Abstract
Chitin and its derivatives are attracting great interest in cosmetic and cosmeceutical fields, thanks to their antioxidant and antimicrobial properties, as well as their biocompatibility and biodegradability. The classical source of chitin, crustacean waste, is no longer sustainable and fungi, a possible alternative, have not been exploited at an industrial scale yet. On the contrary, the breeding of bioconverting insects, especially of the Diptera Hermetia illucens, is becoming increasingly popular worldwide. Therefore, their exoskeletons, consisting of chitin as a major component, represent a waste stream of facilities that could be exploited for many applications. Insect chitin, indeed, suggests its application in the same fields as the crustacean biopolymer, because of its comparable commercial characteristics. This review reports several cosmetic and cosmeceutical applications based on chitin and its derivatives. In this context, chitin nanofibers and nanofibrils, produced from crustacean waste, have proved to be excellent cosmeceutical active compounds and carriers of active ingredients in personal care. Consequently, the insect-based chitin, its derivatives and their complexes with hyaluronic acid and lignin, as well as with other chitin-derived compounds, may be considered a new appropriate potential polymer to be used in cosmetic and cosmeceutical fields.
1. Introduction
In the cosmetics and cosmeceuticals fields, there is a constant search and high demand for innovative and safe materials and new formulations to be used in personal care for hair, nails, lips, and skin in general. Among new materials, the natural-derived polymers, including chitin, are receiving particular and increasing attention because of their low environmental impact and no negative effects on human health [1]. Due to their excellent biological properties, chitin and its derivatives are among the most widely used polymers for several cosmetic/cosmeceuticals applications. Specifically, they are considered of great utility because they act both as carriers and active ingredients [2].
A cosmetic product is defined as a substance applied externally to the human body or in the oral cavity for the purpose of cleaning, protecting, perfuming, improving the appearance or modifying the odour [3]. The term “cosmeceutical” combines the concepts of cosmetic and pharmaceutical, highlighting the additional therapeutic effect of these new body care products [4,5]. In both cosmetics and cosmeceuticals, the use of nanomaterials is constantly growing, since nanocosmeceuticals are considered more efficient carriers, having a better ability of active ingredients than traditional materials [6]. Chitin nanomaterials, mainly nanofibers and nanofibrils, are already being produced from commercially available chitin, mainly derived from crustaceans, for biomedical applications, especially for drug delivery and wound healing [7,8,9]. The use in cosmetics and cosmeceuticals of chitin and its derivatives produced from a more sustainable and growing source, such as insects, could provide a further benefit.
2. Chitin
2.1. Structure and Properties
Chitin and chitosan, its deacetylated derivative, are among the most widely used polysaccharide biopolymers [10,11]. Polysaccharide biopolymers have gained a lot of attention for their physico-chemical properties as biomaterials (i.e., materials of a non-pharmacological nature, which can interact with biological systems efficiently, improving or restoring all the functions of an organ or tissue). Polysaccharide biopolymers have a rather low cost and their similarity to some of the most important biological macromolecules makes them of particular interest for various applications [10,11].
After cellulose, chitin is the second most widespread natural polysaccharide. In 1811, Henri Braconnot discovered chitin as a “material particularly resistant to usual chemicals” in fungi and gave it the name “fungine” [12]. After some years, the French chemist August Odier discovered the same material in both insect cuticle and non-animal tissues, and he gave the name “chitin” to this substance [13]. The physiologist Charles Rouget discovered that chitin, boiled in potassium hydroxide, became its deacetylated and soluble derivative named chitosan [11].
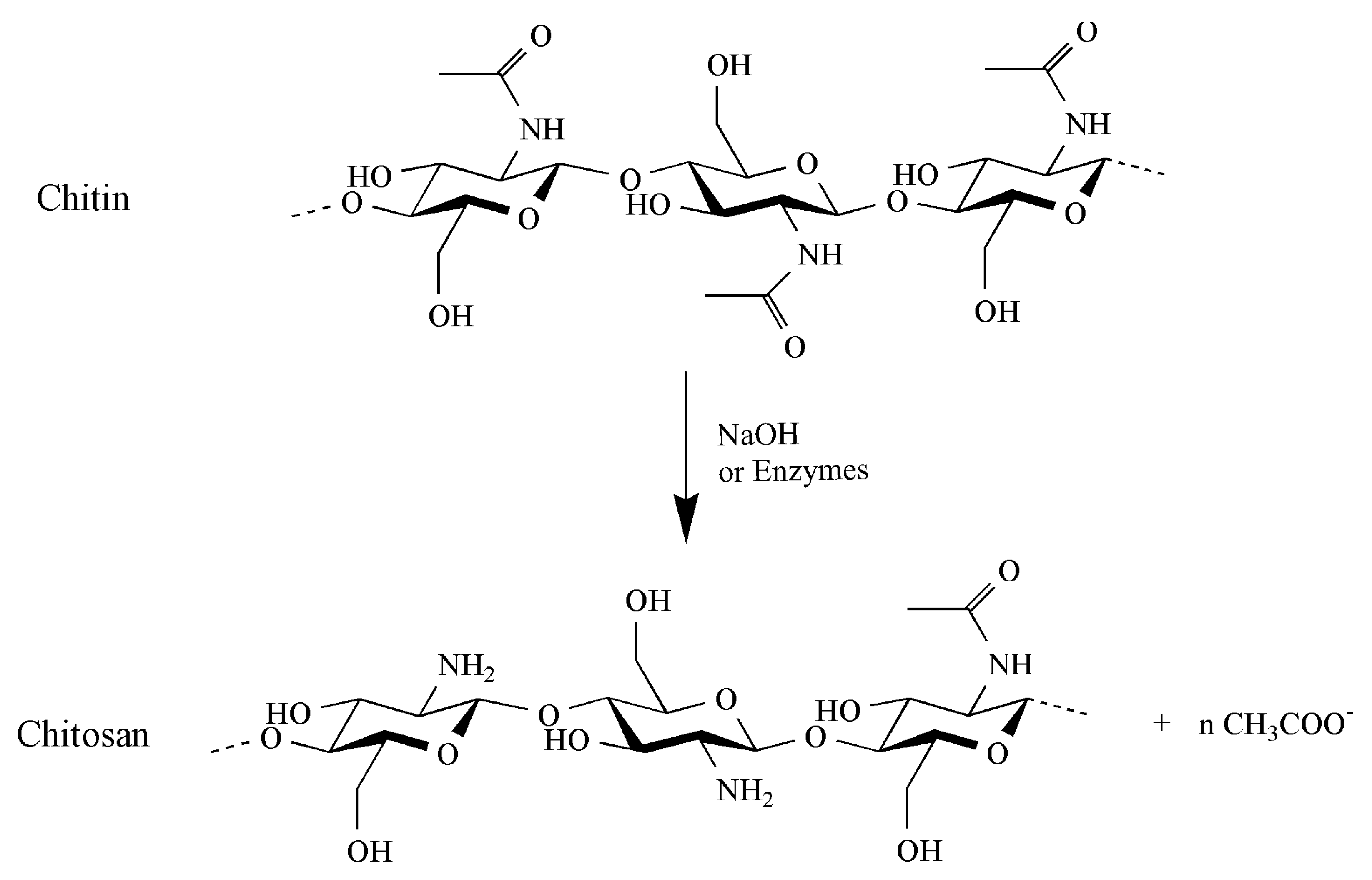
Chitin is composed of β-(1-4)-linked N-Acetyl-D-glucosamine. It is structurally identical to cellulose, but with acetamide groups (-NHCOCH3) at the C-2 positions instead of the hydroxyl group (-OH) (Figure 1). Chitin is a white, hard, inelastic and nitrogenous polysaccharide, with low chemical reactivity, having the same role of collagen in animals and cellulose in plants [14,15,16]. Chitosan is a linear polymer of α-(1-4)-linked 2-amino-2-deoxy-β-D-glucopyranose and is easily derived by N- deacetylation of chitin (Figure 1). Chitin is hydrophobic, it is insoluble in water and common organic solvents and turns out to be soluble only in hexafluoroisopropanol, hexafluoroacetone, chloroalcohols when they are conjugated with aqueous acid solutions and dimethylacetamide in 5% lithium chloride [17,18] and in ionic liquids [19,20]. The amino groups in chitosan improve its solubility at pH values below 6.5, which is their pka value. Therefore, the solubility problem of chitin can be overcome by deacetylating it into chitosan, which is thus soluble at acid pH [11,21]. Like polysaccharides with numerous hydrogen bonds, chitin and chitosan degrade before melting [22]. Different parameters affect the polymer properties, such as deacetylation degree, molecular weight and first of all polydispersity and crystallinity. Other characteristics, such as the purity, the moisturizing capacity and the content of heavy metals, endotoxin and proteins, must be determined for example for applications in food and medical fields [22]. As previously reported, the degree of deacetylation (DD) has a major effect on the solubility of chitin. The lowest deacetylation degree in chitin can be less than 10% [23]. Generally, the more chitin is deacetylated, the more soluble it becomes in acidic solution. A DD of 50% is the commonly proposed threshold above which chitin is defined as chitosan. The molecular weight (MW), as well as the DD, greatly affect many properties of chitin and chitosan, particularly their antimicrobial activity [24]. Chitin has a molecular weight exceeding 1000 kDa, while the chitosan range from 100 to 1000 kDa [10,25,26].
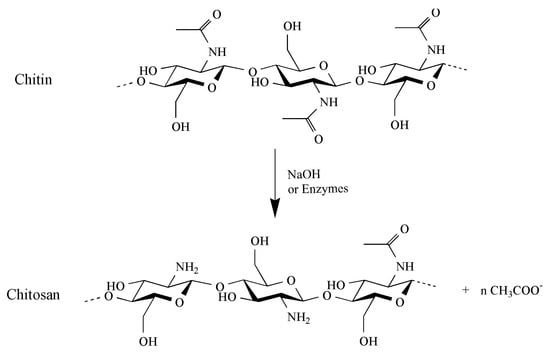
Figure 1.
