Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
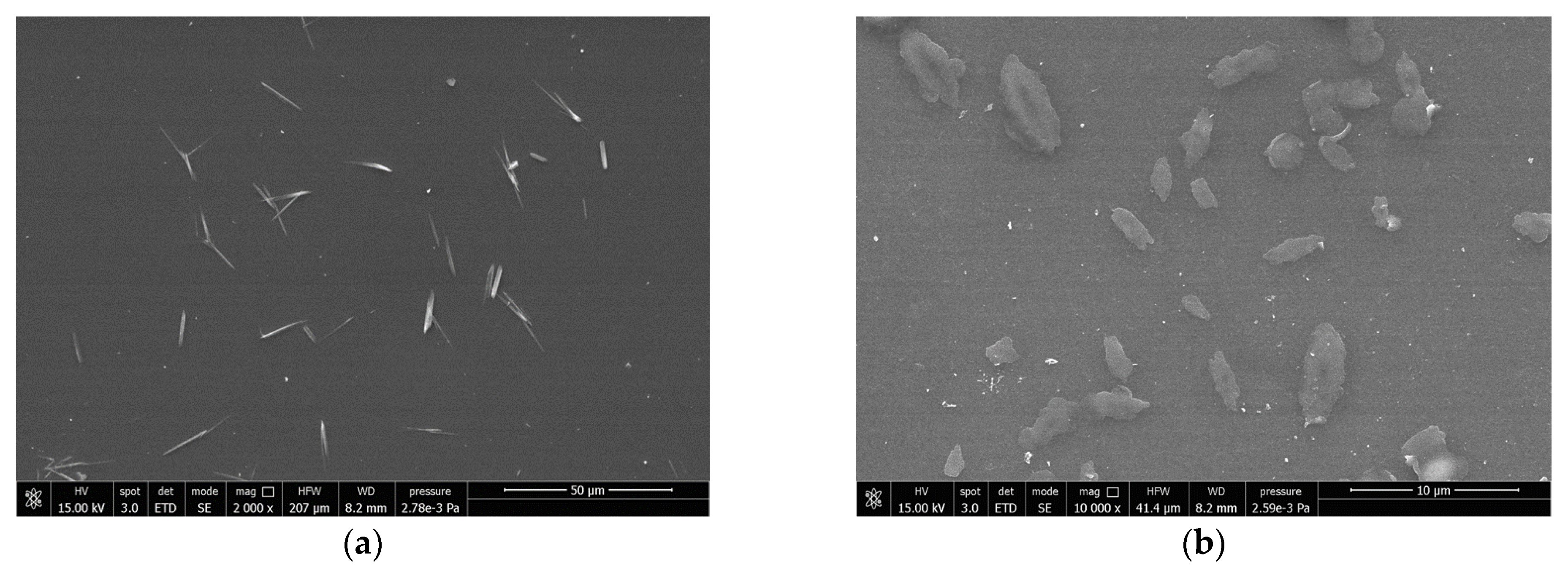
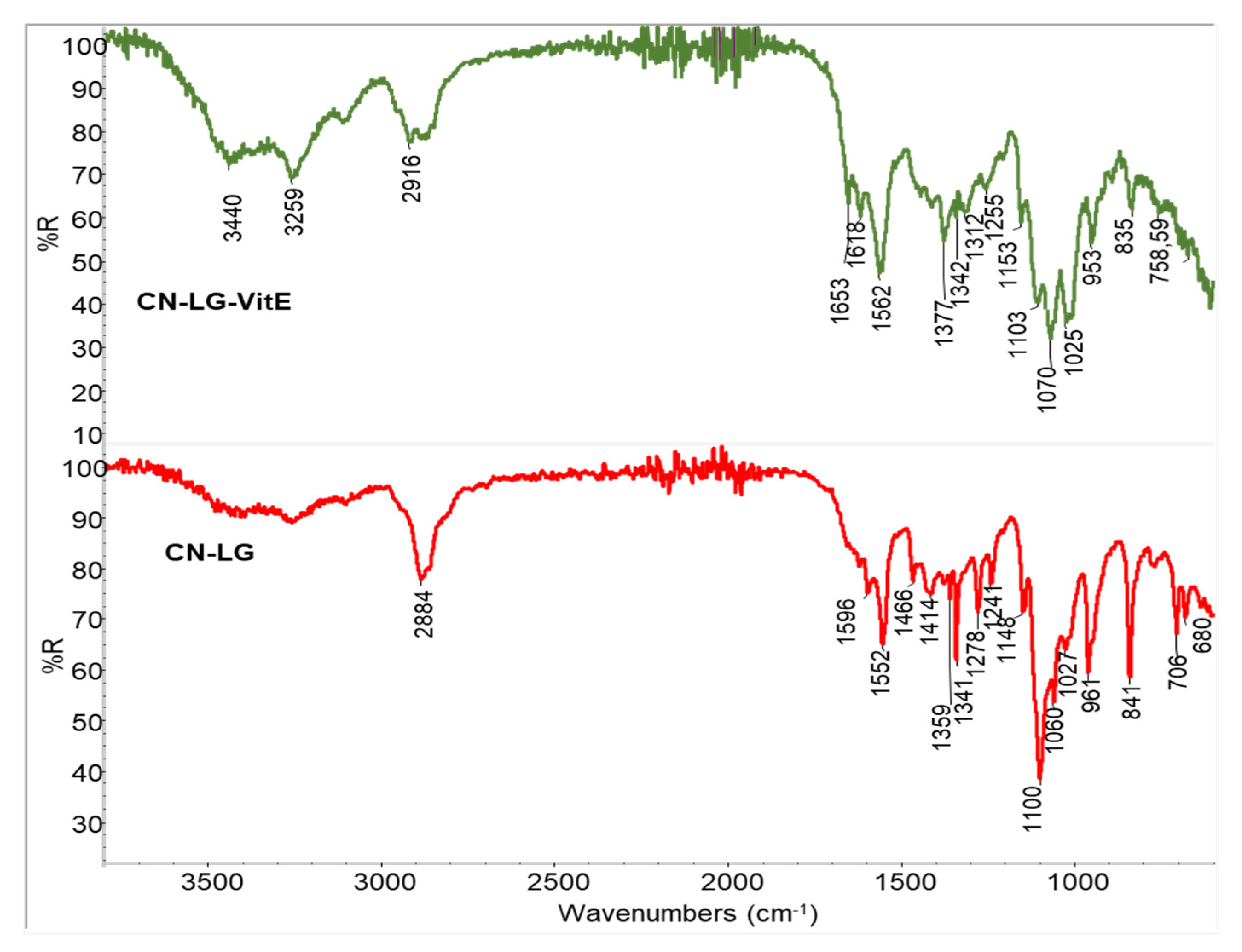
3.1. Characterization of Powders
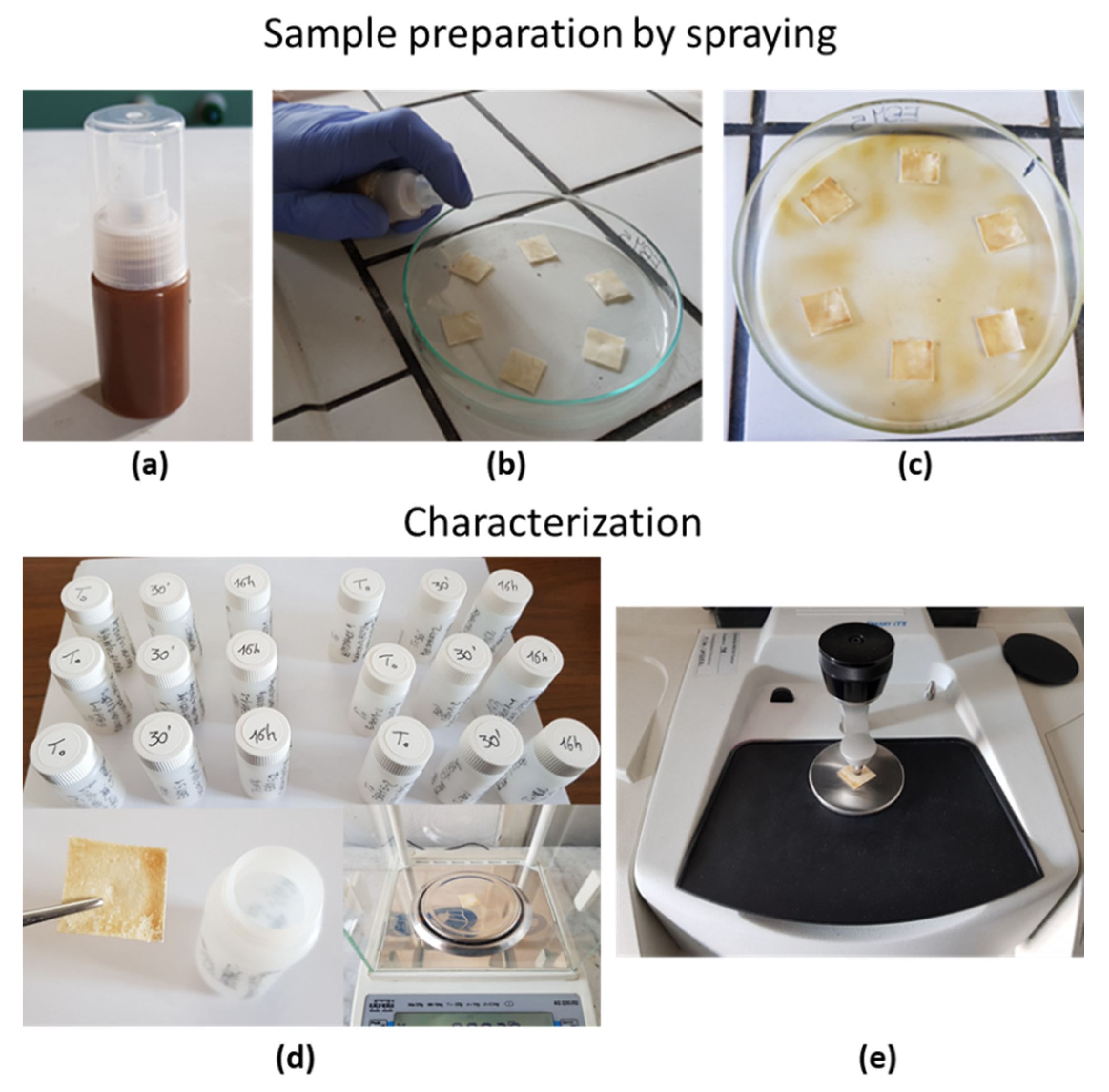
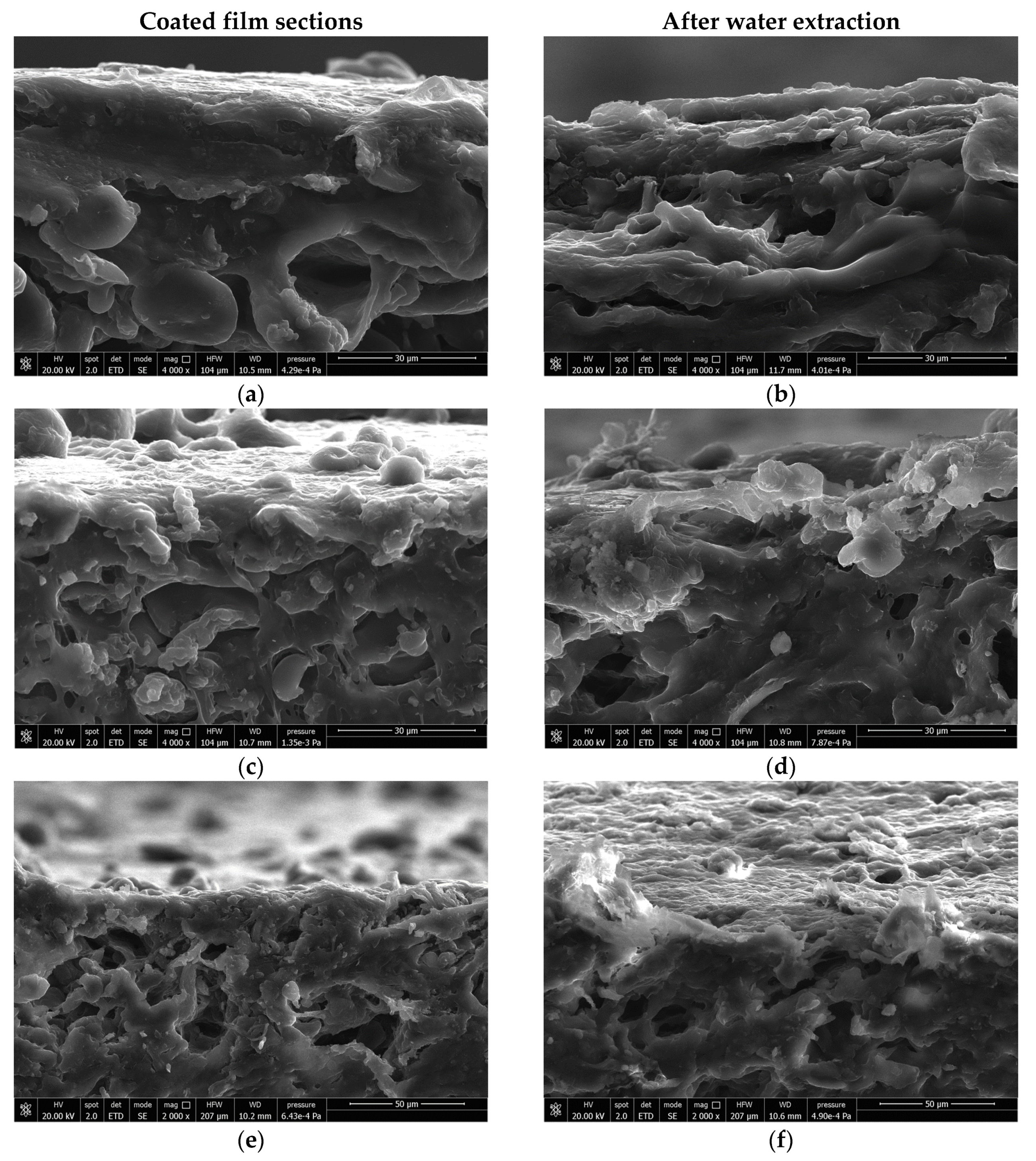
3.2. Coating Preparation and Characterization of the Coated Substrates
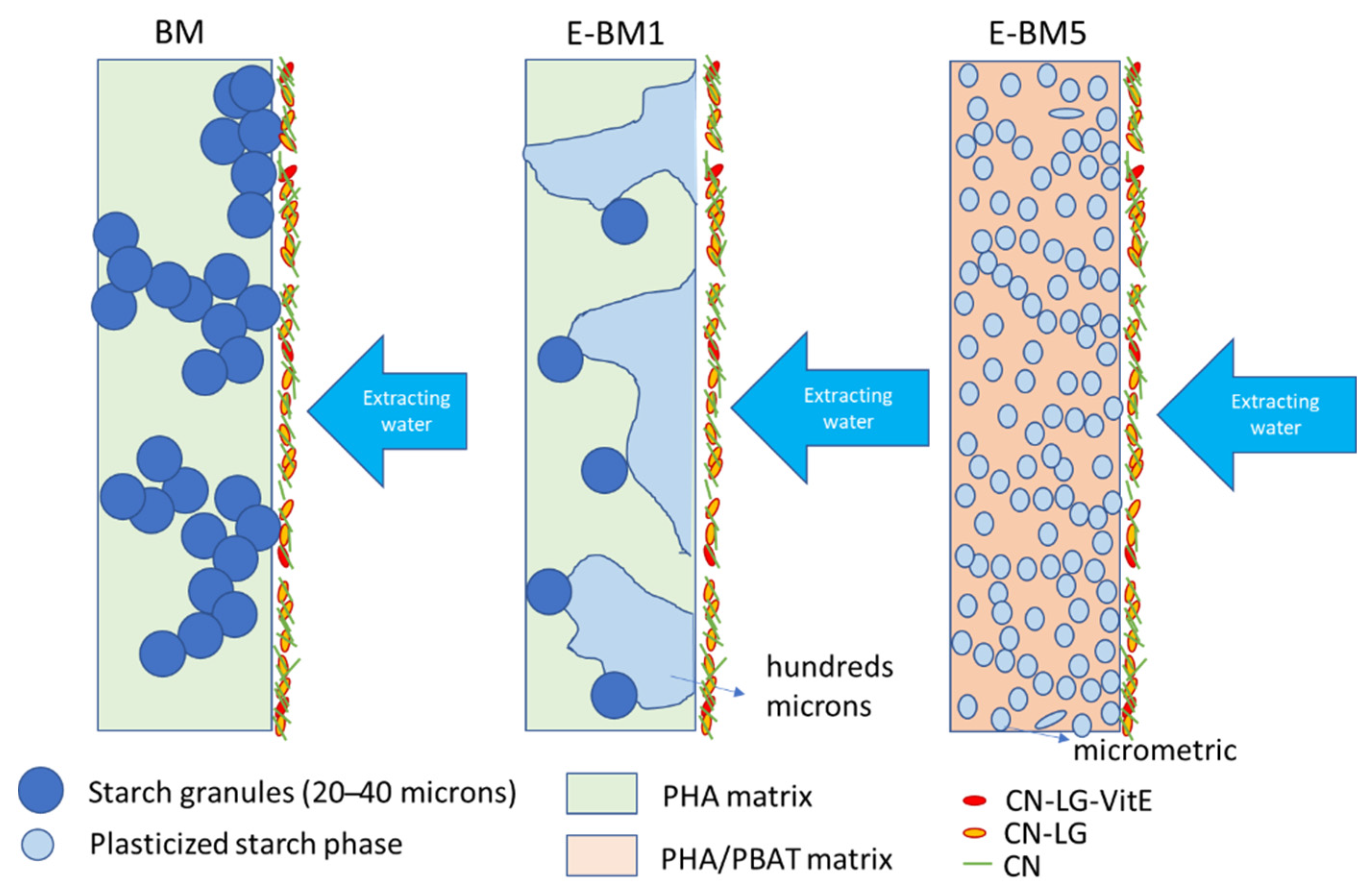
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Renner, G.; Audebert, F.; Burfeindt, J.; Calvet, B.; Caratas-Perifan, M.; Leal, M.E.; Gorni, R.; Long, A.; Meredith, E.; O’Sullivan, Ú.; et al. Cosmetics Europe guidelines on the management of undesirable effects and reporting of serious undesirable effects from cosmetics in the European Union. Cosmetics 2017, 4, 1. [Google Scholar] [CrossRef]
- Effiong, D.E.; Uwah, T.O.; Jumbo, E.U.; Akpabio, A.E. Nanotechnology in Cosmetics: Basics, Current Trends and Safety Concerns—A Review. Adv. Nanoparticles 2020, 9, 1–22. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.-B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Chou, I.N.; Tseng, S.H.; Wu, H.S. Application of biodegradable polyhydroxyalkanoates as surgical films for ventral hernia repair in mice. