Anti-Wrinkle Efficacy of Neuropeptide Substance P-Based Hydrogel in Human Volunteers
Abstract
1. Introduction
2. Methods
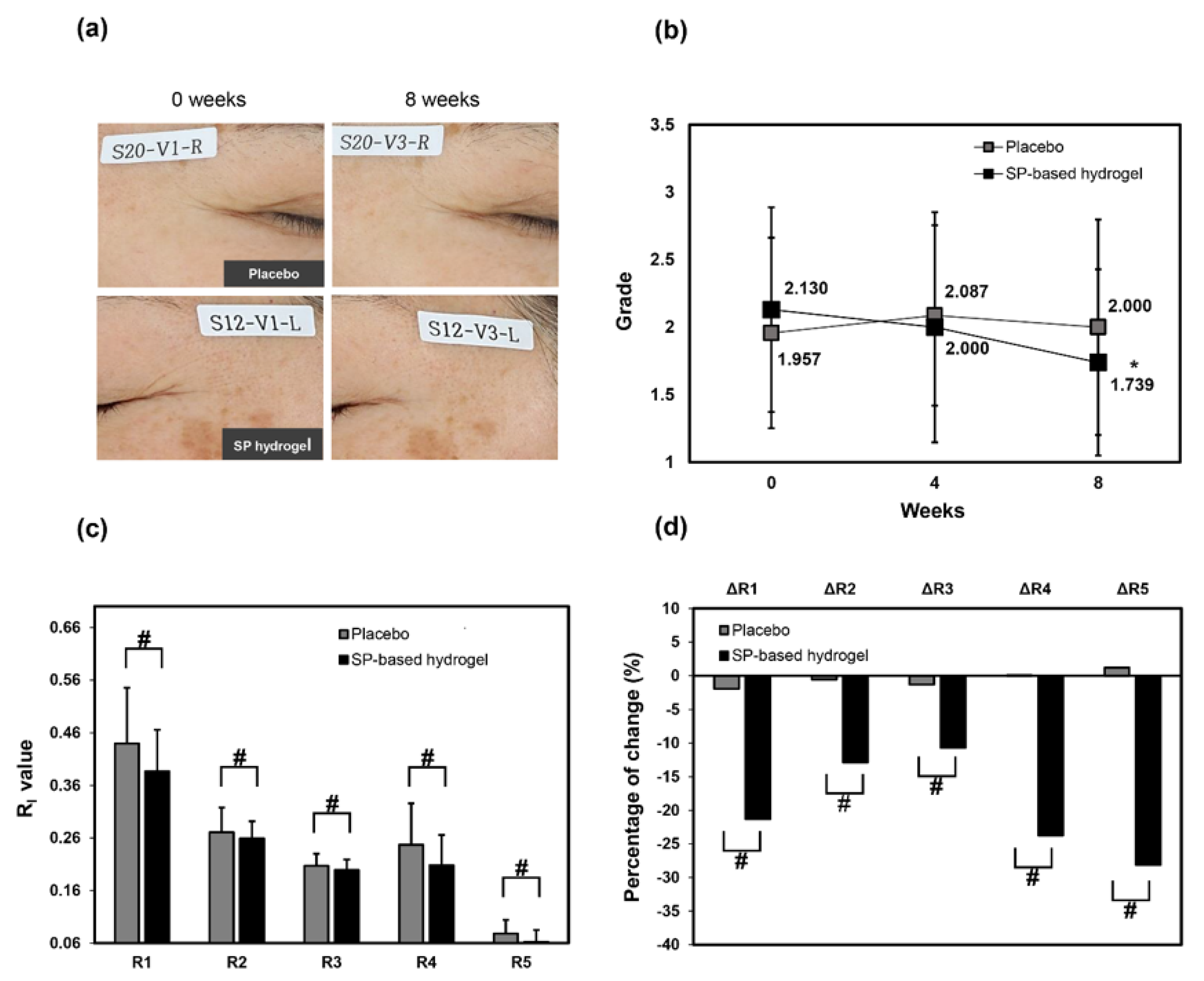
3. Results
Author Contributions
Funding
Conflicts of Interest
References
- Lim, J.E.; Chung, E.; Son, Y. A neuropeptide, substance P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 2017, 7, 9417. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Chang, S.S.; Lee, J. Anti-aging potential of substance P-based hydrogel for human skin longevity. Int. J. Mol. Sci. 2019, 20, 4453. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Jang, J.H.; Jang, S.S.; Lee, J. A novel substance P-based hydrogel for increased wound healing efficiency. Molecules 2018, 23, 2215. [Google Scholar] [CrossRef] [PubMed]
- Lam, X.M.; Yang, J.Y.; Celand, J.L. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER 2. J. Pharm. Sci. 1997, 86, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, B.A. Polysorbate 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Agarkhed, M.; O’Dell, C.; Hsieh, M.C.; Zhang, J.; Goldstein, J.; Srivastava, A. Effect of polysorbate 80 concentration on thermal and photostability of a monoclonal antibody. AAPS PharmSciTech 2013, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; McAuley, A.; Schike, K.F.; McGuire, J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug. Deliv. Rev. 2011, 63, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussein, A.; Gieseler, H. Investigation of the stabilizing effects of hydroxyethyl cellulose on LDH during freeze drying and freeze thawing cycles. Pharm. Dev. Technol. 2015, 20, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J.; Hardas, B.; Kaur, M.; Goertelmeyer, R.; Jones, D.; Rzany, B.; Cohen, J.; Kerscher, M.; Flynn, T.C.; et al. A validated grading scale for crow’s feet. Dermatol. Surg. 2008, 34, S173–S178. [Google Scholar] [CrossRef]