Chemical structure of chitin and chitosan. Chitosan is obtained through deacetylation of chitin using NaOH or enzymes.
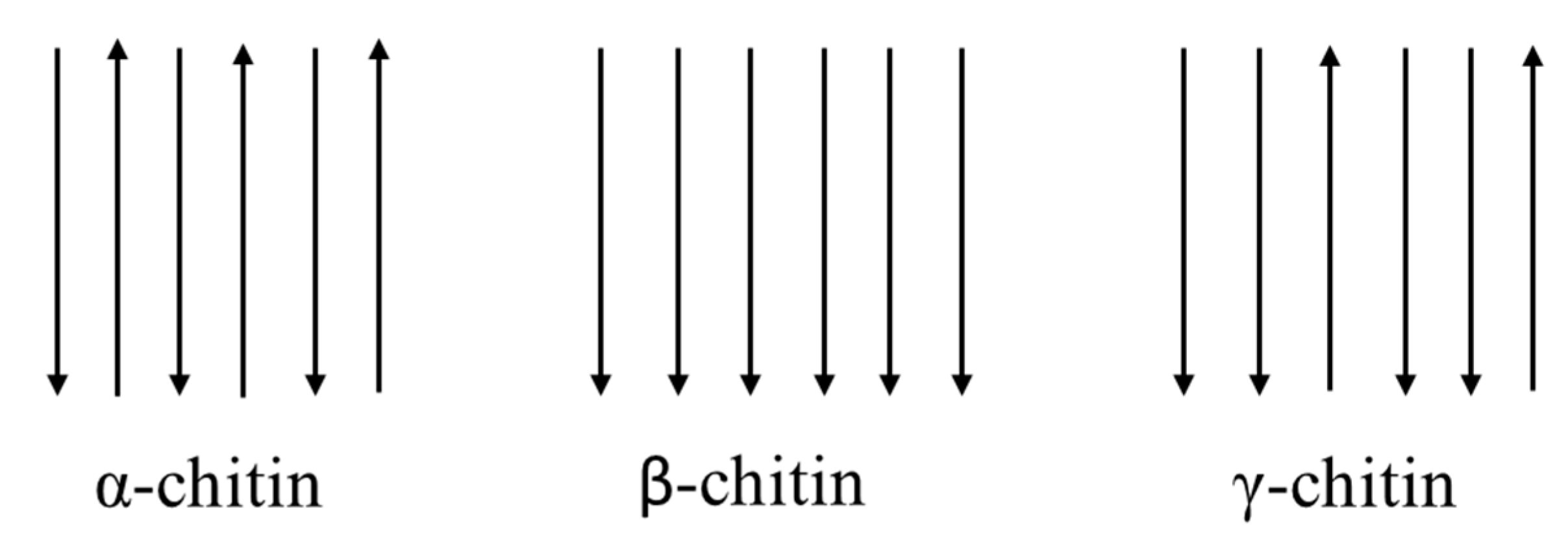
Both chitin and cellulose have a crystalline structure [27] which, reducing the solubility, represents the major limiting factor in its utilization. There are three polymorphic crystalline structures of chitin: α, β, γ [28] (Figure 2). α-chitin for its strong inter-sheet and intra-sheet hydrogen bonding has a compact and crystalline structure, which makes it a robust and recalcitrant material [18]. It is the most common form, mainly present in crustaceans, fungi, yeasts and insects, with an antiparallel orientation of the chains [29]. β-chitin has weak intra-sheet hydrogen bonds, which characterize a weak intermolecular force. Moreover, it has a parallel arrangement of the chains, is less crystalline, with less packaging possibility, being more flexible and more reactive [30]. β-chitin is mainly found in the pen of the Loligo squids. It is interesting to remember that α-chitin can be transformed into β-chitin, but not the reverse [13]. γ-chitin is a mix of forms α and β, combining chains arranged in both parallel and antiparallel ways and can be found in cocoon fibers of the Ptinus beetle and also in the stomach of the Loligo squid [13,28].
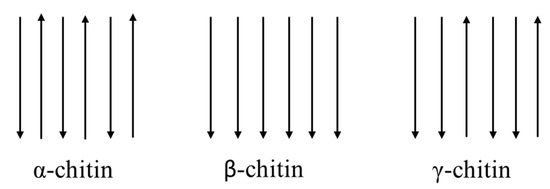
Figure 2.
Representation of the three allomorphic forms of chitin in nature.
The most important biological property of chitin and chitosan is their non-toxicity. However, there are many other important properties, such as biodegradability and biocompatibility, but also hemostaticity, immunostimulating activity and bioadhesivity, which are mostly specific to chitosan [18,22]. Both chitin and chitosan can be hydrolyzed in order to obtain oligosaccharides, which are structurally simpler and with a lower MW, and make them water-soluble, widening their applications. This hydrolysis can be carried out with specific enzymes, such as chitinases and chitosanases, but also with non-specific enzymes, including papain, pepsin and lysozyme, which are able to produce chitosan hydrolysates with no residual proteins [22,31].
Properties of chitin and chitosan can vary with their source, method of preparation, MW and DD [22]. In addition, several authors have reported that both chitin and chitosan show analgesic and antimicrobial activity, properties that seem better in chitosan [22,32], so that is receiving the most attention and is among the most studied properties [22].
2.2. Chitin Fields of Application
Chitin fields of application range from cosmetics, agriculture, tissue engineering to biomedical and pharmaceutical areas [15]. However, the poor solubility of chitin makes its use difficult. Therefore, to make it suitable for more application, it is necessary to modify its structure by adding functional chemical groups [27]. Among chitin derivatives there are carboxymethyl chitin, alkyl chitin, benzyl chitin and hydroxybutyl chitin [27,33,34].
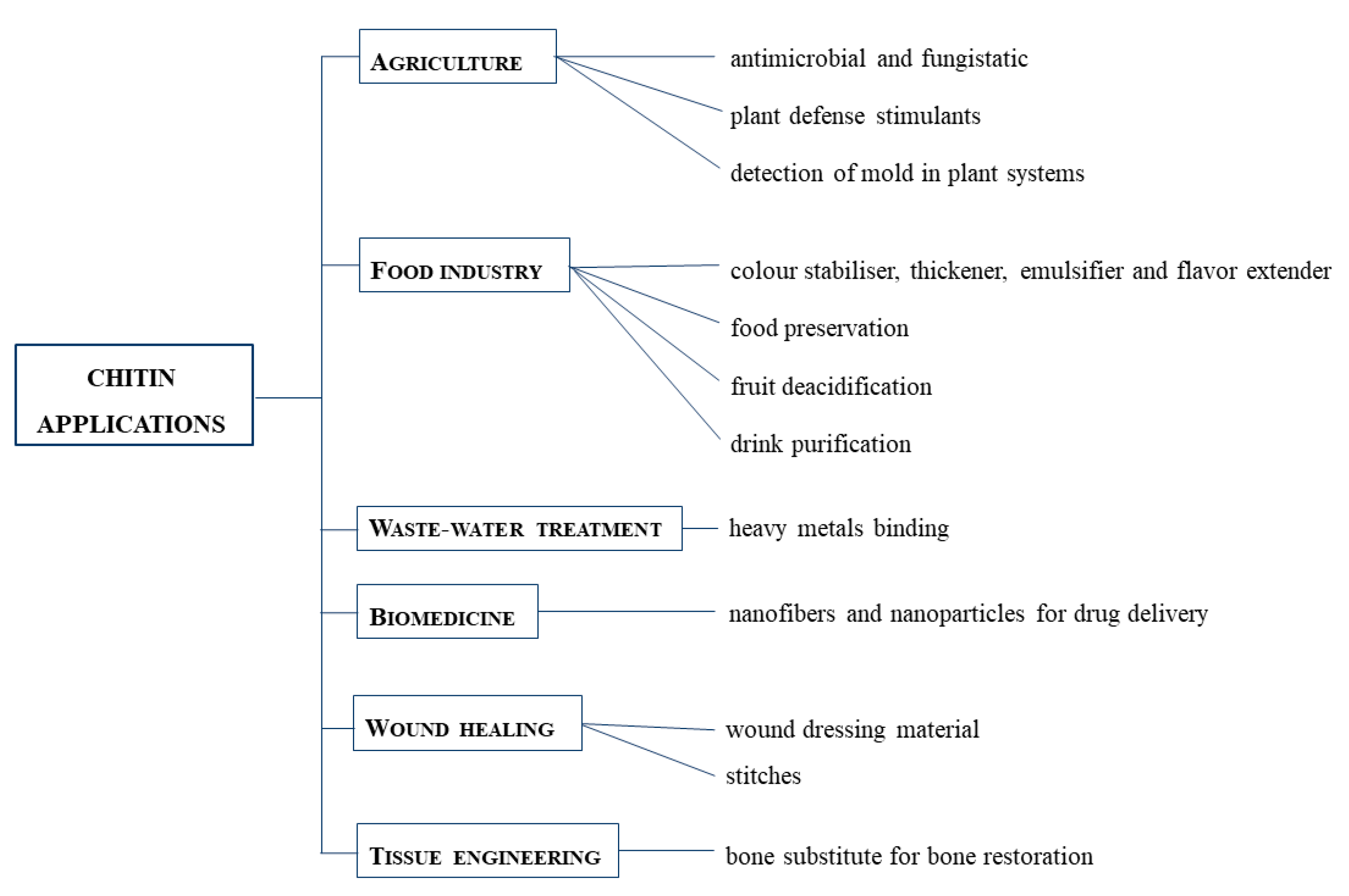
Chitin and its derivatives, for example, can be used for the following applications [10,35] (Figure 3):
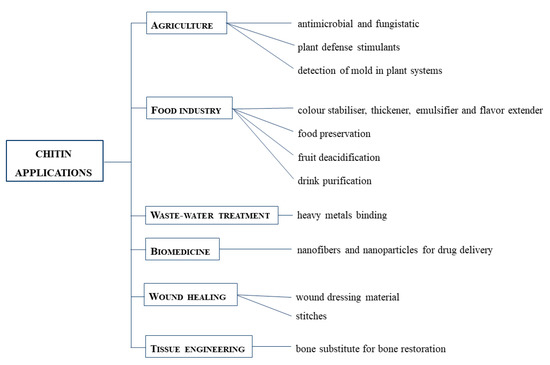
Figure 3.