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.X.L. The greenhouse gas emissions and fossil energy requirement of bioplastics from cradle to gate of a biomass refinery. Environ. Sci. Technol. 2008, 42, 6961–6966. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial degradation behavior in seawater of polyester blends containing poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for Skin Hydrating Cosmetics. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1867–1892. ISBN 978-3-319-16298-0. [Google Scholar]
- De Paepe, K.; Hachem, J.P.; Vanpee, E.; Roseeuw, D.; Rogiers, V. Effect of rice starch as a bath additive on the barrier function of healthy but SLS-damaged skin and skin of atopic patients. Acta Derm. Venereol. 2002, 82, 184–186. [Google Scholar] [CrossRef]
- Maalouf, A.; El-Fadel, M. Effect of a food waste disposer policy on solid waste and wastewater management with economic implications of environmental externalities. Waste Manag. 2017, 69, 455–462. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the implementation of circular economy in the wastewater sector: Challenges and opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Tisserant, A.; Pauliuk, S.; Merciai, S.; Schmidt, J.; Fry, J.; Wood, R.; Tukker, A. Solid Waste and the Circular Economy: A Global Analysis of Waste Treatment and Waste Footprints. J. Ind. Ecol. 2017, 21, 628–640. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Teno, J.; Pardo-Figuerez, M.; Hummel, N.; Bonin, V.; Fusco, A.; Ricci, C.; Donnarumma, G.; Coltelli, M.B.; Danti, S.; Lagaron, J.M. Preliminary studies on an innovative bioactive skin soluble beauty mask made by combining electrospinning and dry powder impregnation. Cosmetics 2020, 7, 96. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS PharmSciTech 2010, 11, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Perugini, P.; Bleve, M.; Redondi, R.; Cortinovis, F.; Colpani, A. In vivo evaluation of the effectiveness of biocellulose facial masks as active delivery systems to skin. J. Cosmet. Dermatol. 2020, 19, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Danti, S.; Trombi, L.; Morganti, P.; Donnarumma, G.; Baroni, A.; Fusco, A.; Lazzeri, A. Preparation of innovative skin compatible films to release polysaccharides for biobased beauty masks. Cosmetics 2018, 5, 70. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Panariello, L.; Morganti, P.; Danti, S.; Baroni, A.; Lazzeri, A.; Fusco, A.; Donnarumma, G. Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. J. Funct. Biomater. 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; de Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed chitin nanofibril/electrospun polyhydroxyalkanoate fiber mesh as functional nonwoven for skin application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef]
- Morganti, P. Chitin Nanofibril and Melatonin to Modulate the Immune Defence System in Plant and Humans. SciFed Nanotech Res. Lett. 2018, 1, 1000001. [Google Scholar]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed]
- Miletić, A.; Ristić, I.; Coltelli, M.B.; Pilić, B. Modification of PLA-based films by grafting or coating. J. Funct. Biomater. 2020, 11, 30. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant cosmetotextiles: Cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef]
- Omerogullari Basyigit, Z.; Kut, D.; Yenilmez, E.; Eyüpoglu, S.; Hocaoglu, E.; Yazan, Y. Vitamin E loaded fabrics as cosmetotextile products: Formulation and characterization. Tekst. Konfeksiyon 2018, 28, 162–169. [Google Scholar]
- Morganti, P.; Muzzarelli, C. Spray-Dried Chitin Nanofibrils, Method for Production and Uses Thereof. U.S. Patent 8,552,164, 8 October 2013. [Google Scholar]
- Morganti, P. Compositional and Material Comprising Chitin Nanofibrils, Lignin and a Co-Polymer and Their Uses. WO2016042471A1, 24 March 2016. [Google Scholar]
- Massey-Brooker, A.D.; Vaccaro, M.; Scialla, S.; Walker, S.J.; Morganti, P.; Carezzi, F.; Benjelloun-Mylayah, B.; Crestini, C.; Lange, H.; Bartzoka, E.; et al. Consumer Goods Product Comprising Chitin, Lignin and a Polymer or Co-Polymer. U.S. Patent Application No. 14/854,121, 17 March 2016. [Google Scholar]
- Fathi, M.; Nasrabadi, M.N.; Varshosaz, J. Characteristics of vitamin E-loaded nanofibres from dextran. Int. J. Food Prop. 2017, 20, 2665–2674. [Google Scholar] [CrossRef]
- Man, Y.C.; Ammawath, W.; Mirghani, M.E.S. Determining α-tocopherol in refined bleached and deodorized palm olein by Fourier transform infrared spectroscopy. Food Chem. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical Review of Its Current Use in Cosmetic and Clinical Dermatology. Dermatol. Surg. 2005, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Panariello, L.; Coltelli, M.