug substances through mycosis-infected nails by matrix assisted laser desorption ionization mass spectrometry imaging. Mycoses 2020, 63, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredeint Review. Dermal Penetration, Absorption, and other Considerations for Babies and Infants in Safety Assessments. Available online: https://www.cir-safety.org/sites/default/files/Infskn092014rep-%20final.pdf (accessed on 7 June 2021).
- Makri, A.; Goveia, M.; Balbus, J.; Parkin, R. Children’s susceptibility to chemicals: A review by developmental stage. J. Toxicol. Environ. Health B Crit. Rev. 2004, 7, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Garg, A. Chronic effects of toxic environmental exposures on children’s health. J. Toxicol. Clin. Toxicol. 2002, 40, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Gundert-Remy, U.; Heinemeyer, G.; Olejniczak, K.; Stahlmann, R.; Kaufmann, W.; Bolt, H.M.; Greim, H.; von Keutz, E.; Gelbke, H.P. Children as a sensitive subgroup and their role in regulatory toxicology. DGPT Workshop Rep. Arch. Toxicol. 2003, 77, 2–6. [Google Scholar] [CrossRef]
- Ficheux, A.S.; Roudot, P.J. Evaluation Probabiliste de L’exposition de la Population Française Aux Produits Cosmétiques; LERCCo, UBO: Brest, France, 2017; Available online: https://www.cert-online.biz/sites/cert-online.biz/files/page/fichiers/lercco_table_des_matieres.pdf (accessed on 7 June 2021).
- Scientific Committee on Consumer Safety (SCCS). Clarification on Opinion SCCS/1348/10 in the Light of the Danish Clause of Safeguard Banning the Use of Parabens in Cosmetic Products Intended for Children under Three Years of Age; SCCS/1446/11; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Osman-Ponchet, H.; Alriquet, M.; Kouidhi, M.; Sevin, K.; Gaborit, A. Use of microneedle device to enhance dermal absorption: Study on ex vivo human skin. J. Dermat. Cosmetol. 2018, 2, 00032. [Google Scholar] [CrossRef][Green Version]
- Osman-Ponchet, H.; Gaborit, A.; Sevin, K.; Bianchi, C.; Linget, J.M.; Wilson, C.E.; Bouvier, G. Preteatment of skin using an abrasive skin preparation pad, a microneedling device or iontophoresis improves absorption of methyl aminolevulinate in ex vivo human skin. Photodiagn. Photodyn. Ther. 2017, 20, 130–136. [Google Scholar] [CrossRef]
- Osman-Ponchet, H.; Gaborit, A.; Kouidhi, M.; Anglars, M.; Marceau-Suissa, J.; Duffy-Roger, O.; Linget, J.M.; Wilson, C.E. Comparison of the effect of skin preparation pads on transepidermal water loss in ex vivo human skin. Dermatol. Ther. (Heidelb.) 2017, 7, 407–415. [Google Scholar] [CrossRef][Green Version]
- OECD. Test No. 404: Acute Dermal Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2015. [Google Scholar] [CrossRef]
- OECD. Test No. 405: Acute Eye Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2020. [Google Scholar]
- OECD. Test No. 492: Reconstructed Human Cornea-like Epithelium (RhCE) Test Method for Identifying Chemicals not Requiring Classification and Labelling for Eye Irritation or Serious Eye Damage, OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Aberdam, E.; Petit, I.; Sangari, L.; Aberdam, D. Induced pluripotent stem cell-derived limbal epithelial cells (LiPSC) as a cellular alternative for in vitro ocular toxicity testing. PLoS ONE 2017, 12, e0179913. [Google Scholar] [CrossRef] [PubMed]
- Report from the Commission to the European Parliament and the Council. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017; COM/2020/16 final; Brussels, 5.2.2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/04a890d4-47ff-11ea-b81b-01aa75ed71a1 (accessed on 7 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Chérouvrier Hansson, A.; Johansson, H.; Lemkine, G.F.; Thénot, J.-P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. https://doi.org/10.3390/cosmetics8020050
Barthe M, Bavoux C, Finot F, Mouche I, Cuceu-Petrenci C, Forreryd A, Chérouvrier Hansson A, Johansson H, Lemkine GF, Thénot J-P, et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics. 2021; 8(2):50. https://doi.org/10.3390/cosmetics8020050
Chicago/Turabian StyleBarthe, Manon, Clarisse Bavoux, Francis Finot, Isabelle Mouche, Corina Cuceu-Petrenci, Andy Forreryd, Anna Chérouvrier Hansson, Henrik Johansson, Gregory F. Lemkine, Jean-Paul Thénot, and et al. 2021. "Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs)" Cosmetics 8, no. 2: 50. https://doi.org/10.3390/cosmetics8020050
APA StyleBarthe, M., Bavoux, C., Finot, F., Mouche, I., Cuceu-Petrenci, C., Forreryd, A., Chérouvrier Hansson, A., Johansson, H., Lemkine, G. F., Thénot, J.-P., & Osman-Ponchet, H. (2021). Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics, 8(2), 50. https://doi.org/10.3390/cosmetics8020050