Chitin applications in different fields: agriculture, food industry, waste-water treatment, biomedicine, wound healing and tissue engineering.
- Agriculture, thanks to their antimicrobial and fungistatic properties [16]. They also stimulate plants to produce defense agents [36]. They can be used to detect the presence of mold in plant systems [37].
- Food and nutrition, used as a food additive, acting like a colour stabiliser, a thickener, an emulsifier and a natural flavor extender [38]. They are also useful for film formation, food preservation, fruit deacidification and water purification [39,40].
- Biomedical applications, used to produce fibers, nanofibers and nanoparticles for drug delivery, but also to produce tissue engineering systems for wounds repair [41], also because chitin sutures resist degradation caused by bile, urine and pancreatic juice [42].
- Tissue engineering, as a bone substitute for bone restoration through modification with hydroxyapatite or bioactive glass ceramics [43].
- Wound dressing, thanks to the antimicrobial, blood clotting, swelling, cell attachment and cytocompatibility aspects of chitin-based composites [44].
- Wastewater treatments, thanks to their non-toxicity. They can bind to water pollutants, especially heavy metals [45].
Moreover, it should be emphasized that all these activities are notably increased when chitin and chitosan are used in their micro/nano size, as reported in many papers of this report.
2.3. Chitin Sources
It has been estimated that up to 1010 to 1012 tons of chitin are annually synthesized in nature, being a major structural component of the exoskeleton of arthropods and the cell wall of fungi and yeasts [35]. The main source for the commercial production of chitin is the exoskeleton of crustaceans, especially crabs, shrimps and prawns. In crustaceans, chitin is deposited together with calcium carbonate in a complex protein network that enables the shell to be formed [46]. Approximately 60% of the body weight of crustaceans consists of waste exoskeleton, which can be recovered for the production of chitin and chitosan [47,48]. The chitin content of this crustacean waste widely ranges from 15% to 40% [47,49]. However, this source presents some limitations, such as seasonality and a generally poor sustainability [50,51]. The geographical limitation to coastal areas, for instance, results in high CO2 emissions to transport the raw material to other areas [50,51]. The growing market of chitin makes it necessary to search for alternative sources in order to cope with the huge demand for chitin, avoiding further depletion of marine resources [52]. Alternative sources for the production of chitin and chitosan include fungi. Fungi can be a valid, cheap source of chitin and chitosan. The average yields of chitin and chitosan isolated from fungi are around 8.5–19.6%, correlating to 1–4% of dry biomass, respectively. The species of fungi that have been mostly investigated for chitin and chitosan production are Pleurotus sajor-caju, Lentinula edodes, Agaricus bisporus, Auricula-judae, Trametes versicolor, Armillaria mellea, Pleurotus ostreatus, and Pleurotus eryngii [53]. Although fungi as an alternative source of chitin are promising, chitin production from this origin has not yet been prepared on an industrial scale [54]. Insects can be an alternative, renewable, sustainable and non-seasonal source of chitin and chitosan [55,56,57,58,59,60,61,62]. Insect farms are spreading worldwide for the production of animal feed combined to waste management. These farms generate a huge amount of insect waste, mainly consisting in exuviae and adults dead at the end of their life cycle, that are rich in chitin and suitable for further purification processing [63].
3. Insect-Based Chitin
There is currently a strong focus on insects as a new source of valuable and useful molecules, such as lipids, (antimicrobial) peptides, polymers, proteins and vitamins, for several applications [64,65,66]. Among the natural polymers, chitin is becoming increasingly important. Chitin is found in the cuticle of insects, of which it can constitute up to 25–60% of the dry weight [67], embedded in a sclerotized protein matrix together with lipoproteins and other materials, both organic and inorganic [68]. Within the cuticle, chitin is embedded in the procuticle (the innermost layer) in the form of nanofibers arranged in fibre bundles, forming an asymmetrical sheet structure responsible for the elasticity and stiffness of the cuticle [69,70].
The usefulness of insects for the production of molecules with high market value, combined with their ease of breeding on a wide range of organic substrates, has led to the increasing development of their large-scale farming. Insect farms are often aimed at both feed production and waste disposal, since the larvae of many species can exploit different organic wastes, bioconverting them into a valuable biomass with high protein and lipid content [53,71]. Among these species with bioconverting ability, Hermetia illucens (Diptera: Stratiomyidae) is the most bred [72,73]. Larval stages of H. illucens are able to feed on organic material, including waste of animal and vegetable origin, converting them into valuable products that can be re-placed on the market [74]. Within the bioconversion process, which corresponds to the life cycle of the larva, H. illucens is able to reduce organic matter with zero value on up to 70–75% (wet weight). At the same time, the conversion into larval biomass is very quick at optimal temperature and humidity conditions of the environment and substrate (around 15 days) [73,75]. Larval biomass could be used for pet food and insectivorous animals or can be transformed into larval meal with high protein content for aquaculture, in accordance with European regulation 893/2017. Even insect frass could be considered a precious element, as they are comparable to soil improver for organic agriculture [76].
The chitin-rich insect waste biomass, generated in huge amounts from the breeding facilities of these species, is the only by-product of a zero-waste process. Particularly, during its life cycle, H. illucens produces larval and pupal exuviae consisting of the exoskeletons shed by the insect as it moults from one developmental stage to the next one. If properly exploited, this biomass could be a valuable source of chitin and its derivatives, making insect breeding a total circular process and economy. Studies on insect chitin in the above-described applications are still at the beginning, but the scientific world is making many efforts to validate the usage of this polymer and its derivatives in several fields. There are ongoing projects aimed at extracting chitin from H. illucens to produce nanofibers for use in the biomedical and cosmetic sectors, particularly for wound healing and formulations of anti-aging products, respectively (unpublished data).
3.1. Chitin Extraction from Insects
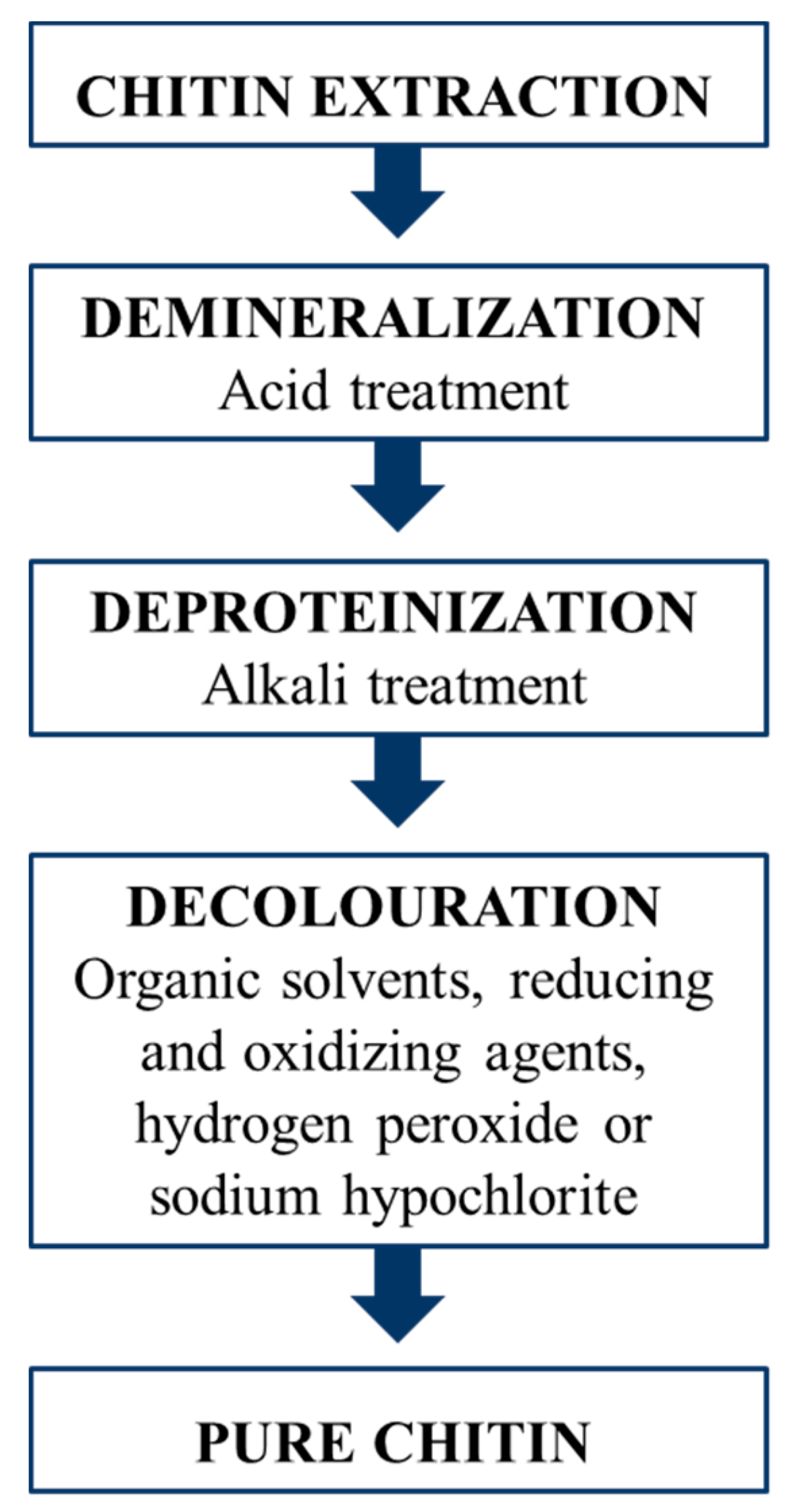
A purification process is required to isolate chitin from the insect biomass, since chitin in the cuticle is also bound to proteins, minerals and pigments. Methods for chitin extraction from insects are almost the same used for the industrial purification from crustacean waste, involving two main steps: demineralization and deproteinization (Figure 4). Minerals, chiefly calcium carbonate, are removed using acidic solutions, while for protein removal an alkaline treatment is required. An additional bleaching step is often performed to whiten the raw chitin [77,78,79]. The current state of chitin production from insects has been reviewed by Hahn et al. [80]. The average yield of chitin extracted from insects ranges between 5% and 15% [80], depending mainly on the insect species, the developmental stage and the body parts of the specimens used [81,82,83,84]. For chitin extracted from crustaceans, a slightly higher average yield ranging from 5 to 32% has been reported [26,85,86,87,88,89].