-B.; Buchignani, M.; Lazzeri, A. Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials. Eur. Polym. J. 2019, 113. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.N.I.; Setty, C.M.; Peter, G. V Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Picchio, V.; Cammisotto, V.; Pagano, F.; Carnevale, R.; Chimenti, I. We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists TOP 1%. Intechopen 2020, 1–15. [Google Scholar]
- Chen, R.; Abdelwahab, M.A.; Misra, M.; Mohanty, A.K. Biobased Ternary Blends of Lignin, Poly(Lactic Acid), and Poly(Butylene Adipate-co-Terephthalate): The Effect of Lignin Heterogeneity on Blend Morphology and Compatibility. J. Polym. Environ. 2014, 22, 439–448. [Google Scholar] [CrossRef]
- Xiong, S.J.; Pang, B.; Zhou, S.J.; Li, M.K.; Yang, S.; Wang, Y.Y.; Shi, Q.; Wang, S.F.; Yuan, T.Q.; Sun, R.C. Economically Competitive Biodegradable PBAT/Lignin Composites: Effect of Lignin Methylation and Compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Xing, Q.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.J. Biodegradable and High-Performance Poly(butylene adipate-co-terephthalate)-Lignin UV-Blocking Films. ACS Sustain. Chem. Eng. 2017, 5, 10342–10351. [Google Scholar] [CrossRef]