- Kim, H.; Kim, N.; Jung, S.; Mun, J.; Kim, J.; Kim, B.; Lee, J.; Ryoo, H.; Jung, H. Improvement in skin wrinkles from the use of photostable retinyl retinoate: A randomized controlled trial. Br. J. Dermatol. 2010, 162, 497–502. [Google Scholar] [CrossRef]
| Component | Placebo (% w/w) | SP Hydrogel (% w/w) |
|---|---|---|
| Water | 75.5–80.5 | 75.5–80.5 |
| Glycerine | 5–6 | 5–6 |
| Dipropylene glycol | 8–9 | 8–9 |
| 1,2-Hexanediol | 2–3 | 2–3 |
| Sodium hyaluronate | 0.2–0.5 | 0.2–0.5 |
| Tromethamine | 0.2–0.5 | 0.2–0.5 |
| Carbomer | 0.2–0.5 | 0.2–0.5 |
| SP-based hydrogel (SP 20 µg/mL) | - | 5 |
| Viscosity (25 °C) | 2000–2500 cPs | 2000–2500 cPs |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.J.; Lee, S.B.; Chang, S.S.; Lee, J. Anti-Wrinkle Efficacy of Neuropeptide Substance P-Based Hydrogel in Human Volunteers. Cosmetics 2020, 7, 37. https://doi.org/10.3390/cosmetics7020037
Kim DJ, Lee SB, Chang SS, Lee J. Anti-Wrinkle Efficacy of Neuropeptide Substance P-Based Hydrogel in Human Volunteers. Cosmetics. 2020; 7(2):37. https://doi.org/10.3390/cosmetics7020037
Chicago/Turabian StyleKim, Da Jung, Seul Bi Lee, Song Sun Chang, and Jungsun Lee. 2020. "Anti-Wrinkle Efficacy of Neuropeptide Substance P-Based Hydrogel in Human Volunteers" Cosmetics 7, no. 2: 37. https://doi.org/10.3390/cosmetics7020037
APA StyleKim, D. J., Lee, S. B., Chang, S. S., & Lee, J. (2020). Anti-Wrinkle Efficacy of Neuropeptide Substance P-Based Hydrogel in Human Volunteers. Cosmetics, 7(2), 37. https://doi.org/10.3390/cosmetics7020037