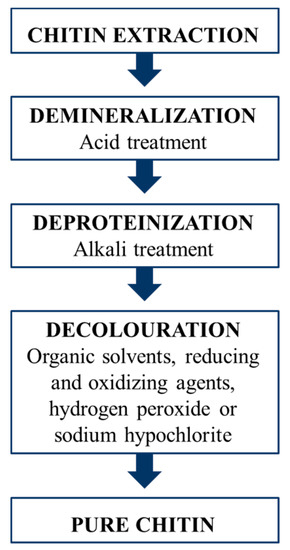
Figure 4.
Schematic representation of the chitin extraction process applied for both crustaceans and insects, involving demineralization, removal of proteins and bleaching.
Characteristics of insect-based chitin are generally similar to the ones of the commercial crustacean-derived chitin [57,90,91]. For instance, the degree of acetylation (i.e., the content of N-acetyl groups) of insect chitin, one of the factors that has a major impact on the performance of the polymer, commonly ranges from 80 to 99% [92], similarly to that of crustacean chitin [90,93,94,95]. Furthermore, chitin extracted from insects has always been determined in the α-form, as the one derived from crustaceans [55,58,92,96,97]. The suitability of chitin for different applications is also greatly affected by its surface morphology. Generally, for biomedical applications, like drug delivery and tissue engineering, a porous structure of the chitin surface is preferred [84]. Within insects, the surface morphology of chitin is mostly related to the species and, within the species, to the body part and developmental stage [55,84,98]. Nonetheless, a similarity in the morphology was observed between insect and crustacean chitin, a combination of nanofibers and pores being the most common morphology [83,87]. All these considerations support and legitimate the use of insects as a source of chitin alternative to crustaceans.
3.2. Insect Chitin Derivatives
Chitosan is the main derivative of chitin and is produced by deacetylation of chitin (i.e., partial removal of the acetyl groups from the polymer chain). Chitosan greatly widens the range of applications of chitin, being much more soluble and reactive thanks to the higher content of free amino groups [99]. Chitin can be deacetylated by chemical or enzymatic treatment. Enzymatic deacetylation involves the use of deacetylases but this does not generally result in an efficient deacetylation because of the crystalline structure of chitin [100,101,102]. Hence, chemical deacetylation, using high concentrated sodium hydroxide solutions at high temperature, is the most performed for chitosan production from both crustaceans and insects [46]. The average yield of chitosan produced from insects ranges from 2 to 8%, related to the initial insect dry biomass [80]. Slightly higher yields are generally obtained from crustacean chitin, ranging from 4 to 15%, related to the original dry biomass, depending on the species and the applied treatment [26,60,86,88,89,103]. It should be noted that crustaceans have a lower protein and fat content than insects, which contributes to a higher yield of chitosan [104].
One of the most important characteristics of chitosan is the degree of deacetylation (DD) (the proportion of glucosamine residues), which can affect its solubility and performance [105]. DD of insect-derived chitosan ranges from around 60 to 98%, depending on the deacetylation parameters applied, entirely similarly to that of commercial chitosan produced from crustaceans [80,106]. The molecular weight (MW) also plays a pivotal role in determining the chitosan performance in several applications, since it greatly affects its biological activity [107,108]. Insect-derived chitosan generally has a MW varying from 26 to 300 kDa [80], lower than that of commercial chitosan (100–1000 kDa) [25,103]. It is worth noting that a low MW of chitosan is often related to a greater antimicrobial activity [107,108]. These values are, however, highly variable, depending on both the chitosan source and the applied deacetylation method [83,109].
Overall, the aforementioned characteristics confirm the similarity between chitin and chitosan derived from insects and those produced from crustaceans. This supports the potential for insect-based chitin and chitosan to be used in the well-established applications for the commercial polymers. Chitosan produced from H. illucens is currently under investigation as a preservative coating for the shelf-life extension of fruits and vegetables. Moreover, tests are ongoing on the production of chitosan nanoparticles derived from H. illucens for applications in drug delivery (unpublished data).
4. Application of Chitin and Its Derivatives in Cosmetics and Cosmeceuticals
In recent years, in response to the desire to maintain youth and beauty, the goal of cosmetology has been the continuous and constant search for innovative compounds for the development of new formulations, including cosmeceuticals, to treat various skin damages and prevent, slow down and/or reduce the undesirable effects of aging [6,110]. These new compounds include biopolymers, such as starch, cellulose, chitin and their derivatives, which have a low environmental impact and no side effects for humans [1,111]. Chitin and its derivatives, thanks to their antioxidant, humectant, antimicrobial, biocompatibility, biodegradability, cleansing and protective properties, are used in cosmetic and cosmeceutical products for hair, nail, oral, lip and, especially, skin care [15]. They are excellent moisturizing and anti-aging agents, which can protect the skin from external hazards, improving important skin functions such as heat regulation, protection, secretion, excretion, sensation and absorption [10,112,113]. Furthermore, due to their specific biological and technological properties, chitin and its derivatives can act as both active ingredients and carriers [2]. Its poor solubility often limits the use of chitin, though [27]. This problem is overcome by converting chitin into its more soluble derivatives. Further, chitin and its derivatives can, in turn, be processed into various conformations, such as micro- and nanoparticles, nanofibers, hydrogels, membranes and films (or complexed with surfactants or polyelectrolytes) in order to broaden the spectrum of possible applications [2,114,115,116].
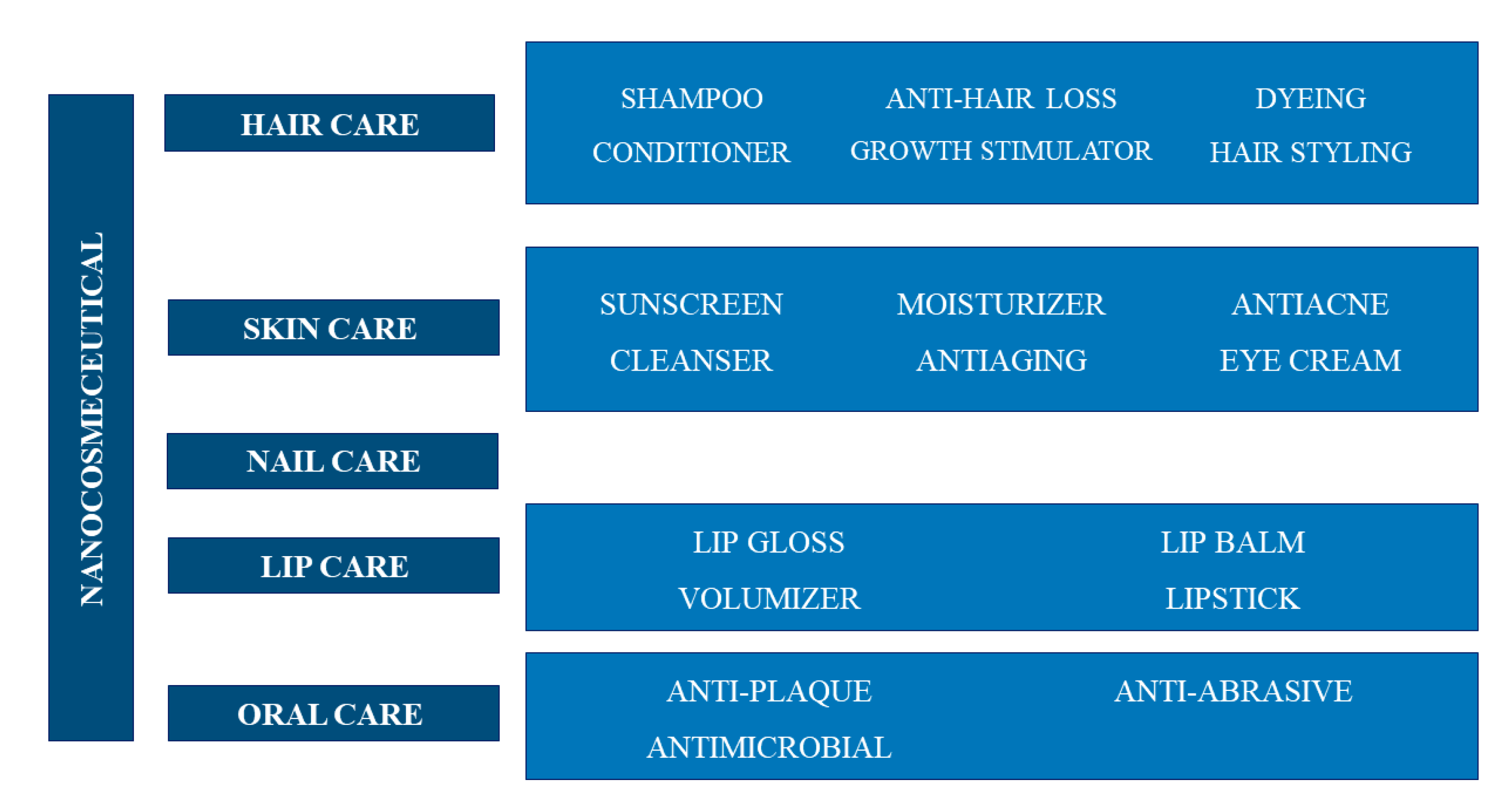
Nanotechnology is one of the leading innovative sectors of the personal care industry. Nanomaterial-based cosmeceuticals are of particular interest due to their improved properties compared to conventional materials. Nanomaterials are used for their ability to release the active ingredients in a targeted and controlled manner at their site of action (mainly the skin), thus leading to a prolongation of the effects, their defined use, and an increased efficacy and bioavailability [6,110]. In Figure 5 the main classes of nanocosmeceuticals are reported, according to their respective roles in skin, hair, nail, lip and oral care [6,10,110].