- Tavares, L.B.; Ito, N.M.; Salvadori, M.C.; dos Santos, D.J.; Rosa, D.S. PBAT/kraft lignin blend in flexible laminated food packaging: Peeling resistance and thermal degradability. Polym. Test. 2018, 67, 169–176. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Galeski, A.; Pawlak, A. PBAT green composites: Effects of kraft lignin particles on the morphological, thermal, crystalline, macro and micromechanical properties. Polymer 2020, 203, 122748. [Google Scholar] [CrossRef]
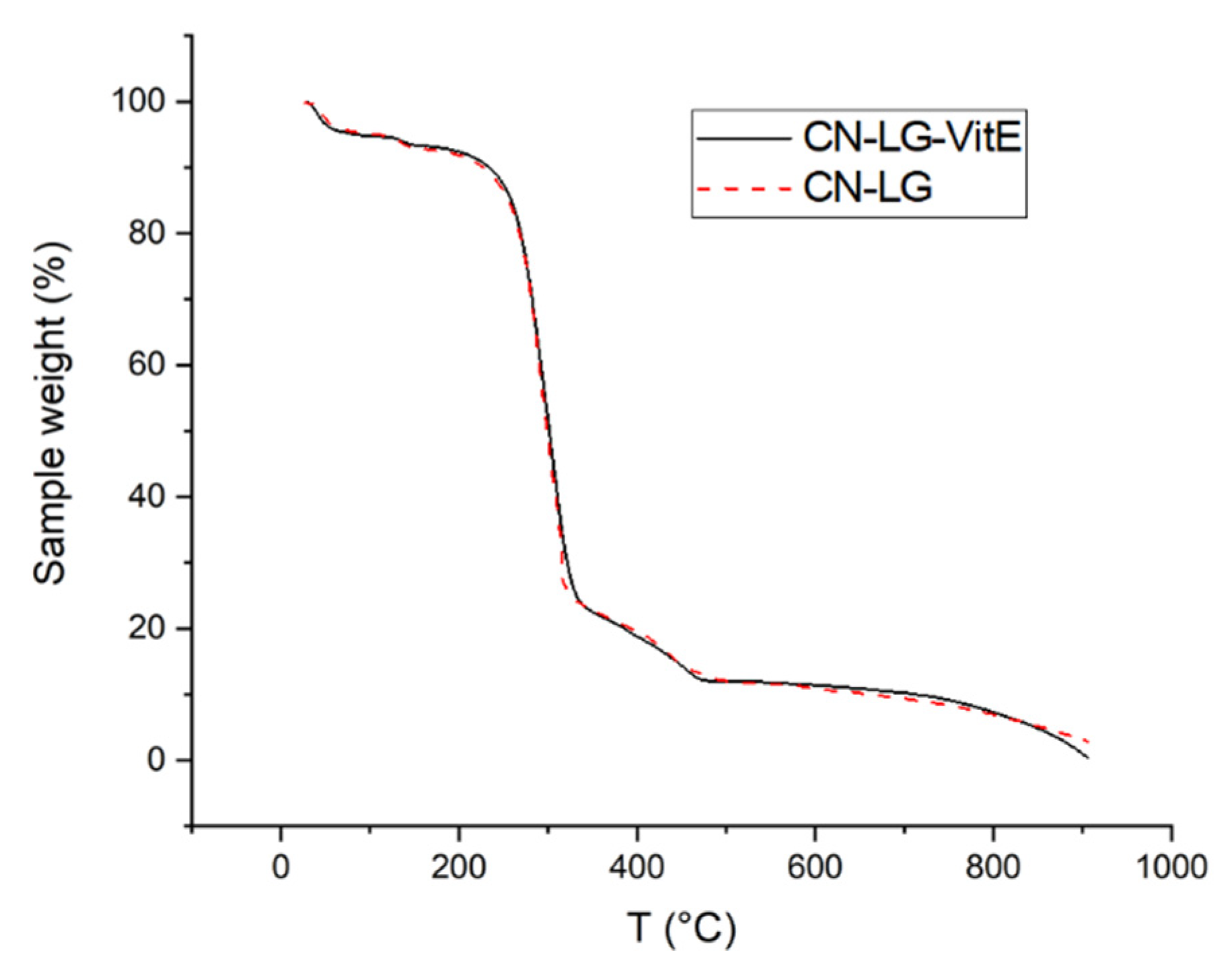


| Entry | Onset 1 3 (°C) | Step 1 (%) | Onset 2 (°C) | Infl. 2 4 (°C) | Step 2 (%) | Onset 3 (°C) | Step 3 (%) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| CN 1 | 45; 140 | −2.4; −2.6 | 277 | 349.1 | −21.2 | 387 | −9.5 | 58.08 |
| CN-LG-PEG 2 | 114 | −2.59 | 266 | 287 | −69.25 | 406 | −10.39 | 12.37 |
| CN-LG-Vit-E 2 | 125 | −2.54 | 264 | 301 | −70.99 | 435 | −10.90 | 11.56 |
| Entry | wt% after 30 min | wt% after 16 h | Δ30 m | Δ16 h |
|---|---|---|---|---|
| COATED BMSMART | −12.0 | −16.4 | 30.2% | 30.2% |
| BMSMART | −17.2 | −23.5 | ||
| COATED EBM1 | −11.7 | −16.5 | 39.4% | 20.7% |
| EBM1 | −19.3 | −20.8 | ||