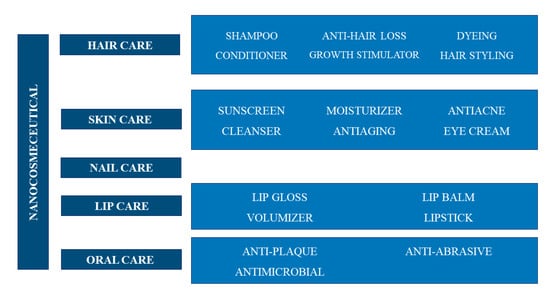
Figure 5.
Nanocosmeceutical applications in personal care.
Within the cosmetics and cosmeceutical research, chitosan remains the most focused and used chitin derivative. Particularly, chitosan-based drug delivery systems are attracting interest as vehicles because they are able to release their active ingredients at the desired rate and body site [117]. For instance, chitosan nanoparticles (CNPs) can be retained in the skin matrix, thanks to the positive charge of these systems, that allow them to interact with skin lipids [118]. CNPs are widely used for topical and transdermal delivery of active ingredients [119]. For example, retinol, a derivative of vitamin A, can be included in the chitin-based nanosystems for the treatment of acne or skin wrinkles [120]. CNPs can be used for sunscreen products, including natural substances like annatto and saffron [121]; chitin nanogels and clobetasol propionate are used to treat psoriasis, avoiding adverse effects such as skin atrophy or steroid acne [118].
Besides chitosan, there are other chitin derivatives that can be solubilized also in water at physiological and alkaline pH, maintaining their characteristics, such as oligosaccharides, complexes with proteins, carotenoids or glucans, and by-products obtained by chemical alkylation and carboxymethylation [2,10,34,85]. All these derivatives can be suitable molecules for cosmeceutical purposes. For instance, the carboxymethylated derivatives of chitin and chitosan (CM-chitin and CM-chitosan respectively), and particularly the 6-O-carboxymethylates derivatives of the same (6-O-CM-chitin and 6-O-CM-chitosan), can promote moisture absorption and retention of the stratum corneum (the outermost layer of the epidermis) comparable to hyaluronic acid (HA), through the formation of a moisturizing film on the skin [122,123]. In addition, other studies have demonstrated that CM-chitin and CM-chitosan, along with chitin oligomers, are promising antioxidants (ascribed to the carboxymethyl groups) and inhibitors of metalloproteases and myeloperoxidases (ascribed to the acetyl groups), alleviating oxidative damage to the skin [114,124,125]. Another form of carboxylated chitin, known as 6-Oxychitin [126] or 6-Carboxychitin [127], was found to have even better properties than the previously mentioned derivatives, when compared to HA. In addition to its regenerative properties for which it is used in the biomedical field [128], 6-Oxychitin has shown to be a valid substitute of HA for its excellent moisture absorption and retention abilities in the formulation of cosmetics and cosmeceuticals [127].
Clinical studies performed by Gautier et al. reported that the presence of chitin-glucan complexes in skin care lotions did not alter the functionality of the epidermal barrier but increased its hydration degree, also showing a reduction in the signs of aging with a final effect of skin rejuvenation [129]. Concerning the application in wound healing, water-soluble chitin (WSC) has been shown to be more effective than chitin and chitosan in accelerating the regenerative process of the skin [130]. Also, the studies performed by Chilarski et al. and Blasinska et al. have highlighted the efficacy and mode of action in the wound healing process of dibutyrylchitin (DBC), a water-soluble derivative of chitin obtained by its reaction with the butyric anhydride [131,132]. Particularly, Chilarski et al. applied DBC non-woven fabrics on non-infected wounds and left them there until the healing process ended, with a positive outcome [132]. Blasinska et al. reported that DBC dressing materials, subcutaneously implanted in rats, contributed to the wound reparative process through an increase in the number of cells and in the amount of glycosaminoglycans, thus contributing to the modification of the extracellular matrix (ECM) and to the increase in the weight of the granulation tissue [131].
To date, there are no examples of applications with insect chitin and its derivatives (including different formulations) in cosmetic and cosmeceutical products, as this field is still unexplored, even the potentiality of chitin from insects is really promising. The physical characteristics of these biopolymers, such as degree of acetylation, the α-form and the surface morphology, are very similar to those of crustaceans and this analogy represents the starting point to replicate the same or similar experiments, in order to validate the use of insect chitin in cosmetic and cosmeceutical fields [133].
5. Chitin-Based Nanomaterials in Personal Care
5.1. Innovation of Chitin Nanofibers and Nanofibrils
Chitin nanofibers and nanofibrils have the same typical microstructure of chitin found in crustacean and insects’ exoskeleton [69,111,134]. Nanofibers are defined by Ifuku et al. as fibers with a diameter of less than 100 nm and an aspect ratio (length/width ratio) of more than 100 [135]. As nanofibrillar material, chitin has a highly ordered crystalline architecture, organized into several increasing structural levels. At the molecular level there are the polysaccharide chains, arranged in anti-parallel orientation (α-chitin), forming nanofibers or nanofibrils with a diameter of approximately 2–5 nm and a length of about 300 nm, each containing 18–25 chitin chains. These fibrils, stabilized by strong hydrogen bonds and wrapped with proteins, assemble into bundles of fibers or fibrils of larger diameter (approximately 50–300 nm). For the next level of chitin hierarchical organization, these bundles of fibrils, embedded into a protein–mineral matrix, align to form branched planes (chitin–protein layers). A twisted plywood-like structure, also called “Bouligand”, is created by helicoidal stacking of these planar layers [134,135,136,137,138]. The structural organization of nanofibers and nanofibrils confers relevant morphological characteristics and mechanical properties (high mechanical strength) and unique optical properties [115,135,138,139].
Thanks to their properties, such as very high surface area (surface/volume ratio) and high porosity, chitin nanofibers represent good candidates as environmentally friendly nanomaterials usable in various applications [134,138]. The main challenges related to these nanofibers and nanofibrils are to develop new preparation methods (optimizing also the existing ones) and to improve their properties through chemical modification, in order to increase their uses in cosmetic, cosmeceutical and biomedical fields. Recently, Ifuku et al. managed to isolate chitin nanofibers with uniform structure, high aspect ratio and a width of 10–20 nm from crabs, shrimps and prawns shells [7,8,140], and from mushrooms [141]. On the other hand, Morganti et al. isolated α-chitin nanocrystals from crustacean waste using a patented industrial process free of environmental impact [142,143]. Chitin nanofibrils (CNs), with an average size of 240 × 5 × 7 nm, represent the crystal-sugar molecular portion of the α-chitin separated from the protein portion. Despite their small size, CNs exhibits a large surface area (400 m2/g) that allows them to interact with different cellular components. Moreover, the size factor and predominantly electropositive surface allow CNs to remain stably suspended in aqueous solution, ready to link other active molecules [144].
5.2. Cosmetic and Cosmeceutical Applications of Chitin-Based Nanomaterial
Recently, chitin nanofibers and nanofibrils have aroused great interest for their application in the biomedical and biotechnological fields as transdermal carriers of drugs and cosmetics, and dressing for wound healing and tissue engineering [9,145,146].
CNs are eco- and bio-compatible as they are natural compounds recognized and metabolized by human enzymes. In addition, they are of great biological value, being able to support hemostasis and regulate the inflammatory response, cell proliferation and the following collagen synthesis in the healing process [1,111]. When CNs come into contact with the stratum corneum, they are hydrolyzed by skin enzymes into smaller units (disaccharides and tetrasaccharides). The latter may undergo the process of re-polymerization once they reach the epidermis’ inner layers and the dermis. The complete hydrolysis of CNs, on the other hand, results in the release of glucosamine and its acetylated derivative, N-acetylglucosamine (NAG), molecules that are involved in several cellular metabolic pathways and are fundamental for one’s body health, particularly for the skin [1]. Glucosamine can, in turn, be further catabolized to supply the cellular metabolism with glucose and glutamic acid, its precursors, or can be used to limit the water loss, by retaining water at the dermal extra cellular matrix (ECM) level. Indeed, glycosaminoglycans, synthesized from glucosamine and N-acetylglucosamine, are involved in the maintenance of hydration in the dermis [1,111,144,147], and, on the other hand, represents the monosaccharide unit responsible for the structural stability of ECM because of the similarity between chitin and HA [148]. N-acetylglucosamine is not only able to reduce skin hyperpigmentation and thus reduce the appearance of spots through the inhibition of melanin production, but it is also effective in reducing wrinkles and the appearance of acne, and increasing skin exfoliation and hydration [149,150]. During the wound healing process, N-acetylglucosamine controls collagen synthesis, promoting tissue granulation and the proper repair of the skin lesions [5,144]. Therefore, CNs as cosmeceutical active compounds are able to promote skin health and beauty, keeping the skin perfectly hydrated by binding many water molecules, and contributing to the maintenance of the skin barrier integrity [1,113]. As a consequence, CNs contribute to two beneficial effects: the increasing of the skin capacity to fix water molecules (active strategy), protecting itself against environmental hazards, and a reduction of the continuous and unconscious loss of water from the body, known as perspiratio insensibilis (passive strategy) [111,144].
Chitin acts as an excellent cosmeceutical and cosmetic carrier as well, by loading the active ingredients ensuring their penetration through the skin, and targeting and releasing them at the right dose and site, thus improving their bioavailability and efficacy with minimal side effects [1,111]. Morganti et al. underlined how the efficacy of a cosmetic product depends not only on the active ingredients bound to the CNs, but also on the type of formulation in which these compounds are integrated. For an emulsion, the most common skin delivery system, the type created (micellar or lamellar, micro or nano-emulsions) is a crucial factor to obtain a transcutaneous, transdermal or transfollicular penetration of the active molecules [144,148]. In this way, it is possible to formulate multifunctional cosmetics capable of performing their action at the level of specific skin layers. For example, sunscreens have to act on the stratum corneum, antioxidants and anti-agings on the epidermis and dermis, deodorants on sweat glands, anti-hair loss or shampoos or similar products on hair, whitening products on melanocytes [144,148]. On the other hand, the combination of positively charged CNs with electronegative polymers, such as HA and lignin (LG), may produce nano/micro-capsules/particles that can load and entrap various active ingredients, both hydro- and lipo-soluble, which can be embedded into emulsions or bound to non-woven tissue fibers. Thus, it is possible to obtain cosmetics and cosmeceuticals that can be used for UV protection, for hair care, but mainly to moisturize, rejuvenate, and whiten the skin, and finally to repair it in case of lesions [148,151,152]. The CN-HA and CN-LG complexes can easily encapsulate many active compounds such as drugs, vitamins, amino acids, antioxidants (lutein, melatonin, vitamin C, α-lipoic acid), immunomodulants (ectoine, ß-glucane) and normalizing agents (creatine, nicotinamide, urea), protecting them from the oxidative phenomena, increasing in their effectiveness and reducing side effects [148,151,152].
Specifically in the case of sunscreens, the main challenge involved the development of multifunctional emulsions in which CNs may act not only as active carriers but as boosters of antioxidant and immunomodulant compounds (such as lutein, melatonin and ectoine), to increase their photoprotective effect against UV-induced damages [144,148]. CNs combined with urocanic acid, a natural shielding agent against UVB radiation, show higher efficacy than conventional products [153]. In addition, Ito et al. demonstrated that the protective effect of CNs nanocrystals against UV radiation is attributable to their abilities to reduce the production of TGF-β (transforming growth factor beta), a multifunctional cytokine. TGF-β regulates the inflammatory processes to maintain the skin hydration, increasing the density of the granular epithelial layer (i.e., the epidermis layer that marks the transition between the innermost portion, consisting of living and active cells, and the outer part, consisting of dead cells) [154].
CNs have proven to have a repairing and anti-aging effect, not only on the skin, but also on the scalp [152]. This polymer is able to penetrate into the hair scales, bind to keratin and repair its fibers in depth, improving shine. The efficacy of shampoos in reducing dandruff and greasy hair and conditioners containing complexes of CNs with metal ions as zinc and other ionic compounds has also been verified by in vitro and in vivo studies [155]. Zinc is a mineral that plays an important role in the sebum regulation, and in the growth and repair of hair tissue. The combined treatment of shampoo and conditioner containing these complexes was found to achieve a stronger, brighter and more manageable hair [155]. The same complexes, thanks to their sebum-regulating and anti-inflammatory properties, are also effective in the treatment of seborrheic dermatitis [156].
Morganti et al. investigated the beneficial effects of both CNs alone and complexed with HA and LG as functional agents in various formulations to obtain skin aging and repairing effectiveness [113,151,152]. Anti-aging cosmetics, in fact, may promote skin renewal through a series of cellular processes including the differentiation and proliferation of keratinocytes and fibroblasts, the increase of their activity, the production of collagen, lipids and other ECM constituents [157]. CNs-based emulsions enriched with antioxidants and immunomodulants, such as lutein, melatonin and ectoine [158,159], as well as α-lipoic acid, urea, creatine and nicotinamide [157], have been effective in reducing wrinkles, spots and roughness, and increasing hydration and elasticity, resulting in younger-looking skin within weeks. CN-HA complexes have shown a greater moisturizing and rejuvenating effect compared to the vehicle alone (CNs). Moreover, they are excellent carriers, enabling the proper delivery of the active ingredients and their interaction with the epidermis layers. Thanks to these properties, once injected, these complexes could represent a valid support to the fillers activity in cosmetic surgery, as demonstrated by the complex of phosphatidylcholine and linoleic acid complexed with HA and CNs, loading, in turn, caffeine, cholesterol, amino acids, vitamins, creatine and melatonin [160]. Among the various molecules tested, the combination consisting of melatonin, vitamin E and ß-glucane (MEB) resulted to be efficient in mitigating the signs of both aging and photoaging. When complexed to form CN-HA nanoparticles (CN-HA-MEB) and administered both in form of capsule (orally) and in form of emulsion (topically) [161], it resulted in a greater increase of the anti-inflammatory activity and collagen production compared to the CN-HA complexes applied topically and hydrocortisone alone [162,163]. Another objective of cosmeceuticals for skin care is to counteract the spots appearance on the skin face, especially when exposed to UV rays and other environmental hazards. In this case, formulations containing whitening agents, such as undecylenoyl phenylalanine, arbutin and diacetyl boldine, complexed to CN-HA, help women with hyperpigmented skin by inhibiting the synthesis and transfer of melanin to keratinocytes [164].
In addition to HA, CN can be combined with another electronegative polymer, such as LG, to form nanoparticles incorporated into emulsions or biofunctional fabrics in order to repair skin damages related to aging, injuries and wounds [151,165]. LG is a natural polyphenolic polymer representing 15–25% of the plant cell wall, having antioxidant, antimicrobial and photoprotective properties. These beneficial properties, combined with the rejuvenating activity of CNs, confer to CN-LG complexes an anti-inflammatory and cicatrizing effect [165,166]. Indeed, these complexes, combining with glycyrrhetinic acid, an anti-inflammatory agent, act on the production of cytokines enhancing their bio-effectiveness [151].
The innovative and relevant aspect of the CN-LG complexes, compared to CN-HA complexes, is their use for the production of smart non-woven tissues that represent a valid alternative delivery system to the emulsion. Their composition (including the presence of CN and LG biopolymers) is biocompatible, biodegradable and free of preservatives and other chemical components that may cause irritation and sensitization. Moreover, they are able to mimic the structure and function of the ECM, promoting skin protection, health and repair [151,165,167]. Nowadays, these smart tissues, impregnated with active ingredients, are the basic materials for the formulation of beauty masks, a category of cosmeceuticals widely used by women for their bio-nature and convenience of use. Formulated as a lotion, cream, gel, sheet or beauty masks, they complement the skin care routine by improving hydration, treating possible skin spots and other disorders and slowing the appearance of various signs of aging and photoaging, depending on the used ingredients [167,168]. For example, vitamin C, melatonin and ß-glucane complexed to CN-LG nanoparticles increased the bio-effectiveness of the mask against photo-aging, maintaining the effect until one month [165]. The presence of poly(ethylene) oxide, on the other hand, in CN-LG non-woven fabric, has shown an in vitro potential. Medications obtained by incorporating CN-LG non-woven fabrics in poly(ethylene) oxide alone [166], and in a chitosan and poly(ethylene) oxide matrix [169], have shown antibacterial activity and shown to promote the repair of injured and burned skin also.
Preparing dressings for wound care, it is important to consider the biochemical and physiological processes that characterize the steps sequence of the healing process, such as hemostasis, inflammation, proliferation and remodeling [170]. Therefore, it has been observed that chitosan has increased its hemostatic properties through the introduction of CNs into the formulation [171]. The synergistic effect of the two biopolymers has been exploited for the design of a new medical material [171,172]. It includes two layers, one external and a non-resorbable one, made of aliphatic copolyamide nanofibers which confers mechanical support, and one internal, made of chitosan nanofibers and CNs. The latter, in contact with the skin damage, is gradually resorbed, facilitating re-epithelialization. The efficacy of these dressings in the treatment of skin injuries and burns, and therefore their potential application in medical practice, has been evaluated and confirmed through in vivo tests on mice [171,172]. Similar effects, controlling also the other phases of the skin regenerative process, besides hemostasis, were observed using three different formulations, such as spray, gel and gauze, based on CN and chitosan glycolate, each acting differently on the wound. The spray was more effective on large superficial wounds, including abrasions, while the gel took longer to aesthetically improve wounds in more delicate areas, such as the face; on the other hand, gauze was the best device in promoting complete healing of deeper wounds, requiring even longer cicatrization times [173,174]. Izumi et al. studied the biomedical efficacy of a promising functional derivative known as superficially deacetylated CNs (SDACNFs), obtained by chemical deacetylation of the CNs surface. The results showed that SDACNFs not only accelerated the early stages of the regenerative process, but also promoted the proliferative (fibroblast migration and collagen synthesis) and final remodeling (re-epithelialization and scar tissue formation) stages, compared with CNs and chitosan in nanofibrillar form [175].
All these applications with CNs and chitin nanofibrils, alone or complexed with other bioactive molecules, including HA and LG, prepared in different formulations for skin aging and repair process, could be reproduced with insect chitin. Although no studies are currently in an advanced phase, insects could be an alternative, innovative and really promising source to extract chitin and transform it into nanofibers or nanofibrils and perform the same experiments performed with chitin from crustacean. As well as the fact that insect chitin and chitosan have the same characteristics as those of crustaceans, both CNs and nanofibers from insects have the same microstructure of those produced from crustacean, allowing to hypothesize very similar properties (antimicrobial, antioxidant, moisturizing and anti-inflammatory characteristics, biocompatibility, biodegradability and wound dressing).