| COATED EBM5 | −5.6 | −5.9 | 68.2% | 73.4% |
| EBM5 | −17.6 | −22.2 |
| Entry | k | n |
|---|---|---|
| COATED BMSMART | 0.131 | 0.0904 |
| BMSMART | 0.188 | 0.0901 |
| COATED EBM1 | 0.119 | 0.0992 |
| EBM1 | 0.353 | 0.0217 |
| COATED EBM5 | 0.107 | 0.0152 |
| EBM5 | 0.229 | 0.0669 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panariello, L.; Vannozzi, A.; Morganti, P.; Coltelli, M.-B.; Lazzeri, A. Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E. Cosmetics 2021, 8, 27. https://doi.org/10.3390/cosmetics8020027
Panariello L, Vannozzi A, Morganti P, Coltelli M-B, Lazzeri A. Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E. Cosmetics. 2021; 8(2):27. https://doi.org/10.3390/cosmetics8020027
Chicago/Turabian StylePanariello, Luca, Alessandro Vannozzi, Pierfrancesco Morganti, Maria-Beatrice Coltelli, and Andrea Lazzeri. 2021. "Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E" Cosmetics 8, no. 2: 27. https://doi.org/10.3390/cosmetics8020027
APA StylePanariello, L., Vannozzi, A., Morganti, P., Coltelli, M.-B., & Lazzeri, A. (2021). Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E. Cosmetics, 8(2), 27. https://doi.org/10.3390/cosmetics8020027