6. Conclusions
Chitin and its derivatives are versatile materials useful for many applications: agriculture, food, the textile sector and wastewater purification are just to report some of the most widespread. However, the biomedical, pharmaceutical and cosmetic fields, as related to personal care, are among those of greatest interest for their use. Nanomaterials based-systems, in which chitin and its derivatives are used (such as nanofibers, nanoparticles), seem to be particularly effective for the delivery of active ingredients, ensuring a site-specific action of cosmetic and cosmeceutical products. Recently, the conventional industrial source of chitin (crustacean waste from the fishing industry) has become no longer sustainable and no longer sufficiently able to cope with the ever-increasing demand for chitin and related products. With zero environmental impact and easy reproducibility, insects are emerging as the most promising source of chitin alternative to crustaceans, although they have still received little attention, especially on an industrial scale. Insect species able to bioconvert organic waste into valuable products, first and foremost H. illucens, are the most advantageous solution and are already widely bred worldwide. Indeed, H. illucens breeding facilities, in addition to producing proteins and lipids, generate a large amount of chitin-rich insect waste, chiefly exuviae of larvae and pupae, and dead adults that could be exploited for chitin extraction, in the frame of a zero-waste production process. Due to the great sustainability of insect breeding, especially of H. illucens larvae, insect chitin could be the best alternative to crustacean source. Moreover, yields and characteristics of insect chitin, and particularly from H. illucens, are comparable to the commercially available chitin derived from crustaceans.
This preludes to its potential use in the same applications already consolidated for crustacean chitin, including cosmetics and cosmeceuticals, also in the form of nanofibers, nanofibrils or nanoparticles, and thus develop increasingly innovative and effective products. However, there are still some points to further investigate for the optimal use of this biopolymer, especially as cosmeceutical. The main challenges for the scientific community regarding insect chitin (and its derivatives) include the optimization and scale-up of its purification process from insect waste, and the development of suitable procedures to improve characterization, not only chemical–physical but also biological, with the support of in vitro and in vivo tests. Moreover, the critical points of the cosmetic production with the addition of chitin and its derivatives will be analyzed and evaluated, related to their properties, solubility and methodology of incorporation in the final formulations, in order to improve their bio-effectiveness.
Author Contributions
Conceptualization: P.F.; writing—original draft preparation: M.T., E.T., A.G., P.F.; writing—review and editing: M.T., E.T., A.G., C.S., T.H., S.Z., R.S., P.F.; supervision: P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Basilicata and Italian Ministry of Education, University and Research (MIUR), within the frameworks of Action I.2 “Attraction and Mobility of Researchers” (PON R&I 2014-2020) and within the Industrial PhD Programme PON/POC 2014-2020 funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest with respect to research, authorship, and publication of this article.
References
- Morganti, P.; del Ciotto, P.; Morganti, G.; Fabien-Soule, V. Application of Chitin Nanofibrils and Collagen of Marine Origin as Bioactive Ingredients. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 267–289. [Google Scholar]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Regulation (CE) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209.
- Elsner, P.; Maibach, H.I. (Eds.) . Cosmeceuticals: Drugs vs. Cosmetics; Marcel Dekker, Inc.: New York, NY, USA, 2000; Volume 23. [Google Scholar]
- Morganti, P. Cosmeceuticals. Clin. Dermatol. 2008, 26, 317. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr. Polym. 2011, 84, 762–764. [Google Scholar] [CrossRef]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Austin, P.R. Chitin solutions and purification of chitin. Methods Enzym. 1988, 161, 403–407. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of lignin and chitin in ionic liquids and their biomedical applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 10. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Rasul, R.M.; Tamilarasi Muniandy, M.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Vaz, J.M.; Taketa, T.B.; Hernandez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. Sci. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Hossain, M.; Iqbal, A. Production and characterization of chitosan from shrimp waste. J. Bangladesh Agric. Univ. 2014, 12, 153–160. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jang, M.K.; Kong, B.G.; Il Jeong, Y.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Pighinelli, L. Methods of Chitin Production a Short Review. Am. J. Biomed. Sci. Res. 2019, 3, 307–314. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawakami, K.; Miyatake, K.; Morimoto, M.; Shigemasa, Y.; Minami, S. Analgesic effects of chitin and chitosan. Carbohydr. Polym. 2002, 49, 249–252. [Google Scholar] [CrossRef]
- Azuma, K.; Nishihara, M.; Shimizu, H.; Itoh, Y.; Takashima, O.; Osaki, T.; Itoh, N.; Imagawa, T.; Murahata, Y.; Tsuka, T.; et al. Biological adhesive based on carboxymethyl chitin derivatives and chitin nanofibers. Biomaterials 2015, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Da Silva, S.B.; De Souza, D.; Lacerda, L.D. Food applications of chitosan and its derivatives. Chitin Chitosan Prop. Appl. 2010, 55, 675–709. [Google Scholar]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. Chitosan A Preserv. Fruits Veg. A Rev. Chem. Antimicrob. Prop. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Yang, Y.M.; Hu, W.; Wang, X.D.; Gu, X.S. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J. Mater. Sci. Mater. Med. 2007, 18, 2117–2121. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gallert, C.; Winter, J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Pittman, S.J.; McAlpine, C.A. Movements of marine fish and decapod crustaceans: Process, theory and application. Adv. Mar. Biol. 2003, 44, 205–294. [Google Scholar] [CrossRef]
- Gillett, R. Global study of shrimp fisheries. In Food and Agriculture Organization of the United Nations Fisheries; Technical Paper No. 475; FAO: Rome, Italy, 2008; p. 331. [Google Scholar]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Bakar, M.F.A. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 1–25. [Google Scholar]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Badawy, R.; Mohamed, H. Chitin extration, Composition of Different Six Insect Species and Their Comparable Characteristics with That of the Shrimp. J. Am. Sci. 2015, 11, 127. [Google Scholar]
- Meky, N.; Badawy, R.; Mohamed, H.; Samir, S. Extraction of chitin from six different insect species as alternative source for biological applications. Afr. J. Biol. Sci. 2017, 13, 197–205. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar]
- Ganguly, S.; Neog, P.; Gogoi, M.; Bordoloi, P.L.; Para, P.A. Edible Insects as Sources of Novel Bioactive Compounds. In Recent Research Trends in Veterinary Sciences and Animal Husbandry; AkiNik Publications: Delhi, India, 2018; pp. 55–69. [Google Scholar]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 1, 3. [Google Scholar]
- Davies, R.G. Insect structure and function. In Outlines of Entomology; Springer: Dordrecht, The Netherlands, 1988; pp. 7–96. [Google Scholar] [CrossRef]
- Gunderson, S.; Schiavone, R. The insect exoskeleton: A natural structural composite. JOM 1989, 41, 60–63. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; La Jeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Derrien, C.; Boccuni, A. Current status of the insect producing industry in Europe. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 471–479. [Google Scholar]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Jucker, C.; Lupi, D.; Moore, C.D.; Leonardi, M.G.; Savoldelli, S. Nutrient recapture from insect farm waste: Bioconversion with Hermetia illucens (L.)(Diptera: Stratiomyidae). Sustainability 2020, 12, 362. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Kaya, M.; Lelešius, E.; Nagrockaite, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS ONE 2015, 10, e115531. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Bulut, E.; Mujtaba, M.; Sivickis, K.; Sargin, I.; Akyuz, B.; Erdogan, S. Gender Influences Differentiation of Chitin Among Body Parts. Arch. Insect Biochem. Physiol. 2016, 93, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Aşan Özüsağlam, M.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Kaya, M. High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. Int. J. Biol. Macromol. 2016, 89, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Shanmugam, A. Extraction of Chitin and Chitosan From Mud Crab Scylla Tranquebarica (Fabricius, 1798). Int. J. Appl. Bio-Eng. 2009, 3, 31–33. [Google Scholar] [CrossRef]
- Kaya, M.; Baublys, V.; Can, E.; Šatkauskienė, I.; Bitim, B.; Tubelytė, V.; Baran, T. Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology 2014, 133, 285–293. [Google Scholar] [CrossRef]
- Oduor-Odeto, P.M.; Struszezyk, M.H.; Peter, M.G. Characterisation of Chitosan from Blowfly Larvae and Some Crustacean Species from Kenyan Marin Waters Prepared Under Different Conditions. West. Indian Ocean J. Mar. Sci. 2007, 4, 99–108. [Google Scholar] [CrossRef][Green Version]
- Bolat, Y.; Bilgin, Ş.; Günlü, A.; Izci, L.; Koca, S.B.; Çetinkaya, S.; Koca, H.U. Chitin-Chitosan Yield of Freshwater Crab (Potamon potamios, Olivier 1804) Shell. Pak. Vet. J. 2010, 30, 227–231. [Google Scholar]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Kaya, M.; Bağriaçik, N.; Seyyar, O.; Baran, T. Comparison of chitin structures derived from three common wasp species (vespa crabro linnaeus, 1758, vespa orientalis linnaeus, 1771 and vespula germanica (FABRICIUS, 1793)). Arch. Insect Biochem. Physiol. 2015, 89, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Draczynski, Z. Honeybee corpses as an available source of chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 25009. [Google Scholar] [CrossRef]
- Majtán, J.; Bíliková, K.; Markovič, O.; Gróf, J.; Kogan, G.; Šimúth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Win, N.N.; Stevens, W.F. Shrimp chitin as substrate for fungal chitin deacetylase. Appl. Microbiol. Biotechnol. 2001, 57, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, J.; Niehues, A.; Cord-Landwehr, S.; Hoßbach, J.; David, L.; Delair, T.; Moerschbacher, B.M. Enzymatic Production and Enzymatic-Mass Spectrometric Fingerprinting Analysis of Chitosan Polymers with Different Nonrandom Patterns of Acetylation. J. Am. Chem. Soc. 2019, 141, 3137–3145. [Google Scholar] [CrossRef]
- Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylation by enzymatic means: Monitoring of deacetylation processes. Carbohydr. Res. 1995, 273, 235–242. [Google Scholar] [CrossRef]
- Cortizo, M.S.; Berghoff, C.F.; Alessandrini, J.L. Characterization of chitin from Illex argentinus squid pen. Carbohydr. Polym. 2008, 74, 10–15. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Ohara, A.; Aguilar, J.G.D.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Zivanovic, S.; Basurto, C.C.; Chi, S.; Davidson, P.M.; Weiss, J. Molecular weight of chitosan influences antimicrobial activity in oil-in-water emulsions. J. Food Prot. 2004, 67, 952–959. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Song, Y.S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.K.; Kang, S.H.; Kim, S.A.; Choi, C.; Jung, W.J. Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Entomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 1–14. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. White Biotechnology in Cosmetics. In Industrial Biorefineries and White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar]
- Morganti, P.; Muzzarelli, R.A.A.; Muzzarelli, C. Multifunctional use of innovative chitin nanofibrils for skin care. J. Appl. Cosmetol. 2006, 24, 105–114. [Google Scholar]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, pp. 15–31. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and Uf-purified annatto and saffron for the preparation of UV protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Carboxymethylated chitins and chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wu, H.; Xiao, L. Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Ahn, B.; Byun, H.G.; Kim, S.K. Carboxymethylations of chitosan and chitin inhibit MMP expression and ROS scavenging in human fibrosarcoma cells. Process Biochem. 2010, 45, 179–186. [Google Scholar] [CrossRef]
- Ngo, D.N.; Kim, M.M.; Kim, S.K. Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohydr. Polym. 2008, 74, 228–234. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Cosani, A.; Terbojevich, M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydr. Polym. 1999, 39, 361–367. [Google Scholar] [CrossRef]
- Sun, L.; Du, Y.; Yang, J.; Shi, X.; Li, J.; Wang, X.; Kennedy, J.F. Conversion of crystal structure of the chitin to facilitate preparation of a 6-carboxychitin with moisture absorption-retention abilities. Carbohydr. Polym. 2006, 66, 168–175. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Nanochitins and Nanochitosans, Paving the Way to Eco-Friendly and Energy-Saving Exploitation of Marine Resources. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 153–164. [Google Scholar]
- Gautier, S.; Xhauflaire-Uhoda, E.; Gonry, P.; Piérard, G.E. Chitin-glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008, 30, 459–469. [Google Scholar] [CrossRef]
- Cho, Y.W.; Cho, Y.N.; Chung, S.H.; Yoo, G.; Ko, S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Blasinska, A.; Drobnik, J. Effects of nonwoven mats of di-o-butyrylchitin and related polymers on the process of wound healing. Biomacromolecules 2008, 9, 776–782. [Google Scholar] [CrossRef]
- Chilarski, A.; Szosland, L.; Krucińska, I.; Kiekens, P.; Błasińska, A.; Schoukens, G.; Cisło, R.; Szumilewicz, J. Novel dressing materials accelerating wound healing made from dibutyrylchitin. Fibres Text. East. Eur. 2007, 15, 77–81. [Google Scholar]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Lin, A.Y.M.; McKittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Raabe, D.; Romano, P.; Sachs, C.; Fabritius, H.; Al-Sawalmih, A.; Yi, S.B.; Servos, G.; Hartwig, H.G. Microstructure and crystallographic texture of the chitin-protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A 2006, 421, 143–153. [Google Scholar] [CrossRef]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10-20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Mavi, S. Preparation of Chitin and Derivatives Thereof for Cosmetic and Therapeutic Use. International Patent PCT/IB2005/053576, 2 November 2005. [Google Scholar]
- Morganti, P. Spray-Dried Chitin Nanofibrils, Method for Production and Uses Thereof. International Patent PCT/IB2006/054403, 23 November 2006. [Google Scholar]
- Morganti, P. Chitin Nanofibrils in Skin Treatment. J. Appl. Cosmetol. 2009, 25, 251–270. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef]
- Jain, T.; Kumar, H.; Dutta, P.K. D-glucosamine and N-acetyl d-glucosamine: Their potential use as regenerative medicine. In Chitin and Chitosan for Regenerative Medicine; Springers: New Delhi, India, 2016; pp. 279–295. [Google Scholar]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Morganti, G.; Nunziata, M.L.; Gao, X.; Tishenko, G.; Yudin, V. Chitin Nanofibrils: A Natural Multifunctional Polymer. Nanobiotechnology 2014, 1, 1–31. [Google Scholar]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Farmer, T.; McPhail, S.; Reichling, T.; Tiesman, J.P.; Juhlin, K.D.; Hurley, G.J.; Robinson, M.K. Genomic expression changes induced by topical N-acetyl glucosamine in skin equivalent cultures in vitro. J. Cosmet. Dermatol. 2007, 6, 232–238. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Morganti, P.; Li, Y.H. Innovation in cosmetic and medical science. The role of chitin nanofibrils composites. J. Appl. Cosmetol. 2015, 33, 9–24. [Google Scholar]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y.; et al. Protective effect of chitin urocanate nanofibers against ultraviolet radiation. Mar. Drugs 2015, 13, 7463–7475. [Google Scholar] [CrossRef]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Cardillo, A.; Del Ciotto, P.; Morganti, G.; Gazzaniga, G. Anti-dandruff and anti-oily efficacy of hair formulations with a repairing and restructuring activity. The positive influence of the Zn-chitin nanofibrils complexes. J. Appl. Cosmetol. 2012, 30, 149–159. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, M.; Cardillo, M.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. Activity of chitin nanofibrils block-copolymers entrapping Zn/AI/SA/allantoin on seborrheic dermatitis. A randomized double-blind placebo controlled study. J. Appl. Cosmetol. 2014, 32, 3–19. [Google Scholar]
- Morganti, P.; Palombo, M.; Palombo, P.; Fabrizi, G.; Cardillo, A.; Carezzi, F.; Morganti, G.; Ruocco, E.; Dzierzgowski, S. Cosmetic Science in Skin Aging: Achieving the Efficacy by the Chitin Nano-Structured Crystallites. SÖFW J. 2010, 136, 14–24. [Google Scholar]
- Morganti, P.; Morganti, G.; Fabrizi, G.; Cardillo, A. A new sun to rejuvenate the skin. J. Appl. Cosmetol. 2008, 26, 159–168. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Ruocco, E.; Cardillo, A.; Morganti, G. Chitin-nanofibrils: A new active cosmetic carrier. J. Appl. Cosmetol. 2008, 26, 113–128. [Google Scholar]
- Morganti, P.; Palombo, P.; Palombo, M.; Fabrizi, G.; Cardillo, A.; Svolacchia, F.; Guevara, L.; Mezzana, P. A phosphatidylcholine hyaluronic acid chitin-nanofibrils complex for a fast skin remodeling and a rejuvenating look. Clin. Cosmet. Investig. Dermatol. 2012, 5, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Guarneri, F.; Cardillo, A.; Morganti, G. New chitin complexes and their anti-aging activity from inside out. J. Nutr. Health Aging 2012, 16, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Svolacchia, F.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. New insights on anti-aging activity of chitin nanofibril-hyaluronan block copolymers entrapping active ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Carezzi, F.; Guarneri, F.; Yeo, Y.J. Skin lightening efficacy of new formulations enhanced by chitin nanoparticles delivery system. Note I. J. Appl. Cosmetol. 2014, 32, 57–71. [Google Scholar]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.L.; Morganti, G.; Cardillo, M.; Chianese, A. Green nanotechnology serving the bioeconomy: Natural beauty masks to save the environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Ciotto, P.; Del Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-inflammatory, immunomodulatory, and tissue repair activity on human keratinocytes by green innovative nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Aging Skin. Biomedicines 2019, 7, 51. [Google Scholar] [CrossRef]
- Morganti, P.; Yudin, V.E.; Morganti, G.; Colte, M.B. Trends in surgical and beauty masks for a cleaner environment. Cosmetics 2020, 7, 68. [Google Scholar] [CrossRef]
- Donnarumma, G.; Fusco, A.; Morganti, P.; Palombo, M. Advanced medications made by green nanocomposites. Int. J. Res. Pharm. Nano Sci. 2016, 5, 261–270. [Google Scholar]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Maevskaia, E.N.; Shabunin, A.S.; Dresvyanina, E.N.; Dobrovol’skaya, I.P.; Yudin, V.E.; Paneyah, M.B.; Fediuk, A.M.; Sushchinskii, P.L.; Smirnov, G.P.; Zinoviev, E.V.; et al. Influence of the Introduced Chitin Nanofibrils on Biomedical Properties of Chitosan-Based Materials. Nanomaterials 2020, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.V.; Lebedeva, I.O.; Shabunin, A.S.; Stoyanovsky, R.G.; Asadulaev, M.S.; Ivan, E.M.; Morganti, P. In vivo study of the nanofiber-based composite wound dressing intended for treatment of deep skin wounds. J. Appl. Cosmetol. 2016, 34, 1–8. [Google Scholar]
- Mattioli-Belmonte, M.; Zizzi, A.; Lucarini, G.; Giantomassi, F.; Biagini, G.; Tucci, G.; Orlando, F.; Provinciali, M.; Carezzi, F.; Morganti, P. Chitin Nanofibrils Linked to Chitosan Glycolate as Spray, Gel, and Gauze Preparations for Wound Repair. J. Bioact. Compat. Polym. 2007, 22, 525–538. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Mattioli Belmonte, M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).